Abstract
Epithelial cells lining the gastrointestinal tract require distinct apical and basolateral domains to function properly. Trafficking and insertion of enzymes and transporters into the apical brush border of intestinal epithelial cells is essential for effective digestion and absorption of nutrients. Specific critical ion transporters are delivered to the apical brush border to facilitate fluid and electrolyte uptake. Maintenance of these apical transporters requires both targeted delivery and regulated membrane recycling. Examination of altered apical trafficking in patients with Microvillus Inclusion disease caused by inactivating mutations in MYO5B has led to insights into the regulation of apical trafficking by elements of the apical recycling system. Modeling of MYO5B loss in cell culture and animal models has led to recognition of Rab11a and Rab8a as critical regulators of apical brush border function. All of these studies show the importance of apical membrane trafficking dynamics in maintenance of polarized epithelial cell function.
Epithelial cells lining the gastrointestinal (GI) tract serve diverse functions including absorption of nutrients and water and formation of a barrier against harmful substances and gut microbiota. The large surface area of the intestine allows for greater contact between the luminal contents and the epithelium, thereby providing efficient absorption of nutrients and water by enterocytes (Helander and Fandriks 2014). This expansive surface area is achieved by the presence of finger-like protrusions called villi. The surface is further amplified by the intestinal brush border of fully differentiated intestinal absorptive epithelial cells, which is composed of tightly packed microvilli of uniform height and diameter (Mooseker and Howe 1982). Microvilli are supported by a core of polarized bundles of actin grouped by villin (Bretscher and Weber 1978; Mooseker and Howe 1982; Crawley et al. 2014a), espin (Bartles 2000; Loomis et al. 2003), and fimbrin (also known as plastin-1) (Fig. 1) (Loomis et al. 2003). Proper membrane trafficking and structural components are necessary to assemble the microvilli of the intestinal epithelium and to facilitate the insertion of the correct compendium of apical transporters and enzymes into the apical brush border.
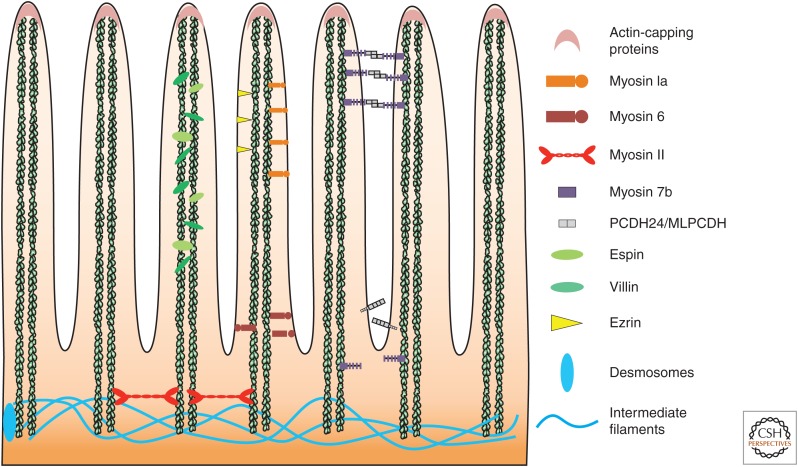
Figure 1.
Structure of the apical brush border. The intestinal microvilli consist of cores of actin filaments bundled by villin and espin. Cross-linking of the membrane to the actin cytoskeleton is regulated by ezrin and myosin 1a. Protocadherin-24 and mucin-like protocadherin form a trans-heterophilic adhesion complex that links the distal ends of microvilli for optimal packing and uniformity of the microvilli.
STRUCTURAL DETERMINANTS OF THE MICROVILLI
Villin
Villin is an epithelial cell-specific actin-modifying protein that is highly expressed in the brush border of the intestine. Villin regulates cell morphology, apoptosis, cell migration, actin capping and severing, actin nucleation, and actin filament bundle assembly. Villin influences microvilli morphology by bundling actin filaments (Bretscher and Weber 1979; Mooseker et al. 1980) in parallel with espin (Bartles et al. 1998; Bartles 2000) and fimbrin (Fig. 1) (Bretscher and Weber 1980). The resulting structure provides enough structural rigidity to deform the membrane, which could not be achieved by single actin filaments alone, while simultaneously increasing the number of force producing ends of actin (Mogilner and Rubinstein 2005; Claessens et al. 2006; Bathe 2008). The parallel actin filaments bundled by villin, espin, and fimbrin are believed to act as tracks for myosin motor proteins, moving cargo to the apex of the microvillar structure, enabling trafficking to the plasma membrane (Fig. 1).
Additionally, villin can remodel the actin cytoskeleton through its ability to sever actin (Ubelmann et al. 2013). Actin remodeling, bundling, capping of barbed end, or severing by villin is regulated by intracellular calcium concentrations and phosphorylation status (Bretscher and Weber 1980; Glenney et al. 1980, 1981). Phosphorylation of tyrosines in villin also regulates villin severing and bundling actions (Zhai et al. 2001, 2002). The dynamic roles of villin in modulating the brush border suggest an essential role for villin in intestinal homeostasis. Interestingly, villin-null mice have no overt alterations in the ultrastructure of the intestinal microvilli (Ferrary et al. 1999). Even more surprising, triple knockout mice lacking villin, espin, and fimbrin (plastin 1) develop microvilli although the brush border is disorganized (Revenu et al. 2012). Collectively, these in vivo models suggest that, although these bundling proteins might stabilize actin bundles in microvilli, the formation of microvilli is not strictly dependent on their actions.
Ezrin
Ezrin (also known as cytovillin or villin-2), radixin, and moesin proteins compose a group of proteins known as ERM proteins. ERM proteins organize complex membrane domains through their ability to act as regulated cross-linkers between the apical membrane and the actin cytoskeleton (Fig. 1) (Fehon et al. 2010). These proteins are composed of three distinct regions: an amino-terminal FERM domain, an α-helical domain, and a carboxy-terminal ERM association domain (C-ERMAD) (Algrain et al. 1993; Chisti et al. 1998). The C-ERMAD domain binds F-actin, whereas the FERM domain tethers ezrin to the plasma membrane and is also responsible for binding other proteins in the apical membrane (Turunen et al. 1994). Ezrin is the only ERM expressed in the intestinal epithelium. In its dormant closed state, Ezrin resides in the cytoplasm with its F-actin and membrane-binding sites masked by an intramolecular interaction (Gary and Bretscher 1995). Ezrin is recruited to the plasma membrane via its phosphatidylinositol 4,5-bisphosphate (PIP2)-binding region on the FERM domain (Barret et al. 2000). Once near the plasma membrane ezrin phosphorylation at threonine residue 567 on the C-ERMAD domain releases the closed confirmation by reducing the affinity between the FERM and C-ERMAD domains (Nakamura et al. 1995). This allows ezrin to bind the cytoskeleton and interact with the nearby plasma membrane in its active confirmation. Mst4 appears to be the major kinase responsible for ezrin phosphorylation in intestinal epithelial cells (ten Klooster et al. 2009; Gloerich et al. 2012).
In its open conformation ezrin is capable of binding numerous apical membrane proteins including CD44, CD43 intercellular adhesion molecule 2, ERM-binding phosphoprotein 50 (EBP50 also known as NHERF1), and NHE3 kinase A regulatory protein (E3KARP or NHERF2) (Fehon et al. 2010). EBP50 increases the diversity of proteins associated with ezrin by binding additional proteins through its PDZ domains (Fig. 2). Well-known examples of these proteins include NHE3, CFTR, β2-adrenergic receptor, PDGFR, EGFR, and PDZK1. Ezrin may be necessary for the trafficking and retention of apical transporters NHE3 and CFTR in the apical membrane (Bagorda et al. 2002). Ezrin’s ability to alter dynamically components of the plasma membrane suggests that it is responsible for orchestrating signaling pathways by positioning cytoskeletal regulatory proteins in close proximity to downstream signaling components. Ezrin-null mice show short microvilli and abnormal villi architecture indicating that ezrin plays an important role in development and maintenance of the intestinal brush border (Saotome et al. 2004).
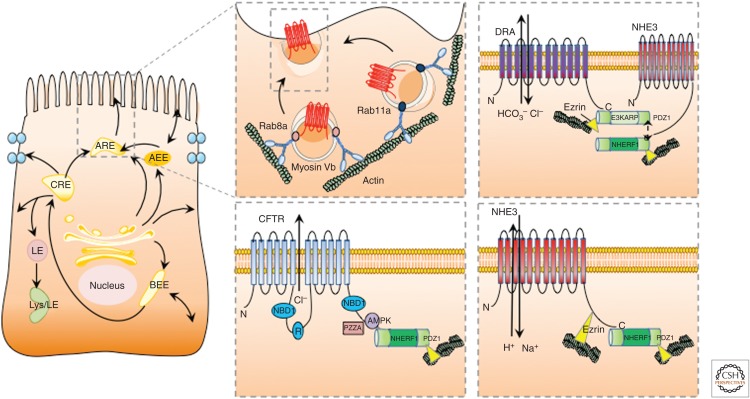
Figure 2.
Trafficking to the apical brush border. On exit from the trans-Golgi network, proteins are targeted for trafficking to the apical, basolateral, or lateral membrane. Apically targeted proteins can be directed to the apical early endosome (AEE) or the apical recycling endosome (ARE) before being delivered to the plasma membrane. Proteins taken up by the cell from the apical membrane can be delivered to the ARE for recycling back to the apical surface or can be taken to the common recycling endosome and late endosome if targeted for degradation.The motor protein, myosin Vb, restricts Rab8a- and Rab11a-containing vesicles within subapical F-actin-containing domains. Cystic fibrosis transmembrane conductance regulator (CFTR), sodium hydrogen exchanger isoform 3 (NHE3), and down-regulated in adenoma (DRA) are transported to the apical membrane for insertion and are reported to interact directly and/or indirectly with ezrin. LE, Late endosome; lys, lysosome; BEE basolateral early endosome.
Myosin 1a
Similar to the ERM proteins, Myosin 1a (myo1a) appears to cross-link the plasma membrane to the underlying actin core of intestinal microvilli (Fig. 1) (Mooseker and Tilney 1975; Howe and Mooseker 1983). Myosin Ia, a class I myosin, is the most abundant class I myosin in the intestinal brush border (McConnell et al. 2011). Myosin 1a interacts with the membrane via a tail homology 1 (TH1) domain that has a moderate affinity for acidic phospholipids (Mooseker and Cheney 1995). Two TH1-membrane-binding motifs (the amino- and carboxy-terminal-binding motifs) create a close proximity between Myosin 1a and the membrane (Mazerik and Tyska 2012), thus ensuring proper motor function. Myosin 1a is required for the maintenance of sucrose isomaltase in the brush border (Tyska and Mooseker 2004). Tyska and colleagues showed the in vivo function of myosin 1a through the generation of myosin 1a–null mice (Tyska et al. 2005). This work showed that myosin 1a is important for microvillar membrane morphology and organization of the brush border. Interestingly, myosin 1c, another short-tailed class I motor, was recruited to the brush border of myosin 1a knockout enterocytes showing functional redundancy among myosin I isoforms and further indicating their importance in the composition and structure of the brush border (Tyska et al. 2005).
Protocadherin-24 (PCDH24)
Extracellular adhesion molecules play an important role in creating a highly ordered intestinal brush border. Microvilli achieve an organized, tightly packed brush border via the linking of microvilli to each other via thread-like links. These links are composed of the cadherin family members, protocadherin-24 (PCDH24), and mucin-like protocadherin (MLPCDH) (Fig. 1) (Crawley et al. 2014b). PCDH24 and MLPCDH form a trans-hetereophilic adhesion complex at the tips of microvilli to physically attach microvilli to each other, facilitating an organized brush border (Crawley et al. 2014b). Knockdown of PCDH24 impaired brush border assembly with disordered microvilli of variable length and fewer microvilli on the apical surface (Crawley et al. 2014b). These findings suggest that intermicrovillar bridges may be critical stabilizers of the enterocyte brush border.
DIRECTIONAL TRAFFICKING OF TRANSPORTERS AND ENZYMES
Intestinal epithelial cells show a polar structure with an apical plasma membrane facing the lumen and a basolateral plasma membrane facing the lamina propria and blood. These two membranes differ in terms of protein and lipid compositions and in the mechanism of transport. Proteins synthesized in the rough endoplasmic reticulum are modified and sorted in the trans-Golgi network (Fig. 2) (Jacob and Naim 2001; Ang et al. 2004). In the trans-Golgi network, proteins are segregated into distinct vesicle populations for membrane delivery based on their sequences or posttranslational modifications (Jacob et al. 1999; Zheng et al. 1999). Proteins can be directed to the apical plasma membrane, basolateral plasma membrane, apical and basolateral sorting endosomes, early/sorting endosomes, late endosomes, apical recycling endosomes, secretory granules, and other select locations. Given the multitude of potential target locations, each cargo must have a distinct set of sorting signals as well as specific docking and fusion factors that are complementary to the acceptor compartment.
Basolaterally transported proteins contain a number of unique amino acid sequences such as tyrosine-based (NPxY or YxxØ), dileucine (D/ExxxLL), or single leucine motifs in their cytosolic domain (Casanova et al. 1991; Hunziker et al. 1991; Le Bivic et al. 1991; Matter et al. 1992; Hunziker and Fumey 1994). In contrast, apical sorting signals occur in the extracellular, the transmembrane, or the cytosolic domain of transmembrane proteins (Scheiffele et al. 1995; Yeaman et al. 1997; Chuang and Sung 1998; Lin et al. 1998; Alfalah et al. 1999). These signals are diverse and can be comprised of oligosaccharides, lipids, or peptide motifs. Some proteins possess N- or O-linked glycans, which are required to convey them to the apical cell membrane (Rodriguez-Boulan and Gonzalez 1999). Protein can also be modified at the carboxyl terminus with glycolipid glycosylphosphatidylinositol (GPI). Cytoplasmic apical sorting signals have been identified, but currently they remain largely uncharacterized (Altschuler et al. 2003; Marzolo et al. 2003; Takeda et al. 2003). Depending on their directional signals, proteins interact with sorting receptors or sorting platforms, which further facilitate their delivery. Small GTPases of the ARF and Rab families, adaptors, GRIP-golgins, and lipids (phosphoinositides, sphingolipids, and cholesterol) serve as key interacting systems. Glycosylated apical cargo can assemble into high-molecular-weight clusters in the presence of the glycan recognizing lectin galectin-3 (Delacour et al. 2007), which promotes transport to the apical membrane. GPI-anchored proteins associate with cholesterol-rich lipid structures, also known as lipid rafts, which serve as sorting platforms for the apical membrane (Simons and Ikonen 1997). Of note, select GPI-anchored proteins require N-glycans to localize apically implicating the possibility of multiple signals cooperatively directing proper apical delivery (Benting et al. 1999).
TRAFFICKING OF APICAL ION TRANSPORTERS IN INTESTINAL ENTEROCYTES
Sodium Hydrogen Exchanger (NHE3)
Na+/H+ exchanger isoform 3 (NHE3, SLC9A3) is an apical sodium-hydrogen transporter found on epithelial cells of the small and large intestine. It is responsible for the majority of NaCl and water absorption that occurs in the intestine (Hoogerwerf et al. 1996; Schultheis et al. 1998). NHE3 consists of two functional domains: the amino and carboxyl terminus. The amino terminus conducts Na+ and H+ exchange, whereas the carboxyl terminus controls the transport rate (Donowitz et al. 2009). It has been speculated that NHEs, including NHE3, may act as dimers with a 2Na+:2H+ stoichiometry (Fuster et al. 2008). NHE3 is normally anchored into the plasma membrane by interaction with the cytoskeleton. NHE3 is known to bind directly to ezrin (Cha et al. 2006; Cha and Donowitz 2008) and bind to the NHERF family of multi-PDZ domain proteins (Fig. 2) (Yun et al. 1997).
Under normal conditions, NHE3 exists in a partially activated state (Brett et al. 2005). During the digestive period, it is speculated that NHE3 is inhibited to increase luminal water and expand the range of digestive enzymes (Donowitz et al. 2009). However, later in the digestive process, NHE3 is likely activated to maintain volume and pH homeostasis, which aid in water and nutrient absorption (Anderson et al. 2004; Anderson and Thwaites 2005; Thwaites and Anderson 2007). In addition to the roles of NHE3 in the plasma membrane, NHE3 is also involved in endosome acidification via a cytoplasmic Na+ gradient (Gekle et al. 1999, 2001). The importance of NHE3 for water absorption was highlighted using animal knockout studies. NHE3 knockout mice show watery diarrhea (Schultheis et al. 1998; Noonan et al. 2005), an altered gut microbiota (Engevik et al. 2013; Larmonier et al. 2013), and a shortened life span (Schultheis et al. 1998).
Rapid regulation of NHE3 is integral to intestinal homeostasis. Short-term regulation of NHE3 relies on alterations in NHE3 trafficking, which controls the concentration of plasma membrane NHE3. Acute control of NHE3 also occurs via changes in the transporter Vmax and K′(H+)i (Levine et al. 1993). Prolonged regulation of NHE3 uses two distinct trafficking mechanisms, both involving NHE3 phosphorylation. One mechanism involves regulated alterations in transporter endocytosis or exocytosis, whereas the other involves alterations in turnover number (D’Souza et al. 1998; Janecki et al. 1998, 2000; Kurashima et al. 1999; Akhter et al. 2000; Hu et al. 2001).
Previous studies have shown that the NHE3 carboxyl terminus is required for all NHE3 regulation described (Zachos et al. 2005; Donowitz and Li 2007). Truncated NHE3 was found to transport Na/H, but was not regulated (Levine et al. 1995). Interestingly, the carboxyl terminus of NHE3 is known to behave as a scaffold and bind proteins involved in NHE3 regulation (Donowitz and Li 2007). These regulator proteins include ezrin, NHERF1, 2, 3, 4, CaM kinase II (CaM KII), CaM, CK2, phospholipase Cγ (PLCγ), CHP, megalin, IP3-receptor-binding protein (IRBIT), Shank2, and PP2A (Yun et al. 1997; Biemesderfer et al. 1999; Girardi et al. 2001, 2004, 2008; Pang et al. 2001; Di Sole et al. 2004; Ben Ammar et al. 2005; Ammar et al. 2006; Han et al. 2006; He et al. 2008; Sarker et al. 2008). Activation or inhibition of NHE3 activity alters the assembly of NHE3 interacting proteins, also known as NHE3 complexes, indicating the NHE3 complexes show dynamic behavior on signaling (Agbemafle et al. 2005). Abnormal inhibition of NHE3 exists in acute inflammation, chronic inflammatory bowel diseases (IBD), and diarrheal disease (Hecht et al. 2004; Sullivan et al. 2009; Engevik et al. 2015), highlighting the key role of NHE3 in intestinal homeostasis.
Down-Regulated in Adenoma (DRA)
The chloride bicarbonate exchanger known as down-regulated in adenoma (DRA or SLC26A3) is an integral membrane transporter located on intestinal epithelial cells. DRA and NHE3 are responsible for electroneutral absorption of NaCl in the intestine. In humans loss of function mutations in DRA results in congenital chloride diarrhea, showing the important role that DRA plays in maintaining balanced ion transport (Hoglund et al. 1996). DRA insertion into the plasma membrane of enterocytes is dependent on the interaction with PDZ adaptor proteins, association with lipid rafts, lipid raft integrity, and the action of PI3-kinase (Fig. 2) (Lissner et al. 2010). DRA undergoes endocytic recycling through early endosomes where it is sorted and returned to the apical membrane (Lissner et al. 2010). In vitro inhibition of endocytosis using Dynasore, a dynamin inhibitor, results in retention of DRA in the plasma membrane. The maintenance of DRA on the cell surface promotes increased association with lipid rafts and enhanced activity of DRA. Furthermore, disruption of microtubules both in vitro and in vivo in mice decreased apical DRA expression through decreased exocytosis showing the need for intact microtubules for proper expression of DRA (Gujral et al. 2015). DRA is also known to bind to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), thereby linking intestinal Cl−/HCO3− exchange to Na+/H+ exchange (Lamprecht et al. 2002).
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
The cystic fibrosis transmembrane conductance regulator (CFTR) (Fig. 2) is responsible for chloride and bicarbonate secretion in intestinal epithelia, airway, and exocrine glands. Within the intestinal epithelium, approximately one-third of the total amount of CFTR is located on the apical membrane of enterocytes, whereas the larger population of CFTR resides in subapical vesicles in the cytoplasm (Ameen et al. 2007). CFTR is cAMP-activated and regulated by phosphorylation. There are four main phases of CFTR trafficking. These include (1) biosynthesis of CFTR, folding and glycosylation of CFTR in the ER and transport from the ER to the Golgi, and, finally, insertion into the plasma membrane. (2) Once located at the plasma membrane CFTR can be endocytosed and trafficked to early endosomes. Internalized CFTR from the plasma membrane can then be (3) recycled directly back to the apical membrane or sent to recycling endosomes, or (4) can be sent to the lysosome for degradation (Ameen et al. 2007).
Correctly folded, glycosylated CFTR exits the Golgi and enters the exocytic pathway for final transport and insertion into the plasma membrane (Ameen et al. 2007). Insertion of CFTR into the plasma membrane and endocytosis are both mediated by the cAMP-dependent protein kinase A (PKA) (Lukacs et al. 1997; Bertrand and Frizzell 2003). Rab11a interacts with the motor protein Myosin 5b and CFTR, facilitating the trafficking of CFTR to the cell surface from recycling endosomes (Prince et al. 1994; Swiatecka-Urban et al. 2007). Internalization of fully functional CFTR occurs rapidly (Bradbury et al. 1994; Prince et al. 1994; Lukacs et al. 1997; Weixel and Bradbury 2000) and CFTR is efficiently recycled back to the plasma membrane, with ∼50% of internalized CFTR being returned to the cell surface (Swiatecka-Urban et al. 2002; Picciano et al. 2003). Chloride secretion stimulated through the cAMP/PKA pathway results in activation of CFTR on the cell surface as well as trafficking of CFTR from intracellular pools that reside in storage vesicles below the plasma membrane (Prince et al. 1999; Swiatecka-Urban et al. 2007).
DEFECTS IN APICAL RECYCLING SYSTEM FUNCTION IN NEONATAL DIARRHEA: LESSONS FROM PATHOPHYSIOLOGY
Studies over the past decade have shown that membrane trafficking proteins involved in recycling membrane to the apical surface of epithelial cells are also responsible for defining the apical lumen surface. These studies have led to the recognition that Rab8a, Rab11a, and MYO5B are all involved in specifying apical lumen formation (Bryant et al. 2010; Roland et al. 2011). These investigations, mostly in MDCK cells, have identified the process of transcytosis, in particular of podocalyxin, which sets up the establishment of epithelial polarity (Mrozowska and Fukuda 2016). Nevertheless, the exact trafficking regulators appear to be distinct depending on culture techniques (Mrozowska and Fukuda 2016). The regulators may also differ based on the particular epithelia involved and the types of apical transporters and apical membrane specializations that are elaborated. In the intestinal epithelium, this trafficking requires both assembly of the apical brush border and the appropriate delivery of apical enzymes and transporters to microvilli. The construction and maintenance of microvilli coincides with coordinated Rab8a- and Rab11a-dependent apical trafficking coupled to MYO5B, which facilitates proper microvilli growth through trafficking of key microvillar components (Sato et al. 2007; Knowles et al. 2014).
Microvillus Inclusion Disease
Much progress in this field has emanated from studies of patients with neonatal diarrhea from Microvillus Inclusion disease (MVID). MVID was initially characterized in 1978 in newborns experiencing chronic, unremitting, secretory diarrhea (Davidson et al. 1978). Studies of these patient biopsies from the small intestine displayed significant villus blunting, crypt hypoplasia, absence of microvilli, malabsorption of glucose, and defective sodium transport. In 2008, reports from Austria and the United States showed that inactivating mutations in the MYO5B gene caused MVID (Erickson et al. 2008; Muller et al. 2008). Numerous mutations in the MYO5B gene have now been identified in MVID patients (van der Velde et al. 2013). Loss of functional MYO5B leads to delocalization of Rab8a and Rab11a from the apical domain of enterocytes in association with loss of ezrin and deficits in microvillar brush border and microvillar trafficking (Fig. 3) (Roland et al. 2011; Knowles et al. 2014). Loss of apical microvilli and transporters in MVID patients has also been associated with decreases in polarity regulators as well as activated cdc42 (Knowles et al. 2014; Michaux et al. 2016).
Figure 3.
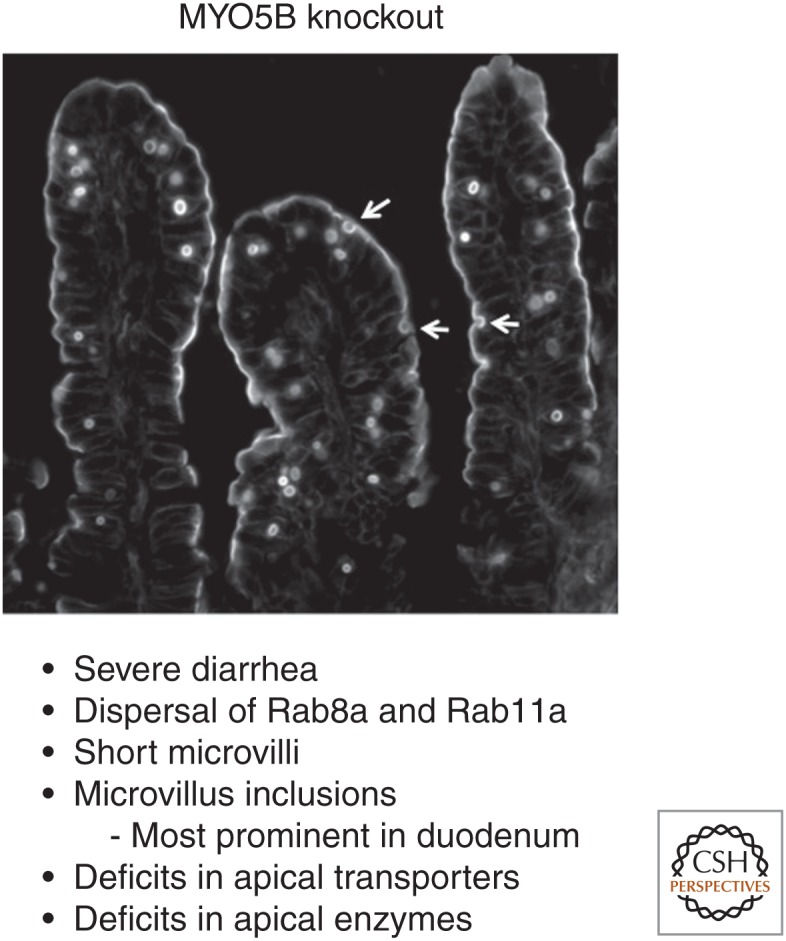
Altered trafficking associated with deletion of MYO5B in intestinal enterocytes. Loss of myosin Vb (MYO5B) in MYO5B germline-null mice results in the formation of inclusions from the apical cell surface of intestinal enterocytes. Phalloidin staining shows the presence of intracellular inclusions positive for F-actin, showing that the inclusions originate from the brush border. Arrows indicate the formation of inclusions by invagination of the plasma membrane. F-actin staining at the apical membrane is diminished but still presents in short microvilli.
The vast majority, if not all, of MVID patients have disabling mutations in MYO5B that disrupt the function of this motor or induce premature termination before the tail domain (Muller et al. 2008; Ruemmele et al. 2010; Szperl et al. 2011; van der Velde et al. 2013). Recently, mutations in STX3 and STXBP2 (Munc18-2) have been implicated as the causative agents for what has been termed an atypical form of MVID (Muller et al. 2008; Stepensky et al. 2013; Wiegerinck et al. 2014), characterized by loss of apical microvilli, but additionally with lateral microvilli formation. Thus, this anomaly really represents a separate class of trafficking defects, distinct from MVID. Interestingly, previous studies have identified STX3 as a critical regulator of apical recycling in gastric parietal cells (Calhoun et al. 1998; Karvar et al. 2005; Lapierre et al. 2007). These rare but devastating neonatal diarrhea syndromes show that aberrations in apical trafficking can have functional implications for epithelial cell physiology. It is important to note however that, although MYO5B and STX3 are ubiquitously expressed in multiple epithelial cells, these children manifest their major symptoms focally in the intestine. Thus, some epithelial cells must be able to compensate for loss-of-function mutations in apical trafficking proteins better than others.
Cell Culture Modeling of MVID
A number of studies have attempted to model MYO5B loss in either parental Caco-2 cells or in the Caco-2BBE cell line that was selected as a subclone with highly developed brush border (Peterson and Mooseker 1992). These studies have provided varying levels of confirmation of findings in patient samples. Alterations in brush border structure and maturation have been identified in Caco-2 cells with knockdown of MYO5B expression or CRISPR-mediated gene mutation (Muller et al. 2008; Thoeni et al. 2013; Dhekne et al. 2014; Knowles et al. 2014; Kravtsov et al. 2014, 2016; Vogel et al. 2015). All of these studies have observed either disruption or denudation of the microvilli attendant with loss of MYO5B expression in Caco-2 cell lines. These studies have also documented alterations in apical resident proteins including DPP-IV and alkaline phosphatase. Although NHE3 trafficking to the apical membrane appears to be impaired by loss of MYO5B, there has been controversy over whether CFTR is lost from the apical membrane with MYO5B knockdown (Vogel et al. 2015; Kravtsov et al. 2016). Some of the variability in findings may be because of variable expression of NHE3, CFTR, and DRA, in particular, Caco-2 cell lines. It is notable that none of these transporters are expressed at the high levels observed in duodenal enterocytes.
The observation of microvillus inclusions has varied among the studies of Caco-2 cells. In studies using parental Caco-2 cells, which are a mixture of cell clones, some inclusions were observed. In Caco-2BBE cells with knockdown, however, inclusions were not readily apparent, even though trafficking defects were readily observable. Knowles et al. (2014) did observe inclusions when either the P660L Navajo mutation or a MYO5B mutation that could not bind Rab11a (MYO5B(YE/QR)) were re-expressed. All of these studies also showed varying degrees of losses of polarity. Alterations included redistribution of E-cadherin to the apical membranes, changes in claudins in tight junctions, and alterations in cdc42 activation and Par protein distribution.
MOUSE MODELS OF TARGETED DELETION OF TRAFFICKING REGULATORS IDENTIFY COMMON THEMES FOR REGULATION OF THE ENTEROCYTE APICAL BRUSH BORDER
Over the past 5 years, a number of investigations have examined the effects of targeted deletion of putative regulators of apical trafficking. These new mouse models have included intestinally targeted deletion of cdc42, MYO5B, Rab8a, and Rab11a. All of these models have shown somewhat different phenotypes that can be merged to make important conclusions about the pathways that regulate how the brush border components are assembled and how they are maintained.
Mouse Models of MVID
Mouse models have been developed to model inactivation of MYO5B observed in children with MVID. These models have sought to evaluate the effects of either germline or intestinally targeted deletion of MYO5B. Two models of germline deletion have been reported. In one with gene trap deletion of MYO5B, mice died soon after birth, but did show short microvilli and a failure to deliver apical enzymes to the apical membrane (Carton-Garcia et al. 2015). Complete examination of these mice was hampered by their early postnatal demise. A second gene-interruption germline deletion on a partial CD-1 background allowed analysis of MYO5B-null mice out to 5–7 d postbirth (Weis et al. 2016). As in the gene trap model, the mice showed short microvilli and deficits in the delivery of apical enzymes and transporters to the brush border. The mice also displayed numerous microvillus inclusions in the duodenal enterocytes (Fig. 3). Both germline knockout models noted the presence of large numbers of membrane vesicles below the apical membranes of MYO5b-null mice, and expansion of lysosomes often associated with microvillus inclusions was also noted. Importantly, in MYO5B-null mice the normal distribution of Rab11a- and Rab8a-containing vesicles below the apical membrane was not observed. This dispersal of Rab11a- and Rab8a-containing vesicles was also associated with a loss of subapical activated cdc42 in Caco-2-BBE MYO5B knockdown cells (Knowles et al. 2014). Interestingly, examination of the distribution of microvillus inclusions and apical transporters suggested that the observation of microvillus inclusions and losses in apical transporters was attenuated in the distal intestines of MYO5B-null mice (Weis et al. 2016). No apparent effects were observed in the colonic mucosa.
Intestinally directed deletions of MYO5B have also been reported. Constitutive deletion of MYO5B using Villin-Cre on a C57BL/6 background led to a phenotype in the intestinal enterocytes that was essentially identical to the germline-null mice with short microvilli, loss of duodenal transporters, severe diarrhea, and early demise by 5–7 d of age (Weis et al. 2016). The constitutive intestinal deletion mice also showed attenuation of microvillus inclusions in the distal intestine. Two models of inducible MYO5B deletion directed by tamoxifen inducible Villin-CreERT2 showed similar induction of diarrhea and loss of apical transporters in adult enterocytes (Schneeberger et al. 2015; Weis et al. 2016). The appearance of microvillus inclusions in these models appears controversial. Although Weis et al. saw only few microvillus inclusions following induction of deletion in adults, Schneeberger et al. reported their presence after tamoxifen. Importantly, Weis et al. (2016) showed significant numbers of inclusions if neonatal mice were induced with tamoxifen, so the differences in these observations may relate to the level of maturity at the time of MYO5B deletion. Weis et al. have provided evidence that microvillus inclusions form from invaginations of the apical plasma membrane into apical bulk endosomes, a process that appears to be enhanced to the postnatal, preweaning period in duodenal enterocytes (Knowles et al. 2014; Weis et al. 2016). Importantly, these results suggest that, although the microvillus inclusions are pathognomonic for MVID, the true diarrheal pathology may relate more to the lack of delivery and maintenance of apical transporters in enterocytes.
It is important to compare the results that have been identified in these mouse models of MYO5B deletion with those observed in both human MVID patient material and in Caco-2 cell–modeling studies. These studies are highly relevant to the interpretation of alterations in polarity. All of the studies in human tissue, Caco-2 cell lines, and in mouse models of MYO5B deletion have shown prominent alterations in the presence of appropriate transporters and enzymes in the apical brush border. Thus, there is agreement concerning an alteration in apical trafficking associated with loss of MYO5B. In contrast, marked disruption of the brush border microvillus integrity or maturation was observed in both patient samples (at least in the enterocytes at the tips of intestinal villi) and in Caco-2 cells. But, whereas microvilli in the MYO5B-null enterocytes were short, they were assembled into an organized apical brush border (Weis et al. 2016). Similarly, both Caco-2 cells and human MVID duodenal biopsies have indicated that more gross alteration in cell polarity are present after MYO5B loss, including alterations in E-cadherin distribution and changes in intercellular junctions (Knowles et al. 2014). In contrast, the predominance of the data in MYO5B-null mice did not show alterations in E-cadherin or remarkable alterations in cell junctions (Carton-Garcia et al. 2015; Weis et al. 2016). Thus, the pathology related to loss of MYO5B seems dominated by deficits in trafficking. In the case of Caco-2 cells, whether parenteral or the more selected Caco-2BBE lines, more impressive loss of brush border structure may suggest that other inherent trafficking defects are present, as might be expected in a colon cancer cell line whose phenotype mimics duodenal enterocytes. In the case of the human tissue samples from MVID patients, the differences may relate to the timing of observations. In the case of infants with MVID, initial duodenal biopsies are usually not taken until 6–8 wk of life after long-term support on total parenteral nutrition, usually without the presence of enteral feedings. Thus, the phenotype represents a hybrid view of the effects of the loss of MYO5B along with the effects of villus atrophy because of a lack of enteral feeding. Because loss of junctional integrity has been associated with fasting (Feng et al. 2009), a process that might be exacerbated by MYO5B loss, the observed alterations in E-cadherin and junctional proteins may represent a treatment epiphenomenon. In contrast, observations in mouse models are made in untreated subjects at their initial presentation in the early postnatal period.
Other Mouse Models of Trafficking Protein Regulator Deletion
Although not directly linked to specific mutations in humans, a number of mouse deletion models have recently examined the roles of specific deletions in putative trafficking regulators on the intestinal enterocyte. An interesting commonality of all of these mouse models is that they produce enterocytes with short microvilli. Constitutive (Villin-Cre) targeted deletion of Rab8a in enterocytes leads to severe diarrhea and early demise in mice with the observation of microvillus inclusions and numerous vesicular lysosomal elements in the subapical region of cells. Although these mice showed intact, albeit short, microvilli they also showed alterations in the delivery of apical transporters to the brush border (Sato et al. 2007). A similar phenotype was also observed in mice with intestinally directed deletion of cdc42 (Sakamori et al. 2012). In contrast, intestinally directed deletion of Rab11a led to short apical microvilli as well as ectopic microvilli assembled at the lateral membrane (Knowles et al. 2015). These findings appeared to more closely match the phenotype observed in children with truncation mutations in Syntaxin-3 (Wiegerinck et al. 2014). Interestingly, microvillus inclusions were not observed in these animals, although accumulation of lysosomal membranes was observed (Knowles et al. 2015). In these mice, the most prominent phenotypes appear to relate to alterations in vesicle-trafficking organization and alterations to delivery of cargoes to the appropriate membranes (Yu et al. 2014; Knowles et al. 2015). Taken together, all of these studies show that an orderly coordination of Rab11a- and Rab8a-dependent trafficking pathways is necessary for assembly and maintenances of an organized functional enterocyte brush border.
REMAINING QUESTIONS IN THE PATHOLOGY OF INTESTINAL POLARITY
It is perhaps impressive that deletion or mutation of trafficking proteins can elicit relatively small alterations in enterocyte function. It is indeed surprising that a truncation mutation of Syntaxin-3 that eliminates its association with membranes would have a clinical phenotype that is less prominent than that for patients with inactivating mutations in MYO5B. It would thus appear that the intestinal mucosa has a strong ability to overcome deficiencies and maintain the epithelial mucosal function. This may occur through adaptation in different gut regions, but also may accrue from compensation by parallel trafficking pathways. Thus, the unusual impact of MYO5B mutations may reflect both a lack of compensatory pathways caused by lack of MYO5A expression in enterocytes as well as the disruption of both Rab11a- and Rab8a-dependent trafficking pathways by MYO5B mutations. It also seems likely that the present standard of care, which uses a lack of enteral feeding to control diarrhea in neonates, likely promotes exacerbation of trafficking defects in enterocytes. Thus, future studies must focus on reactivation of proper trafficking pathways or engagement of adaptation pathways that can compensate for deficits in particular apical transporters.
Footnotes
Editor: Keith E. Mostov
Additional Perspectives on Cell Polarity available at www.cshperspectives.org
REFERENCES
- Agbemafle BM, Oesterreicher TJ, Shaw CA, Henning SJ. 2005. Immediate early genes of glucocorticoid action on the developing intestine. Am J Physiol Gastrointest Liver Physiol 288: G897–G906. [DOI] [PubMed] [Google Scholar]
- Akhter S, Cavet ME, Tse CM, Donowitz M. 2000. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry 39: 1990–2000. [DOI] [PubMed] [Google Scholar]
- Alfalah M, Jacob R, Preuss U, Zimmer KP, Naim H, Naim HY. 1999. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr Biol 9: 593–596. [DOI] [PubMed] [Google Scholar]
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. 1993. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol 120: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler Y, Hodson C, Milgram SL. 2003. The apical compartment: Trafficking pathways, regulators and scaffolding proteins. Curr Opin Cell Biol 15: 423–429. [DOI] [PubMed] [Google Scholar]
- Ameen N, Silvis M, Bradbury NA. 2007. Endocytic trafficking of CFTR in health and disease. J Cyst Fibros 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar YB, Takeda S, Hisamitsu T, Mori H, Wakabayashi S. 2006. Crystal structure of CHP2 complexed with NHE1-cytosolic region and an implication for pH regulation. EMBO J 25: 2315–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Thwaites DT. 2005. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1). J Cell Physiol 204: 604–613. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, et al. 2004. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: An intestinal nutrient/drug transporter in human and rat. Gastroenterology 127: 1410–1422. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagorda A, Guerra L, Di Sole F, Hemle-Kolb C, Cardone RA, Fanelli T, Reshkin SJ, Gisler SM, Murer H, Casavola V. 2002. Reciprocal protein kinase A regulatory interactions between cystic fibrosis transmembrane conductance regulator and Na+/H+ exchanger isoform 3 in a renal polarized epithelial cell model. J Biol Chem 24: 21480–21488. [DOI] [PubMed] [Google Scholar]
- Bartles JR. 2000. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol 12: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles JR, Zheng L, Li A, Wierda A, Chen B. 1998. Small espin: A third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol 143: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. 2000. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol 151: 1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathe M. 2008. A finite element framework for computation of protein normal modes and mechanical response. Proteins 70: 1595–1609. [DOI] [PubMed] [Google Scholar]
- Ben Ammar Y, Takeda S, Sugawara M, Miyano M, Mori H, Wakabayashi S. 2005. Crystallization and preliminary crystallographic analysis of the human calcineurin homologous protein CHP2 bound to the cytoplasmic region of the Na+/H+ exchanger NHE1. Acta Crystallogr 61: 956–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benting JH, Rietveld AG, Simons K. 1999. N-glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin–Darby canine kidney cells. J Cell Biol 146: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand CA, Frizzell RA. 2003. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol 285: C1–C18. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D, Nagy T, DeGray B, Aronson PS. 1999. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274: 17518–17524. [DOI] [PubMed] [Google Scholar]
- Bradbury NA, Cohn JA, Venglarik CJ, Bridges RJ. 1994. Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J Biol Chem 269: 8296–8302. [PubMed] [Google Scholar]
- Bretscher A, Weber K. 1978. Localization of actin and microfilament-associated proteins in the microvilli and terminal web of the intestinal brush border by immunofluorescence microscopy. J Cell Biol 79: 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Weber K. 1979. Villin: The major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci 76: 2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Weber K. 1980. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20: 839–847. [DOI] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R. 2005. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16: 1396–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. 2010. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun BC, Lapierre LA, Chew CS, Goldenring JR. 1998. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol 275: C163–C170. [DOI] [PubMed] [Google Scholar]
- Carton-Garcia F, Overeem AW, Nieto R, Bazzocco S, Dopeso H, Macaya I, Bilic J, Landolfi S, Hernandez-Losa J, Schwartz S, et al. 2015. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep 5: 12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. 1991. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell 66: 65–75. [DOI] [PubMed] [Google Scholar]
- Cha B, Donowitz M. 2008. The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol 35: 863–871. [DOI] [PubMed] [Google Scholar]
- Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. 2006. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: Dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell 17: 2661–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, et al. 1998. The FERM domain: A unique module involved in the linkage of cytoplasmic proteins to membrane. Trends Biochem Sci 23: 281–282. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Sung CH. 1998. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol 142: 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens MM, Bathe M, Frey E, Bausch AR. 2006. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat Mater 5: 748–753. [DOI] [PubMed] [Google Scholar]
- Crawley SW, Mooseker MS, Tyska MJ. 2014a. Shaping the intestinal brush border. J Cell Biol 207: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley SW, Shifrin DA Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, et al. 2014b. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson GP, Cutz E, Hamilton JR, Gall DG. 1978. Familial enteropathy: A syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75: 783–790. [PubMed] [Google Scholar]
- Delacour D, Greb C, Koch A, Salomonsson E, Leffler H, Le Bivic A, Jacob R. 2007. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic 8: 379–388. [DOI] [PubMed] [Google Scholar]
- Dhekne HS, Hsiao NH, Roelofs P, Kumari M, Slim CL, Rings EH, van Ijzendoorn SC. 2014. Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J Cell Sci 127: 1007–1017. [DOI] [PubMed] [Google Scholar]
- Di Sole F, Cerull R, Babich V, Quinones H, Gisler SM, Biber J, Murer H, Burckhardt G, Helmle-Kolb C, Moe OW. 2004. Acute regulation of Na/H exchanger NHE3 by adenosine A1 receptors is mediated by calcineurin homologous protein. J Biol Chem 279: 2962–2974. [DOI] [PubMed] [Google Scholar]
- Donowitz M, Li X. 2007. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872. [DOI] [PubMed] [Google Scholar]
- Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. 2009. NHE3 regulatory complexes. J Exp Biol 212: 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. 1998. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem 273: 2035–2043. [DOI] [PubMed] [Google Scholar]
- Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ, Worrell RT. 2013. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am J Physiol Gastrointest Liver Physiol 305: G697–G711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR, Worrell RT. 2015. Human Clostridium difficile infection: Inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol 308: G497–G509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP, Larson-Thome K, Valenzuela RK, Whitaker SE, Shub MD. 2008. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A 146A: 3117–3119. [DOI] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. 2010. Organizing the cell cortex: The role of ERM proteins. Nat Rev Mol Cell Biol 11: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Sun X, Yang H, Teitelbaum DH. 2009. Dissociation of E-cadherin and β-catenin in a mouse model of total parenteral nutrition: A mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol 587: 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrary E, Cohen-Tannoudji M, Pehau-Arnaudet G, Lapillonne A, Athman R, Ruiz T, Boulouha L, El Marjou F, Doye A, Fontaine JJ, et al. 1999. In vivo, villin is required for Ca2+-dependent F-actin disruption in intestinal brush borders. J Cell Biol 146: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster D, Moe OW, Hilgemann DW. 2008. Steady-state function of the ubiquitous mammalian Na/H exchanger (NHE1) in relation to dimer coupling models with 2Na/2H stoichiometry. J Gen Physiol 132: 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Bretscher A. 1995. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell 6: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M, Drumm K, Mildenberger S, Freudinger R, Gassner B, Silbernagl S. 1999. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol 520: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M, Freudinger R, Mildenberger S. 2001. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol 531: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. 2001. Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Knauf F, Demuth HU, Aronson PS. 2004. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. 2008. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422. [DOI] [PubMed] [Google Scholar]
- Glenney JR Jr, Bretscher A, Weber K. 1980. Calcium control of the intestinal microvillus cytoskeleton: Its implications for the regulation of microfilament organizations. Proc Natl Acad Sci 77: 6458–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney JR Jr, Kaulfus P, Weber K. 1981. F actin assembly modulated by villin: Ca++-dependent nucleation and capping of the barbed end. Cell 24: 471–480. [DOI] [PubMed] [Google Scholar]
- Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL. 2012. Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 14: 793–801. [DOI] [PubMed] [Google Scholar]
- Gujral T, Kumar A, Priyamvada S, Saksena S, Gill RK, Hodges K, Alrefai WA, Hecht GA, Dudeja PK. 2015. Mechanisms of DRA recycling in intestinal epithelial cells: Effect of enteropathogenic E. coli. Am J Physiol Cell Physiol 309: C835–C846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG. 2006. Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem 281: 1461–1469. [DOI] [PubMed] [Google Scholar]
- He P, Zhang H, Yun CC. 2008. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G, Hodges K, Gill RK, Kear F, Tyagi S, Malakooti J, Ramaswamy K, Dudeja PK. 2004. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 287: G370–G378. [DOI] [PubMed] [Google Scholar]
- Helander HF, Fandriks L. 2014. Surface area of the digestive tract—Revisited. Scand J Gastroenterol 49: 681–689. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. 1996. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, et al. 1996. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol 270: G29–G41. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mooseker MS. 1983. Characterization of the 110-kdalton actin-calmodulin-, and membrane-binding protein from microvilli of intestinal epithelial cells. J Cell Biol 97: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. 2001. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: Dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. 1994. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J 13: 2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter K, Mellman I. 1991. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell 66: 907–920. [DOI] [PubMed] [Google Scholar]
- Jacob R, Naim HY. 2001. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol 11: 1444–1450. [DOI] [PubMed] [Google Scholar]
- Jacob R, Preuss U, Panzer P, Alfalah M, Quack S, Roth MG, Naim H, Naim HY. 1999. Hierarchy of sorting signals in chimeras of intestinal lactase-phlorizin hydrolase and the influenza virus hemagglutinin. J Biol Chem 274: 8061–8067. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. 1998. Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Protein kinase C-mediated inhibition of the exchanger. J Biol Chem 273: 8790–8798. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Janecki M, Akhter S, Donowitz M. 2000. Basic fibroblast growth factor stimulates surface expression and activity of Na+/H+ exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J Biol Chem 275: 8133–8142. [DOI] [PubMed] [Google Scholar]
- Karvar S, Zhu L, Crothers J Jr, Wong W, Turkoz M, Forte JG. 2005. Cellular localization and stimulation-associated distribution dynamics of syntaxin-1 and syntaxin-3 in gastric parietal cells. Traffic 6: 654–666. [DOI] [PubMed] [Google Scholar]
- Knowles BC, Roland JT, Krishnan M, Tyska MJ, Lapierre LA, Dickman PS, Goldenring JR, Shub MD. 2014. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest 124: 2947–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles BC, Weis VG, Yu S, Roland JT, Williams JA, Alvarado GS, Lapierre LA, Shub MD, Gao N, Goldenring JR. 2015. Rab11a regulates Syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci 128: 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov D, Mashukova A, Forteza R, Rodriguez MM, Ameen NA, Salas PJ. 2014. Myosin 5b loss of function leads to defects in polarized signalling: Implication for Microvillus Inclusion disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol 307: G992–G1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov DV, Ahsan MK, Kumari V, van Ijzendoorn SC, Reyes-Mugica M, Kumar A, Gujral T, Dudeja PK, Ameen NA. 2016. Identification of intestinal ion transport defects in Microvillus Inclusion disease. Am J Physiol Gastrointest Liver Physiol 311: G142–G155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima K, D’Souza S, Szaszi K, Ramjeesingh R, Orlowski J, Grinstein S. 1999. The apical Na+/H+ exchanger isoform NHE3 is regulated by the actin cytoskeleton. J Biol Chem 274: 29843–29849. [DOI] [PubMed] [Google Scholar]
- Lamprecht G, Heil A, Baisch S, Lin-Wu E, Yun CC, Kalbacher H, Gregor M, Seidler U. 2002. The down regulated in adenoma (dra) gene product binds to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), potentially linking intestinal Cl−/HCO3− exchange to Na+/H+ exchange. Biochemistry 41: 12336–12342. [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. 2007. Characterization of immunoisolated human gastric parietal cells tubulovesicles: Identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292: G1249–G1262. [DOI] [PubMed] [Google Scholar]
- Larmonier CB, Laubitz D, Hill FM, Shehab KW, Lipinski L, Midura-Kiela MT, McFadden RM, Ramalingam R, Hassan KA, Golebiewski M, et al. 2013. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 305: G667–G677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. 1991. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol 115: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SA, Montrose MH, Tse CM, Donowitz M. 1993. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem 268: 25527–25535. [PubMed] [Google Scholar]
- Levine SA, Nath SK, Yun CH, Yip JW, Montrose M, Donowitz M, Tse CM. 1995. Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J Biol Chem 270: 13716–13725. [DOI] [PubMed] [Google Scholar]
- Lin S, Naim HY, Rodriguez AC, Roth MG. 1998. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol 142: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner S, Nold L, Hsieh CJ, Turner JR, Gregor M, Graeve L, Lamprecht G. 2010. Activity and PI3-kinase dependent trafficking of the intestinal anion exchanger downregulated in adenoma depend on its PDZ interaction and on lipid rafts. Am J Physiol Gastrointest Liver Physiol 299: G907–G920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis PA, Zheng L, Sekerkova G, Changyaleket B, Mugnaini E, Bartles JR. 2003. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol 163: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. 1997. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem J 328: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo MP, Yuseff MI, Retamal C, Donoso M, Ezquer F, Farfan P, Li Y, Bu G. 2003. Differential distribution of low-density lipoprotein-receptor-related protein (LRP) and megalin in polarized epithelial cells is determined by their cytoplasmic domains. Traffic 4: 273–288. [DOI] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. 1992. Basolateral sorting of LDL receptor in MDCK cells: The cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71: 741–753. [DOI] [PubMed] [Google Scholar]
- Mazerik JN, Tyska MJ. 2012. Myosin-1A targets to microvilli using multiple membrane binding motifs in the tail homology 1 (TH1) domain. J Biol Chem 287: 13104–13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell RE, Benesh AE, Mao S, Tabb DL, Tyska MJ. 2011. Proteomic analysis of the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol 300: G914–G926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux G, Massey-Harroche D, Nicolle O, Rabant M, Brousse N, Goulet O, Le Bivic A, Ruemmele FM. 2016. The localisation of the apical Par/Cdc42 polarity module is specifically affected in microvillus inclusion disease. Biol Cell 108: 19–28. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Rubinstein B. 2005. The physics of filopodial protrusion. Biophys J 89: 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. 1995. Unconventional myosins. Annu Rev Cell Dev Biol 11: 633–675. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Howe CL. 1982. The brush border of intestinal epithelium: A model system for analysis of cell-surface architecture and motility. Methods Cell Biol 25: 143–174. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Tilney LG. 1975. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol 67: 725–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Graves TA, Wharton KA, Falco N, Howe CL. 1980. Regulation of microvillus structure: Calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol 87: 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozowska PS, Fukuda M. 2016. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol 213: 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Rollinghoff B, Kohler H, et al. 2008. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 40: 1163–1165. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Amieva MR, Furthmayr H. 1995. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem 270: 31377–31385. [DOI] [PubMed] [Google Scholar]
- Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. 2005. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol 288: R685–R691. [DOI] [PubMed] [Google Scholar]
- Pang T, Su X, Wakabayashi S, Shigekawa M. 2001. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem 276: 17367–17372. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Mooseker MS. 1992. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600. [DOI] [PubMed] [Google Scholar]
- Picciano JA, Ameen N, Grant BD, Bradbury NA. 2003. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 285: C1009–C1018. [DOI] [PubMed] [Google Scholar]
- Prince LS, Workman RB Jr, Marchase RB. 1994. Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci 91: 5192–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, Collawn JF. 1999. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tryosine-based signal. J Biol Chem 274: 3602–3609. [DOI] [PubMed] [Google Scholar]
- Revenu C, Ubelmann F, Hurbain I, El-Marjou F, Dingli F, Loew D, Delacour D, Gilet J, Brot-Laroche E, Rivero F, et al. 2012. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol Biol Cell 23: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Gonzalez A. 1999. Glycans in post-Golgi apical targeting: Sorting signals or structural props? Trends Cell Biol 9: 291–294. [DOI] [PubMed] [Google Scholar]
- Roland JTE, Bryant DM, Datta A, Itzen A, Mostov KE, Goldenring JR. 2011. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci 108: 2789–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruemmele FM, Muller T, Schiefermeier N, Ebner HL, Lechner S, Pfaller K, Thoni CE, Goulet O, Lacaille F, Schmitz J, et al. 2010. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat 31: 544–551. [DOI] [PubMed] [Google Scholar]
- Sakamori R, Das S, Yu S, Feng S, Stypulkowski E, Guan Y, Douard V, Tang W, Ferraris RP, Harada A, et al. 2012. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest 122: 1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. 2004. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6: 855–864. [DOI] [PubMed] [Google Scholar]
- Sarker R, Gronborg M, Cha B, Mohan S, Chen Y, Pandey A, Litchfield D, Donowitz M, Li X. 2008. Casein kinase 2 binds to the C terminus of Na+/H+ exchanger 3 (NHE3) and stimulates NHE3 basal activity by phosphorylating a separate site in NHE3. Mol Biol Cell 19: 3859–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. 2007. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448: 366–369. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. 1995. N-glycans as apical sorting signals in epithelial cells. Nature 378: 96–98. [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Vogel GF, Teunissen H, van Ommen DD, Begthel H, El Bouazzaoui L, van Vugt AH, Beekman JM, Klumperman J, Muller T, et al. 2015. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci 112: 12408–12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, et al. 1998. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. 1997. Functional rafts in cell membranes. Nature 387: 569–572. [DOI] [PubMed] [Google Scholar]
- Stepensky P, Bartram J, Barth TF, Lehmberg K, Walther P, Amann K, Philips AD, Beringer O, Zur Stadt U, Schulz A, et al. 2013. Persistent defective membrane trafficking in epithelial cells of patients with familial hemophagocytic lymphohistiocytosis type 5 due to STXBP2/MUNC18-2 mutations. Pediatr Blood Cancer 60: 1215–1222. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, et al. 2009. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: Potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. 2002. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 277: 40099–40105. [DOI] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, et al. 2007. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 282: 23725–23736. [DOI] [PubMed] [Google Scholar]
- Szperl AM, Golachowska MR, Bruinenberg M, Prekeris R, Thunnissen AM, Karrenbeld A, Dijkstra G, Hoekstra D, Mercer D, Ksiazyk J, et al. 2011. Functional characterization of mutations in the myosin Vb gene associated with microvillus inclusion disease. J Pediatr Gastroenterol Nutr 52: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Yamazaki H, Farquhar MG. 2003. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol 284: C1105–C1113. [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Colland F, de Koning J, Maurice MM, Hornbeck P, et al. 2009. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell 16: 551–562. [DOI] [PubMed] [Google Scholar]
- Thoeni CE, Vogel GF, Tancevski I, Geley S, Lechner S, Pfaller K, Hess MW, Muller T, Janecke AR, Avitzur Y, et al. 2013. Microvillus inclusion disease: Loss of myosin Vb disrupts intracellular traffic and cell polarity. Traffic 15: 22–42. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CM. 2007. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 92: 603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen O, Wahlstrom T, Vaheri A. 1994. Ezrin has a COOH terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol 126: 1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS. 2004. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol 165: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. 2005. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 16: 2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelmann F, Chamaillard M, El-Marjou F, Simon A, Netter J, Vignjevic D, Nichols BL, Quezada-Calvillo R, Grandjean T, Louvard D, et al. 2013. Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. Proc Natl Acad Sci 110: E1380–E1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde KJ, Dhekne HS, Swertz MA, Sirigu S, Ropars V, Vinke PC, Rengaw T, van den Akker PC, Rings EH, Houdusse A, et al. 2013. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum Mutat 34: 1597–1605. [DOI] [PubMed] [Google Scholar]
- Vogel GF, Klee KM, Janecke AR, Muller T, Hess MW, Huber LA. 2015. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J Cell Biol 211: 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis GV, Knowles BC, Choi E, Goldstein AE, Williams JA, Manning EH, Roland JT, Lapierre LA, Goldenring JR. 2016. Loss of MYO5B in mice recapitulates Microvillus Inclusion disease and reveals an apical trafficking pathway distinct to neonatal duodenum. Cell Mol Gastroenterol Hepatol 2: 131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixel KM, Bradbury NA. 2000. The carboxyl terminus of the cystic fibrosis transmembrane conductance regulator binds to AP-2 clathrin adaptors. J Biol Chem 275: 3655–3660. [DOI] [PubMed] [Google Scholar]
- Wiegerinck CL, Janecke AR, Schneeberger K, Vogel GF, van Haaften-Visser DY, Escher JC, Adam R, Thoni CE, Pfaller K, Jordan AJ, et al. 2014. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147: 65–68. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. 1997. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol 139: 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Nie Y, Knowles B, Sakamori R, Stypulkowski E, Patel C, Das S, Douard V, Ferraris RP, Bonder EM, et al. 2014. TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. EMBO J 33: 1882–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. 1997. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci 94: 3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, Tse M, Donowitz M. 2005. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443. [DOI] [PubMed] [Google Scholar]
- Zhai L, Zhao P, Panebra A, Guerrerio AL, Khurana S. 2001. Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J Biol Chem 276: 36163–36167. [DOI] [PubMed] [Google Scholar]
- Zhai L, Kumar N, Panebra A, Zhao P, Parrill AL, Khurana S. 2002. Regulation of actin dynamics by tyrosine phosphorylation: Identification of tyrosine phosphorylation sites within the actin-severing domain of villin. Biochemistry 41: 11750–11760. [DOI] [PubMed] [Google Scholar]
- Zheng X, Lu D, Sadler JE. 1999. Apical sorting of bovine enteropeptidase does not involve detergent-resistant association with sphingolipid-cholesterol rafts. J Biol Chem 274: 1596–1605. [DOI] [PubMed] [Google Scholar]