Abstract
Background
Individuals who use cocaine have fewer cognitive resources needed to maintain abstinence. This is evidenced by blunted brain function during cognitive control tasks and reduced communication between brain regions associated with cognitive function. For instance, relapse vulnerability is heightened in individuals with less communication between the right and left frontoparietal executive control network (ECN). Given that recent cocaine use enhances such communication, it is plausible that recency of cocaine use influences interhemispheric ECN communication. However, it is unclear whether ECN communication weakens over the course of early cocaine abstinence, which may then enhance relapse risk.
Methods
In ten men with cocaine use disorder, we conducted a preliminary assessment of the relationship between the number of days since last cocaine use (1–3 days) and interhemispheric ECN coupling using resting state functional magnetic resonance imaging (fMRI).
Results
Reduced interhemispheric ECN coupling was associated with increasing days since last cocaine use; weaker coupling was also associated with lower urine cocaine metabolite concentrations. This association was more prominent in prefrontal than parietal ECN-subregions.
Conclusions
Preliminary results indicate that resting state interhemispheric ECN coupling weakens within the first few days following last cocaine use. Because of the known link between reduced ECN interhemispheric coupling and relapse vulnerability, these results suggest that relapse risk may increase the longer an individual abstains during an early quit attempt. Treatments focused on reversing this coupling deficit may facilitate abstinence.
Keywords: cocaine, abstinence, executive control network, interhemispheric, resting state functional connectivity, fMRI
1. Introduction
Disrupted brain function plays a major role in maintaining chronic cocaine use (Goldstein and Volkow, 2011). For instance, in comparison to non-drug users, individuals with cocaine use disorder (CUD) show reduced engagement of brain regions involved in executive control during cognitive tasks (Bolla et al., 2003; Hester and Garavan, 2004; Kaufman et al., 2003). Additionally, the communication between executive control brain regions is reduced in abstinent individuals with CUD relative to healthy controls (Kelly et al., 2011). This is particularly relevant since increased interhemispheric coupling in frontoparietal regions is associated with better executive functioning and attention (He et at., 2007; Kelly et al., 2011; McHugh et al., 2016; Wang et al., 2013). These interhemispheric coupling deficits are associated with drug-related behavior as cocaine relapse vulnerability is heightened in those with reduced interhemispheric coupling of the executive control network (ECN; McHugh et al., 2016), a resting state network comprised of two primary hubs underling cognitive control: dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC) (Bressler and Menon, 2010). Due to this networks’ anatomy and function, it is sometimes also referred to as the frontoparietal network (Janes et al., 2012; Laird et al., 2011). In contrast, interhemispheric coupling within other resting state networks (default mode and salience networks) was not related to time to relapse (McHugh et al., 2016), indicating that the ECN may play a unique role in abstinence from cocaine.
Though chronic cocaine use is associated with reduced functional connectivity of brain regions involved in executive control, acute cocaine use can temporarily enhance such interhemispheric coupling (Reid et al., 2008). In contrast, it is plausible that in the initial days following cocaine use, such interhemispheric communication declines, impairing the cognitive resources needed to prevent relapse. Though prior research has shown that reduced interhemispheric coupling after the first few weeks of abstinence is associated with relapse (McHugh et al., 2016), the extent to which interhemispheric coupling strength varies with the days since last cocaine use is unknown. Understanding how recency of use impacts interhemispheric coupling may clarify the neurobiological factors that influence the ability to sustain cocaine abstinence during early withdrawal.
As a preliminary step, we assessed whether the number of days since last cocaine use, during the first three days of abstinence, was associated with weaker connectivity between the right and left lateralized ECN. We focused on the ECN given the prior link between reduced interhemispheric ECN connectivity and relapse (McHugh et al., 2016). We subsequently assessed whether the relationship between ECN coupling and days since last cocaine use was driven by the PFC or PPC ECN-subregions, which are thought to be involved in shared and also distinct cognitive functions (Zhou et al., 2012). The PFC is associated with top-down volitional attentional control, whereas the PPC is thought to have additional involvement with bottom-up attentional processing (Miller and Buschman, 2013). Since these regions have been associated with different patterns of coupling during attentional control tasks (Friese et al., 2016), the extent to which resting state interhemispheric coupling is related to recency of cocaine use may also vary between the PFC and PPC hubs of the ECN. We hypothesized that coupling in the PFC subregion would have the strongest relationship with recency of use, because deficits in the PFC are a key feature of addiction (Goldstein and Volkow, 2011).
2. Material and Methods
2.1 Participants
Participants were ten treatment-seeking cocaine-using men who had a history of using cocaine via the smoked and intranasal routes. Eligibility criteria included current cocaine abuse or dependence per the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) and DSM-IV-TR criteria, having used cocaine at least four times in the past month, and a urine sample positive for benzoylecgonine (BE; >300 ng/mL; Alere iScreen DX CLIA Waived 12 Panel Instant Drug Test Dip Card) to verify recent cocaine use. Positive urine screens were then quantified for BE metabolite concentration (Quest Diagnostics, Cambridge, MA); one data point was missing due to sample leakage during transit. Exclusion criteria included current DSM-IV-TR psychotic disorder or drug dependence (except cocaine, nicotine, marijuana, and alcohol), magnetic resonance contraindications, head injury with cognitive impairments, and seizure or other neurological disorders. Participants provided informed consent for the study, and procedures were approved by the Partners Human Research Committee.
2.2 Functional Magnetic Resonance Imaging (fMRI) Parameters
Brain imaging data were collected on a Siemens 3 Tesla TIM Trio MR imaging system (Erlangen, Germany) with a 32-channel phased-array radio frequency head coil. High-resolution, multiecho multiplanar rapidly acquired gradient-echo (ME-MPRAGE) anatomical scans were collected with a T1-weighted scan with TR=2.1 sec, TE=3.3 msec, matrix size: 256 × 256 mm, flip angle: 7°, 128 slices, and 1.0 × 1.0 × 1.33 mm voxels. Resting state fMRI scans were collected using multiband echo planar imaging with TR=0.72 sec, TE=32 msec, matrix size: 85 × 85 mm, flip angle: 66°, 64 slices, and 2.5 mm3 voxels. Transversal interleaved slices were aligned to the anterior and posterior commissures with phase encoding from posterior to anterior to avoid prefrontal signal loss. During the 6-minute fMRI scans, participants were instructed to keep their eyes open, look at a fixation cross on a screen, and remain as still as possible.
2.3 fMRI Pre-processing and Denoising
Data analysis was conducted using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Functional data preprocessing included deleting the first 10 volumes, motion correction (MCFLIRT; Jenkinson et al., 2002), brain extraction (BET; Smith, 2002), slice timing correction, spatial smoothing (Gaussian kernel of 6 mm full-width half-maximum), and Gaussian-weighted least-squares straight line fitting high-pass temporal filter (100 sec). Denoising involved identifying and removing motion and other artifactual components from each participant’s resting state data using Multivariate Exploratory Linear Decomposition into Independent Components (MELODIC). After all independent components were identified in each participant, components were visually inspected for noise (Janes et al., 2015; Kelly et al., 2010; Putcha et al., 2015), and noise components were regressed out of the resting state data using fsl_regfilt. Individual participant data were affine-registered to standard space at 2 × 2 × 2 mm resolution (MNI152 2 mm3; Montreal Neurological Institute, Montreal, QC, Canada) using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
2.4 Interhemispheric Coupling
The average time courses for each participant were extracted using fslmeants for each bilateral region of interest (ROIs). The right and left ECN were defined using the frontoparietal ROIs in Smith et al. (2009, Fig. 1.). The lateralized masks were further divided to separately examine the ECN-subregions: PFC and PPC, which did not include overlap with the opposite hemisphere. For each ROI, the average time course was demeaned, detrended, and Hamming windowed (Oppenheim and Schafer, 1975). The time courses for the right and left ROIs were then time-lagged cross-correlated to establish one maximum correlation value (r) for each participant (Janes et al., 2015), which was exported to SPSS 24 for further analysis.
Fig. 1.
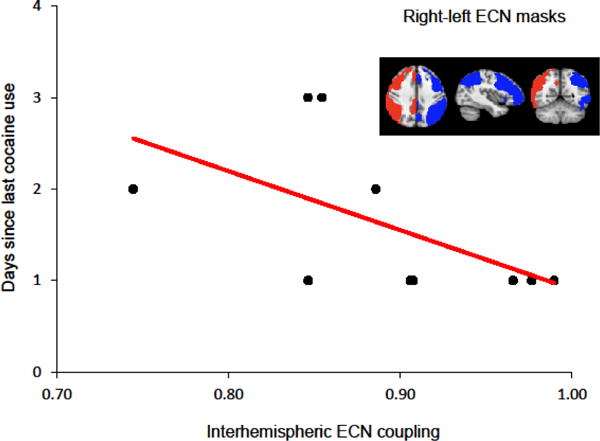
Correlation between interhemispheric coupling and duration of cocaine abstinence. Weaker interhemispheric coupling in the executive control network (ECN) is associated with more days since last cocaine use (r = −0.69, p = 0.028). The right and left ECN ROIs are shown in red and blue respectively.
2.5 Measures and Data Analyses
Participants completed demographics and substance use history questionnaires, and the Cocaine Craving Questionnaire – Brief (Sussner et al., 2006). Two-tailed Spearman’s rank order correlations were conducted to assess the a priori relationship between reduced interhemispheric ECN coupling and the number of days since last cocaine use. Relationships between recency of cocaine use and interhemispheric coupling were then examined separately for each ECN-subregion (PFC, PPC).
3. Results
3.1 Interhemispheric Coupling
Reduced interhemispheric ECN coupling was significantly correlated with greater number of days since last cocaine use (r = −0.69, p = 0.028, Fig. 1). Our follow-up analyses indicated that number of days since last cocaine use was significantly associated with reduced interhemispheric coupling of the PFC subregion of the ECN (r = −0.80, p = 0.006), but not the PPC ECN-subregion (r = −0.39, p = 0.271). The link between ECN and PFC interhemispheric coupling and duration of self-reported cocaine abstinence was supported biochemically as lower cocaine metabolite concentrations (BE; n = 9) were associated with less interhemispheric coupling in the ECN (r = 0.72, p = 0.030) and PFC (r = 0.80, p = 0.010).
3.2 Demographic and Clinical Characteristics
Demographic and clinical characteristics are provided in Table 1. Age, education, severity of use (cocaine use days in past 7 and 30 days), cocaine craving, and tobacco smoking variables (smoking status, average daily cigarettes smoked, expired carbon monoxide prior to scanning) were not significantly associated with interhemispheric coupling in the ECN, PFC, or PPC (all ps > 0.05).
Table 1.
Demographic and clinical characteristics
| N | % | |
|---|---|---|
| Male | 10 | 100 |
| African American | 6 | 60 |
| Caucasian | 3 | 30 |
| Multiple racial backgrounds | 1 | 10 |
| Tobacco smoker | 7 | 70 |
|
| ||
| Mean | SD | |
|
| ||
| Age | 50.90 | 4.12 |
| Education (years) | 13.60 | 2.50 |
| Days since last cocaine use | 1.60 | 0.84 |
| Days of cocaine use (past 7 days)* | 5 | 1.69 |
| Days of cocaine use (past 30 days)* | 24 | 10.15 |
| CCQ-Brief | 2.78 | 1.82 |
| Cocaine metabolites BE ng/mL* | 11,708.00 | 15,429.62 |
| Daily cigarettes smoked* | 7.17 | 6.71 |
| Expired CO prior to scanning | 15.60 | 13.74 |
One missing data point.
CCQ-Brief = Cocaine Craving Questionnaire – Brief; BE = benzoylecgonine; CO = carbon monoxide; N = number; SD = standard deviation
4. Discussion
The present pilot study offers evidence that reduced interhemispheric ECN coupling is associated with longer duration of cocaine abstinence. Beyond the current work, reduced interhemispheric coupling has been linked with other abused substance use (Orr et al., 2013; Qiu et al., 2017) and psychiatric disorders with high rates of comorbid substance use (Dixon et al., 1999, Swendsen and Merikangas, 2000), such as depression (Guo et al., 2013a, 2013b; Wang et al., 2013) and schizophrenia (Li et al., 2014; Spencer et al., 2003). Together, these studies suggest a strong link between substance use and reduced interhemispheric connectivity.
The current work also fits with prior data indicating that recent cocaine use (Reid et al., 2008) and cigarette smoking (Viswanath et al., 2015) acutely enhances interhemispheric coupling. Psychostimulants may mediate such interhemispheric communication via a dopaminergic mechanism as dopamine terminals directly synapse onto PFC neurons involved in interhemispheric communication (Carr and Sesack, 2000). Thus, interhemispheric communication may fluctuate due to dopaminergic changes following acute drug administration and early abstinence. The role of dopamine in PFC interhemispheric connectivity also fits with our follow-up analysis, which showed a relationship between days since last cocaine use and interhemispheric connectivity of the PFC ECN-subregion but not PPC ECN-subregion. However, future work is needed to directly test the mechanism for cocaine-induced changes in ECN interhemispheric connectivity.
Collectively, our work suggests the need to mitigate the reduced interhemispheric coupling that evolves over early abstinence. It is plausible that novel techniques such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) may hold promise. For instance, tDCS targeting of the DLPFC, a key ECN node, has been shown to increase ECN interhemispheric coupling in healthy controls (Park et al., 2013). While the influence of TMS on interhemispheric coupling in individuals with CUD has not been tested, TMS in those with CUD improves cognition (Boggio et al., 2006) and reduces cocaine use and craving (Batista et al., 2015; Terraneo et al., 2016). Whether these beneficial effects in those with CUD are associated with enhanced interhemispheric ECN coupling is still unclear.
The current pilot study confirms that there is a relationship between interhemispheric ECN coupling and the duration of short-term cocaine abstinence. However, these preliminary results are limited by a small, cross-sectional, single sex sample. Further, correlation analyses lack the ability to prove that progressing through the first days of cocaine abstinence caused interhemispheric coupling reductions, though this is likely given prior work showing that acute cocaine use strengthens interhemispheric communication (Reid et al., 2008). Additionally, though cigarette smoking can enhance interhemispheric coupling, smoking intensity was minimal in our sample and smoking related variables had no significant effects on interhemispheric ECN coupling strength. Despite these limitations, the current results provide initial evidence linking recency of cocaine use with disrupted ECN coupling, which may compromise the ability to maintain abstinence (McHugh et al., 2016). Future longitudinal studies using larger sample sizes that include both sexes and are powered to address effects of co-occurring substance use are needed to determine the trajectory of interhemispheric ECN coupling during the course of abstinence to assess if/when this disruption resolves and whether treatments that reverse such a coupling deficit lead to abstinence.
Highlights.
Interhemispheric executive control network (ECN) coupling declines in cocaine abstinence.
Coupling correlates were strongest in the prefrontal subregion of the ECN.
Lower cocaine metabolite concentrations were correlated with less ECN coupling.
Acknowledgments
We would like to thank the participants who made this study possible.
Role of Funding Source
This work was supported by the National Institute of Drug Abuse grants R21DA036047 and T32 DA015036 (SEL); K01 DA029645 (ACJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
JMM completed fMRI data analysis/interpretation and drafted the manuscript. CSZ contributed to study design, data collection, and manuscript preparation. JMS and ND completed data collection/management and contributed to manuscript preparation. SEL was responsible for study design, overseeing research procedures, and contributed to manuscript preparation. ACJ contributed to the study design, oversaw data analysis/interpretation and drafting the manuscript. All authors have reviewed and approved the final manuscript.
Conflict of Interest
No conflict declared.
References
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychophamacol. 2015;18:pyv066–11. doi: 10.1093/ijnp/pyv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/S1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J Comp Neurol. 2000;425:275–283. doi: 10.1002/1096-9861(20000918)425:2<275::AID-CNE9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. doi: https://doi.org/10.1016/S0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Friese U, Daume J, Göschl F, König P, Wang P, Engel AK. Oscillatory brain activity during multisensory attention reflects activation, disinhibition, and cognitive control. Sci Rep. 2016;6:32775. doi: 10.1038/srep32775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:1–18. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013a;41:24–29. doi: 10.1016/j.pnpbp.2012.11.003. doi: https://doi.org/10.1016/j.pnpbp.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Chen H, Zhao J. Decreased interhemispheric coordination in treatment-resistant depression: A resting-state fMRI study. PloS One. 2013b;8:e71368. doi: 10.1371/journal.pone.0071368. doi: https://doi.org/10.1371/journal.pone.0071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. doi: https://doi.org/10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, de B Frederick B, Lukas SE. Insula–dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, de B Frederick B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. doi: https://doi.org/10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/S1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TH, Stein EA. Cingulate hypoactivity in cocaine users during a GO–NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckman CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. doi: http://doi.org/10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Xu Y, Zhang KR, Hoptman MJ, Zuo XN. Homotopic connectivity in drug‐naïve, first‐episode, early‐onset schizophrenia. J Child Psychol Psychiatry. 2015;56:432–443. doi: 10.1111/jcpp.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Gu H, Yang Y, Adinoff B, Stein EA. Executive control network connectivity strength protects against relapse to cocaine use. Addict Biol. 2016 doi: 10.1111/adb.12448. [DOI] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr Opin Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. doi: http://dx.doi.org/10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Digital Signal Processing. Prentice-Hall International; London: 1975. [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, Kelly C, Weierstall K, Watts R, Smyth B, Garavan H. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39:372–381. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Park JY, Shin YI, Kim ST, Kim YH. Transcranial direct current stimulation increases resting state interhemispheric connectivity. Neurosci Lett. 2013;539:7–10. doi: 10.1016/j.neulet.2013.01.047. doi: http://dx.doi.org/10.1016/j.neulet.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Putcha D, Ross RS, Cronin-Golomb A, Janes AC, Stern CE. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. NeuroImage Clin. 2015;7:449–455. doi: 10.1016/j.nicl.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YW, Lv XF, Jiang GH, Su HH, Ma XF, Tian JZ, Zhuo FZ. Larger corpus callosum and reduced orbitofrontal cortex homotopic connectivity in codeine cough syrup-dependent male adolescents and young adults. Eur Radiol. 2017;27:1161–1168. doi: 10.1007/s00330-016-4465-5. [DOI] [PubMed] [Google Scholar]
- Reid M, Flammino F, Howard B, Nilsen D, Prichep L. Cocaine cue versus cocaine dosing in humans: Evidence for distinct neurophysiological response profiles. Pharmacol Biochem Behav. 2008;91:155–164. doi: 10.1016/j.pbb.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubler Y, Harnett-Sheehan K, Amorim PA, Janavs J, Weiller EHT, Hergueta T, Baker RDG, Dunbar GCL. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. doi: https://doi.org/10.1016/S0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath H, Velasquez KM, Savjani R, Molfese DL, Curtis K, Molfese PJ, Eagleman DM, Baldwin PR, Frueh BC, Fowler JC, Salas R. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015;92:63–68. doi: 10.1016/j.neuropharm.2014.12.030. doi: http://dx.doi.org/10.1016/j.neuropharm.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang QE, Zeng YW, Jin Z, Dai WJ, Su YA, Wang G, Tan YL, Yu X, Si TM. Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: a resting state fMRI study. PLoS One. 2013;8:e60191. doi: 10.1371/journal.pone.0060191. doi: https://doi.org/10.1371/journal.pone.0060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Katsuki F, Qi XL, Constantinidis C. Neurons with inverted tuning during the delay periods of working memory tasks in the dorsal prefrontal and posterior parietal cortex. J Neurophysiol. 2012;108:31–38. doi: 10.1152/jn.01151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


