Abstract
In this study, the role of known Parkinson’s disease (PD) genes was examined in families with autosomal recessive (AR) parkinsonism to assist with the differential diagnosis of PD. Some families without mutations in known genes were also subject to whole genome sequencing with the objective to identify novel parkinsonism-related genes. Families were selected from 4000 clinical files of patients with PD or parkinsonism. AR inheritance pattern, consanguinity, and a minimum of two affected individuals per family were used as inclusion criteria. For disease gene/mutation identification, multiplex ligation-dependent probe amplification, quantitative PCR, linkage, and Sanger and whole genome sequencing assays were carried out. A total of 116 patients (50 families) were examined. Fifty-four patients (46.55%; 22 families) were found to carry pathogenic mutations in known genes while a novel gene, not previously associated with parkinsonism, was found mutated in a single family (2 patients). Pathogenic mutations, including missense, nonsense, frameshift, and exon rearrangements, were found in Parkin, PINK1, DJ-1, SYNJ1, and VAC14 genes. In conclusion, variable phenotypic expressivity was seen across all families.
Keywords: Early-onset, Parkinson’s disease, Pathogenic mutations, Genotype-phenotype correlations
Introduction
Parkinson’s disease (PD; MIM# 168600) is the second most common neurodegenerative disorder, with the prevalence of about 1% in people over 60 years of age [1]. PD results from degeneration of dopaminergic neurons in the substantia nigra pars compacta and the presence of proteinaceous inclusions called Lewy bodies (LBs) in the surviving neurons [2]. PD is characterized by the presence of motor symptoms, including resting tremor, bradykinesia, rigidity, postural instability, stooped posture, and freezing, as well as non-motor symptoms, such as fatigue, cognitive, behavioral and sensory phenotypes, sleep disorders, and autonomic dysfunction [3, 4]. Despite that the majority of PD is sporadic and is thought to be caused by a combination of genetic and environmental risk factors, approximately 5–10% of patients have monogenic forms of the disease with either an autosomal dominant (AD) or autosomal recessive (AR) Mendelian pattern of inheritance [5, 6]. To date, several genes have been found to be mutated in monogenic PD, with nine genes being involved in the pathogenesis of AR PD (ARPD) and/or juvenile parkinsonism (ARJP). These include Parkin [6q26; MIM# 600116], PINK1 [1p36.12; MIM# 605909], DJ-1 [1p36; MIM# 606324], ATP13A2 [1p36; MIM# 606693], FBXO7 [22q12.3; MIM# 260300], PLA2G6 [22q12.3; MIM# 612953], DNAJC6 [1p31.3; MIM# 608375], SYNJ1 [21q22.2; MIM# 615530], and VPS13C [15q.22.2; MIM# 616840] [7–16]. Overall, missense, nonsense, splice site, frameshift mutations, and whole exon and gene deletions/duplications have been identified in all forms of PD, including AD, AR, and sporadic PD/parkinsonism [17–19].
In this study, we investigated the known autosomal recessive PD/parkinsonism genes in 50 Iranian consanguineous families with ARPD or ARJP and identified VAC14 as a novel gene for hereditary progressive dystonic tremor and disabling dystonia.
Materials and Methods
Subjects
As part of a large multi-center study, we investigated 4000 clinical files, which belonged to Iranian patients with a diagnosis of parkinsonism. Tables 1 and 2 include information about the age at onset, pattern of inheritance, and the presence of consanguinity of all clinical files examined. Early-onset was considered when the disease symptoms begun before age 46, while patients with late-onset disease developed their symptoms at the age of 46 or later [20]. A total of 50 recessive families (116 patients) with at least two patients being born to consanguineous parents were selected to be examined. Parents did not show any sign of parkinsonism or other movement disorder. Selected families were from various parts of Iran, and they belonged to different ethnicities. Expert neurologists from different clinical centers examined selected families and confirmed their diagnosis. The local ethics committees at each participating medical center approved this study, and informed consent, according to the Declaration of Helsinki, was obtained from all participants. DNA samples from ethnicity-matched neurologically normal individuals were also available (n = 96). DNA samples from all participants were isolated from whole blood, using standard procedures.
Table 1.
Distribution of patients according to the age of onset and recurrence in the family
| Age of onset | Familial (%) | Sporadic (%) | Total (%) | |
|---|---|---|---|---|
|
| ||||
| AD | AR | |||
| Early onset | 22 (2.2) | 215 (22.2) | 736 (75.6) | 973 (100) |
| Late onset | 272 (9) | 160 (5.3) | 2595 (85.7) | 3027 (100) |
| Total | 294 (7.3) | 375 (9.4) | 3331 (83.3) | 4000 (100) |
AD autosomal dominant, AR autosomal recessive
Table 2.
Distribution of patients according to parents’ consanguinity and number of patients in the family
| Marriage | One affected (%) | More than one affected (%) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Late | Early | Sum | Late | Early | Sum | |
| Consanguineous | 151 (22.8) | 512 (77.2) | 663 (100) | 45 (19.3) | 188 (80.7) | 233 (100) |
| Non-consanguineous | 2444 (91.6) | 224 (8.4) | 2668 (100) | 387 (88.8) | 49 (11.2) | 436 (100) |
| Total | 2595 (77.9) | 736 (22.1) | 3331 (100) | 432 (64.6) | 237 (35.4) | 669 (100) |
Genetic Analysis
Multiplex Ligation-Dependent Probe Amplification and Quantitative PCR Assays
The salsa multiplex ligation-dependent probe amplification (MPLA) kit P051-c1/P52-c1 (MRC-Holland, Amsterdam, The Netherlands) was used to detect large deletions or duplications, which were later confirmed by using gap PCR or relative quantification of implicated exons. The relative quantification was performed and analyzed by using the Eco Real-Time PCR System following the 2−ΔΔCt method and as described elsewhere [21, 22].
Linkage Analysis
Families who showed no mutations in the MLPA assays were subject to linkage analyses. Linkage analyses were carried out by using short tandem repeat (STR) markers, covering the nine known genes involved in ARPD or AJP. At least six different markers were selected for each gene to be examined. STR markers were amplified by PCR, and the products were analyzed by polyacrylamide gel electrophoresis (8%).
To reduce the number of candidate genes in one family without mutations in known PD genes (F23), all available family members were subject to genome-wide SNP genotyping (HumanOmniExpress Exome arrays v1.3; Illumina Inc., San Diego, CA, USA) and genotyping data was used to perform homozygosity mapping as previously described [15, 23].
Whole Genome Sequencing
Two affected individuals from three different families (n = 6) without mutations in known PD genes were subject to whole genome sequencing (WGS) analyses. WGS was carried out at the New York Genome Center (NYGC). Sequencing libraries were constructed with the TruSeq PCR-Free Library kit (Illumina) following the manufacturer’s recommended protocol. Libraries were sequenced on the Illumina HiSeq X instruments, with 2 × 150 bp paired reads, to a minimum coverage of >30×. Sequencing data was processed with NYGC’s automated analysis pipeline, which includes alignment to GRCh37 using BWA-MEM (v0.78) [24], and further processing with GATK Best Practices, including the marking of duplicates with Picard (v1.83, http://picard.sourceforge.net) and GATK (v3.2.2) [25]. Single-nucleotide variations (SNVs) and indels were called by using the GATK HaplotypeCaller and were jointly genotyped. Deletions were called by using Genome STRiP (v2.0) [26] and were jointly called by using 17 HapMap individuals (CEPH Platinum Genomes pedigree). All deletions annotated as PASS in the Genome STRiP results were further filtered by using custom scripts to remove redundant calls and breakpoints overlapping repeat regions, or with extensive mapping ambiguity. SplazerS, which identifies and split-aligns reads that cross structural variant breakpoints, was further used to determine the breakpoints of candidate deletions [27]. First, all reads mapping to the candidate region were extracted, and then by using SplazerS, they were mapped back to the region to identify and confirm the breakpoint locations. Annotations of variants included predictions of the effect of nucleotide change on protein sequence using SnpEff; variant frequencies in different populations from the 1000 Genomes Project, the NHLBI GO Exome Sequencing Project, cross-species conservation scores from PhyloP, Genomic Evolutionary Rate Profiling (GERP), and PhastCons; functional prediction scores from PolyPhen-2, SIFT, and Combined Annotation Dependent Depletion (CADD) [28]; variant disease associations from OMIM, ClinVar, and Genetic Association Database (GAD); regulatory annotations from ENCODE, RegulomeDB, ORegAnno, and KEGG pathway annotations; transcription factor binding sites from the TRANSFAC database; and Gene Ontology (GO) annotations for biological process, cellular component, and molecular function.
Sanger Sequencing
Direct Sanger sequencing was used to examine the PD genes found to be associated with disease in one or more families and to validate the mutations identified through WGS. Primers, covering all exons and intron-exon boundaries of the genes of interest (Parkin, PINK1, SYNJ1, VAC14), or flanking the identified mutations in Parkin and DJ-1 genes, were designed by using a public primer design website (http://ihg.gsf.de/ihg/ExonPrimer.html; primer sequences available upon request). PCR products were then purified, sequenced, and analyzed as previously described [15, 23].
Computational Prediction of Mutation Pathogenicity
The pathogenicity of the novel missense mutations identified within the SYNJ1 and VAC14 genes was predicted by several computational methods, including MutPred (http://mutpred.mutdb.org/) and SNPs&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go/), previously evaluated as most efficient [29], as well as MutationTaster (http://www.mutationtaster.org/), SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and CADD [28]. The allele frequency of novel mutations was also investigated in the recent released “The Genome Aggregation Database” (gnomAD; http://gnomad.broadinstitute.org/).
Results
Genetic Analyses
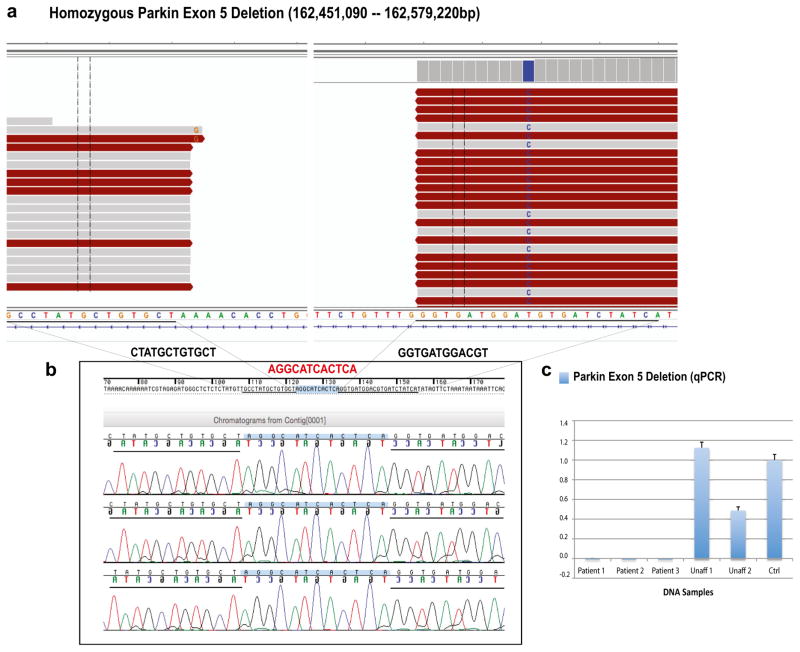
Twenty-two out of 50 (44%) consanguineous families with parkinsonism were found to have PD due to mutations in known genes. The most prevalent mutated gene was Parkin, being found mutated in 18 different families. Exon deletions within the Parkin gene were the most common mutations, being present in 15 different PD families (Table 3). MLPA assays identified large Parkin deletions in 14 different PD families while a homozygous Parkin exon 5 deletion was identified through WGS in a single family (Family F3; Fig. 1a). Deletions in exons 3 and 5, respectively identified in four and three different families, were the most common deletions. The breakpoints of the Parkin exon 5 deletion identified through WGS were determined by using both Genome STRiP [26] and SplazerS [27]. A large deletion of 128 kb in size was identified in all affected family members. Validation of this deletion through Sanger sequencing additionally revealed the presence of a small 12-bp insertion within the deletion breakpoints in all affected individuals (Fig. 1b). QPCR analyses confirmed its segregation with disease status (Fig. 1c). Additional families with mutations in Parkin include two families with the same missense mutation (p.Arg42Pro) and one family with a novel single nucleotide deletion (p.Thr414Profs*20; Table 3). The p.Arg42Pro mutation was already reported to be pathogenic [30, 31], while the novel p.Thr414Profs*20 mutation causes a premature stop codon, further supporting its pathogenicity. All Parkin mutations were found to segregate with disease status, being found in homozygous state in the affected patients and in heterozygous state or absent in the unaffected family members.
Table 3.
Detailed clinical description of patients with mutations in PD/parkinsonism genes
| Patient (gender) | AAO | Age | Gene mutation | Start location | First symptom | Tremor | Progression | Parkinsonism | Rigidity | Postural instability | Dystonia | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1P1 (F) | 26 | 32 | PINK1 | Hands | Resting tremor | Rest, intention, limbs, head, chin | Fast | Sy | N | N | N | |||
| F1P2 (F) | 15 | 55 | c.736C>T; | Hands | Resting tremor | Rest | Slow | As | N | N | N | |||
| F1P3 (F) | 22 | 40 | p.R246X | Hands | Resting tremor | Whole body | Slow | Sy | Y | La | Y | |||
| F1P4 (F) | 33 | 35 | Right hand | Resting tremor | Limbs, head, chin, voice | Fast | As | N | La | Y | ||||
| F1P5 (M) | 30 | 38 | Hands | Resting tremor | Whole body | Fast | Sy | Y | N | N | ||||
| F1P6 (M) | 40 | 46 | Hands | Resting tremor | Intention, limbs | Slow | Sy | N | N | N | ||||
| F2P1 (F) | 16 | 52 | PINK1 | Hands | Resting tremor | Rest | Slow | Sy | Y | Ea | Y | |||
| F2P2 (M) | 24 | 50 | c.1366C>T; | Hands | Resting tremor | Rest | Slow | As | N | La | N | |||
| F2P3 (F) | 24 | 42 | p.Q456X | Hands | Resting tremor | Rest | Slow | As | Y | La | N | |||
| F2P4 (M) | 39 | 45 | Hands | Resting tremor | Rest | Slow | As | Y | La | N | ||||
| F3P1 (M) | 13 | 62 | Parkin | Limbs | Resting tremor | Rest, intention, limbs | Slow | Sy | Y | La | Y | |||
| F3P2 (M) | 13 | 54 | Deletion | Limbs | Resting tremor | Intention, limbs, chin | Slow | Sy | N | La | N | |||
| F3P3 (M) | 13 | 50 | exon 5 | Whole body | Whole body tremor | Rest, intention, limbs | Slow | Sy | Y | La | N | |||
| F4P1 (M) | 24 | 29 | Parkin | Leg | Resting tremor | Limbs, head, neck | Fast | Sy | N | N | N | |||
| F4P2 (F) | 23 | 35 | Deletion | Leg | Resting tremor | Limbs, voice | Slow | Sy | Y | La | N | |||
| F4P3 (F) | 27 | 42 | exon 3 | Limbs | Resting tremor | Rest, intention, limbs, chin, voice | Slow | N | N | La | N | |||
| F5P1 (F) | 34 | 42 | Parkin | Hands | Resting tremor | Intention, limbs | Slow | Sy | Y | La | N | |||
| F5P2 (F) | 17 | 37 | Deletion | Hands | Resting tremor | Intention, limbs | Fast | Sy | N | La | N | |||
| F5P3 (M) | 20 | 49 | Exons 5 and 6 | Whole body | Whole body tremor | Rest, intention, limbs, voice | Fast | Sy | Y | La | N | |||
| F6P1 (M) | 23 | 30 | Parkin | Limbs | Resting tremor | Rest, limbs, head, chin | Slow | As | Y | La | N | |||
| F6P2 (M) | 24 | 34 | Deletion | Limbs | Resting tremor | Rest, limbs, head, chin | Slow | As | Y | La | N | |||
| F6P3 (F) | 35 | 38 | Exon 5 | Limbs | Resting tremor | Rest, limbs, head, chin | Slow | As | Y | La | N | |||
| F7P1 (F) | 27 | 42 | Parkin | Limbs | Resting tremor | Limbs | Slow | Sy | N | La | N | |||
| F7P2 (M) | 27 | 60 | Deletion Exons 3 and 4 |
Left leg | Resting tremor | Limbs | Slow | Sy | Y | La | N | |||
| F8P1 (F) | 28 | 31 | Parkin | Legs | Resting tremor | Rest, intention, limbs | Fast | N | N | N | N | |||
| F8P2 (M) | 30 | 33 | Deletion Exon 3 |
Left hand | Resting tremor | Rest, intention, limbs | Fast | N | Y | N | N | |||
| F9P1 (M) | 44 | 47 | Parkin | Legs | Resting tremor | Rest | Fast | Sy | Y | La | N | |||
| F9P2 (M) | 37 | 44 | Deletion Exon 4 |
Left upper limb | Myoclonus | Other | Moderate | As | Y | La | Y | |||
| F10P1 (F) | 27 | 32 | Parkin | Legs | Resting tremor | Rest | Slow | As | N | Ea | Y | |||
| F10P2 (F) | 35 | 45 | Deletion Exon 7 |
Legs | Resting tremor | Rest | Slow | As | N | Ea | N | |||
| F11P1 (F) | 45 | 50 | Parkin | Hands | Resting tremor | Rest, limbs | Slow | Sy | Y | Ea | Y | |||
| F11P2 (F) | 45 | 52 | Deletion Exon 5 |
Hands | Resting tremor | Rest | Slow | Sy | Y | Ea | Y | |||
| F12P1 (M) | 35 | 47 | Parkin | Left hand | Resting tremor | Limbs | Slow | As | Y | La | N | |||
| F12P2 (F) | 32 | 45 | c.1240delA; p.T414PfsX20 | Lower limbs | Stiffness of the lower limbs | Non | Very Slow | Sy | Y | N | N | |||
| F13P1 (M) | 22 | 41 | Parkin | Un | Bradykinesia | Rest, limbs | Slow | As | Y | La | N | |||
| F13P2 (M) | 22 | 40 | Deletion Exons 5 and 6 |
Un | Slowness | Non | Slow | Sy | N | N | N | |||
| F14P1 (F) | 14 | 42 | Parkin | Hands | Resting tremor | Rest | Slow | Sy | Y | Ea | Y | |||
| F14P2 (F) | 40 | 54 | c.125G>C; p.R42P | Hands | Resting tremor | Rest | Slow | Sy | Y | La | N | |||
| F15P1 (F) | 14 | 28 | Parkin | Un | Bradykinesia | Rest, limbs | Slow | As | Y | La | Y | |||
| F15P2 (M) | 12 | 30 | Deletion Exons 3 to 7 |
Un | Bradykinesia | Rest, limbs | Fast | As | Y | Ea | Y | |||
| F16P1 (M) | 19 | 23 | Parkin | Left limbs | Resting tremor | Intention, limbs, neck, chin, voice, tongue | Fast | Sy | Y | N | N | |||
| F16P2 (M) | 15 | 32 | c.125G>C; p.R42P | Hands | Resting tremor | Rest, limbs, chin, voice | Fast | Sy | Y | Ea | Y | |||
| F17P1 (M) | 14 | 35 | Parkin | Un | Bradykinesia | Rest, limbs | Slow | As | Y | La | Y | |||
| F17P2 (M) | 15 | 46 | Deletion Exons 5 to 7 |
Un | Bradykinesia | Rest, limbs | Fast | As | Y | Ea | Y | |||
| F18P1 (F) | 25 | 35 | Parkin | Left hand | Resting tremor | Intention, limbs, head, voice | Slow | As | Y | La | Y | |||
| F18P2 (M) | 24 | 48 | Deletion Exon 9 |
Un | Bradykinesia | Intention, limbs, chin, voice | Fast | As | Y | Ea | Y | |||
| F19P1 (M) | 30 | 38 | Parkin | Left hand | Resting tremor | Intention, limbs, voice | Fast | Sy | N | La | N | |||
| F19P2 (M) | 21 | 42 | Deletion Exon 3 |
Whole body | Resting tremor | Intention, limbs, chin, voice | Fast | Sy | Y | La | N | |||
| F20P1 (M) | 23 | 52 | Parkin | Un | Bradykinesia | Non | Slow | Sy | Y | Y | Y | |||
| F20P2 (M) | 24 | 47 | Deletion Exon 3 |
Hands | Resting tremor | Rest | Slow | Sy | Y | Y | N | |||
| F21P1 (M) | 27 | 42 | DJ-1 | Un | Oromandibular dystonia then parkinsonism | Non | Slow | Sy | Y | La | Y | |||
| F21P2 (M) | 27 | 35 | c.70delA; p.D24MfsX3 | Un | Oromandibular dystonia then parkinsonism | Non | Slow | Sy | Y | La | Y | |||
| F22P1 (M) | 24 | 30 | SYNJ1 | Right hand | Tremor, rigidity | Rest | Fast | As | Y | Y | N | |||
| F22P2 (F) | 27 | 47 | c.2515C>T; p.R839C | Whole body | Tremor, rigidity | Rest, limbs | Fast | As | Y | Y | N | |||
| F23P1 (M) | 13 | 24 | VAC14 | Lower limbs and gait | Dystonic gait | Whole body dystonic tremor | Fast | Sy | Y | n/a | Y | |||
| F23P2(M) | 8 | 13 | Lower limbs and gait | Dystonic gait | No | Fast | Sy | N | N | Y | ||||
|
| ||||||||||||||
| Patient (gender) | Bradykinesia | Hypokinesia | Autonomic dysfunction | Pyramidal signs | Stride and sleep apnea | REM sleep behavior disorder | Falling | Response to levodopa | Other symptoms | |||||
|
| ||||||||||||||
| F1P1 (F) | Y | Y | Y | N | Y | N | N | Y | Eyelid apraxia or blepharospasm, amnesia, insomnia, impaired smell | |||||
| F1P2 (F) | Y | Y | Y | N | Y | N | Y | Y | Wheelchair dependency, impaired smell | |||||
| F1P3 (F) | Y | Y | N | Sp | Y | N | Y | Y | Incontinence, wheelchair dependency, insomnia | |||||
| F1P4 (F) | Y | Y | N | Sp | N | Y | N | Y | Amnesia, insomnia | |||||
| F1P5 (M) | Y | Y | Y | N | N | N | Y | Y | Amnesia, insomnia | |||||
| F1P6 (M) | Y | Y | N | N | N | Y | N | Y | Amnesia | |||||
| F2P1 (F) | Y | Y | N | N | N | N | N | Y | – | |||||
| F2P2 (M) | N | N | N | N | NN | N | N | Y | – | |||||
| F2P3 (F) | Y | Y | N | N | N | N | N | N | – | |||||
| F2P4 (M) | Y | Y | N | N | N | N | N | Y | – | |||||
| F3P1 (M) | Y | Y | Y | N | N | N | Y | Y | Eyelid apraxia or blepharospasm, incontinence, sensory polyneuropathy, impaired smell | |||||
| F3P2 (M) | Y | Y | N | N | N | N | Y | Y | Incontinence, sensory polyneuropathy | |||||
| F3P3 (M) | Y | Y | N | N | N | N | N | Y | Sensory polyneuropathy | |||||
| F4P1 (M) | Y | Y | N | Sp | Y | N | N | Y | – | |||||
| F4P2 (F) | Y | Y | Y | Sp | Y | N | N | Y | Amnesia | |||||
| F4P3 (F) | N | N | Y | N | Y | N | Y | Y | Insomnia | |||||
| F5P1 (F) | N | N | Y | Sp | N | N | N | N | – | |||||
| F5P2 (F) | Y | Y | N | N | N | N | Y | N | Amnesia, insomnia | |||||
| F5P3 (M) | N | N | Y | Sp | Y | Y | Y | Y | Amnesia, insomnia, seizure | |||||
| F6P1 (M) | Y | Y | Y | Sp | Y | Y | Y | Y | Incontinence, wheelchair dependency, insomnia, impaired smell | |||||
| F6P2 (M) | Y | Y | Y | Sp | Y | Y | Y | Y | Incontinence, wheelchair dependency, insomnia, impaired smell | |||||
| F6P3 (F) | Y | Y | Y | Sp | Y | Y | Y | Y | Incontinence, wheelchair dependency, insomnia, impaired smell | |||||
| F7P1 (F) | Y | Y | Y | N | N | N | Y | Y | Amnesia, insomnia | |||||
| F7P2 (M) | Y | Y | Y | Sp | N | N | Y | Y | Incontinence, wheelchair dependency, insomnia, seizure, impaired smell | |||||
| F8P1 (F) | N | N | Y | N | Y | N | N | Y | Amnesia | |||||
| F8P2 (M) | N | N | Y | N | N | Y | N | Y | – | |||||
| F9P1 (M) | Y | Y | Y | Sp | Y | Y | N | Y | Incontinence, amnesia, insomnia, seizure | |||||
| F9P2 (M) | Y | Y | N | N | N | N | N | Y | Off dystonia | |||||
| F10P1 (F) | Y | N | N | N | N | N | N | Y | – | |||||
| F10P2 (F) | N | N | N | N | N | N | Y | Y | Insomnia | |||||
| F11P1 (F) | N | N | N | N | N | N | N | Y | Insomnia | |||||
| F11P2 (F) | Y | N | N | N | N | N | Y | Y | – | |||||
| F12P1 (M) | Y | Y | N | N | N | Y | N | Y | Psychosis, insomnia, paranoid ideas with pramipexole | |||||
| F12P2 (F) | Y | Y | N | Sp | N | N | N | Y | Psychiatric symptoms (depression, anxiety, and panic attacks) | |||||
| F13P1 (M) | Y | Y | Y | Ba | Y | Y | Y | Y | Incontinence, depression, suicidal attempt, drug induced dyskinesias, insomnia, impaired smell | |||||
| F13P2 (M) | Y | Y | N | N | N | N | N | Y | Psychosis, dyskinesias, insomnia | |||||
| F14P1 (F) | Y | Y | N | N | N | Y | N | Y | Incontinence | |||||
| F14P2 (F) | Y | Y | Y | N | N | N | N | Y | – | |||||
| F15P1 (F) | Y | Y | N | N | N | N | N | Y | Impaired smell | |||||
| F15P2 (M) | Y | Y | N | N | N | N | N | Y | Impaired smell | |||||
| F16P1 (M) | Y | Y | Y | Sp | N | N | N | Y | – | |||||
| F16P2 (M) | Y | Y | Y | N | Y | N | Y | Y | Wheelchair dependency, impaired smell | |||||
| F17P1 (M) | Y | N | N | N | N | N | N | Y | – | |||||
| F17P2 (M) | Y | Y | N | N | N | N | N | Y | Wheelchair dependency | |||||
| F18P1 (F) | Y | Y | Y | Sp | N | Y | Y | Y | Eyelid apraxia or blepharospasm, psychosis, amnesia, insomnia, seizure, impaired smell | |||||
| F18P2 (M) | Y | Y | Y | Sp | N | Y | Y | Y | Eyelid apraxia or blepharospasm, wheelchair dependency, psychosis, amnesia, insomnia, seizure, impaired smell | |||||
| F19P1 (M) | Y | Y | Y | Sp | N | N | Y | Y | Incontinence, insomnia, impaired smell | |||||
| F19P2 (M) | Y | Y | Y | Sp | N | N | Y | Y | Insomnia, impaired smell | |||||
| F20P1 (M) | Y | N | N | N | N | N | Y | Y | – | |||||
| F20P2 (M) | Y | N | N | N | N | N | Y | Y | – | |||||
| F21P1 (M) | Y | Y | N | N | N | N | Y | Y | Wheelchair dependency, psychosis, bilateral cataract | |||||
| F21P2 (M) | Y | Y | N | N | N | N | Y | Y | Psychosis | |||||
| F22P1 (M) | Y | Y | N | N | N | N | N | N | Seizure, chin tremor, longitudinal fissured tongue | |||||
| F22P2 (F) | Y | Y | N | N | N | N | N | N | This patient was not alive to be examined for the tongue feature | |||||
| F23P1 (M) | Y | Y | N | N | N | N | N | N | Striatal toe, normal intellectual function | |||||
| F23P2(M) | N | N | N | N | N | N | N | N | Normal intellectual function | |||||
F1P1 family 1, patient 1, M male, F female, AAO age at onset, Sy symmetric, As asymmetric, N no, Y yes, Ea early, La late, Sp spasticity, Ba Babinski, Un unknown, n/a not available
Fig. 1.
Parkin exon 5 deletion (128-kb). a WGS reads were visualized by using the Integrative Genomics Viewer (IGV). Two different plots that represent the Parkin exon 5 deletion breakpoints are shown. b Sanger chromatograms of the three affected individuals, who carried the 128-kb Parkin exon 5 deletion, are shown. Deletion breakpoints are highlighted in black (12 bp of the flanking regions), while the 12-bp insertion identified within the deletion breakpoints is highlighted in red. c Validation of the Parkin exon 5 deletion through qPCR analyses. Y-axis represents PRKN/GPR15 ratios; x-axis: DNA samples analyzed. Affected individuals showed no copy of Parkin exon 5 (ratio = 0.0), whereas unaffected individuals showed either two copies (Unaff 1; ratio = 1.0–1.2) or one copy (Unaff 2; ratio = 0.4–0.6). Unaff unaffected family member, Ctrl control DNA sample (color figure online)
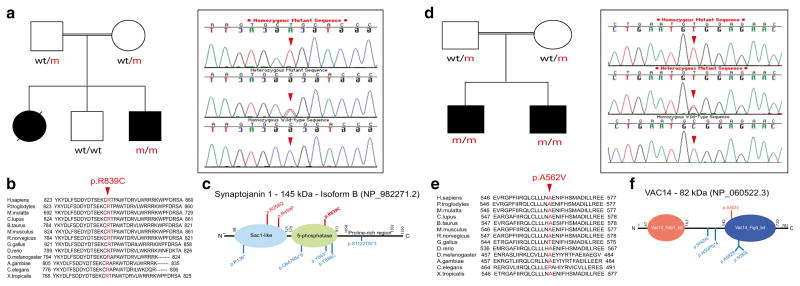
We also identified two families, respectively carrying previously reported nonsense PINK1 mutations (p.Arg246Stop; p.Gln456Stop), one family carrying a novel frameshift DJ-1 mutation (p.Asp24Metfs*3) and another family carrying a novel SYNJ1 mutation (p.Arg795/800/839His) (Table 3). Both PINK1 mutations segregate with disease status: the p.Arg246Stop mutation was found in homozygous state in the six patients and in heterozygous state in both parents as well as two unaffected siblings, while the p.Gln456Stop mutation was found in homozygosis in the three affected members, in heterozygosis in the unaffected mother, and absent in the only unaffected sibling. The novel DJ-1 mutation, located in exon 1 and resulting in a premature stop codon, was identified by WGS. It did segregate with disease status, being found in homozygosis in both the affected patients, heterozygosis in unaffected mother and one unaffected sibling, and absent in another unaffected sibling. The novel SYNJ1 mutation did segregate with disease status, was found to be absent in 192 ethnicity-matched control chromosomes, and was shown to be conserved among other orthologs (Fig. 2a, b). It is not described in public databases, such as the NHLBI GO Exome Sequencing Project (http://evs.gs.washington.edu/EVS/) and gnomAD, and it was predicted to be pathogenic by various computational methods (MutPred score: 0.818; SNPs&GO effect: disease; MutationTaster: disease causing; SIFT: deleterious; PolyPhen-2: probably damaging; and CADD_phred: 35). It is located in exon 19 of the SYNJ1 gene and lies within the inositol-5-phosphatase domain of Synaptojanin 1 (Fig. 2c) which is known to dephosphorylate a variety of lipids, such as PI(4,5)P2 and PI(3,4,5)P3 [15, 32].
Fig. 2.
SYNJ1 p.Arg839Cys and VAC14 p.Ala562Val mutations. a Pedigree structure of the family carrying the SYNJ1 p.R839C mutation is shown on the left side. Affected family members are represented with black circle (female) or square (male). Wt/m heterozygous mutation carriers; m/m homozygous mutation carriers; wt/wt non-carriers. Sanger chromatogram sequences belonging to the SYNJ1 p.R839C mutation (red arrow) are shown on the right side. b Conservation of the SYNJ1 p.R839C mutation across different orthologs. c Diagram of the Synaptojanin 1 protein structure. The three SYNJ1 mutations identified to date in patients with parkinsonism are represented in red, whereas the SYNJ1 mutations identified in patients with seizures and severe neurodegeneration are shown in blue. The mutation identified in the current study is highlighted in bold. d Pedigree structure of the family carrying the VAC14 p.A562V mutation is shown on the left side. Affected family members are represented with black squares (males). Wt/m heterozygous mutation carriers; m/m homozygous mutation carriers; wt/wt non-carriers. Sanger chromatogram sequences for the VAC14 p.A562V mutation (red arrow) are shown on the right side. e Conservation of the VAC14 p.A562V mutation across different species. f Diagram of the Vac14 protein structure showing the mutation identified in patients with dystonic tremor and disabling dystonia at the top (bold) and the mutations identified in patients with striatonigral neurodegeneration at the bottom
Lastly, a novel gene, VAC14 (MIM #604632), was found to be mutated in a single family by combining both WGS and HM approaches. Briefly, WGS performed on the two affected siblings (Fig. 2d) identified 10 different homozygous genetic variants, not reported or present with low frequency in public databases, in both affected individuals. No common compound heterozygous variants were identified. Genetic variants were located on chromosomes 3, 16, and 19. To assist with disease gene identification and reduce the number of candidate variants, HM was then performed and eight different homozygous segments, on chromosomes 5, 7, 11 (n = 3), 16, 18, and 21 (data not shown), were identified to be shared exclusively by both affected siblings. Four genetic variants were localized on the largest autozygous region located on chromosome 16 (21 Mb) and flanked by rs8049176 (57,655,792 bp) and rs16949243 (79,053,995 bp) SNVs. Among these, the ZFHX3 p.Pro2304Thr (p.Pro3218Thr) variant was also found to be present in 5 out 16,510 South Asian alleles (http://exac.broadinstitute.org/variant/16-72822523-G-T). Two variants (p.Asp1849Val, p. Pro2304Thr) located in the same gene (ZFHX3) were predicted non-deleterious, while the other two located in CES2 (p.Phe127Leu) and VAC14 (p.Ala562Val) genes were predicted to be highly pathogenic. However, VAC14 was the only gene found to be highly expressed in brain tissues (http://gtexportal.org/home/gene/VAC14) and therefore selected as the disease candidate gene. No dystonia genes were found to be mutated in affected patients subject to WGS analyses. The novel VAC14 mutation (c.1685C>T) was found to segregate with disease status, was absent in 188 ethnicity-matched control chromosomes, was shown to be highly conserved across other species (Fig. 2d, e), and was predicted to be pathogenic by SNPs&GO (disease), MutationTaster (disease causing), SIFT (deleterious), PolyPhen-2 (possibly damaging), and CADD_phred (29.8). Despite being absent in public databases, it was recently described with very low frequency (1/252,016) in gnomAD database. The Vac14 protein contains two binding domains, the lipid kinase PIKFYVE (MIM# 609414; Fab1) and the phosphatase Fig4 (MIM# 609390), through which it regulates the synthesis of the signaling lipid PI(3,5)P2 [33, 34]. The VAC14 p.Ala562Val mutation is located in exon 15 which encodes part of the Fig4-binding domain. The entire VAC14 gene was additionally examined in 20 familial cases with dystonia-parkinsonism phenotypes, but no pathogenic mutation was identified.
Clinical Findings
A detailed description of all observed phenotypic features is presented in Table 3.
Families with Parkin Mutations
The mean age of onset (± standard deviation) of the patients with Parkin mutations was 25.3 ± 9.29 (mean age in males = 22.6, mean age in females = 29.25). The earliest AAO was 12 (F15P2) while the latest AAO was 45 years (F11P1, F11P2). The first symptom in the majority of patients was resting tremor (28/40: 70%). The progress of the disease was slow (meaning that symptoms continue and worsen over a period of years) in 27 patients (67.5%), but fast, with motor symptoms progressing very quickly, in 13 (32.5%). Rigidity was seen in 30 patients (75%). Parkin families showed variable phenotypic expressivity, including the presence of sensory polyneuropathy in one family (F3).
Families with PINK1 Mutations
The mean age of onset (± standard deviation) for patients with PINK1 mutations was 26.9 ± 8.06 (mean age in males = 33.25, mean age in females = 22.6). The earliest AAO was 15 (F1P2) while the latest AAO was 40 years (F1P6). Resting tremor was the first symptom in all patients. Seven patients (70%) showed slow disease progression, while the disease progressed quickly in three patients (30%). Variable phenotypic expressivity was seen in both families.
Family Carrying a Novel DJ-1 Mutation
The novel DJ-1 mutation (c.70delA) was detected in two brothers who displayed oromandibular dystonia and profound psychosis at the age of 27. The disease progressed slowly to a marked symmetric parkinsonism with rigidity and bradykinesia. There was no significant tremor in these patients. They had frequent falling attacks and good response to levodopa therapy. One of the brothers showed bilateral cataract.
Family Carrying a Novel SYNJ1 Mutation
The novel SYNJ1 mutation (c.2515C>T) was identified in a patient who manifested asymmetric parkinsonism and seizures at 24 years of age. The disease manifested with tremor and rigidity in the right hand, but became generalized with tremor and rigidity in all limbs within a year. The patient showed chin tremor and significant dysarthria. He received phenytoin for generalized tonic clonic seizures and showed poor response to levodopa therapy. There was no falling or autonomic dysfunction, and rapid eye movement (REM) sleep behavior disorder was absent as well. He also showed a longitudinal fissured tongue. As this seems to be an uncommon phenomenon, we re-examined our previously reported patients carrying the SYNJ1 p.Arg258Gln mutation [15], and then observed the same feature in their tongue. There was also a female patient in the family who died from liver cancer during the early stage of this study.
Family Carrying a Novel VAC14 Mutation
Both siblings carrying the novel VAC14 mutation were reported as having parkinsonism; however, a follow-up clinical characterization revealed that both patients presented with dystonic gait affecting both lower limbs at a young age. Moreover, the disease progression was very severe in one patient (F23P1), with dystonia spreading to upper limbs and trunk. He manifested dystonic action tremors and became bedridden 5 years after disease presentation, and now had profound hypokinesia and bradykinesia. Patients’ speech was impaired due to severe dysarthria and dystonia, but their mental state was normal. In both patients, the disease progressed to marked generalized and disabling dystonia. Brain MRI was normal and there was no clinical response to levodopa treatment.
Discussion
In this study, we describe the phenotypic and genetic features of 23 consanguineous recessive families, featuring either ARPD or ARJP. In total, 56 patients were clinically examined. The majority (91.07%) of our patients with mutations reported their first symptom before the age of 40, with only five patients (8.93%) manifesting the disease at the age of 40 or later. The most common first symptom was resting tremor, being present in 67.85% of the patients with pathogenic mutations. Psychiatric features were seen only in three different families carrying either Parkin (F12, F13) or DJ-1 mutations (F21). Seizures were seen in five Parkin and one SYNJ1 mutation carriers. Sensory polyneuropathy, previously not reported in patients with Parkin mutations, was observed in three Parkin patients (F3). And marked generalized and disabling dystonia was the main phenotype observed in VAC14 mutation carriers (F23).
The most common mutated gene was Parkin, with exon deletions being the most prevalent mutations. In concordance with previous reports, most of the exon rearrangements fell into the genomic region between exons 2 and 8 of the Parkin gene, further confirming this region as a mutational hotspot. Novel mutations were also reported in DJ-1 and SYNJ-1 genes. The DJ-1 mutation was identified in a family presented with oromandibular dystonia, parkinsonism, and phenotypic features similar to those observed in previously reported DJ-1 mutation carriers [8, 35]. The SYNJ1 mutation identified in this study represents the third SYNJ1 mutation reported to date in patients with parkinsonism and the first one reported in the inositol-5-phosphatase domain [36], further confirming the role of SYNJ1 in the pathogenesis of parkinsonism (Fig. 2c). Like in our patient, seizures were previously reported in patients carrying the SYNJ1 p.Arg258Gln mutation [15, 37], as well as in patients with early-onset epilepsy, progressive spastic quadriplegia, severe intellectual disability, visual impairments, and feeding problems [38, 39]. Given the variable phenotypic expressivity of SYNJ1 mutation carriers, it has been postulated that variants with complete loss of SYNJ1 dual phosphatase activity (nonsense, frameshift mutations) lead to severe progressive neurodegeneration, while reduced SYNJ1 enzymatic activity (caused by missense mutations) leads to a milder phenotype associated with parkinsonism and increased seizure susceptibility [39].
Lastly, we identified a novel gene (VAC14) to be mutated in patients with progressive and disabling dystonia (F23). Although mutations in VAC14 have recently been identified in pediatric patients with striatonigral degeneration [40], some differences in clinical presentation and course were observed in our family when compared with the previously reported VAC14 patients. In our family, disease presentation occurred at older age, ranging from 8 to 13 years, versus 18 months and 3 years; no psychomotor regression was observed; and despite the marked abnormality in the basal ganglia reported in patients with VAC14 mutations, our patients showed normal brain MRI with no obvious abnormality in the striatum. However, the oldest patient (F23P1) showed profound hypokinesia and bradykinesia at a later age. The VAC14 p.Ala562Val mutation we identified is very likely to result in PI(3,5)P2 deficiency, as it lies in the protein’s Fig4-binding domain that is required for Vac14 dimerization and is thought to regulate Fab1 activity to maintain normal levels of PI(3)P, PI(3,5)P2, and PI(5)P [41]. Low levels of PI(3,5)P2 have already been reported in mice exhibiting central and peripheral nervous system neurodegeneration due to Vac14 deficiency and in mice and patients with pathogenic FIG4 mutations [34, 42]. Moreover, both patients and mice with VAC14 mutations have been reported to exhibit vacuolation in both cultured fibroblasts and affected neurons that are thought to arise from defects in the intracellular membrane trafficking, particularly in the retrograde transport from late endosomes to the trans-golgi network (TGN) [34, 40]. Taken together, the VAC14 p.Ala562Val mutation might also result in vacuolation and impaired retrograde transport from late endosomes to the TGN, likely supporting its pathogenicity.
We concluded that Parkin is the most common mutated gene in our population, being found mutated in 71.42% (n = 40) of our examined patients with mutations in known genes (n = 56). Despite the observed, variable phenotypic expressivity in our patients with Parkin or PINK1 mutations, the phenotype observed in the majority of Parkin and PINK1 mutation carriers was indistinguishable from one another. It was mainly characterized by an early-onset presentation, resting tremor of the limbs as an onset symptom, and slow disease progression. Given the presence of seizures in three different families with SYNJ1 mutations, we propose that seizures should be considered in prospective subjects with SYNJ1 mutations and parkinsonism. Lastly, given the progressive dystonic phenotype observed in patients with VAC14 mutations, we suggest nominating VAC14 as DYT27 gene and categorizing its phenotype as hereditary progressive dystonia with dystonic gait as a symptom of onset, followed by parkinsonian symptoms.
The finding of VAC14 as a novel gene for hereditary progressive dystonia is very interesting as it sums up to the large list of parkinsonism-related proteins, including alpha-synuclein, Lrrk2, VPS35, parkin, auxilin, and Synaptojanin 1, that act as important regulators of synaptic vesicle endocytosis and trafficking pathways at synapses. This together suggests the polyphosphoinositide signaling pathway as a relevant therapeutic target for neurodegenerative diseases such PD, parkinsonism, and now dystonia.
Acknowledgments
We are deeply grateful to all patients and relatives for their generous contribution to this study.
Funding This work was in part supported by the Shahid Beheshti University of Medical Sciences, the American Parkinson Disease Association (APDA; CPR), and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01NS079388; CPR).
Footnotes
Compliance with Ethical Standards The local ethics committees at each participating medical center approved this study, and informed consent, according to the Declaration of Helsinki, was obtained from all participants.
Conflict of Interest The authors declare that they have no competing interests.
References
- 1.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 2.Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18(2):101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliday G, Lees A, Stern M. Milestones in Parkinson’s disease—clinical and pathologic features. Mov Disord. 2011;26(6):1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- 4.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C. The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev. 2009;19(3):254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Kalinderi K, Bostantjopoulou S, Fidani L. The genetic background of Parkinson’s disease: current progress and future prospects. Acta Neurol Scand. 2016 doi: 10.1111/ane.12563. [DOI] [PubMed] [Google Scholar]
- 7.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 8.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 9.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 11.Shojaee S, Sina F, Banihosseini SS, Kazemi MH, Kalhor R, Shahidi GA, Fakhrai-Rad H, Ronaghi M. Genome-wide linkage analysis of a parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am J Hum Genet. 2008;82(6):1375–1384. doi: 10.1016/j.ajhg.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, et al. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65(1):19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P, et al. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord. 2010;25(12):1791–1800. doi: 10.1002/mds.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One. 2012;7(5):e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, Di Paolo G, Walker RH, et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive parkinsonism with generalized seizures. Hum Mutat. 2013;34(9):1200–1207. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A, Cormier-Dequaire F, Hassoun SM, et al. Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin-dependent mitophagy. Am J Hum Genet. 2016;98(3):500–513. doi: 10.1016/j.ajhg.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darvish H, Movafagh A, Omrani MD, Firouzabadi SG, Azargashb E, Jamshidi J, Khaligh A, Haghnejad L, et al. Detection of copy number changes in genes associated with Parkinson’s disease in Iranian patients. Neurosci Lett. 2013;551:75–78. doi: 10.1016/j.neulet.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Ambroziak W, Koziorowski D, Duszyc K, Gorka-Skoczylas P, Potulska-Chromik A, Slawek J, Hoffman-Zacharska D. Genomic instability in the PARK2 locus is associated with Parkinson’s disease. J Appl Genet. 2015;56(4):451–461. doi: 10.1007/s13353-015-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez P, Lesage S, Janin S, Lohmann E, Durif F, Destee A, Bonnet AM, Brefel-Courbon C, et al. Alpha-synuclein gene rearrangements in dominantly inherited parkinsonism: frequency, phenotype, and mechanisms. Arch Neurol. 2009;66(1):102–108. doi: 10.1001/archneurol.2008.555. [DOI] [PubMed] [Google Scholar]
- 20.Periquet M, Latouche M, Lohmann E, Rawal N, De Michele G, Ricard S, Teive H, Fraix V, et al. Parkin mutations are frequent in patients with isolated early-onset parkinsonism. Brain. 2003;126(Pt 6):1271–1278. doi: 10.1093/brain/awg136. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Karkheiran S, Krebs CE, Darvish H, Asadian M, Shahidi GA, Paisan-Ruiz C. Variable phenotypic expression in families with early-onset parkinsonism due to PRKN mutations. J Neurol. 2014;261(6):1223–1226. doi: 10.1007/s00415-014-7360-5. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez E, Darvish H, Mesias R, Taghavi S, Firouzabadi SG, Walker RH, Tafakhori A, Paisan-Ruiz C. Identification of a large DNAJB2 deletion in a family with spinal muscular atrophy and parkinsonism. Hum Mutat. 2016;37(11):1180–1189. doi: 10.1002/humu.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handsaker RE, Korn JM, Nemesh J, McCarroll SA. Discovery and genotyping of genome structural polymorphism by sequencing on a population scale. Nat Genet. 2011;43(3):269–276. doi: 10.1038/ng.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emde AK, Schulz MH, Weese D, Sun R, Vingron M, Kalscheuer VM, Haas SA, Reinert K. Detecting genomic indel variants with exact breakpoints in single- and paired-end sequencing data using SplazerS. Bioinformatics. 2012;28(5):619–627. doi: 10.1093/bioinformatics/bts019. [DOI] [PubMed] [Google Scholar]
- 28.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thusberg J, Olatubosun A, Vihinen M. Performance of mutation pathogenicity prediction methods on missense variants. Hum Mutat. 2011;32(4):358–368. doi: 10.1002/humu.21445. [DOI] [PubMed] [Google Scholar]
- 30.Chaugule VK, Burchell L, Barber KR, Sidhu A, Leslie SJ, Shaw GS, Walden H. Autoregulation of Parkin activity through its ubiquitin-like domain. EMBO J. 2011;30(14):2853–2867. doi: 10.1038/emboj.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25(3):302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 32.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99(2):179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 33.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem. 2007;282(33):23878–23891. doi: 10.1074/jbc.M611678200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann Neurol. 2003;54(3):283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- 36.Kirola L, Behari M, Shishir C, Thelma BK. Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile parkinsonism. Parkinsonism Relat Disord. 2016 doi: 10.1016/j.parkreldis.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Olgiati S, De Rosa A, Quadri M, Criscuolo C, Breedveld GJ, Picillo M, Pappata S, Quarantelli M, et al. PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics. 2014;15(3):183–188. doi: 10.1007/s10048-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 38.Dyment DA, Smith AC, Humphreys P, Schwartzentruber J, Beaulieu CL, Bulman DE, Majewski J, et al. Consortium FC. Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology. Neurobiol Aging. 2015;36(2):e1221–e1225. doi: 10.1016/j.neurobiolaging.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Hardies K, Cai Y, Jardel C, Jansen AC, Cao M, May P, Djemie T, Hachon Le Camus C, et al. Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain. 2016;139(Pt 9):2420–2430. doi: 10.1093/brain/aww180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenk GM, Szymanska K, Debska-Vielhaber G, Rydzanicz M, Walczak A, Bekiesinska-Figatowska M, Vielhaber S, Hallmann K, et al. Biallelic mutations of VAC14 in pediatric-onset neurological disease. Am J Hum Genet. 2016;99(1):188–194. doi: 10.1016/j.ajhg.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alghamdi TA, Ho CY, Mrakovic A, Taylor D, Mao D, Botelho RJ. Vac14 protein multimerization is a prerequisite step for Fab1 protein complex assembly and function. J Biol Chem. 2013;288(13):9363–9372. doi: 10.1074/jbc.M113.453712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]