Abstract
Background
Hippocampal volume loss is a hallmark of clinical depression. Chronic stress produces volume loss in the hippocampus in humans and atrophy of CA3 pyramidal cells and suppression of adult neurogenesis in rodents.
Methods
To investigate the relationship between decreased adult neurogenesis and stress-induced changes on hippocampal structure and volume, we compared the effects of chronic unpredictable restraint stress and inhibition of neurogenesis in a rat pharmacogenetic model.
Results
Chronic unpredictable restraint stress over 4 weeks decreased total hippocampal volume, reflecting loss of volume in all hippocampal subfields and in both dorsal and ventral hippocampus. In contrast, complete inhibition of adult neurogenesis for 4 weeks led to volume reduction only in the dentate gyrus. With prolonged inhibition of neurogenesis, for 8 or 16 weeks, volume loss spread to the CA3 region, but not CA1. Combining stress and inhibition of adult neurogenesis did not have additive effects on the magnitude of volume loss but did produce a volume reduction throughout the hippocampus. One month of chronic unpredictable restraint stress and inhibition of adult neurogenesis both led to atrophy of pyramidal cell apical dendrites in dorsal CA3, and neuronal reorganization in ventral CA3. Stress significantly affected granule cell dendrites as well.
Discussion
The findings suggest that adult neurogenesis is required to maintain hippocampal volume but is not responsible for stress-induced volume loss.
Keywords: Stress, adult neurogenesis, hippocampal volume, depression, MRI, CA3 atrophy
Introduction
The hippocampus has long been recognized as important for learning and memory, but studies suggest that it plays a role in regulating mood as well (1). MRI studies indicate that hippocampal volume is decreased in patients with major depression (2; 3) – one of the only detectable physical changes associated with this illness – and patients with more depressive episodes show greater hippocampal volume loss (4). Following antidepressant treatment or electroconvulsive therapy, hippocampal volume can be normalized, correlating with a decrease of symptoms (5; 6). Similar volume losses seen in individuals with adverse life events (7–10) and glucocorticoid treatment (11; 12) suggest that stress may be involved in this volume decrease.
In rodent models, chronic stress is often used as a model of depression as it leads to depressive-like behaviors including learned helplessness, anhedonia, and social withdrawal (13; 14). Chronic stress and corticosterone administration in rodents also reduce hippocampal volume (15–19), though the timing, location, and magnitude of the effects are variable and in some cases undetectable (20; 21). There are many cellular changes that could contribute to volume loss, including loss of dendritic length or dendritic spines, slower neurogenesis, decreased glial size or number, and constriction of extracellular space (22–24). CA3 pyramidal cell dendritic atrophy is seen in rats following chronic stress or glucocorticoid treatment (25–32) and has been implicated in depressive-like behavior (33–35). Generation of new dentate gyrus (DG) granule neurons can also be inhibited by stress and corticosterone, though the effects of chronic stress on adult neurogenesis are complex and not fully understood (36–41). The impact of stress on adult neurogenesis is of particular interest because inhibition of adult neurogenesis impacts stress-induced anxiodepressive-like behavior in rodents (42; 43) and is suggested to play a role in depression in humans as well (44).
Although stress has many effects on hippocampal neurons, it is unclear whether they lead to volume loss in the hippocampus or its subfields (20; 37; 45). It is also unknown whether changes in adult neurogenesis and CA3 dendritic morphology are linked or independent of one another, though one study in mice suggests that inhibiting adult neurogenesis for several months can lead to CA3 atrophy (46). To investigate the relationship between adult neurogenesis, CA3 dendritic atrophy, and volume loss throughout the hippocampus, we inhibited adult neurogenesis using a rat pharmacogenetic model (47) and stressed rats using a chronic unpredictable restraint paradigm. We then used high resolution MRI and computer-aided tracing methods to assess the volume of hippocampal subfields and morphology of DG and CA3 neurons.
Methods and Materials
Animals and Experimental Design
Adult male Long Evans rats were used for all experiments. For the initial chronic restraint experiment, rats were purchased (Charles River, Germantown, MD) and given ad libitum access to water and food. All other experiments involved inhibition of adult neurogenesis and therefore used transgenic rats expressing Herpes Simplex Virus Thymidine Kinase (HSV-TK) under the control of the human glial fibrillary acidic protein (GFAP) promoter on a Long Evans background bred in house (47; 48). All transgenic rats and wildtype littermate controls were meal fed from the time of weaning (15–16g chow/rat/day) and given ad libitum access to water. The anti-viral drug, valganciclovir (VGCV), was added to a peanut butter/chow mixture (4mg VGCV/rat) and fed to all wildtype (WT) and transgenic (TK) rats twice a week beginning at 8 weeks of age. VGCV, when phosphorylated by HSV-TK, interferes with DNA replication and prevents cell division in the GFAP+ radial cells that generate new neurons, without affecting post-mitotic astrocytes (47). Rats were maintained on a 12-hour reverse light-dark cycle (lights on at 9am). All procedures followed the Institute of Laboratory Animal Research guidelines and were approved by the Animal Care and Use Committee of the NIMH.
Chronic stress, like VGCV treatment, began at 8 weeks of age. Rats were weighed daily during the chronic restraint paradigm. For Experiment 1, testing the effects of stress alone, 10 rats underwent restraint stress daily for 4-weeks, after which they were sacrificed and processed for volume analysis (6 for MRI, all 10 for Nissl). Ten control rats were weighed daily and placed back into their cages. For Experiment 2, testing the effects of neurogenesis ablation alone, VGCV treatment was given to WT and TK rats for 4- or 16-weeks, after which rats were sacrificed and processed for volume analysis (N = 4–6). For Experiment 3, combining stress and inhibition of neurogenesis, WT and TK rats were given VGCV for 8 weeks, and half of the TK rats were also subjected to stress for the last 4 weeks of VGCV treatment before being sacrificed and processed for volume analysis (N = 4–6). For Experiment 4, testing depressive-like behavior and dendritic atrophy following stress and inhibition of neurogenesis, VGCV and chronic stress (in half of the rats) began at the same time and continued for 4 weeks (N = 10–11). Rats in this experiment were assessed the day after stress in the novelty-suppressed feeding test, and on sucrose preference test thereafter. Directly following completion of sucrose preference test, all rats were perfused and brains were processed for Golgi analysis. See Supplemental Information for details on restraint stress procedure, behavior testing, volume measurements, histology, and statistical methods.
Results
Experiment 1: Effects of chronic unpredictable restraint stress on hippocampus volume and neurogenesis
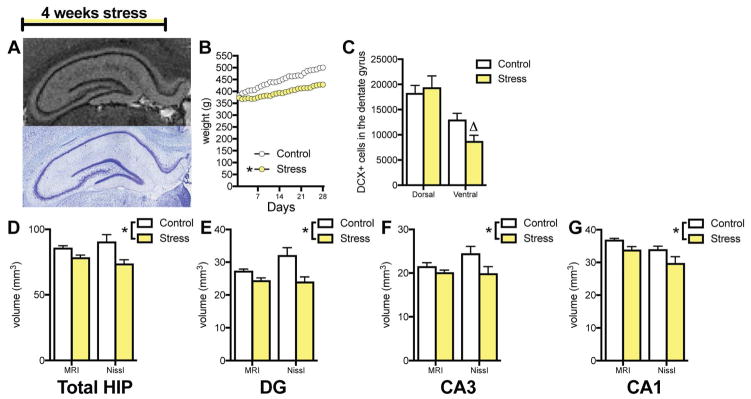
To measure the effects of chronic stress on hippocampal volume, rats were restrained daily for 4 weeks, followed by MRI on perfused brains and reconstruction of Nissl-stained sections (Figure 1A). Stress affected weight gain (Figure 1B), with stressed rats showing significantly lower weight by day 4 (p=.03 by Sidak post hoc test). Restraint stress decreased DCX+ cell number by 33% in the ventral DG but had no effect in the dorsal DG (Figure 1C).
Figure 1.
Effects of chronic unpredictable restraint stress on hippocampal volume. A. Representative hippocampal images from MRI-scanned (top) and Nissl-stained (bottom) sections. B. Chronic unpredictable restraint stress attenuates normal weight gain in ad lib fed rats (2-way ANOVA, stress x day interaction: F(27,252) = 2.693, p < .0001; slope comparison: F(1,556) = 66.05, p < .0001, * p < .05 for slope compared to control) C. Chronic stress affects neurogenesis differently in the dorsal and ventral dentate gyrus (2-way ANOVA, stress x region interaction: F(1,18) = 6.10, p = .02, Δ p = .12 in Holm-Sidak post-hoc test D. Chronic stress reduces overall hippocampal volume (2-way ANOVA, main effect of stress: F(1,10) = 11.82, p = .006). E. Chronic stress reduces dentate gyrus volume (2-way ANOVA, main effect of stress: F(1,10) = 12.46, p = .005). F. Chronic stress reduces CA3 volume (2-way ANOVA, main effect of stress: F(1,10) = 8.54, p = .015). G. Chronic stress reduces CA1 volume (2-way ANOVA, main effect of stress: F(1,10) = 8.62, p = .015). N = 6 for each group in all graphs; all graphs represent means+SEM.
Restraint stress for 4 weeks decreased overall hippocampal volume as measured by 14.1T MRI and 3D-reconstruction of Nissl-stained sections (Figure 1D). Subfield analysis showed that stress reduced volume in the DG, CA3, and CA1 (Figures 1E–G), as measured by MRI and Nissl reconstruction. If the analysis was run using a single technique alone, MRI was less likely to detect significant shrinkage (DG and CA1 p<.05, CA3 p>.05) than Nissl reconstruction (DG, CA3, CA1 all p<.05). However, there were no main effects of volume measurement technique or technique x stress interactions in any subfield (p>.05) in the two-way analysis. There were no differential effects of stress (stress x region interactions) in left versus right hemisphere or in dorsal versus ventral region in DG, CA3, or CA1 subfields (Supplemental Table 1).
Experiment 2: Effects of adult neurogenesis on hippocampal volume
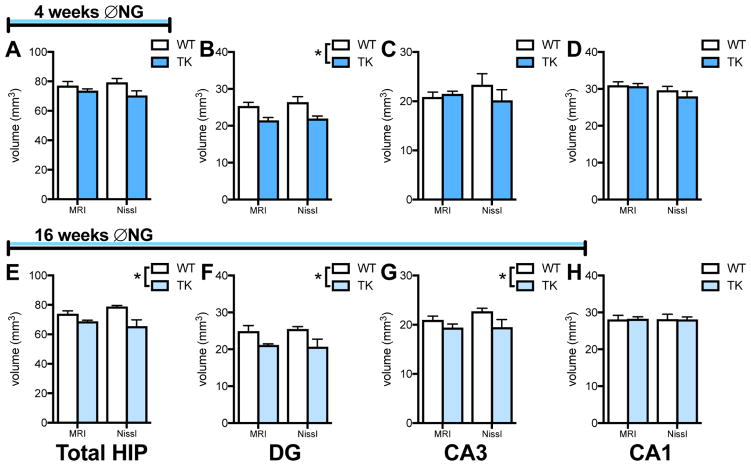
To determine the effects of chronic inhibition of adult neurogenesis on hippocampal volume, WT and TK rats were given VGCV for multiple weeks, and hippocampal volume was assessed as above. Astrocyte density appeared unaffected by VGCV treatment (Supplemental Figure 1A,B), as previously demonstrated in this line (47), as was blood vessel density (Supplemental Figure 1C,D). Hippocampal volume was first measured after 4 weeks of VGCV treatment, to match the stress time course. At this time point, TK rats showed no significant change in total hippocampal volume using MRI or section reconstruction (Figure 2A). Subfield analysis showed that volume was reduced in the DG, as measured both by MRI and Nissl-stained section reconstruction (Figure 2B), but no significant differences were found in CA3 or CA1 at this time point (Figure 2C,D). There were no differential effects of treatment in left versus right hemisphere or in dorsal versus ventral region in any subfield (Supplemental Table 2).
Figure 2.
Effects of 4- and 16-weeks of inhibiting neurogenesis on hippocampal volume. A. 4 weeks of inhibiting neurogenesis has no effect on overall hippocampal volume (2-way ANOVA, main effect of genotype: F(1,8) = 4.25, p = .07). B. 4 weeks of inhibiting neurogenesis reduces dentate gyrus volume (2-way ANOVA, main effect of genotype: F(1,8) = 13.23, p = .007). C. 4 weeks of inhibiting neurogenesis has no effect on CA3 volume (2-way ANOVA, main effect of genotype: F(1,8) = 0.56, p = .48). D. 4 weeks of inhibiting neurogenesis has no effect on CA1 volume (2-way ANOVA, main effect of genotype: F(1,8) = 0.56, p = .47). E. 16 weeks of inhibiting neurogenesis reduces overall hippocampal volume (2-way ANOVA, main effect of genotype: F(1,6) = 12.16, p = .013). F. 16 weeks of inhibiting neurogenesis reduces dentate gyrus volume (2-way ANOVA, main effect of genotype: F(1,6) = 10.46, p = .018). G. 16 weeks of inhibiting neurogenesis reduces CA3 volume (2-way ANOVA, main effect of genotype: F(1,6) = 8.30, p = .028). H. 16 weeks of inhibiting neurogenesis has no effect on CA1 volume (2-way ANOVA, main effect of genotype: F(1,6) = 0.68, p = .44). N = 5/group for 4-week experiment and n = 4/group for 16-week experiment; all graphs represent means+SEM.
To determine whether prolonged inhibition of adult neurogenesis produced more widespread volume reductions, we measured hippocampal volume after 16 weeks of VGCV treatment. At this time point, TK rats had reduced overall hippocampal volume seen with both MRI and Nissl reconstruction (Figure 2E). Inhibiting adult neurogenesis reduced volume in both the DG (Figure 2F) and CA3 (Figure 2G) but produced no changes in CA1 (Figure 2H). There were no effects of volume measurement technique or technique x genotype interactions in any brain region. However, if measurement techniques were assessed individually, MRI was less likely to detect significant shrinkage in CA3 with the relatively small sample size used here. There were no differential effects of treatment (no genotype x region interactions) in the left versus right hemisphere or dorsal versus ventral region in any subfield (Supplemental Table 2).
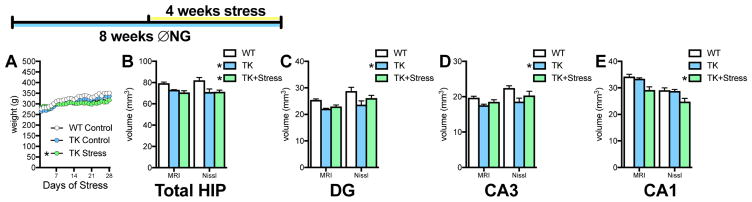
Experiment 3: Combined effects of neurogenesis ablation and stress on hippocampal volume
To assess whether effects of stress and new neuron loss on hippocampal volume are additive, rats were given VGCV for 4 weeks, followed by 4 weeks of chronic unpredictable restraint stress along with VGCV. Unstressed TKs did not differ in weight from WTs (Figure 3A), however stressed TK rats had significantly lower weights than rats in the other two groups starting on day 15 of stress (p=.02 by Sidak post hoc test). Inhibiting adult neurogenesis for 8 weeks reduced overall hippocampal volume, with no additional effect of stress (Figure 3B). Volume was reduced in TK rats in both the DG and CA3, however stress attenuated this volume loss (Figure 3C,D). The effect in CA1 differed, showing no effect of ablation alone, consistent with observations at other time points, but a significant decrease in volume with stress plus ablation (Figure 3E). There were no differential effects of treatment based on hemisphere of dorsal-ventral analysis in any subfields (Supplemental Table 3). Nissl-stained section tracing enabled layer-specific volume analysis for all experiments, but this analysis yielded no specific pattern of layer reductions across experiments to suggest atrophy of particular neuronal components (Supplemental Table 4).
Figure 3.
Effects of 8 weeks of inhibiting neurogenesis and chronic stress on hippocampal volume. A. Genotype has no effect on weight gain, but stress attenuates normal weight gain in food restricted rats (2-way ANOVA, stress x day interaction: F(54,324) = 13.66, p < .0001; slope comparison: F(2,414) = 34.62, p < .0001, * p < .05 for slope compared to WT and TK control). B. 8 weeks of inhibiting neurogenesis with and without chronic stress reduces overall hippocampal volume (2-way ANOVA, main effect of genotype: F(2,12) = 13.40, p = .0009; * p < .05 versus WT with Tukey post-hoc test). C. 8 weeks of inhibiting neurogenesis reduces dentate gyrus volume, but adding stress attenuates volume loss (2-way ANOVA, main effect of genotype: F(2,12) = 8.45, p = .005; * p < .05 versus WT with Tukey post-hoc test). D. 8 weeks of inhibiting neurogenesis reduces CA3 volume but adding stress attenuates volume loss (2-way ANOVA, main effect of genotype: F(2,12) = 9.57, p = .003; * p < .05 versus WT with Tukey post-hoc test). E. 8 weeks of inhibiting neurogenesis has no effect on CA1 volume, but chronic stress reduces CA1 volume in neurogenesis-depleted rats (2-way ANOVA, main effect of genotype: F(2,12) = 15.91, p = .0004; * p < .05 versus WT and TK with Tukey post-hoc test). N = 4–6 for all graphs; all graphs represent means+SEM.
Experiment 4: Effects of stress and neurogenesis on behavior and neuronal morphology
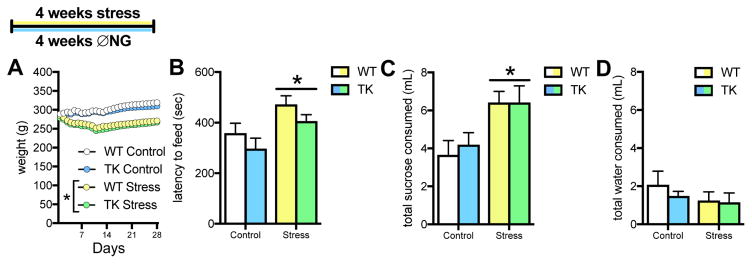
To determine whether our chronic stress model and inhibition of neurogenesis affect depressive-like behavior, rats were subjected to 4 weeks of stress and/or VGCV treatment, followed by testing on novelty-suppressed feeding (NSF) and sucrose preference. Neuronal morphology was then assessed in these rats using Golgi analysis.
Weight and depressive-like behavior
Inhibition of neurogenesis alone did not affect weight gain (Figure 4A), however stress significantly inhibited weight gain by day 3 (p=.01 by Sidak post hoc test), which is notable because diet was controlled in this experiment.
Figure 4.
Effects of chronic stress and inhibiting neurogenesis on depressive-like behavior. A. Unpredictable restraint stress decreases weight, while genotype has no effect in food restricted rats (3-way ANOVA, genotype x day interaction: F(27,1134) = 0.52, p = .98; stress x day interaction: F(27,1134) = 100.30, p < .0001; stress slope comparison: F(1,1284) = 66.39, p < .0001, * p < .05 for slope compared to controls B. Chronic stress increases latency to feed in the novelty-suppressed feeding test, but inhibiting neurogenesis does not have a significant effect (2-way ANOVA, main effect of stress: F(1,40) = 9.89, p = .003; main effect of genotype: F(1,40) = 3.26, p = .08; stress x genotype interaction: F(1,40) = 0.01, p = .94). C,D. Chronic stress increases sucrose consumption (2-way ANOVA, main effect of stress F(1,31) = 11.69, p = .002), but does not affect water consumption (2-way ANOVA, main effect of stress: F(1,31) = 1.30, p = .26). For all graphs, WT control = white, TK control = blue, WT stress = yellow, TK stress = green. N = 9–10 for all graphs; all graphs represent means+SEM.
Stressed rats had longer latencies to eat in the NSF test (Figure 4B), an indication of increased depressive-like behavior. Inhibiting adult neurogenesis appeared to decrease latency to eat, but this did not reach statistical significance. There was no interaction between neurogenesis and stress. There were no effects on latency to approach the food, and all rats showed similar food consumption in the home cage, suggesting similar hunger levels (Supplemental Figure 2A,B). Neither restraint stress nor inhibition of neurogenesis affected sucrose consumption during habituation (Supplemental Figure 2C). In a 10-minute test, sucrose consumption was greater in rats that were stressed, independent of genotype (Fig 4C), with no effect on water consumption (Fig 4D). This resulted in a significant increase in sucrose preference in stressed rats, irrespective of genotype (Supplemental Figure 2D).
Dentate gyrus granule cell morphology
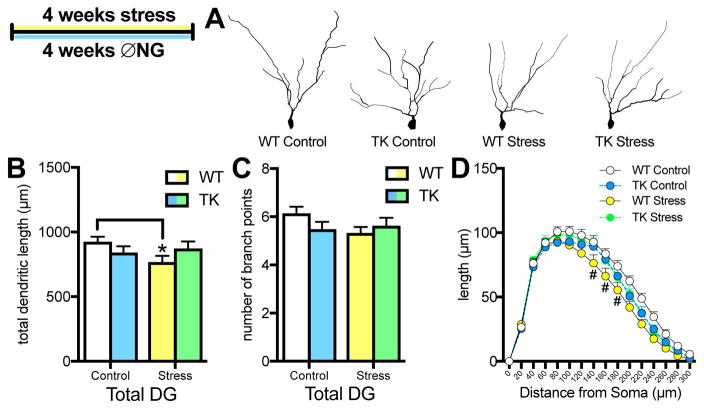
Morphologic analysis of DG granule cells showed no effect of dorsal versus ventral location (stress x region interaction: F(1,41)=0.61, p=.44), so data were aggregated for dendritic analysis. A stress x genotype interaction indicated that chronic stress significantly decreased granule cell dendritic length in WT rats but not in TKs (Figure 5B). The same pattern was suggested for the number of branch points but did not reach significance (Figure 5C). Sholl analysis showed that stressed WTs had reduced distal dendritic length, 140–180μm from the soma (Figure 5D).
Figure 5.
Effects of chronic stress and inhibiting neurogenesis on granule cell morphology. A. Representative examples of granule cell traces in the dentate gyrus. B. Chronic stress reduces total dendritic length of dentate gyrus granule cells only in WT rats (2-way ANOVA, interaction: F(1,41) = 1.87, p = .049; * p < .05 versus WT control with Tukey post-hoc test). C. Chronic stress did not significantly affect the number of branch points (2-way ANOVA, interaction: F(1,41) = 2.79, p = .10; p = .09 for WT stress versus WT control with Tukey post-hoc test. D. Sholl analysis of dendritic length in dentate gyrus granule cells. Stress reduces dendritic length 140–180μm away from soma (Three-way ANOVA genotype x stress interaction: F(1,41) = 4.37, p = .04; distance x stress interaction: F(14,574) = 2.70, p = .0008; # p < .05 for WT stress versus WT control in Newman-Keuls post-hoc tests). For all graphs, WT control = white, TK control = blue, WT stress = yellow, TK stress = green. N = 11–12 for all graphs; all graphs represent means+SEM.
CA3 pyramidal cell morphology
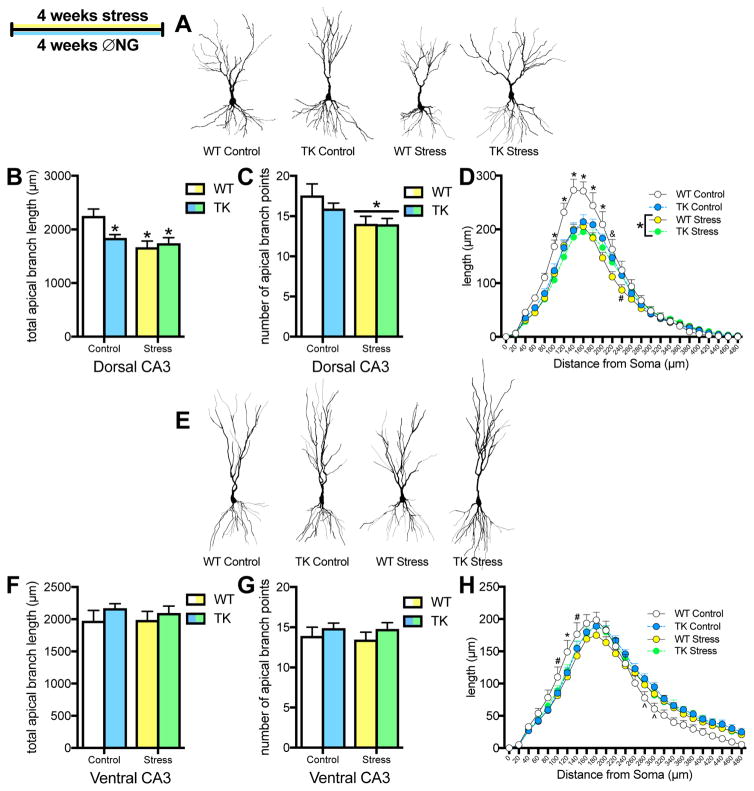
Consistent with previous studies (26; 31), 4 weeks of restraint stress led to shrinkage of the apical dendrites in dorsal CA3 pyramidal cells, reducing both the number of branch points and total dendritic length (Figure 6B,C). Inhibition of adult neurogenesis alone also reduced apical dendritic length in dorsal CA3 pyramidal cells, but did not significantly affect branch points. Sholl analysis revealed that all treatments decreased dendritic length proximal to the soma (≤200μm away), relative to unstressed WTs (Figure 6D). while stressed WTs had an broader effect.
Figure 6.
Effects of chronic stress and inhibiting neurogenesis on dendritic morphology of pyramidal cells in dorsal and ventral CA3. A. Representative examples of pyramidal cell traces in dorsal CA3. B. Chronic stress and inhibiting neurogenesis reduce overall length of apical dendrites in dorsal CA3 pyramidal neurons (2-way ANOVA, interaction: F(1,38) = 4.94, p = .03; main effect of stress: F(1,38) = 9.76, p = .003; * p < .05 versus WT control with Tukey post-hoc test). C. Chronic stress reduces branching of apical dendrites in dorsal CA3 pyramidal neurons (2-way ANOVA, main effect of stress: F(1,38) = 7.27, p = .01). D. Sholl analysis of apical dendritic length in dorsal CA3 pyramidal cells. Stress reduces dendritic length 100–220μm away from the soma, inhibition of neurogenesis reduces dendritic length from 100–180μm away from the soma, and stress plus inhibition of neurogenesis reduces dendritic length from 100–200μm away from the soma (3-way ANOVA, main effect of stress: F(1,38) = 7.27, p = .01; distance x genotype x stress interaction: F(23,874) = 1.78, p < .01; * p < .05 for WT control versus all other conditions, & p < .05 for WT control versus WT+TK stress conditions, # p < .05 for WT control versus WT stress with Newman-Keuls post-hoc tests). E. Representative examples of pyramidal cell traces in ventral CA3. F,G. Chronic stress and inhibition of neurogenesis have no effect on overall total length or branch points in ventral CA3 pyramidal neurons (2-way ANOVA branch points: main effect stress: F(1,36) = 0.10, p = .76; main effect of genotype: F(1,36) = 1.68, p = .20; 2-way ANOVA length: main effect of stress: F(1,36) = 0.06, p = .80; main effect of genotype: F(1,36) = 1.53, p = .22). H. Sholl analysis of length of apical dendrites in ventral CA3 pyramidal cells. Chronic stress and inhibition of neurogenesis reduce dendritic length proximal to the soma, but inhibition of neurogenesis increases dendritic length distally (3-way ANOVA: distance x genotype x stress interaction: F(23,828) = 3.02, p = .000003; * p < .05 for WT control versus all other conditions, # p < .05 for WT control versus WT stress, ^ p < .05 for WT control versus TK control with Newman-Keuls post-hoc tests). For all graphs, WT control = white, TK control = blue, WT stress = yellow, TK stress = green. N = 11–12 for all graphs; all graphs represent means+SEM.
In the ventral hippocampus, there were no effects on total apical dendritic length or branch points (Figure 6F,G). However, Sholl analysis indicated a reorganization of dendritic branching with a 3-way interaction (Figure 6H). Stress and inhibition of adult neurogenesis decreased dendritic length proximal to the soma (100–140 μm) but inhibition of adult neurogenesis also increased dendritic length more distally (280–300 μm from the soma).
Short-shaft pyramidal neurons were more complex than long-shaft neurons in both dorsal and ventral CA3, as expected (49), but the pattern of effects of stress and inhibition of neurogenesis on dendritic length and branch point was the same for both neuronal subtypes (Supplemental Figure 3A–F). None of the treatments affected the basal dendrites throughout CA3 (Supplemental Table 5).
Discussion
Our results show that inhibiting adult neurogenesis in GFAP-TK rats leads to a measurable decrease in hippocampal volume. Dentate gyrus volume decreased within 4 weeks, while CA3 volume decreased significantly only after 8 weeks, and CA1 volume was unaffected even after 16 weeks. Chronic unpredictable restraint stress over 4 weeks decreased neurogenesis by 33% specifically within the ventral portion of the dentate gyrus but reduced the volume of the dentate gyrus, CA3, and CA1 throughout dorsal and ventral subfields. At a cellular level, both stress and inhibition of adult neurogenesis decreased apical dendritic length of pyramidal neurons in dorsal, but not ventral CA3. Chronic unpredictable restraint stress decreased dorsal and ventral granule cell dendritic length, while direct inhibition of adult neurogenesis had no discernable effect on the morphology of the remaining granule cells. Taken together, these findings demonstrate that adult neurogenesis is required for maintaining the normal structure of the hippocampus but suggest that decreased adult neurogenesis is not responsible for the hippocampal volume loss that occurs with stress.
Comparison of MRI and Nissl-stained section reconstruction methods
High resolution MRI of fixed brains and 3D-reconstruction of Nissl-stained sections showed similar results, indicating that both of these methods are valid for future volumetric studies in rats. We found higher variability using Nissl-stained section reconstruction, possibly due to greater opportunity for human error during sectioning, staining, and tracing steps. However, it was feasible to analyze more Nissl-stained brains due to lower cost and time constraints relative to high-resolution MRI. When using the larger sample (N=10 versus N=6 per group), the section reconstruction was more likely to detect significant shrinkage if one test was used alone. In addition, section reconstruction allowed analysis of layer-specific changes in hippocampal subfields, although we did not detect any localization of changes to particular layers, possibly because neurons are able to shift slightly as they or neighboring cells shrink. MR scanning at 14.1T provided high resolution images in which the hippocampal subfields could be resolved more readily than in previous studies using 3.0T– 9.4T scanners (17; 19). However, a major advantage of using MRI, the ability to detect within-subject changes over time, is not currently possible at the resolution used here.
Regional effects of stress and inhibition of adult neurogenesis
Regional differences in volume loss have been seen in human studies, with depression-associated changes typically detected only in the posterior hippocampus (equivalent to the dorsal hippocampus in rodents) (50; 51). Although most rodent studies of stress-induced volume changes have not separated dorsal and ventral hippocampus, one study found significant volume loss only in one portion of the dorsal hippocampus (52) and another found no volume loss with stress in the ventral hippocampus, the only region that was examined (20). Somewhat surprisingly, therefore, the current study found that volume loss was similar in the dorsal and ventral portion of all subfields (Supplemental Table 1–3). However, we did find that stress-induced atrophy of CA3 pyramidal cells was restricted to the dorsal hippocampus. This regional effect on pyramidal cell dendrites is consistent with one recent study (52), but not with an earlier study, which showed similar atrophy of CA3 neurons in both dorsal and ventral hippocampus (53). The apparent inconsistencies within the rodent literature can potentially be explained by differences in the severity of the stress procedure. The studies suggesting volume changes limited to the dorsal hippocampus used chronic mild stress procedures, which have smaller effects on weight than chronic restraint, suggesting that they are in fact milder stressors. The study that found ventral as well as dorsal effects on CA3 dendritic atrophy (53), on the other hand, used immobilization stress, which is a stronger stressor (49) and produced greater effects on weight than our model – though this study also used mice rather than rats, suggesting a species difference as an alternative explanation.
If regional effects of stress are seen only with mild stress, this suggests that the dorsal hippocampus is more susceptible than the ventral portion but that strong enough stress can produce similar effects throughout the hippocampus. One study that directly compared milder and more aversive stress did not examine hippocampal volume or dendritic atrophy but found synapse loss restricted to dorsal CA3 following mild stress and throughout CA3 following more severe stress (54), consistent with this possibility. Glucocorticoid receptors are found at high concentration throughout the hippocampus (55), so stress hormones seem unlikely to be responsible for differences in regional susceptibility. However, differential inputs from the amygdala, a region that shows dendritic growth with chronic stress (31; 56; 57), to dorsal and ventral portions of the hippocampus (58; 59) may enhance or buffer the effects of chronic glucocorticoid release on dendritic architecture.
There is also some suggestion that hippocampal volume loss in depression is more pronounced in the left hemisphere than right (60–65), though the two hemispheres are usually not directly compared. Here we found equal volume loss in both hemispheres in all experiments, perhaps reflecting less laterality in the rodent brain or, alternatively, enhanced detection capability due to greater imaging resolution. A recent report found faster shrinkage in left than right hippocampus during chronic restraint in rats (66), but these differences were transient and would have been missed in our study.
Cellular effects underlying volume reduction
Our findings suggest that inhibition of neurogenesis contributes little to stress-induced volume change, based on differences in the timing and subfield localization of the two effects. Complete ablation of neurogenesis resulted in CA3 volume loss and detectable total hippocampal volume loss only after 8 weeks – long after similar volume changes were detected with stress. In addition, CA1 was completely unaffected by inhibition of neurogenesis, even after 16 weeks. Since CA1 comprises almost two-thirds of the human hippocampal formation (67), shrinkage of DG and CA3 would have to be even greater to be detectable as a change in total hippocampal volume in humans. Finally, the partial inhibition of adult neurogenesis seen with stress would likely have an even more limited effect on dendritic atrophy and volume than complete ablation, which was used here to determine the maximum possible effect of adult neurogenesis.
Dendritic atrophy also seems to be only partially responsible for stress-induced loss of hippocampal volume. Although DG and dorsal CA3 exhibited dendritic atrophy with stress that could potentially explain decreased volume in those areas, the volume of the ventral CA3 region decreased with no corresponding change in pyramidal cells. If neither change in neurogenesis nor in dendritic arborization can explain the observed volume loss, it is still unclear what cellular changes are responsible. Several other known effects of stress, including decreased numbers of inhibitory interneurons throughout the hippocampus (68), decreased numbers of glia in the neuropil (69), and decreased hippocampal microvasculature (70) are potential factors. Glial cells, particularly astrocytes, are reduced in number and size in both human depression and animal models of chronic stress (71–73), suggesting a potential contribution to volume change.
New neurons may protect against stress effects
A few of the current findings hint at the possibility that loss of new neurons may actually attenuate the structural effects of stress in the hippocampus. First, stress had no detectable additive effects on DG or CA3 volume or dendritic morphology in TK rats. Although this may reflect a floor effect, several measures in stressed TK rats suggest a slight increase in volume relative to unstressed TK rats (e.g., Fig 3C,D) and less dendritic atrophy than in stressed wildtype rats (e.g., Fig 5B,D). In addition, stress decreased neurogenesis only in the ventral portion of the hippocampus, where CA3 dendritic atrophy was curbed. These patterns do not demonstrate any causative relationship, they are consistent with current thinking on the effects of new neurons on hippocampal circuits and the causes of pyramidal cell dendritic atrophy. Young neurons appear to enhance excitability in the hippocampus (74–78), and inhibiting neurogenesis can decrease overall hippocampal activity during unpredictable stress (42). Work on dendritic remodeling following stress suggests that CA3 pyramidal neuron atrophy results from glutamate excitotoxicity associated with DG hyperexcitability (26; 79), which could potentially be exacerbated by the presence of excitable new neurons. Additional studies, possibly with a milder stress paradigm, will be required to determine whether inhibition of neurogenesis does in fact have a protective role on hippocampal structure and whether this produces, or is mediated by, changes in behavior.
Behavioral changes following stress and inhibition of adult neurogenesis
Many behavioral consequences of chronic stress may reflect ethologically protective adaptations. For example, the increased latency to eat in the NSF test in stressed rats observed here could reflect increased caution in foraging and eating, which is likely to be protective when frequently encountering stressors. Somewhat surprisingly, we found that stress increased consumption in another reward-motivated task, the sucrose preference test. This contradicts several reports that stress, typically chronic mild stress, decreases sucrose preference (13; 80; 81). However, the originators of the test have pointed out that null effects or increases in sucrose consumption are sometimes observed in their own lab as well as others (82). One factor contributing to this lack of reliability is stress-induced weight loss, which can enhance sucrose consumption, thereby masking or reversing the anhedonia-related decrease in sucrose preference (82; 83). Our restraint stress paradigm produced greater effects on weight than chronic mild stress typically does, supporting this possibility. Several studies have observed increased consumption of sweet foods following chronic restraint stress (84–87) consistent with the idea that restraint stress increases caloric drive. Both decreased NSF and increased sucrose consumption, therefore, may reflect increased protective behavior, adaptively balancing caution and feeding in predictable and unpredictable environments, following chronic unpredictable stress.
In rodents, decreased hippocampal volume and dendritic atrophy have been associated with increased anxiodepressive-like behavior (19; 30; 52; 88). However, the specific relationship between behavioral and structural effects of stress is complex and difficult to address, because morphology and volume cannot be manipulated directly. Adult neurogenesis can be specifically inhibited, but this manipulation has few behavioral effects under baseline testing conditions. Complete inhibition of adult neurogenesis had no effect on NSF in this study, consistent with previous findings that new neurons only affect behavior in this test under stressful conditions (42; 43; 89). Adult neurogenesis also had no effect on sucrose preference, possibly because ablation of new neurons did not affect weight, as stress did. Previous studies have suggested that inhibition of adult neurogenesis can exacerbate both behavioral and endocrine responses to acute stress (43; 90; 91). Previous work comparing chronic stress and exogenous corticosterone effects suggests that CA3 dendritic atrophy affects behavior by increasing endocrine response to the acute stress of behavioral testing (33), suggesting a possible common function for neurogenic and dendritic/structural changes. In humans, progressive hippocampal volume shrinkage and decreases in perceived stress levels are associated with increasing numbers of prior depressive episodes (4; 92), suggesting that structural changes may eventually diminish stress perception in the context of depressive illness. Similarly, continued neurogenesis may function to bias perception of, or response to, the potentially threatening experiences (42), suggesting that ongoing plasticity may enable more resilient and flexible behavior (93; 94). Future studies will be needed to understand exactly how the interacting microcircuits within the hippocampus function to affect behavior in the face of chronic stressors.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Program of the NIH, National Institute of Mental Health, ZIAMH002784 (H.A.C).
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cameron HA, Glover LR. Adult Neurogenesis: Beyond Learning and Memory*. Annu Rev Psychol. 2015;66:53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal Atrophy in Recurrent Major Depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, et al. Hippocampal volume in geriatric depression. Biological Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 4.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness Progression, Recent Stress, and Morphometry of Hippocampal Subfields and Medial Prefrontal Cortex in Major Depression. Biological Psychiatry. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheline YI, Gado MH, Kraemer HC. Untreated Depression and Hippocampal Volume Loss. American Journal of Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 6.Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. 2014;4:e483–7. doi: 10.1038/tp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic Resonance Imaging-Based Measurement of Hippocampal Volume in Posttraumatic Stress Disorder Related to Childhood Physical and Sexual Abuse-A Preliminary Report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- 10.Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: A longitudinal voxel-based morphometry study. Stress. 2010;14:227–232. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- 11.Starkman MN, Giordani B, Gebarski SS, Berent S. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biological Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 12.Brown ES, Jeon-Slaughter H, Lu H, Jamadar R, Issac S, Shad M, et al. Hippocampal Volume in Healthy Controls Given 3-Day Stress Doses of Hydrocortisone. 2015;40:1216–1221. doi: 10.1038/npp.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 14.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nature Publishing Group. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. European Journal of Pharmacology. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Jarome T, Li S-J, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. NeuroReport. 2009;20:1554–1558. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Shu X-J, Chen F-Y, Zhu C, Sun X-H, Liu L-J, et al. Tianeptine reverses stress-induced asymmetrical hippocampal volume and N-acetylaspartate loss in rats: An in vivo study. Psychiatry Research: Neuroimaging. 2011;194:385–392. doi: 10.1016/j.pscychresns.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Cao Z, Wang D, Wu L, Li Y, Sun W, Zhu Y. Dynamic study of the hippocampal volume by structural MRI in a rat model of depression. Neurol Sci. 2014;35:1777–1783. doi: 10.1007/s10072-014-1837-y. [DOI] [PubMed] [Google Scholar]
- 20.Jayatissa MN, Henningsen K, Nikolajsen G, West MJ, Wiborg O. A reduced number of hippocampal granule cells does not associate with an anhedonia-like phenotype in a rat chronic mild stress model of depression. Stress. 2009;13:95–105. doi: 10.3109/10253890902951786. [DOI] [PubMed] [Google Scholar]
- 21.Xi G, Hui J, Zhang Z, Liu S, Zhang X, Teng G, et al. Learning and Memory Alterations Are Associated with Hippocampal N-acetylaspartate in a Rat Model of Depression as Measured by 1H-MRS. In: Hashimoto K, editor. PLoS ONE. Vol. 6. 2011. pp. e28686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Eur Arch Psychiatry Clin Neurosc. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 23.Wennström M, Hellsten J, Ekstrand J, Lindgren H, Tingström A. Corticosterone-Induced Inhibition of Gliogenesis in Rat Hippocampus is Counteracted by Electroconvulsive Seizures. Biological Psychiatry. 2006;59:178–186. doi: 10.1016/j.biopsych.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Gould E, Daniels DC, Cameron H. Tianeptine attenuates stress-induced morphological changes in the hippocampus. European Journal of Pharmacology. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 28.Galea L, McEwen BS, Tanapat P, Deak T. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 29.Magariños AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Research. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- 30.Sousa N, Lukoyanov NV, Madeira MD, Almeida O. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 31.Vyas A, Mitra R, Rao B. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of …. 2002 doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson KM, McLaughlin KJ, Wright RL, Ortiz JB, Anouti DP, Mika A, et al. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiology of Learning and Memory. 2012;97:250–260. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad CD. What Is the Functional Significance of Chronic Stress-Induced CA3 Dendritic Retraction Within the Hippocampus? Behavioral and Cognitive Neuroscience Reviews. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Workman JL, Brummelte S, Galea LAM. Postpartum Corticosterone Administration Reduces Dendritic Complexity and Increases the Density of Mushroom Spines of Hippocampal CA3 Arbours in Dams. Journal of Neuroendocrinology. 2013;25:119–130. doi: 10.1111/j.1365-2826.2012.02380.x. [DOI] [PubMed] [Google Scholar]
- 35.Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, et al. Vulnerability to Depression: From Brain Neuroplasticity to Identification of Biomarkers. Journal of Neuroscience. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 37.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 38.Czéh B, Müller-Keuker JIH, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic Social Stress Inhibits Cell Proliferation in the Adult Medial Prefrontal Cortex: Hemispheric Asymmetry and Reversal by Fluoxetine Treatment. Neuropsychopharmacology. 2006;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 39.Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS. Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharmacology. 2011;217:239–253. doi: 10.1007/s00213-011-2279-3. [DOI] [PubMed] [Google Scholar]
- 40.Tanapat P, Hastings NB, Rydel TA, Galea LAM, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. Journal of Comparative Neurology. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 41.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Experimental Neurology. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glover LR, Schoenfeld TJ, Karlsson R-M, Bannerman DM, Cameron HA. Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. In: Enikolopov G, editor. PLoS Biol. Vol. 15. 2017. pp. e2001154–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–460. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenfeld TJ, Cameron HA. Adult Neurogenesis and Mental Illness. Neuropsychopharmacology. 2014;40:112–127. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olesen MV, Wörtwein G, Pakkenberg B. Electroconvulsive stimulation, but not chronic restraint stress, causes structural alterations in adult rat hippocampus-A stereological study. Hippocampus. 2014;25:72–80. doi: 10.1002/hipo.22351. [DOI] [PubMed] [Google Scholar]
- 46.Schloesser RJ, Jimenez DV, Hardy NF, Paredes D, Catlow BJ, Manji HK, et al. Atrophy of pyramidal neurons and increased stress-induced glutamate levels in CA3 following chronic suppression of adult neurogenesis. Brain Struct Funct. 2013;219:1139–1148. doi: 10.1007/s00429-013-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder JS, Grigereit L, Russo A, Seib DR, Brewer M, Pickel J, Cameron HA. A Transgenic Rat for Specifically Inhibiting Adult Neurogenesis. eNeuro. 2016;3:1–29. doi: 10.1523/ENEURO.0064-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soumier A, Carter RM, Schoenfeld TJ, Cameron HA. New Hippocampal Neurons Mature Rapidly in Response to Ketamine But Are Not Required for Its Acute Antidepressant Effects on Neophagia in Rats. eNeuro. 2016;3:1–13. doi: 10.1523/ENEURO.0116-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conrad CD, Ortiz JB, Judd JM. Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiology & Behavior. 2016:1–16. doi: 10.1016/j.physbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological Psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Sivakumar PT, Kalmady SV, Venkatasubramanian G, Bharath S, Reddy NN, Rao NP, et al. Volumetric analysis of hippocampal sub-regions in late onset depression: A 3 tesla magnetic resonance imaging study. Asian Journal of Psychiatry. 2015;13:38–43. doi: 10.1016/j.ajp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Pinto V, Costa JC, Morgado P, Mota C, Miranda A, Bravo FV, et al. Differential impact of chronic stress along the hippocampal dorsal–ventral axis. Brain Struct Funct. 2014;220:1205–1212. doi: 10.1007/s00429-014-0713-0. [DOI] [PubMed] [Google Scholar]
- 53.Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Molecular Psychiatry. 2014;19:811–822. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- 56.Mitra R, Jadhav S, McEwen BS. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences; Presented at the Proceedings of the …; 2005. pp. 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aggleton JP. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Experimental Brain Research. 1986;64:515–526. doi: 10.1007/BF00340489. [DOI] [PubMed] [Google Scholar]
- 59.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. Journal of Comparative Neurology. 1999;403:229–260. [PubMed] [Google Scholar]
- 60.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal Volume Reduction in Major Depression. American Journal of Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 61.Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychological Medicine. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 62.Vasic N, Walter H, Höse A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: A voxel-based morphometry study. Journal of Affective Disorders. 2008;109:107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Kronmüller K-T, Schröder J, Köhler S, Götz B, Victor D, Unger J, et al. Hippocampal volume in first episode and recurrent depression. Psychiatry Research: Neuroimaging. 2009;174:62–66. doi: 10.1016/j.pscychresns.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Tae WS, Kim SS, Lee KU, Nam EC, Choi JW, Park JI. Hippocampal Shape Deformation in Female Patients with Unremitting Major Depressive Disorder. American Journal of Neuroradiology. 2011;32:671–676. doi: 10.3174/ajnr.A2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han K-M, Won E, Sim Y, Tae W-S. Hippocampal subfield analysis in medication-naïve female patients with major depressive disorder. Journal of Affective Disorders. 2016;194:21–29. doi: 10.1016/j.jad.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 66.Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O’Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016:1–15. doi: 10.1038/srep29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joelving FC, Billeskov R, Christensen JR, West M, Pakkenberg B. Hippocampal neuron and glial cell numbers in Parkinson’s disease—A stereological study. Hippocampus. 2006;16:826–833. doi: 10.1002/hipo.20212. [DOI] [PubMed] [Google Scholar]
- 68.Czéh B, Varga ZKK, Henningsen K, Kovács GL, Miseta A, Wiborg O. Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus. 2014;25:393–405. doi: 10.1002/hipo.22382. [DOI] [PubMed] [Google Scholar]
- 69.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: Relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 70.Czéh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: No effect of fluoxetine treatment. Hippocampus. 2009 doi: 10.1002/hipo.20599. NA–NA. [DOI] [PubMed] [Google Scholar]
- 71.Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial Plasticity in the Hippocampus is Affected by Chronic Psychosocial Stress and Concomitant Fluoxetine Treatment. Neuropsychopharmacology. 2005;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 72.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Current Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cobb JA, O’Neill K, Milner J, Mahajan GJ, Lawrence TJ, May WL, et al. Density of GFAP-immunoreactive astrocytes is decreased in left hippocampi in major depressive disorder. Neuroscience. 2016;316:209–220. doi: 10.1016/j.neuroscience.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 75.Mongiat LA, Espósito MS, Lombardi G, Schinder AF. Reliable Activation of Immature Neurons in the Adult Hippocampus. In: Reh TA, editor. PLoS ONE. Vol. 4. 2009. pp. e5320–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder JS, Kee N, Wojtowicz JM. Effects of Adult Neurogenesis on Synaptic Plasticity in the Rat Dentate Gyrus. Journal of Neurophysiology. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 77.Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, et al. Ablation of Hippocampal Neurogenesis Impairs Contextual Fear Conditioning and Synaptic Plasticity in the Dentate Gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA. Experience-Dependent Regulation of Dentate Gyrus Excitability by Adult-Born Granule Cells. Journal of Neuroscience. 2015;35:11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magariños AM, McEwen BS, Flügge G. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. The Journal of …. 1996 doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 81.Pothion S, Bizot J-C, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural Brain Research. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 83.D’Aquila PS, Newton J, Willner P. Diurnal variation in the effect of chronic mild stress on sucrose intake and preference. Physiology & Behavior. 1997;62:421–426. doi: 10.1016/s0031-9384(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 84.Ely DR, Dapper V, Marasca J, Correa JB. Effect of restraint stress on feeding behavior of rats. Physiology & Behavior. 1997;61:395–398. doi: 10.1016/s0031-9384(96)00450-7. [DOI] [PubMed] [Google Scholar]
- 85.Silveira PP, Xavier MH, Souza FH, Manoli LP, Rosat RM, Ferreira MBC, Dalmaz C. Interaction between repeated restraint stress and concomitant midazolam administration on sweet food ingestion in rats. Brazilian Journal of Medical and Biological Research. 2000;33:1343–1350. doi: 10.1590/s0100-879x2000001100013. [DOI] [PubMed] [Google Scholar]
- 86.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic Stress Promotes Palatable Feeding, which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 87.Handy C, Yanaga S, Reiss A, Zona N, Robinson E, Saxton KB. Stress during Adolescence Alters Palatable Food Consumption in a Context-Dependent Manner. In: McCutcheon JE, editor. PLoS ONE. Vol. 11. 2016. pp. e0148261–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zalosnik MI, Pollano A, Trujillo V, Suárez MM, Durando PE. Effect of maternal separation and chronic stress on hippocampal-dependent memory in young adult rats: evidence for the match-mismatch hypothesis. Stress. 2014;17:445–450. doi: 10.3109/10253890.2014.936005. [DOI] [PubMed] [Google Scholar]
- 89.Santarelli L, Saxe M, Gross C, Surget A. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 90.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Molecular Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yun S, Donovan MH, Ross MN, Richardson DR, Reister R, Farnbauch LA, et al. Stress-Induced Anxiety- and Depressive-Like Phenotype Associated with Transient Reduction in Neurogenesis in Adult Nestin-CreERT2/Diphtheria Toxin Fragment A Transgenic Mice. In: Lucassen PJ, editor. PLoS ONE. Vol. 11. 2016. pp. e0147256–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Samuels BA, Leonardo ED, Hen R. Hippocampal Subfields and Major Depressive Disorder. Biological Psychiatry. 2015;77:210–211. doi: 10.1016/j.biopsych.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glasper ER, Schoenfeld TJ, Gould E. Adult neurogenesis: Optimizing hippocampal function to suit the environment. Behavioural Brain Research. 2012;227:380–383. doi: 10.1016/j.bbr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends in Cognitive Sciences. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.