Abstract
Background
Medication and psychotherapy treatments for posttraumatic stress disorder (PTSD) provide insufficient benefit for many patients. Substantial preclinical and clinical data indicate abnormalities in the hypothalamic-pituitary-adrenal axis, including signaling by corticotropin-releasing factor, in the pathophysiology of PTSD.
Methods
We conducted a double-blind, placebo-controlled, randomized, fixed-dose clinical trial evaluating the efficacy of GSK561679, a corticotropin-releasing factor receptor type 1 (CRF1) antagonist in adult women with PTSD. The trial randomized 128 participants, of whom 96 completed the six-week treatment period.
Results
In both the intent-to-treat and completer samples, GSK561679 failed to show superiority over placebo on the primary outcome of change in Clinician Administered PTSD Scale total score. Adverse event frequencies did not significantly differ between GSK561679- and placebo-treated subjects. Exploration of the CRF1 SNP rs110402 found response to GSK561679 and placebo did not significantly differ by genotype alone. However, subjects who had experienced a moderate or severe history of childhood abuse and who were also GG homozygotes for rs110402 showed significant improvement after treatment with GSK561679 (n=6) but not with placebo (n=7) on the PTSD Symptom Scale, Self-Report.
Conclusions
The results of this trial, the first evaluating a CRF1 antagonist for the treatment of PTSD, combined with other negative trials of CRF1 antagonists for major depressive disorder, generalized anxiety disorder, and Dunlop social anxiety disorder, suggest that CRF1 antagonists lack efficacy as monotherapy agents for these conditions.
ClinicialTrials.gov
Evaluation of GSK561679 in Women With Post-Traumatic Stress Disorder; https://clinicaltrials.gov/ct2/show/NCT01018992?term=NCT01018992&rank=1; NCT01018992
Keywords: clinical trial, adrenocorticotropic hormone, women, pharmacogenetics, dexamethasone, child abuse
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a common psychiatric syndrome afflicting individuals who have been exposed to traumatic events (1). The symptomatology of PTSD is multiplex, encompassing components of intrusive re-experiencing of the traumatic event, avoidance of reminders of the event, negative or reduced range of mood, and hyperarousal and excessive reactivity to the environment. The pathophysiology of PTSD is broad, including abnormalities in fear processing (2), sympathetic nervous system (SNS) hyperactivity (3), and disturbed hypothalamic-pituitary-adrenal (HPA) axis functioning (4). Excessive fear processing is targeted by two established forms of PTSD treatment: exposure-based psychotherapies and selective serotonin reuptake inhibitors (SSRIs). Excessive SNS activity, as measured by systolic blood pressure, is targeted by prazosin and perhaps by atypical antipsychotics, which have some efficacy for certain PTSD symptoms (5). However, response rates to existing interventions are less than 60%, with only 20–30% of patients achieving remission with medication (6), indicating the need for additional therapeutic options. A wealth of studies implicating HPA axis disruption in PTSD pathophysiology suggests that directly targeting this system may prove to be a fruitful approach (7).
Activation of the HPA axis in response to stress begins with the release of corticotropin-releasing factor (CRF)from the hypothalamus. CRF is a 41-amino acid peptide neurotransmitter that mediates the stress response via its effects on neuroendocrine, immune, autonomic, and behavioral systems (8). CRF binding to CRF type 1 receptors (CRF1) in the pituitary stimulates release of adrenocorticotropin (ACTH), which enters the systemic circulation and induces release of cortisol from the adrenal cortex. In healthy subjects, the acute actions of cortisol produce negative feedback to the HPA axis via glucocorticoid receptors in the pituitary and hypothalamus. Abnormalities of the HPA axis in patients with PTSD include low circulating levels of ACTH and cortisol, and hyper-suppression of these hormones after low-dose dexamethasone administration (4). Elevated CRF concentrations are present in the cerebrospinal fluid of PTSD patients (9–11), though mildly ill patients may not show this abnormality (12). Outside the hypothalamus and anterior pituitary, CRF1 are expressed widely in the cortex and cerebellum, hippocampus, amygdala, and bed nucleus of the stria terminalis (BNST) (13). Activation of CRF receptor binding in the amygdala induces fear responses (14), and administration of CRF in animal models produces PTSD-relevant anxiety behaviors, including heightened acoustic startle response, sleep disturbance, and increased conditioned fear response (15). Early life stress in animal models produces hyperactivity of CRF neurons and chronic activation of limbic brain regions (16,17).
Several CRF1 antagonists studied in animal models have demonstrated potential therapeutic value for stress-related disorders (30). An early human trial suggested efficacy of CRF1 antagonism for major depression (31) and another CRF1 antagonist produced anxiolytic effects in healthy adults given 7.5% CO2 (32). However, larger trials examining several CRF1 antagonists in clinical populations have not found efficacy for the treatment of major depression, generalized anxiety disorder, or social anxiety disorder (29).
GSK561679 is an orally active, selective CRF1 antagonist that demonstrates anxiolytic effects in animal models (33). The investigator brochure for GSK561679 reports that in healthy adults, GSK561679 dose-dependently suppressed ACTH response to stress in the Trier Social Stress Test and after intravenous administration of CRF, but only inconsistently reduced cortisol responses in these challenge tests. In social anxiety disorder patients, a single 400 mg dose of GSK561679 reduced reactivity in the amygdala after exposure to facial expressions, similar to a single dose of alprazolam. The drug achieves good brain penetration in rodents and is not a substrate for p-glycoprotein transport. The primary route of metabolism is through cytochrome P450 3A4. Preclinical studies found GSK561679 caused changes to the testes and seminiferous epithelium in male animals, thereby limiting human clinical trials to female participants.
We aimed to determine whether GSK561679 was efficacious for PTSD. Secondary aims were to evaluate the tolerability of GSK561679 and its effects on depressive symptoms. We also examined the potential moderating impact of HPA axis-related genes implicated in the development of PTSD. The clinical trial reported here is a component of the National Institute of Mental Health (NIMH) National Cooperative Drug Discovery/Development Groups (NCDDG) program, which aims to facilitate partnerships between academic clinical and preclinical researchers and industry to support the discovery of drug development tools and apply ‘first in human, first in patient testing.’
METHODS AND MATERIALS
Study Overview
A detailed description of the study rationale, methods, and design was previously published and is summarized here (34). The study design was a randomized, double-blind, placebo-controlled, parallel-group clinical trial of GSK561679, which enrolled patients from January, 2010 to June, 2014. After a screening phase lasting 1–4 weeks, patients entered a 6-week double blind treatment phase, followed by a one-month off-drug follow-up phase to monitor safety and durability of any clinical changes. Four academic sites conducted the study: Emory University, Icahn School of Medicine at Mount Sinai, Baylor College of Medicine, and the University of California San Francisco. Approval to conduct the study was obtained from the institutional review board of each university and its affiliated Veterans Affairs Hospitals, if applicable. The study was conducted in accordance with the Helsinki Declaration of 1975 and its amendments and is listed as NCT01018992 at Clinicaltrials.gov.
Participants
All participants provided written informed consent prior to beginning study procedures. Recruitment was conducted by advertising and clinic referral. Eligible participants were women aged 18–65 years who met DSM-IV TR criteria for PTSD, chronic, determined using the Structured Clinical Interview for DSM-IV (SCID) (35) and confirmed through a clinical interview with a study psychiatrist. For patients with multiple DSM-IV-qualifying traumas, we defined the “index” trauma as the trauma currently causing the greatest distress or impairment to the patient, identified from Parts 1 and 2 of the Posttraumatic Diagnostic Scale (PDS) (36). PTSD had to be at least moderately severe at the screening and baseline visits, defined as Clinician Administered PTSD Scale for DSM-IV (CAPS) (37) past-month and past-week total scores ≥50. Important exclusion criteria included: any current or past diagnosis of schizophrenia or other psychotic disorder, bipolar disorder, or obsessive compulsive disorder; current substance abuse or dependence; use of a psychotropic agent, other than a non-benzodiazepine hypnotic; use of a systemic steroid medication; significant uncontrolled medical conditions, or current clinically significant suicidal or homicidal ideation; current participation in a structured psychotherapy targeting PTSD symptoms; and any current or planned litigation regarding the traumatic event.
Randomization
Randomization to GSK 561679 or placebo was 1:1 with permuted blocks generated separately for each site by a statistician who was not involved in the analysis of the data (see 34). The investigational pharmacist assigned the eligible patient to the treatment indicated by the randomization list at the baseline visit.
Study Medication
The selected dose of 350 mg/d of GSK561679 was based on the tolerability and biological activity observed during Phase 1 testing. Study medication was dispensed in two bottles containing 100 mg or 50 mg white tablets of GSK561679 or matching placebo. Patients took three 100 mg tabs and one 50 mg tab each evening between 1800 and 2000, and recorded the time in a dosing diary.
Study Visits and Assessments
The PDS and CAPS were administered at screening to assess trauma severity. Patients completed the PTSD Symptom Scale, Self-report (PSS-SR) (38), the Childhood Trauma Questionnaire (CTQ) (39), the Montgomery-Asberg Depression Rating Scale (MADRS) (40,41), the Quick Inventory of Depressive Symptomatology, Self-report (QIDS-SR) (42), the Clinical Global Impression of Severity (CGI-S) (43), the Sheehan Disability Scale (SDS) (44), and the clinician-administered version of the Columbia Suicide Severity Rating Scale (C-SSRS) (45). An electrocardiogram, laboratory testing, urine drug screen, medical history and a physical exam were conducted to ensure medical appropriateness for the study. Adverse events were captured by open-ended questions and via the Patient Rated Inventory of Side Effects (PRISE) (46) at each post-screening visit.
On the day prior to the baseline (randomization) visit patients underwent phlebotomy for measurement of ACTH and cortisol, and baseline laboratory tests, and took 0.5 mg of dexamethasone at 23:00 that evening for the low-dose dexamethasone suppression test. Patients returned the next morning to repeat phlebotomy for post-dexamethasone ACTH and cortisol concentrations. Ratings visits occurred at baseline and weeks 1, 2, 4, and 6 post-randomization, with administration of past-week CAPS, MADRS and the self-report symptom measures. Neuroendocrinological testing was repeated during the fifth week post-randomization. Plasma samples for GSK561679 concentrations were collected at weeks 1, 2, 4, and 6. Methods for DNA genotyping are presented in the online Supplement.
The primary outcome was change in past-week CAPS total score from baseline to week 6, assessed at weeks 1, 2, 4 and 6. CAPS raters were initially trained through use of a scoring guide and watching a training video interview. Interrater reliability was assessed annually via independent scoring of standardized videotaped CAPS interviews. Raters whose scores were >4 points from the median for each interview underwent additional training until reliability was achieved.
Statistical Analyses
All analyses used R v.3.2 (https://www.r-project.org). Generalized linear models evaluated the effects of treatment on univariate outcomes; multilevel models examined treatment effects on longitudinal outcomes. Analyses evaluated treatment effects with and without adjustment for site effects. Since inclusion of site as a covariate failed to alter any conclusion derived from models without site, the results are based on the more parsimonious unadjusted models. Primary analyses utilized intention-to-treat principles with multilevel models maximizing the use of all available data using restricted maximum likelihood estimation, and dichotomous outcomes imputed as negative/non-responsive to treatment.
CRF1 SNP rs110402 was the main focus of the genetic analysis. Direct genotypes were taken from the HumanOmniExpress-24 array (rs110402 MAF =0.401, HWE test p-value = 0.52), with patients categorized according to rs110402 A allele carrier status (GG=33 carriers and 53 A-allele carriers, of which 38 patients had the AG genotype and 15 were homozygous for the A allele, Table S2). To assess A-allele carrier main effects as well as interaction of the carrier status with childhood abuse on change in psychiatric symptoms, we performed linear regression models adjusted for age, baseline symptom severity and ancestry PC (Figure S8), with the percent change in CAPS score, PSS score and MADRS score as outcomes. Individuals were categorized as having experienced either no or only mild abuse versus having experienced at least one type of moderate to severe abuse (56=abused, 30=non-abused) as previously described using the CTQ (47). To conserve power, we refrained from testing three-way interactions of SNP x child abuse x treatment on symptom changes, but analyzed two-way interactions of SNP x child abuse on outcome, stratified by treatment status. Significance was considered at p<0.05 and due to limited power, all genetic analyses are considered exploratory only, so no correction for multiple testing was applied.
RESULTS
The CONSORT diagram (Figure S1) depicts the overall participant flow for the trial, with n=266 enrolled, n=128 participants randomized, and n=96 completing treatment. The mean age of the sample was 40.5±12.1 years; only three participants identified combat as their index trauma. The baseline demographic and clinical characteristics of the sample are presented in Table 1.
Table 1.
Demographic and clinical variables at baseline
| Variable | Placebo n=65 n (%) | GSK561679 n=63 n (%) |
|---|---|---|
| Race | ||
| White/Caucasian | 32 (49) | 40 (64) |
| African American | 28 (43) | 18 (29) |
| Other | 5 (8) | 5 (8) |
| Hispanic | 5 (8) | 8 (13) |
| Current Major Depression | 43 (66) | 41 (65) |
| Education (n=125) | ||
| <High School | 4 (6) | 7 (11) |
| High School degree/Some college | 29 (45) | 24 (38) |
| College degree | 15 (23) | 19 (30) |
| Graduate degree | 16 (25) | 11 (18) |
| Current Smoker | 17 (26) | 12 (19) |
| Time since primary trauma (n=125) | ||
| ≤6 months | 5 (8) | 6 (10) |
| 6 months – 3 years | 15 (24) | 11 (18) |
| 3–5 years | 11 (18) | 5 (8) |
| ≥5 years | 32 (51) | 39 (64) |
| Mean (SD) | Mean (SD) | |
|
|
||
| Age (yrs) | 40.4 (12.3) | 40.6 (11.8) |
| Traumatic events, lifetime | 3.7 (2.2) | 3.5 (1.6) |
| CAPS Past Month Total | 79.8 (15.6) | 82.0 (12.5) |
| CAPS Past Week Total | 74.8 (17.6) | 77.5 (14.3) |
| PSS-SR Total | 30.0 (9.3) | 31.1 (7.1) |
| MADRS | 25.1 (8.3) | 26.5 (7.0) |
| QIDS-SR | 13.6 (4.5) | 13.3 (4.1) |
| CTQ Total | 75.9 (23.9) | 79.3 (27.2) |
| SDS | 16.3 (7.1) | 15.5 (7.1) |
| CGI-S | 4.7 (0.7) | 4.7 (0.7) |
CAPS: Clinician-administered PTSD Scale; CGI-S: Clinician Global Impression-Severity; CTQ: Childhood Trauma Questionnaire; MADRS: Montgomery Asberg Depression Rating Scale; PSS-SR: PTSD Symptom Scale – Self-report; QIDS-SR: Quick Inventory of Depressive Symptomatology-Self-report; SDS: Sheehan Disability Scale;
Retention and Treatment Compliance
Kaplan-Meier survival curves failed to demonstrate differential attrition as a function of treatment group χ2(1)=0.2, p=0.647. Among individuals (n=91) who completed treatment and who demonstrated compliance with the medication regimen (verified via serum levels at the week 6 or early termination visit only in the GSK561679 condition) retention did not differ as a function of treatment group (Placebo n=49, GSK561679 n=42; χ2(1)=1.183, p=0.278). The mean week 6 concentration of GSK561679 among compliant patients receiving the active drug was 923±603 ng/ml.
CAPS Outcomes
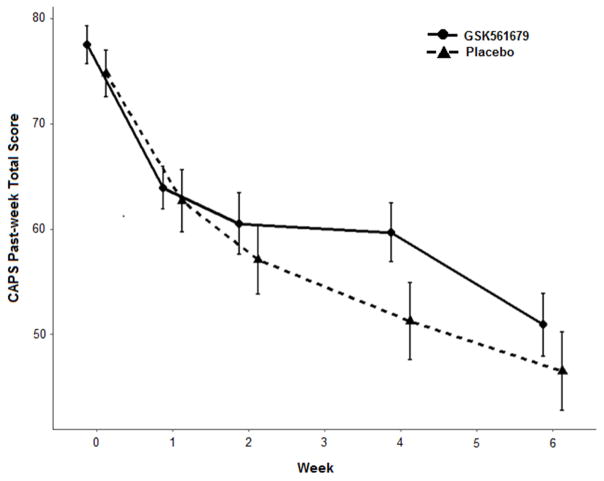
Evaluation of the CAPS past week total score as a function of time, treatment, and their interaction found no differential change over time between GSK561679 and placebo (t(435) = 0.713, p≤0.477) (Figure 1). The three CAPS-derived symptom clusters of re-experiencing, avoidance, and hyperarousal, also found no differential change over time for GSK561679 versus placebo (all p>.05).
Figure 1. Change in CAPS past-week total scores by treatment group.
S.E. bars represent ± 1 S.E. CAPS, Clinician Administered PTSD Scale
Response rates did not differ between treatments, whether defined as a 50% decrease from baseline (Placebo: 18 (27.7%), GSK561679: 14 (22.2%); χ2(1)=0.543, p=.305) or 30% decrease (Placebo: 34 (52.3%), GSK561679: 28 (44.4%); (χ2(1)=0.384, p=.238).
MADRS Outcomes
Longitudinal modeling of MADRS scores found no differential change over time between the treatments (t(425) = −0.693, p≤0.489) (Figure S2).
Completers and Compliers
Reanalysis of symptom outcomes (i.e., CAPS and MADRS) using all completers, as well as the completers and compliers sample, failed to substantively alter any conclusions. Among the completers and compliers sample who received GSK561679, the mean week 6 serum concentrations between responders (≥30% improvement from baseline) and non-responders did not differ (Responders: 852±427 ng/ml; Non-responders: 706± 419 ng/ml; F(1,35) = 1.1, p≤0.301).
Secondary Outcomes
Multilevel modeling evaluated several secondary outcomes as a function of time, treatment and their interaction. Change in PSS-SR Total scores over time did not reveal a treatment-by-time interaction (t(436)=−0.022, p=0.983). Similar null results were found for the re-experiencing (t(438)=−0.016, p=0.987), hyperarousal (t(437)=0.300, p=0.764), and avoidance (t(436)= −0.263, p=0.793) subscales of the PSS-SR. The QIDS-SR (t(427)=0.748, p=0.455), CGI-S (t(411)=1.126, p=0.207), and SDS (t(188)= −0.440, p=0.660) also failed to show differential change for GSK561679 over placebo.
Treatment Outcome Moderators
We conducted post-hoc exploratory evaluation of potential clinical moderators to account for potential heterogeneity in treatment response. We found no significant moderation of the results by patient age, time since traumatic event, comorbid MDD, CAPS score at screening, or CTQ total score.
Genotype by childhood abuse interaction on symptom change stratified by treatment
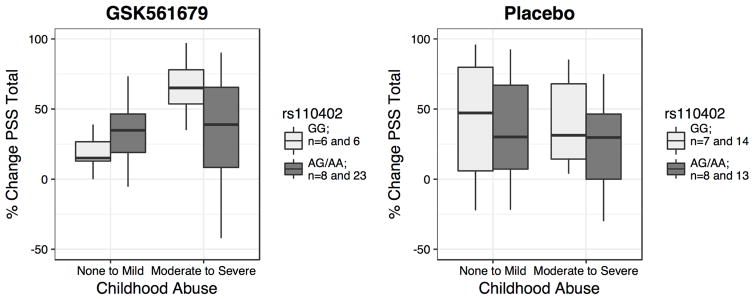
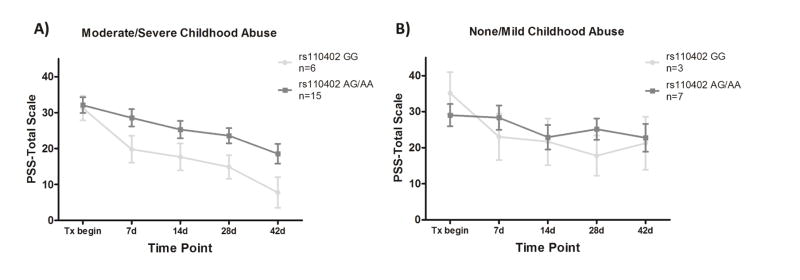
We first tested the interaction effect of SNP rs110402 carrier status and childhood abuse on the percent change of CAPS score, as well as PSS-SR score, separately in GSK561679-treated and placebo-treated patients. rs110402 carrier status showed no significant main effect on the percent change of PTSD symptoms from pre- to post-treatment (p>0.05), or on CAPS score change over treatment (p>0.05), in either treatment group. However, childhood abuse as well as the interaction of genotype by child abuse significantly predicted PSS-SR percent change in the GSK561679 group (Abuse: s=1.534, p=0.021; SNP x abuse: s=−1.904, p=0.043) but not in the placebo group (Abuse: s=−0.629, p=0.53; s=0.421, SNP x abuse p=0.68). More specifically, GG genotype carriers who had experienced childhood abuse showed the highest PSS-SR percent change after GSK561679 treatment (Figure 2). Plotting PSS-SR scores by group over time showed that among the patients with childhood abuse, GG homozygotes who received GSK561679 had consistently lower symptom scores over all 5 post-baseline time-points (Figure 3A and 3B).
Figure 2. Significant interaction effect of rs110402 and childhood abuse on percent change PSS-SR score.
The boxplots describe the mean percent change of PSS total score in abused and non-abused patients treated with the GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Higher PSS percent change corresponds to improvement (reduction) in PTSD symptoms from baseline to endpoint. rs110402 A carrier status by childhood abuse exposure showed a significant interaction effect on PSS score percent change over treatment in subjects treated with GSK561679 (s=-1.904, p=0.043) but not in subjects treated with placebo (s=0.421, p=0.68). rs110402 GG carriers exposed to child abuse displayed the highest percent change of PSS symptoms following GSK561679 treatment. PSS-SR, PTSD Symptom Scale, Self-Report
Figure 3. PSS-SR score change over time among patients treated with GSK561679 by abuse level.
Mean (± SEM) PSS-SR total score at 5 time points during treatment with GSK561679 in: A) patients with a history of childhood abuse and, B) patients with mild/no childhood abuse, stratified by rs110402 carrier status (GG in light grey, AA/AG in dark grey). When treated with GSK561679, the GG genotype carriers that experienced childhood abuse showed consistently lower symptom scores over all 5 time points compared to abused AG/AA carriers while this genotype effect is not observed in the non-abused group. PSS-SR, PTSD Symptom Scale, Self-Report
Interestingly, the interaction of genotype by child abuse on PSS total score was most pronounced for the two PSS-SR subscales of re-experiencing and arousal. Significant interaction effects for the re-experiencing (GSK561679: s=−2.472; p= 0.006; placebo: s=0.075; p=0.92) and arousal subscales (GSK561679: s=2.034; p= 0.019; placebo: s=0.054; p=0.94) emerged in subjects treated with GSK561679, but not for the PSS-SR avoidance subscale (GSK561679: s=−0.945; p= 0.36; placebo: s=0.565 p=0.44) (Figures S3, S4, and S5).
Genotype by childhood abuse interaction on depressive symptoms stratified by treatment
Due to the implications of rs110402 for depression after childhood abuse we also tested the interaction effect of rs110402 and child abuse on the percent change in MADRS scores. There was no main effect of child abuse, nor was there an interaction effect of genotype by abuse, in either of the treatment groups (p>0.05 for all).
Analysis of treatment or genotype effect on blood cortisol and ACTH levels and interaction effects of treatment x cortisol/ACTH levels on psychiatric symptom change
We tested for main effects of GSK561679 as well as rs110402 A-allele carrier status on change in cortisol concentrations over treatment time. There was no significant effect of GSK561679 compared to placebo on morning basal plasma cortisol concentrations after 5 weeks of treatment (p>0.05) (Figure S6). There was also no significant effect on cortisol suppression following the dexamethasone suppression test (DST) at baseline, nor a significant difference in cortisol suppression at baseline compared to week 5 (p>0.05) (Table 2, Figure S7). Genotype analyses of rs110402 carrier status showed similar null results (p>0.05 for all). Neither the interaction of treatment by morning cortisol levels at baseline, nor treatment by change of morning cortisol levels from pre to 5 weeks, were correlated with pre to post percent change of psychiatric symptoms (CAPS, PSS, MADRS). Further there was no interaction effect of treatment with changes in the dexamethasone suppression test from pre to 5 weeks on percent change of psychiatric symptoms (p>0.05 for all). For ACTH analyses, we used the same models replacing cortisol by plasma ACTH concentrations. No significant main or interaction effects were observed (p>0.05 for all).
Table 2.
Morning plasma cortisol and ACTH concentrations before and after five weeks of treatment
| Pre-Treatment | Week 5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Dex | Post-Dex | Pre-Dex | Post-Dex | |||||
| Cortisol (μg/dl) | ||||||||
| Placebo | 10.3±3.8 | p=0.65 | 2.2±2.9 | p=0.50 | 10.7±4.2 | p=0.77 | 2.9±2.9 | p=0.16 |
| GSK561679 | 10.7±3.4 | 1.8±2.0 | 10.4±3.1 | 1.8±3.0 | ||||
|
| ||||||||
| ACTH (pg/ml) | ||||||||
| Placebo | 21.9±15.3 | p=0.18 | 11.6±7.2 | p=0.39 | 20.0±12.8 | p=0.041 | 8.7±4.8 | p=0.32 |
| GSK561679 | 26.5±14.7 | 10.2±6.4 | 27.5±14.4 | 11.0±11.1 | ||||
ACTH: Adrenocorticotropin; Dex: dexamethasone
Safety and Tolerability
One serious adverse event occurred in each treatment arm, and both were considered unrelated to the study medication. Evaluation of suicidal ideation and behavior using the C-SSRS did not find differential levels of either ideation or behavior. No adverse events occurred significantly more frequently in the GSK561679 than placebo arm (Tables S3, S4).
DISCUSSION
This clinical trial found that a potent CRF1 antagonist provided no benefit for reduction of PTSD symptoms beyond those achieved with placebo. The failure of the GSK561679 to demonstrate efficacy is unlikely due to aspects of symptom severity or placebo responsiveness of the sample. The baseline CAPS total score of 76 was very similar to baseline scores in positive trials of SSRIs and venlafaxine, and the mean change in CAPS score of 28 points in the placebo arm was similar to the degree of placebo improvement in those trials, which ranged from 23.2–26.2 points (48–52).
One explanation for the trial’s failure to show benefit of GSK561679 may be found in our analysis of the rs110402 SNP of the CRF1 gene. Among patients with a history of childhood abuse, GG homozygotes at this locus, in contrast to A-allele carriers, demonstrated significant improvements in self-reported hyperarousal and re-experiencing symptoms with GSK561679 treatment, which were absent in the placebo-treated patients. Thus, the responsiveness of patients to CRF receptor antagonism may depend on their genetic endowment and environmental exposures, which could be linked to an increased activity of the CRF system in these individuals. However, this abuse by allele status analysis was exploratory, the number of patients in each arm was relatively small, and the effect was observed on the PSS-SR, not the primary CAPS scale, so this finding requires replication in larger samples before definitive conclusions about this association can be made.
Some data do not support the model that disruptions in CRF signaling are associated with anxiety disorders, raising the possibility that the negative result is a consequence of poor target selection. Adult wild-type and CRF knock-out mice demonstrate similar behavioral responses to stressors, even though CRF knock-outs fail to activate the HPA axis in response to stressors (53). In the central nucleus of the amygdala, CRF1 activation reduces glutamate-mediated excitatory postsynaptic currents (EPSCs) and increases EPSCs in the lateral septum (54). Conflicting data exist on whether chronic antidepressant administration impacts basal CRF mRNA expression in the paraventricular nucleus (55–57), or diminishes stress-induced CRF gene expression in the paraventricular nucleus (57). Other data suggest antidepressants reduce CRF1 mRNA expression in the amygdala (56), but this finding has not been replicated (57). Finally, a small study of PTSD patients who achieved remission with paroxetine found no significant pre- to post-treatment change in cerebrospinal fluid (CSF) CRF concentrations (12). In combat veterans with PTSD, observation of trauma reminder stimuli resulted in unexpected reductions in CSF CRF concentrations (58).
Another alternative is that the negative results of this study may be due to the differential anxiolytic and anxiogenic effects of CRF1 activation by brain region. In the forebrain, CRF1 increases anxiety by amplifying activity in the hippocampal formation via increased firing frequency of glutamatergic inputs. Stress increases CRF concentrations in the locus ceruleus, which can induce anxiety-like behavior in animals (59), and CRF receptor antagonists applied to the locus ceruleus diminish NE release to the hippocampus (60) and prefrontal cortex (61). CRF activity at CRF1 in the dorsal raphe reduces activity of serotonergic neurons (62). In the PFC, CRF acting through CRF1 sensitizes post-synaptic 5HT-2 receptors that mediate anxiety behaviors in mice (63). In contrast, to these effects, loss of CRF1 signaling in midbrain dopaminergic neurons increases anxiety by inhibiting dopamine release in the prefrontal cortex (64).
Another consideration for the trial’s negative results is the potential sex-specific responses to CRF and CRF1 antagonists. In contrast to male mice, who show clear behavioral and HPA axis responses to infusion of either CRF or a CRF1 antagonist into the dorsal raphe, female mice demonstrate modest changes (65). Because participation in the current trial was limited to women, the potential efficacy of GSK561679 in men could not be assessed. Design of future human studies of CRF1 antagonists should prospectively consider possible sex-specific effects of CRF-modulating drugs.
As part of this NCDDG program, a study evaluating the anxiolytic effects of GSK561679 was conducted in healthy adults using a startle paradigm (66). Contrary to expectations, a single 400 mg dose of GSK561679 increased startle in response to a stimulus predictive of electric shock (i.e., increased fear), but had no effect on unpredictable shock (i.e., anxiety), although GSK561679 also reduced baseline startle, which complicates interpretation of the startle potentiation results. In contrast, alprazolam in this study was found to reduce anxiety but did not impact fear. Although these results did not support preclinical rodent data suggesting that CRF1 antagonism decreases anxiety measures, they were consistent with the rodent data suggesting that CRF1 antagonism can increase startle responses potentiated by cued fears (67). Taken together, these data suggest that CRF1 antagonism can inhibit the BNST, thereby reducing the “brake” that BNST exerts on the reactivity of the central nucleus of the amygdala to fear stimuli (68), but that this inhibitory effect is inadequate to reduce behavioral expressions of anxiety. These different regional actions of CRF1 antagonism within the central nervous system (CNS) may have yielded competing effects on patients’ anxiety levels. In addition, the current trial used only a fixed dose of 350 mg/day; employing higher doses may have produced different effects.
Alternatively, if CRF overactivity is truly present in PTSD, the negative study result may indicate that once PTSD is established, blockade of CRF’s extrahypothalamic sensitization effects on anxiety signaling are insufficient to alter expression of anxiety behaviors. The great majority of animal studies implicating the role of CRF1 activation in anxiety responses are based on short-term stressors and drug exposures. For example, in mice, CRF1 antagonism immediately after a predator stressor successfully blocks the initiation and consolidation of the stressor’s effects on startle (69). In human adults affected with depression, PTSD, or anxiety disorders, CRF activation at the time of stress may produce circuit-level changes that, once established, are only weakly responsive to further modulation of CRF signaling. Indeed, chronic over-expression of CRF in adult mice produces only modest effects on behavior (70). Under this model, CRF1 antagonists may prove more efficacious as preventative treatments immediately post-trauma, rather than as monotherapy treatments for established conditions.
While this trial was underway, GSK561679 was found to be ineffective in the treatment of major depressive disorder (71) and one study in social anxiety disorder was completed with undisclosed results (72). The negative result in the current trial suggests that CRF1 receptor antagonists are unlikely to prove useful for the treatment of anxiety disorders, despite the wealth of suggestive preclinical data (29). Our preliminary data on attempting to subtype patients according to possible CRH-system hyperactivity, suggest, however, that CRF1 antagonists may be effective in specific biological subgroups of patients. This observation needs to be confirmed by additional, larger studies. Other possible explanations for the failure of CRF1 antagonists include inadequate CNS penetration of the compounds, inadequate treatment duration, abnormal concentrations of CRF binding protein in the CNS (73), competing actions by urocortins (54), or strong compensatory systems that oppose any anxiolytic effect of CRF1 antagonism (74). The effects of CRF2 activation in the presence of CRF1 antagonism are unknown (75), although existing data suggest that preserved CRF2 signaling in the absence of CRF1 activation should have provided a protective effect against anxiety (76,77). Despite the failures of CRF1 antagonists in mood and anxiety disorders, this mechanism of action may find clinical value in other areas of psychiatry.
Supplementary Material
Table S1. CONSORT Checklist
Table S2. Ethnicity and allele frequency of CRF1 SNP rs110402
Table S3. Spontaneously reported adverse events
Table S4. Patient Rated Inventory of Side Effects (PRISE) symptom counts
Figure S1. CONSORT flow diagram
Figure S2. Change in MADRS scores over time
S.E. bars represent ± 1 S.E.
Figure S3. Significant interaction effect of rs110402 and childhood abuse on percent change in PSS re-experiencing score
The boxplots describe the mean % change of PSS re-experiencing score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed a significant interaction effect on PSS re-experiencing score % change over treatment in subjects treated with GSK561679 (−β= −2.472; p=0.006) but not in subjects treated with placebo (β= −0.075; p=0.92). rs110402 GG carriers exposed to child abuse displayed the highest % change of PSS symptoms following GSK561679 treatment.
Figure S4. Significant interaction effect of rs110402 and childhood abuse on percent change in PSS arousal score
The boxplots describe the mean % change of PSS arousal score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed a significant interaction effect on PSS arousal score % change over treatment in subjects treated with the GSK561679 (β= −2.034; p= 0.019) but not in subjects treated with placebo (β=0.054; p=0.94). rs110402 GG carriers exposed to child abuse displayed the highest % change of PSS symptoms following GSK561679 treatment.
Figure S5. Lack of interaction effect of rs110402 and childhood abuse on percent change in PSS avoidance score
The boxplots describe the mean % change of PSS avoidance score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed no significant interaction effect on PSS avoidance score % change over treatment in subjects treated with either GSK561679 (β= −0.945; p=0.36) or placebo (β=0.565; p=0.44).
Figure S6. Lack of effect of GSK561679 and placebo on morning cortisol
Non-significant change in morning cortisol from baseline to week 5 between patients treated with GSK561679 or placebo (p<.05).
Figure S7. Lack of effect of GSK561679 on change in morning plasma cortisol levels after dexamethasone suppression
Change of 8:00am plasma cortisol levels before and after administration of 0.5mg dexamethasone in subjects treated with the GSK561679 or placebo. a) Pre-treatment; b) after 5 weeks of treatment. At both time points no significant difference was observed between the two treatments groups (p>0.05 for all; Pre-treatment: n= 36 GSK561679, 33 placebo; 5 weeks after treatment: n= 29 GSK561679, 26 placebo).
Figure S8. PCA Plot
PCA plot of samples shows good concordance between self-reported ethnicity (legend) and estimated ethnicity by principal component analysis. African-American (AfrAm), Asian South Central (Asian SC), Asian South East (Asian SE), Hawaiian Pacific Islands (Haw PacIsl) Multiple (Mult), Unknown (Unk), White Arabic (White A), White Caucasian (White C).
Footnotes
FINANCIAL DISCLOSURES
Funding for the study was provided from a grant from the National Institute of Mental Health, U19 MH069056 (BWD, HM). Additional support was received from K23 MH086690 (BWD) and VA CSRD Project ID 09S-NIMH-002 (TCN). The GSK561679 compound was currently licensed by Neurocrine Biosciences. GlaxoSmithKline contributed the study medication and matching placebo, as well as funds to support subject recruitment and laboratory testing, and Neurocrine Biosciences conducted the pharmacokinetic analyses. GlaxoSmithKline and Neurocrine Biosciences were not involved in the data collection, data analysis or interpretation of findings.
This work was supported with resources and the use of facilities at the Michael E DeBakey VA Medical Center, Houston, TX. Disclaimer: the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Dr. Dunlop has received research support from Acadia, Assurex, Axsome, Bristol-Myers Squibb, Janssen, GlaxoSmithKline, NIMH, Otsuka, Pfizer, and Takeda. He has served as a consultant to Pfizer and Medavante.
Dr. Binder receives research funding from NIH, the European Community, the German Ministry of Health (BMBF) as well as Bohringer-Ingelheim Inc.
In the past five years, Dr. Iosifescu has consulted for Avanir, Axome, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, and Sunovion and he has received grant/research support through the Icahn School of Medicine at Mount Sinai from Alkermes, Astra Zeneca, Brainsway, Euthymics, Neosync, Roche, Shire.
Dr. Mathew has received research funding from the NIH, Department of Veterans Affairs, Johnson Family Chair, and Janssen Research & Development. He has served as a consultant to Acadia, Alkermes, Cerecor, Otsuka, and Valeant, and serves on an Advisory Board for VistaGen Therapeutics. Dr. Neylan has received research support from the NIMH, Department of Defense, and Department of Veterans Affairs. In the past three years he has served as a consultant to Resilience Therapeutics and Insys Therapeutics.
Dr. Green has received research support from the NIH, Cancer Prevention Institute of Texas, Department of Veterans Affairs, and Stryker.
Dr. Grigoriadis serves as Chief Research Officer and is a full-time employee of Neurocrine Biosciences, Inc.
Dr. Rothbaum receives funding from Wounded Warrior Project, Department of Defense, the National Institute of Mental Health Grant, the Brain and Behavior Research Foundation, and McCormick Foundation. Dr. Rothbaum receives royalties from Oxford University Press, Guilford, APPI, and Emory University and has received one advisory board payment from Genentech.
Dr. Nemeroff has received research support from the NIMH, Department of Defense, and Department of Veterans Affairs. In the past three years he has served as a consultant to Resilience Therapeutics and Insys Therapeutics.
Dr. Mayberg has received consulting fees from St. Jude Medical Neuromodulation and Eli Lilly (2013 only) and intellectual property licensing fees from St. Jude Medical Neuromodulation. Dr. Carrillo-Roa, Dr. Kinkead, and Dr. Pape declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodriguez P, Holowka DW, Marx BP. Assessment of posttraumatic stress disorder-related functional impairment: A review. J Rehabil Res Dev. 2012;49:649–666. doi: 10.1682/jrrd.2011.09.0162. [DOI] [PubMed] [Google Scholar]
- 2.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 4.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 5.Raskind MA, Millard SP, Petrie EC, Peterson K, Williams T, Hoff DJ, et al. Biol Psychiatry. 2016;80:736–742. doi: 10.1016/j.biopsych.2016.03.2108. [DOI] [PubMed] [Google Scholar]
- 6.Stein D, Ipser J, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006;1:CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim C, Nemeroff CB. Neurobiological pathways involved in fear, stress and PTSD. In: Liberzon I, Ressler KJ, editors. Neurobiology of PTSD. Oxford University Press; New York: 2016. pp. 220–238. [Google Scholar]
- 8.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 9.Bremner JD, Licino J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker D, West S, Nicholson W, Ekhator N, Kasckow J, Hill K, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 11.Sautter F, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 12.Bonne O, Gill JM, Luckenbaugh DA, Collins C, Owens MJ, Alesci S, et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J Clin Psychiatry. 2011;72:1124–1128. doi: 10.4088/JCP.09m05106blu. [DOI] [PubMed] [Google Scholar]
- 13.Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 14.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laryea G, Arnett MG, Muglia JL. Behavioral studies and genetic alterations in CRH neurocircuitry: insights into human psychiatric disorders. Behav Sci. 2012;12:135–171. doi: 10.3390/bs2020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladd C, Huot R, Thrivikraman K, Nemeroff C, Meaney M, Plotsky P. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 17.Plotsky P, Thrivikraman K, Nemeroff C, Caldji C, Sharma S, Meaney M. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 18.Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- 19.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 21.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety – insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halldorsdottir T, Binder EB. Gene x environment interactions: From molecular mechanisms to behavior. Ann Rev Psychol. :68. doi: 10.1146/annurev-psych-010416-044053. (in press) Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Glaser YG, Zubieta JK, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM, et al. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J Neurosci. 2014;34:4099–4107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicchetti D, Rogosch FA, Oshri A. Interactive effects of CRHR1, 5-HTTLPR, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Dev Psychopathol. 2011;23:1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl) 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinol. 2014;43:71–80. doi: 10.1016/j.psyneuen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on HPA axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Disc. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- 30.Kehne JH, Cain CK. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: Evidence from animal models. Pharmacol Ther. 2010;128:460– 487. doi: 10.1016/j.pharmthera.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 32.Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt M, van der Ark P, et al. Preliminary evidence of anxiolytic effects of the CRF(1) receptor antagonist R317573 in the 7.5% CO(2) proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–1206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- 33.Fabio RD, St-Denis Y, Sabbatini FM, Andreotti D, Arban R, Bernasconi G, et al. Synthesis and pharmacological characterization of novel drug like corticotropin-releasing factor 1 antagonists. J Med Chem. 2008;51:7370–7379. doi: 10.1021/jm800744m. [DOI] [PubMed] [Google Scholar]
- 34.Dunlop BW, Rothbaum BO, Binder EB, Duncan E, Harvey PD, Jovanovic T, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient. 20. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. SCID-I/P, Version. [Google Scholar]
- 36.Foa E, Riggs D, Dancu C, Rothbaum B. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;5:395–399. [Google Scholar]
- 37.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress 1995. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 38.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of post-traumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assessment. 1997;9:445–451. [Google Scholar]
- 39.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery SA, Asberg A new depression scale designed to be sensitive to change. Br J Psychiatry 1979. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 41.Williams JBW, Kobak KA. Development and Reliability of the SIGMA: a structured interview guide for the Montgomery-Asberg Depression Rating Scale (MADRS) Br J Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 42.Rush A, Trivedi M, Ibrahim H, Carmody T, Arnow B, Klein D, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician-Rating (QIDS-C), and Self-Report (QIDS-SR) A Psychometric evaluation in patients with major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 43.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education and Welfare, National Institute of Mental Health; Bethesda, MD: 1976. Clinical global impressions; pp. 217–222. [Google Scholar]
- 44.Sheehan DV. Sheehan Disability Scale. In: Rush AJ, Pincus HA, First MB, Blacker D, Endicott J, Keith SJ, Phillips KA, Ryan ND, Smith GR, Tsuang MT, Widiger TA, Zarin DA, editors. Handbook of Psychiatric Measures. American Psychiatric Association; Washington, D.C: 2000. pp. 13–115. [Google Scholar]
- 45.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia classification algorithm of suicide assessment (C-CASA) Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity and burden of side effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 49.Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 50.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 51.Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 52.Davidson J, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry. 2006;63:1158–1165. doi: 10.1001/archpsyc.63.10.1158. [DOI] [PubMed] [Google Scholar]
- 53.Dunn AJ, Swiergiel AH. Behavioral response to stress are intact in CRF-deficient mice. Brain Res. 1999;845:14–20. doi: 10.1016/s0006-8993(99)01912-5. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, et al. Corticotropin-releasing factor and Urocortin 1 modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brady LS, Gold PW, Herkenham M, Lynn AB, Whitfield HJ., Jr The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing-hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res. 1992;572:117–125. doi: 10.1016/0006-8993(92)90459-m. [DOI] [PubMed] [Google Scholar]
- 56.Aubry JM, Possoli G, Vale WW. Chronic treatment with the antidepressant amitriptyline decreases CRF-R1 receptor mRNA levels in the rat amygdala. Neurosci Lett. 1999;266:197–200. doi: 10.1016/s0304-3940(99)00295-5. [DOI] [PubMed] [Google Scholar]
- 57.Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharm Exp Therapeutics. 2002;300:1085–1092. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- 58.Geracioti TD, Jr, Baker DG, Kasckow JW, Strawn JR, Mulchahey J, Dashevsky BA, et al. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinol. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 60.Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–13. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 61.Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- 62.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. Erratum in: Neuropsychopharmacology 2000 22: 449. [DOI] [PubMed] [Google Scholar]
- 63.Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, et al. CRF receptor 1 regulates anxiety behavior via sensitization of 5HT2 receptor signaling. Nat Neurosci. 2010;5:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 65.Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, et al. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75:873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacol. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker DL, Miles L, Davis M, Miles LA. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haufler D, Nagy F, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, et al. Forebrain-specific CRF overproduction during development is sufficient to induce enduring anxiety and startle abnormalities in adult mice. Neuropsychopharmacol. 2014;39:1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.GlaxoSmithKline. Results Summary for CRS106139. [date last accessed 12 January 2017];A six-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study evaluating the efficacy, safety, and tolerability of GSK561679 compared to placebo in female subjects, diagnosed with major depressive disorder. 2014 Available at: http://download.gsk-clinicalstudyregister.com/files/ee490a01-8433-4cba-a027-9c486b866a67.
- 72.GlaxoSmithKline. Protocol Summary for CRH108571. [date last accessed 12 January 2017];Double-blind, randomized, placebo and Alprazolam-controlled three-period crossover incomplete block design study to compare putative anxiolytic-like fRMI activity of GW876008 and GSK561679 after single-dose administration in subjects with Social Anxiety Disorder (SAD) 2013 Available at: https://www.gsk-clinicalstudyregister.com/study/CRH108571?search=study&#ps.
- 73.Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 74.Gresack JE, Risbrough VB. Corticotropin-releasing factor and noradrenergic signaling exert reciprocal control over startle reactivity. Int J Neuropsychopharmacol. 2011;14:1179–1194. doi: 10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf EJ, Mitchell KS, Logue MW, Baldwin CT, Reardon AF, Humphries DE, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30:1161–1169. doi: 10.1002/da.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bale TL, Contarion A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat Genet. 2000;25:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 77.Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2002;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CONSORT Checklist
Table S2. Ethnicity and allele frequency of CRF1 SNP rs110402
Table S3. Spontaneously reported adverse events
Table S4. Patient Rated Inventory of Side Effects (PRISE) symptom counts
Figure S1. CONSORT flow diagram
Figure S2. Change in MADRS scores over time
S.E. bars represent ± 1 S.E.
Figure S3. Significant interaction effect of rs110402 and childhood abuse on percent change in PSS re-experiencing score
The boxplots describe the mean % change of PSS re-experiencing score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed a significant interaction effect on PSS re-experiencing score % change over treatment in subjects treated with GSK561679 (−β= −2.472; p=0.006) but not in subjects treated with placebo (β= −0.075; p=0.92). rs110402 GG carriers exposed to child abuse displayed the highest % change of PSS symptoms following GSK561679 treatment.
Figure S4. Significant interaction effect of rs110402 and childhood abuse on percent change in PSS arousal score
The boxplots describe the mean % change of PSS arousal score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed a significant interaction effect on PSS arousal score % change over treatment in subjects treated with the GSK561679 (β= −2.034; p= 0.019) but not in subjects treated with placebo (β=0.054; p=0.94). rs110402 GG carriers exposed to child abuse displayed the highest % change of PSS symptoms following GSK561679 treatment.
Figure S5. Lack of interaction effect of rs110402 and childhood abuse on percent change in PSS avoidance score
The boxplots describe the mean % change of PSS avoidance score in abused and non-abused patients treated with GSK561679 or placebo. GG carriers are shown in light grey and AA/AG in dark grey. Black dots indicate outliers. rs110402 A carrier status by childhood abuse exposure showed no significant interaction effect on PSS avoidance score % change over treatment in subjects treated with either GSK561679 (β= −0.945; p=0.36) or placebo (β=0.565; p=0.44).
Figure S6. Lack of effect of GSK561679 and placebo on morning cortisol
Non-significant change in morning cortisol from baseline to week 5 between patients treated with GSK561679 or placebo (p<.05).
Figure S7. Lack of effect of GSK561679 on change in morning plasma cortisol levels after dexamethasone suppression
Change of 8:00am plasma cortisol levels before and after administration of 0.5mg dexamethasone in subjects treated with the GSK561679 or placebo. a) Pre-treatment; b) after 5 weeks of treatment. At both time points no significant difference was observed between the two treatments groups (p>0.05 for all; Pre-treatment: n= 36 GSK561679, 33 placebo; 5 weeks after treatment: n= 29 GSK561679, 26 placebo).
Figure S8. PCA Plot
PCA plot of samples shows good concordance between self-reported ethnicity (legend) and estimated ethnicity by principal component analysis. African-American (AfrAm), Asian South Central (Asian SC), Asian South East (Asian SE), Hawaiian Pacific Islands (Haw PacIsl) Multiple (Mult), Unknown (Unk), White Arabic (White A), White Caucasian (White C).