Abstract
Background
The limited neurobiological understanding of PTSD has been partially attributed to the need for improved animal models. Stress-enhanced fear learning (SEFL) in rodents recapitulates many PTSD-associated behaviors, including stress-susceptible (SS) and –resilient (SR) subgroups in outbred rats. Identification of subgroups requires additional behavioral phenotyping, a confound to mechanistic studies.
Methods
We employed a SEFL paradigm in inbred male and female C57BL/6 that combines acute stress with fear conditioning to precipitate “traumatic” memories. Extinction and long-term retention of extinction were examined after SEFL. Further characterization of SEFL effects on male mice was performed with additional behavioral tests, determination of regional activation by Fos immunofluorescence and RNA-sequencing of the basolateral amygdala (BLA).
Results
Stressed animals displayed persistently elevated freezing during extinction. While more uniform in females, SEFL produced male subgroups with differential susceptibility that were identified without post-training phenotyping. Additional phenotyping of males revealed PTSD-associated behaviors, including extinction-resistant fear memory, hyperarousal, generalization and dysregulated corticosterone in SS males. Altered Fos activation was also seen in the infralimbic cortex and BLA of SS males after remote memory retrieval. Key behavioral outcomes, including susceptibility, were replicated by two independent laboratories. RNA-sequencing of the BLA revealed transcriptional divergence between the male subgroups, including genes with reported polymorphic association to PTSD patients.
Conclusions
This SEFL model provides a tool for development of PTSD therapeutics that is compatible with the growing number of mouse-specific resources. Furthermore, use of an inbred strain allows for investigation into epigenetic mechanisms that are expected to critically regulate susceptibility and resilience.
Keywords: memory, stress, learning, traumatic, extinction, resilience
Introduction
A history of stress increases the likelihood of developing post-traumatic stress disorder (PTSD) (1, 2), defined by persistent recollections of a trauma, with hyperarousal, avoidance of trauma-related cues and negative changes in mood and cognition (3). Antidepressants, the only FDA-approved pharmaceutical for PTSD, have limited efficacy over placebo and low remission (4, 5). Further, many patients fail to respond to behavior-based treatments, such as exposure therapy (ET), or even experience an exacerbation of symptoms (6). Among those that do respond, the majority retain their PTSD diagnosis (7, 8). At the crux of this issue lies a lack of neurobiological understanding of the disorder. For instance, what are the long-term brain changes that result from a PTSD-inducing traumatic experience? And why, when the majority of the population experiences at least one traumatic event in their lifetime, is the prevalence of PTSD only approximately 7% (9)?
Because of the numerous limitations inherent to using human postmortem tissue, animal models with a PTSD-like phenotype are valuable. Several rodent paradigms recapitulate core features of the disorder (10), including a stressor (e.g. predator exposure (11), restraint or forced swimming (12)), Pavlovian fear conditioning (FC) (13–15), or a combination of the two in the form of stress-enhanced fear learning (SEFL) (16). SEFL has been used extensively in rodents to produce extinction resistance (for review see (16)).
Many recent reviews have called for improved PTSD models (17, 18), suggesting that therapeutic discovery cannot progress without improving validity. Several features have been highlighted in recent reviews as key elements needed in a PTSD-like model (17, 18). These include a brief, rather than chronic stressor, to differentiate from major depressive disorder (MDD); persistent manifestation of the response; hyperarousal; interindividual variability where resilient and susceptible subgroups result from the same protocol (10, 17–19), and assessment in both sexes, as the risk of PTSD may be higher among females (see 9, but also (20, 21)). At the neurochemical level, it is suggested that validity should be based on insights from human studies in which irregular brain activation (e.g. amygdala, prefrontal cortex, hippocampus, thalamus), aberrant signaling of neurotransmitter systems, and neuroendocrine dysfunction have been identified in PTSD patients (17). Some current models produce interanimal variability thought to be akin to resilience and susceptibility in humans (11, 22–26). However, they require behavioral phenotyping after the stressor to identify individuals that are susceptible to negative effects of the stress. These manipulations induce their own neuromolecular changes, precluding studies of the mechanisms underlying the genesis and perpetuation of the PTSD-like phenotype. Furthermore, outbred lines are used in all cases of differential susceptibility, limiting the ability to isolate epigenetic contributors, which are expected to be highly relevant to individual variability in susceptibility (27–30). Here we present a protocol that addresses these challenges, while retaining the behavioral phenotypes and neurochemical dysregulation demonstrated by existing PTSD-like models.
Materials and Methods
Animals
Adult male and female C57BL/6 mice were used for all studies. 8–10 weeks of age.
SEFL: restraint stress, auditory fear conditioning and extinction
Please see Supplemental Materials and Methods for details.
Acoustic startle response and anxiety Tests
Male animals that did not undergo extinction after SEFL were tested in the following order: open field (OFT), elevated plus maze (EPM) and acoustic startle response (ASR).
Gene expression analyses
RNA extraction, sequencing and qPCR validation were performed as previously described (31) on BLA tissue from males following SEFL and remote memory retrieval (no extinction).
Immunohistochemistry
90min after remote memory retrieval (no extinction), males were perfused for immunolabeling of Fos.
Corticosterone (CORT) measurement
Plasma CORT levels were measured immediately after stress or after SEFL training at: 1) 30min after FC, 2) 6hrs after IP saline, 4d post-SEFL training (no extinction), 3) 6hrs after IP dexamethasone (100μg/ml, Sigma, St. Louis, MO) or saline 30d post-SEFL training (no extinction).
Statistics
A D’Agostino-Pearson omnibus normality test was applied to determine data distribution. For data with a Gaussian distribution, one-way analysis of variance (ANOVA) was performed to determine effects in 3 or more groups, followed by post hoc Tukey tests. Nonparametric Kruskal-Wallis tests were applied to data not evenly distributed. For comparisons between 2 data points within the same group, two-tailed paired t-tests were performed on data with a normal distribution and Mann-Whitney tests on data that required nonparametric analyses. For extinction, repeated measures ANOVA was used to determine significant treatment by bin interactions. For correlations, Pearson correlation coefficient was used. For contingency graphs, one-tailed chi-square tests were performed. For comparisons of variance between two groups, a Brown-Forsythe Test was used. Significant outliers were identified by the outlier function of SPSS software. Statistical significance was set at P < 0.05. Additional details can be found in the supplemental methods section.
Results
Prior stress exposure results in phenotypic variability in fear memory among male mice
A SEFL model commonly used in rats combines an initial stressor with subsequent auditory FC to produce extinction resistance (32–45). Associated behavior has been shown to be analogous to the extinction resistance and memory persistence that PTSD patients experience (46). We modified this paradigm for use in male (Figure 1A) and female (Figure 2) mice. Auditory FC was performed one week after a single restraint session. Four days later, a strong extinction protocol was employed (60 tones over 2d; Figure 1B and Supplemental Figure S1). Freezing differed between stress+FC (SEFL) and FC (control) males over extinction (RM-ANOVA: interaction F(1,37)=6.16, p=0.018). While FC animals froze less by the end of Day 1, the stress+FC group’s freezing to the final tone (Tone 30) did not differ from the first (Paired t-test FC: t(17)=3.07, p=0.007; stress+FC: t(20)=0.572, p=0.574) (Figure 1C), indicating extinction resistance. Freezing at the end of extinction was highly variable in stress+FC males, as evidenced by a high standard deviation (Tone 30 SD: FC=13.25, Stress+FC=29.26) and difference in variance compared to FC mice (Brown-Forsythe: F(1,39)=6.57, p=0.014).
Figure 1. Prior stress increases the prevalence of extinction resistance in male mice.

(A) Overview of SEFL behavioral paradigm. (B) Course of extinction in SEFL (Stress+FC) and FC controls over two days. Shown are five bins (6 tones each) of CS presentations during extinction. (C) Measure of extinction that occurred on Day 1. (D) Unsupervised cluster analysis of average freezing during extinction. (E–G) Distribution analysis of average freezing over extinction days 1 and 2, separated by treatment and cluster group. Cluster 1, grey bars; Cluster 2, blue bars. FC n=18, Stress+FC n=21. (H) Plasma corticosterone levels were measured in stressed or naïve (handled) mice immediately after restraint. Naïve n=7, Stress n=8. *p<.05, ****p<.0001. Error indicates s.e.m.
Figure 2. Prior stress in female mice exaggerates learned fear.
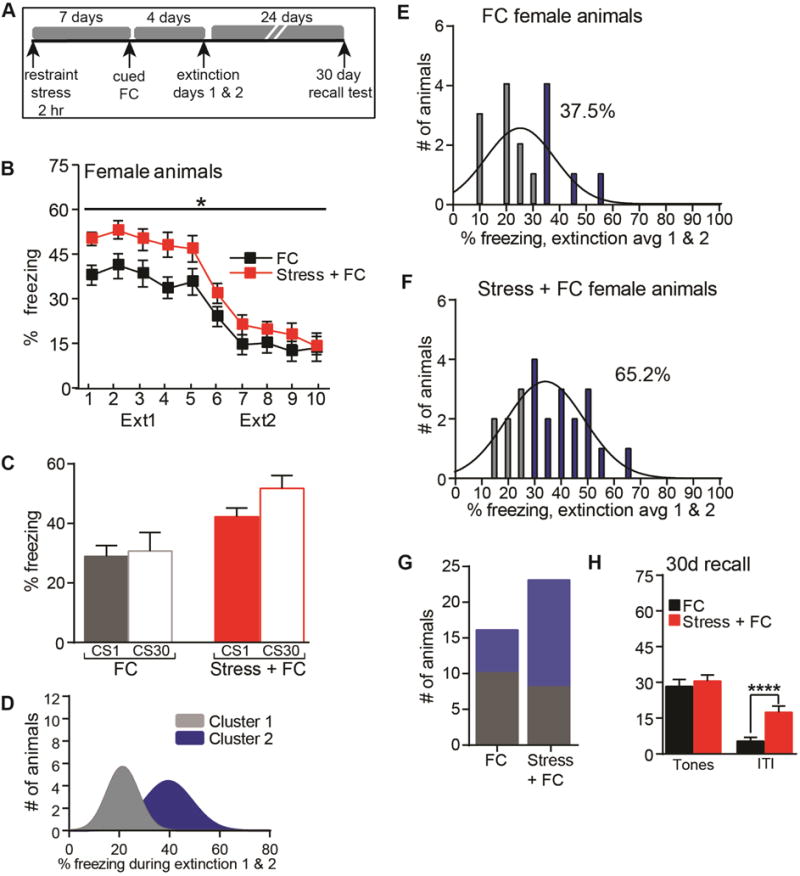
(A) The SEFL behavioral paradigm was performed in female mice. (B) Course of extinction in SEFL (Stress+FC) and FC females over two days. Shown are five bins (6 tones each) of CS presentations during extinction. (C) Measure of extinction that occurred on Day 1. (D) Unsupervised cluster analysis of average freezing during extinction. (E–G) Distribution analysis of average freezing over extinction days 1 and 2, separated by treatment and cluster group. Cluster 1, grey bars; Cluster 2, blue bars. (H) Average freezing during a 5 tone recall test performed 24 days after the last extinction session. *p<.05, ****p<.0001. FC n=16, Stress+FC n=23. Error indicates s.e.m.
To further examine this variability and how the response of stress+FC males deviated from typical variability of FC alone, we performed an unsupervised cluster analysis on the average freezing rate of all animals across extinction. The analysis revealed two clusters (Figure 1D), with a subset freezing at high levels (blue, Cluster 2). A distribution analysis of each treatment group separately indicated most FC mice (89.9%) belonged to Cluster 1 (grey bars), indicating the typical response of males to this FC protocol (Figure 1E). In contrast, there was a rightward shift for stress+FC distribution, with 42.9% in Cluster 2 (blue bars) (Figure 1F). Indeed, stress+FC males were more likely to display high freezing and belong to Cluster 2 (x2=3.70, p=0.027) (Figure 1G). Variability in the response of stressed animals may manifest over time, as restraint alone elevated levels of CORT in all mice well above controls (t-test: t(13)=16.40, p<0.00001), with a lower coefficient of variance in the stressed group (Control CV=37.6%, Stressed CV=13.9%) and no difference in variance between the groups (Brown-Forsythe: F(1,13)=2.46, p=0.141; Figure 1H). This low variability in interindividual stress responses is consistent with previous reports (47–49) and indicates that incubated stress likely interacts with FC to produce variability.
Prior stress results in exaggerated learned fear responses in females
Like stress+FC males, stress+FC females (Figure 2A) froze more than FC females during extinction (RM-ANOVA: interaction F(1,37)=4.16, p=0.049; Figure 2B) and this was most pronounced on Day 1. However, both groups showed extinction resistance on Day 1, with virtually no change from tone 1 to 30 (Figure 2C). Similar to males, an unbiased cluster analysis of average freezing resulted in 2 clusters (Figure 2D). However, consistent with the extinction resistance displayed by females of both groups, there was a greater representation of both FC (37.5%) and stress+FC (65.2%) in the high freezing cluster (Cluster 2, blue bars) (Figure 2E–F). As a result, the prevalence of high freezing did not differ between the two groups (x2=1.71, p=0.088) (Figure 2G). The stress-associated enhancement (Figure 2B) did not persist into a remote memory test given 30 days post-SEFL training. Lastly, stress+FC females displayed heightened freezing during the ITIs of the 30 day recall test (t-test: p<0.01; Figure 2H), suggesting the potential development of fear generalization beyond the tone.
Training performance can predict stress susceptibility in males
Given the potential value for mechanistic studies, we determined if stress susceptible (SS) males could be identified without post-training phenotyping, to avoid additional, stressful experiences that would alter the molecular landscape of SEFL. We examined behavior during the final minute of FC training. Despite the variance in freezing during training for FC controls, average freezing across extinction was clustered and did not correlate with training (Figure 3A). In contrast, stress+FC males’ training correlated to their varied response to extinction (Pearson correlation: FC r=0.269, p=0.281; stress+FC r=0.636, p=0.002) (Figure 3B). We then separated each group into high and low freezing based on their FC training and assessed the extinction profiles. Animals that froze above their group’s mean during the final minute of training (FC mean: 10.28±2.3%; Stress+FC mean: 6.43±1.1%) were classified as high and those below the mean as low (Figure 3C–D). Because of the potentially arbitrary nature of a “mean split” approach, we addressed the validity by determining how this division corresponded to extinction-based cluster analysis (Figure 1D–F). The only two FC animals in Cluster 2 (Figure 1E; high extinction freezing) also froze above the group mean during training (Figure 3C, inset). More importantly, 90% of the stress+FC animals that froze below the group mean during training belonged to Cluster 1 (Figure 1F; low extinction freezing) and 70% of stress+FC animals that froze above the group mean during training belonged to Cluster 2 (Figure 1F), suggesting a mean split based on training is valid to predict extinction performance. As expected, there was no difference in the extinction profiles of FC controls when separated by training [RM-ANOVA FC: interaction F(1,15)=3.74, p=0.072] (Figure 3C). However, the extinction profiles of low and high stress+FC males (Training mean: SR: 3.1±0.8%, SS: 10.1±1.5%) differed (RM-ANOVA stress+FC: interaction F(1,19)=8.88, p=0.008) (Figure 3D), indicating that stress+FC males that freeze at high levels during training also freeze more during extinction. Further, the extinction profile of high freezing stress+FC males differed from FC males (RM-ANOVA: interaction F(1,26)=13.96, p=0.001). Conversely, low freezing stress+FC mice were indistinguishable from their FC counterparts, suggesting the possibility of resilience (SR) to the effects of stress on fear memory. The same pattern of SS versus SR persisted into a remote memory test 30 days post-SEFL training, with SS males freezing more than both FC and SR males (ANOVA: F(2,35)=6.29, p=0.005; Post hoc Tukey tests: p<0.05 for SS vs. FC, p<0.05 for SS vs. SR; Figure 3D–E). The SS group also froze more than FC and SR groups in the ITI (ANOVA: F(2,35)=4.90, p=0.013; Post hoc Tukey tests: p<0.05 for SS vs. SR; Figure 3E), which may suggest generalization of fear beyond the specific auditory stressor association, a symptom present in PTSD patients.
Figure 3. Performance at the end of the fear conditioning portion of the SEFL protocol can be used to identify stress-susceptible males.

(A–B) Correlations between freezing during the last minute of FC training and average freezing over two days of extinction for FC and stress+FC males. Dotted line indicates trend. (C–D) Within each treatment group, animals were split into high (grey, red) or low (black, pink) categories based on the amount of time spent freezing during the final minute of fear conditioning training. Shown are the extinction profiles based on their training classification. Inset box identifies which animals from each treatment group comprise clusters identified in Figure 1, separated by training. (E) Average freezing during the tones or ITIs of a 5 tone recall session 30 days after SEFL training. Stress+FC animals were split into resilient (low training freezer, SR) and susceptible groups (high training freezer, SS). **p<.01, FC high n=10; FC low n=8, Stress+FC high n=10, Stress+FC low n=11. (F) Startle response of SEFL mice that did not undergo extinction in response to a 120 dB noise burst. Animals were identified as SS or SR by FC performance. **p<.01. Naïve n=8; FC n=11; Stress only n=8, SR n=11, SS n=7. Error indicates s.e.m.
The measures of training and extinction freezing did not correlate in stress+FC females (Supplemental Figure S2A–B). Accordingly, there were no differences in extinction profiles between animals that froze above and below the mean during training for either group (Supplemental Figure S2C–D). A qualitative assessment of sex differences suggests average freezing in females, regardless of stress history, resembled SS males in extinction and at the 30 day test, with both female groups freezing more than FC males (ANOVA Ext2: F(2,56)=9.77, p=0.0002; 30d: F(2,55)=9.42, p=0.0003; post hoc Tukey tests). This latter point suggests the lack of difference between females at 30 days may be due to incubation of fear in the controls.
To further characterize the validity of this SEFL paradigm of differential susceptibility in males as a model of PTSD-like behaviors, we examined startle response and anxiety without extinction (Figure 3F and Supplemental Figure S3). SS mice displayed elevated acoustic startle response (ASR) (Naïve, FC, Stress only, SR; ANOVA: F(4,40)=4.05, p=0.0075; post hoc Tukey tests: p<0.05 vs FC, p<0.05 vs. SR, p<0.05 vs. stress only; Figure 3F). This effect on SS arousal is consistent with work by others using ASR to identify SS outbred rats (11). Importantly, this further supports the predictive validity of using training to separate animals into SR and SS subgroups. Relative to naïve handled mice, a mildly “anxious” phenotype was observed in all other groups (restraint only, FC or stress+FC) in both open field and elevated plus maze (Supplemental Figure S3). Thus, this SEFL protocol produces an SS population in males with alterations in fear memory extinction and retention, and startle response, but not generalized anxiety.
Reducing the severity of the stressor decreases the likelihood of developing PTSD (50). And indeed, males stressed for one tenth of the duration (12min; Supplemental Figure S4) displayed no differences in extinction rate or retention between stress+FC and FC groups.
Independent replication of variable stress vulnerability in males
We next assessed reproducibility with two groups, the TSRI Behavior Core and the Shumyatsky Laboratory (51). We had no prior history of collaboration with the latter, located at Rutgers University. Using an electronic copy of the protocol, the TSRI Behavior Core (Supplementary Figure S5, top) and Shumyatsky Laboratory (Supplementary Figure S5, bottom) obtained results similar to core features seen by our group: (1) relationship between FC training and extinction specific to stress+FC males (TSRI FC Pearson=0.245, p=0.38; stress+FC=0.319, p=0.05; Shumyatsky FC Pearson=0.539, p=0.21, stress+FC=0.679, p=0.004; Supplementary Figure S5B, D, G, I); (2) segregation of the stress+FC, but not FC, population into SS and SR phenotypes (RM-ANOVA TSRI stress+FC: interaction F(1,36)=5.07, p=0.031; Shumyatsky stress+FC: interaction F(1,14)=10.56, p=0.006, Supplementary Figure S5E,J); and (3) higher freezing in SS relative to FC (RM-ANOVA SS vs FC, TSRI: interaction F(1,28)=2.51, p=0.124; Shumyatsky: interaction F(1,11)=15.99, p=0.002, Supplementary Figure S5E,J).
Heightened memory and CORT levels in SS males one month after SEFL
Given that PTSD is not manifested and rarely identified immediately after trauma, we examined the longer-term consequences of SEFL on memory and neuroendocrine function. SS males displayed heightened fear in response to 5 tones and ITIs compared to FC and SR animals 30 days post-SEFL training (Figure 4A; RM-ANOVA FC vs SS: interaction F(1,36)=15.13, p=0.001; SS vs SR: interaction F(1,37)=7.74, p=0.008; ANOVA ITI: F(2, 56)=4.82, p=0.012, post hoc Tukey tests: p<0.05 vs naive, p<0.05 vs FC, p<0.05 vs SR) (Figure 4B–C). Furthermore, SS mice had delayed extinction (RM-ANOVA FC vs SS: interaction F(1,19)=13.30, p=0.002; SS vs SR: interaction F(1,20)=5.96, p=0.024; Figure 4D). Stress+FC females also displayed enhanced freezing (RM-ANOVA: interaction F(1,30)=8.22, p=0.008) (Supplementary Figure S6). These data indicate this SEFL protocol produces long-lasting enhancement of fear memory in both SS males and stress+FC females.
Figure 4. Exaggerated remote memory and neurochemical dysregulation in SS mice.

(A) Male mice were tested for remote memory retrieval and underwent two days of extinction 30 days after SEFL training. (B–C) Fear expression during the first 5 tones and ITIs in FC and stress+FC (SR or SS) animals. *p<.05. (D) Extinction performance over the course of two days in a subset of animals. FC n=11, SS n=10, SR n=12. (E–F) CORT levels were measured at three time points after SEFL training in male mice that did not undergo extinction. ****p<.0001, ***p<.001. n=4–8/group. Error indicates s.e.m.
Because the robust response of females to FC, regardless of stress history, precluded the appearance of susceptible and resilient populations, we further characterized SS males. PTSD patients have enhanced suppression of cortisol in response to the synthetic glucocorticoid dexamethasone (dex) and heightened reactivity to stressful situations, indicating dysfunction of the hypothalamic pituitary axis (HPA) and neuroendocrine system (52). We assessed dex-induced suppression of CORT one month after SEFL (no extinction) by administering dex 6hrs before blood collection. This moderate dose was too effective, suppressing plasma CORT below the assay’s linear range of detection (data not shown). However, we also determined CORT levels in Naïve, FC, SR and SS males at three timepoints post-SEFL training: immediately, 4d or 30d. 6hrs prior to 4 and 30d collection, controls received saline injections to allow for comparison to the dex group (Figure 4E). No differences were observed in SEFL animals at 30min or 4d (ANOVA 30min: F(3, 22)=14.95, p<0.0001; post hoc Tukey tests: p<.05 vs naïve; 4d: F(3, 22)=2.52, p=0.085; Figure 4F), indicating CORT is unlikely to drive behavioral differences (Figures 1, 3). However, CORT was elevated at 30d in SS males relative to all other groups, suggesting HPA-associated effects of SEFL may increase over time (ANOVA 30d: F(3, 19)=8.93, p=0.0007; post hoc Tukey tests: p<0.05 vs naive, p<0.05 vs FC, p<0.05 vs SR).
Neuroanatomical and molecular systems implicated in PTSD are altered in SS males
To assess the neuroanatomical substrates that may contribute to the behavioral phenotypes of SS males, we analyzed Fos expression following a remote memory test (Figure 5A). Of the 14 regions analyzed, changes specific to SS animals were seen in two (Figure 5B–C and Supplemental Figure S7). Fos was decreased in the posterior IL (ANOVA postIL: F(3,22)=16.65, p<0.0001; post hoc Tukey tests: p<0.05 vs naive, p<0.05 vs FC, p<0.05 vs SR), a critical regulator of extinction (53). This hypoactivation is consistent with the extinction-resistance produced by the SEFL protocol in SS mice. Fos was increased in the BLA (ANOVA BLA: F(3,22)=70.67, p<0.0001; post hoc Tukey tests: p<0.05 vs naive, p<0.05 vs FC, p<0.05 vs SR), which participates in fear memory storage and expression (54–58). Importantly, a signature of PTSD is dysfunctional activation and connectivity of these regions (59, 60).
Figure 5. Differential recruitment of molecular pathways in SS and SR male populations.

(A) Overview of experimental design to examine Fos activation and transcriptional profiles in SEFL males after a 5 tone remote memory test (30 days post-training). (B–C) Fos+ cell counts in the posterior infralimbic cortex (postIL) and basolateral amygdala (BLA) after the 5 tone recall test and their corresponding representative micrographs. ****p<.0001. Stress only n=7, FC n=7–8, SR n=6–7, SS n=5. (D–G) BLA RNA from SS and SR male mice was sequenced to identify molecular changes associated with these behavioral phenotypes. (D) Significant genes that have the greatest fold change between SS and SR mice. Fold change and p-value were calculated with DESeq2. (E) Technical validation of sequencing data by qPCR for 5 differentially express genes: adcyap1 (adenylate cyclase activating polypeptide 1), drd2 (dopamine receptor 2), pdyn (prodynorphin), ptpn14 (protein tyrosine phosphatase, non-receptor type 14) and tac1 (tachykinin precursor 1). ****p<.0001, ***p<.001, *p<.05. n=3/group. (F) The top 15 functional annotations that are significantly different between SS and SR mice were identified by IPA analysis. (G) Known PTSD-associated genes that are differentially expressed between SS and SR mice. Fold change and corrected p-values were calculated with DESeq2. Error indicates s.e.m.
Given the importance of the amygdala in PTSD and memory persistence, as well as elevated Fos in SS males, we examined the BLA’s molecular profile in SS and SR mice by RNA-Seq with remote memory retrieval. Specificity of the BLA subdissection was first established by ensuring low expression of the central amygdala (CeA)-specific marker, Tac2 (61), compared to adjacent CeA punches (Supplemental Figure S8). RNA-Seq identified 61 differentially expressed genes (DEG) between SS and SR (Figure 5D). Nine were increased and 52 were decreased in SS mice (Figure 5D). Three genes, one that was upregulated in SS and two downregulated, were randomly selected for technical validation by qPCR, prodynorphin (pdyn), protein tyrosine phosphatase, non-receptor type 14 (ptpn14); and tachykinin precursor 1 (tac1). All three validated [Unpaired t-tests: pdyn: t(4)=4.59, p=0.010; ptpn14: t(4)=10.07, p=0.001; tac1: t(4)=3.76, p=0.020] (Figure 5E). The top 15 functional annotations identified by Ingenuity Pathway Analysis (Figure 5F) included relevant categories, (e.g. Cognition, Learning, Memory). A slightly more generous DEG cutoff (p<0.001) resulted in 194 DEGs. This included genes associated with neurochemical systems implicated in PTSD (Figure 5G) and three with polymorphisms in PTSD patients that also validated by qPCR: Adcyap1 (also known as PACAP; Unpaired t-test: t(4)=6.39, p=0.003; 62), Bdnf (63) and Drd2 (t(4)=2.96, p=0.041; 64, 65; Figure 5E).
Discussion
Existing PTSD models that employ predator odor or single prolonged stress (SPS) in outbred rats have reported susceptible and resilient populations through post-training phenotyping (11, 23, 24). However, these models often measure anxiety as a readout of PTSD-like behaviors and few protocols address both stress and the disorder’s prevalent memory component. Molecular characterization of susceptibility factors in these models has not been described, presumably due to the confound introduced by phenotypic tests required to identify the susceptible population.
By developing a SEFL paradigm in C57BL/6 mice, an inbred strain, with susceptibility to stress among females and a subgroup of males (identified without additional phenotyping), a well-annotated genome is available and a growing number of tools can be utilized (e.g. cell type-specific targeting, genetically-driven circuit tracing, CRISPR) for mechanistic PTSD-based research aimed at identifying causative and protective factors. The present results establish its construct and face validity for a number of PTSD-associated behavioral and neuromolecular pathologies. Indeed, FC studies in PTSD patients reported enhanced fear acquisition and startle response, which are thought to contribute to exaggerated fear responses and extinction impairments (66). PTSD-like phenotypes were observed here, in SEFL females and SS males, with stronger memory expression and generalization of a fear response beyond the discrete tone to the ITI. Further, SS males displayed enhanced startle and altered IL and BLA activation, regions strongly implicated in PTSD through functional imaging studies of patients (59). Importantly, the core behavioral results were replicated by two independent groups.
Although SEFL was not run in males and females at the same time, qualitative comparisons indicate that females were initially more sensitive to SEFL and did not segregate into resilient and susceptible populations. However, failure of training to correlate with extinction in SEFL females introduces the possibility they are expressing their response to foot shock through non-freezing behaviors. For instance, darting is described as a coping mechanism that correlates with extinction retention in female rats (67). Alternatively, females could be displaying active avoidance/escape behaviors. In contrast to SS males’ delayed extinction and poor extinction retention at every test, enhanced fear in SEFL females was restricted to extinction Day 1, regardless of test timing (4 or 30 days). Current efforts are exploring protocol adjustments with the goal of producing a temporally persistent difference between FC and stress+FC females, parameters that parse out SS and SR, and identification of relevant, nonfreezing behaviors, such as darting.
Development of an extinction-resistant “traumatic” memory in SS males is unique from FC alone, as well as in SR males that underwent identical SEFL training. This suggests recruitment of unique molecular processes; perhaps experience-dependent epigenetic modifications put in place prior to or during SEFL training (e.g. social hierarchy, stress adaptability). DNA methylation and histone modifications have been studied in the stress (68) and memory fields (13), and epigenetic interactions are likely after successive stressful events to confer resilience or susceptibility (27). Epigenetics may also impart gender-specific extinction impairments, as expression of DNA methyltransferase 3a confers resilience to “depression” in a variable stress paradigm (69). Indeed, RNA-Seq on BLA following a remote memory test one month post-SEFL training (Figure 5) revealed divergent transcriptional profiles between SS and SR males that we will interrogate further to identify epigenetic regulation and functional contribution. Among these were several PTSD-associated genes, either through polymorphisms associated with PTSD (Adcyap1 [Pacap], Bdnf and Drd2), or as members of neuromolecular systems known to be dysregulated in PTSD patients, such as catecholamines (TH, Drd1/2, DARP-32). In a PTSD metastudy, the rs1800497 polymorphism in DRD2 was associated with PTSD diagnosis (70). Bdnf and adcyap1 were also dysregulated in the amygdala of rats following a 15-shock SEFL protocol (45). We also identified DEGs not previously implicated in the PTSD. For example, tachykinin1, a gene downregulated in SS animals, is widely expressed in the brain and contributes to stress responses (71), but has not been explored in the context of “traumatic” memory. Because the human literature relies primarily on GWAS data that identifies SNP associations or DNA methylation changes and does not take into account dynamic transcript levels, the sequencing results presented here may help to identify novel regulators that have not been explored in PTSD.
Rather than resilience to the effects of stress, SR males may freeze less because they are employing active avoidance. While it cannot be fully discounted, resilience appears more likely because of the differences between SS and SR mice in other measures. SR mice did not display the hyperarousal, elevated CORT in response to a mild stressor, or dysregulation of IL or BLA Fos with remote memory that was seen in SS males. The behavioral and neuromolecular phenotype of SR mice is more consistent with an animal that has not experienced stress, simply fear conditioning.
We did not observe a strong anxiety-like phenotype in SS males. While anxiety is a symptom that PTSD patients may experience, PTSD was reclassified in the DSMV from an ‘Anxiety Disorder’ to a ‘Trauma and Stressor-related Disorder’. Moreover, the diagnosis criteria for PTSD specify that prolonged distress be exhibited in response to trauma-related cues, stimuli or reminders (72). Therefore, it is important that a PTSD-like model not cross over into a phenotype more akin to MDD (17). Therefore, we applied a single acute stressor to mitigate the possibility of creating an overly anxious or depressive-like phenotype. Further, our results are consistent with the findings of some rat SEFL studies demonstrating an incubation effect that occurs by temporally separating stress and FC (73), perhaps driven by the increase in amygdala spine density that occurs several days after restraint (74). Building on this, we have demonstrated that SS pathology continues to develop over the course of a month after SEFL training in males. Together, these data indicate that SS mice have prolonged behavioral and neurochemical changes related to stress and memory in ways relevant to PTSD. Selective perseverance of a “traumatic” memory in SS mice enables future studies to identify mechanisms that promote resilience and susceptibility, with the goal of ultimately pinpointing epigenetic and molecular targets that may be suitable for alleviating the persistence of traumatic memories in both genders.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institute of Mental Health MH105400 and MH105400-02 (Diversity Supplement) (CM), National Institute on Drug Abuse DA041469 (SS) and the Brain and Behavior Foundation-NARSAD Young Investigator Award (SS). We thank the Scripps Florida Genomics Core for sequencing services, Mohammad Fallahi and the Bioinformatics Core for data analysis, all members of the Miller and Rumbaugh Labs for their technical assistance and thoughtful discussions and Jan Tuma for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Delahanty DL, Raimonde AJ, Spoonster E, Cullado M. Injury severity, prior trauma history, urinary cortisol levels, and acute PTSD in motor vehicle accident victims. J Anxiety Disord. 2003;17(2):149–64. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 2.Boasso AM, Steenkamp MM, Nash WP, Larson JL, Litz BT. The relationship between course of PTSD symptoms in deployed U.S. Marines and degree of combat exposure. Journal of traumatic stress. 2015;28(1):73–8. doi: 10.1002/jts.21988. [DOI] [PubMed] [Google Scholar]
- 3.Association AP. Diagnostic and statistical manual of mental disorders. 5. Washington, D.C.: 2013. [Google Scholar]
- 4.Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93–100. doi: 10.1192/bjp.bp.114.148551. [DOI] [PubMed] [Google Scholar]
- 5.Alexander W. Pharmacotherapy for Post-traumatic Stress Disorder In Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents. P T. 2012;37(1):32–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71(2):134–68. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- 7.Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for Military-Related PTSD: A Review of Randomized Clinical Trials. Jama. 2015;314(5):489–500. doi: 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
- 8.Shalev AY, Ankri Y, Gilad M, Israeli-Shalev Y, Adessky R, Qian M, et al. Long-term outcome of early interventions to prevent posttraumatic stress disorder. J Clin Psychiatry. 2016;77(5):e580–7. doi: 10.4088/JCP.15m09932. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goswami S, Rodriguez-Sierra O, Cascardi M, Pare D. Animal models of post-traumatic stress disorder: face validity. Front Neurosci. 2013;7:89. doi: 10.3389/fnins.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen H, Matar MA, Joseph Z. Animal models of post-traumatic stress disorder. Curr Protoc Neurosci. 2013:45. doi: 10.1002/0471142301.ns0945s64. Chapter 9:Unit 9. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26(12):1110–7. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 13.Kwapis JL, Wood MA. Epigenetic mechanisms in fear conditioning: implications for treating post-traumatic stress disorder. Trends in neurosciences. 2014;37(12):706–20. doi: 10.1016/j.tins.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in neurosciences. 2012;35(1):24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and biobehavioral reviews. 2005;29(8):1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Maren S, Holmes A. Stress and Fear Extinction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41(1):58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriksen H, Olivier B, Oosting RS. From non-pharmacological treatments for post-traumatic stress disorder to novel therapeutic targets. Eur J Pharmacol. 2014;732:139–58. doi: 10.1016/j.ejphar.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Holly EN, Miczek KA. Capturing Individual Differences: Challenges in Animal Models of Posttraumatic Stress Disorder and Drug Abuse. Biological psychiatry. 2015;78(12):816–8. doi: 10.1016/j.biopsych.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013;38(9):1895–911. doi: 10.1016/j.psyneuen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361–82. doi: 10.1037/a0027649. [DOI] [PubMed] [Google Scholar]
- 21.Haskell SG, Gordon KS, Mattocks K, Duggal M, Erdos J, Justice A, et al. Gender differences in rates of depression, PTSD, pain, obesity, and military sexual trauma among Connecticut War Veterans of Iraq and Afghanistan. J Womens Health (Larchmt) 2010;19(2):267–71. doi: 10.1089/jwh.2008.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biological psychiatry. 2001;50(12):994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- 23.Toledano D, Gisquet-Verrier P. Only susceptible rats exposed to a model of PTSD exhibit reactivity to trauma-related cues and other symptoms: an effect abolished by a single amphetamine injection. Behav Brain Res. 2014;272:165–74. doi: 10.1016/j.bbr.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Le Dorze C, Gisquet-Verrier P. Sensitivity to trauma-associated cues is restricted to vulnerable traumatized rats and reinstated after extinction by yohimbine. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 25.McGuire JL, Bergstrom HC, Parker CC, Le T, Morgan M, Tang H, et al. Traits of fear resistance and susceptibility in an advanced intercross line. The European journal of neuroscience. 2013;38(9):3314–24. doi: 10.1111/ejn.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro-Gomes V, Bergstrom HC, McGuire JL, Parker CC, Coyner J, Landeira-Fernandez J, et al. A dendritic organization of lateral amygdala neurons in fear susceptible and resistant mice. Neurobiology of learning and memory. 2016;127:64–71. doi: 10.1016/j.nlm.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Blouin AM, Sillivan SE, Joseph NF, Miller CA. The potential of epigenetics in stress-enhanced fear learning models of PTSD. Neurobiology of learning and memory. 2016 doi: 10.1101/lm.040485.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadet JL. Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications. Mol Neurobiol. 2016;53(1):545–60. doi: 10.1007/s12035-014-9040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zannas AS, Provencal N, Binder EB. Epigenetics of Posttraumatic Stress Disorder: Current Evidence, Challenges, and Future Directions. Biological psychiatry. 2015;78(5):327–35. doi: 10.1016/j.biopsych.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Zannas AS, West AE. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience. 2014;264:157–70. doi: 10.1016/j.neuroscience.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumbaugh G, Sillivan SE, Ozkan ED, Rojas CS, Hubbs CR, Aceti M, et al. Pharmacological Selectivity Within Class I Histone Deacetylases Predicts Effects on Synaptic Function and Memory Rescue. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40(10):2307–16. doi: 10.1038/npp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA. Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res. 2012;232(1):37–43. doi: 10.1016/j.bbr.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 34.Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;44(4):338–45. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 35.Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiology of learning and memory. 2010;94(2):240–6. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Giachero M, Calfa GD, Molina VA. Hippocampal dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus. 2015;25(5):545–55. doi: 10.1002/hipo.22409. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD. Chronic stress, cyclic 17beta-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiology of learning and memory. 2010;94(3):422–33. doi: 10.1016/j.nlm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman AN, Lorson NG, Sanabria F, Foster Olive M, Conrad CD. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiology of learning and memory. 2014;112:139–47. doi: 10.1016/j.nlm.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiology of learning and memory. 2006;85(3):213–8. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Mitra R, Sapolsky RM. Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress. 2009;12(4):305–12. doi: 10.1080/10253890802379955. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(38):8725–34. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102(2):329–39. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 43.Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, et al. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–31. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Rosenkranz JA. Repeated restraint stress enhances cue-elicited conditioned freezing and impairs acquisition of extinction in an age-dependent manner. Behav Brain Res. 2013;248:12–24. doi: 10.1016/j.bbr.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponomarev I, Rau V, Eger EI, Harris RA, Fanselow MS. Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(6):1402–11. doi: 10.1038/npp.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biological psychiatry. 2015;78(5):E15–27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flint MS, Tinkle SS. C57BL/6 mice are resistant to acute restraint modulation of cutaneous hypersensitivity. Toxicol Sci. 2001;62(2):250–6. doi: 10.1093/toxsci/62.2.250. [DOI] [PubMed] [Google Scholar]
- 48.Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PloS one. 2015;10(2):e0117503. doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–61. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steenkamp MM, Dickstein BD, Salters-Pedneault K, Hofmann SG, Litz BT. Trajectories of PTSD symptoms following sexual assault: is resilience the modal outcome? Journal of traumatic stress. 2012;25(4):469–74. doi: 10.1002/jts.21718. [DOI] [PubMed] [Google Scholar]
- 51.McNutt M. Journals unite for reproducibility. Science. 2014;346(6210):679. doi: 10.1126/science.aaa1724. [DOI] [PubMed] [Google Scholar]
- 52.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40(6):550–67. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(15):3810–5. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M, Davis M. Lack of a temporal gradient of retrograde amnesia in rats with amygdala lesions assessed with the fear-potentiated startle paradigm. Behav Neurosci. 1993;107(6):1088–92. doi: 10.1037//0735-7044.107.6.1088. [DOI] [PubMed] [Google Scholar]
- 56.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110(4):718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. The European journal of neuroscience. 2005;22(7):1775–83. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learning & memory. 2007;14(9):634–44. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Rooij SJ, Kennis M, Vink M, Geuze E. Predicting Treatment Outcome in PTSD: A Longitudinal Functional MRI Study on Trauma-Unrelated Emotional Processing. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41(4):1156–65. doi: 10.1038/npp.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel R, Girard TA, Pukay-Martin N, Monson C. Preferential recruitment of the basolateral amygdala during memory encoding of negative scenes in posttraumatic stress disorder. Neurobiology of learning and memory. 2016;130:170–6. doi: 10.1016/j.nlm.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83(2):444–54. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Molecular psychiatry. 2014;19(1):8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 64.Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A, Noble EP, et al. The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress Anxiety. 2009;26(1):28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 65.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21(3):180–5. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 66.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of learning and memory. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(50):16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Bao Y, He S, Wang G, Guan Y, Ma D, et al. The Association Between Genetic Variants in the Dopaminergic System and Posttraumatic Stress Disorder: A Meta-Analysis. Medicine (Baltimore) 2016;95(11):e3074. doi: 10.1097/MD.0000000000003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–72. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- 72.Levin AP, Kleinman SB, Adler JS. DSM-5 and posttraumatic stress disorder. The journal of the American Academy of Psychiatry and the Law. 2014;42(2):146–58. [PubMed] [Google Scholar]
- 73.Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC. Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37(7):1588–99. doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


