Abstract
Until recently, patients with advanced thyroid cancers had limited options for systemic treatment. With the introduction of tyrosine kinase inhibitors (TKIs) as a promising new class of targeted therapies for thyroid cancer, suddenly patients with advanced disease were given new options to extend survival. Guidelines worldwide have been updated to include general indications for these newer agents, but questions remain regarding which agent(s) to select, when to begin treatment, and how long therapy should continue. Additionally, the true impact of TKIs on overall survival and quality-of-life in thyroid cancer patients needs further clarification. As familiarity with approved agents and longer-term data become available, better strategies for implementation of these targeted drugs will evolve to optimize benefit for patients living with metastatic disease.
1. Introduction
According to the American Cancer Society, an estimated 805,750 people are living with thyroid cancer in the US, with 64,300 new cases and 1,980 thyroid-cancer related deaths expected in 2016 [1]. While overall prognosis is excellent for most patients, outcomes depend highly on disease histology and the presence of regional or systemic metastasis [2]. Histologically almost 95% of thyroid cancers are well-differentiated subtypes (DTC), while 2–5% may be medullary thyroid cancers (MTC), and another 1–3% will be undifferentiatied or anaplastic thyroid cancers (ATC) [3]. DTCs originate in the thyroid follicles and include papillary thyroid cancers (PTC), comprising 80% of DTCs, follicular thyroid cancers (FTC) that comprise another 10–15%, and Hurthle cell cancers (HCC) making up the remainder [3,5]. By comparison, MTCs originate in the parafollicular ‘C’ cells of the thyroid. Finally, ATCs, while rare, are the most aggressive subtype with abysmal 5-year overall survival rates of less than 5% [3–5,95]. Surgical thyroidectomy is the standard initial management for patients with thyroid cancer and most patients with DTC can be fully treated with either surgery and thyrotropin (TSH) suppression or with addition of adjuvant radioiodine ablation (RAI) for select patients [3,6,8]. Efficaciousness of TSH suppression and RAI depends upon the presence of sufficiently differentiated follicular cells, and these modalities are ineffective and not recommended for use in MTC or ATC [3,5,6,8]. In addition to its utility in the adjuvant setting, RAI can also be used as effective systemic therapy for patients with unresectable or metastatic DTC, so long as tumor cells maintain the ability to take up and concentrate 131I [3,6,8]. In 5–15% of patients with DTC, however, this ability is lost and the tumor is classified as refractory to RAI (RAIR) [9–12]. Although outcomes in DTC are generally excellent, with 5-year overall survival reported at 97.8 % [2], the 5-year disease specific survival for patients with RAIR DTC is 66%, and 10-year survival is only 10% [9]. Patients with RAIR DTC and distant metastasis survive approximately 2.5 – 3.5 years [9,94].
Poorly differentiated thyroid cancer (PDTC) is an aggressive rare form of thyroid cancer that carries a high risk of recurrence and metastatic spread to lung and bones. Patients are often treated with a combination of surgery, radioactive iodine and/or radiation therapy and molecular targeted therapies as these tumors are frequently insensitive to RAI. [139] Although anaplastic thyroid cancer (ATC) is commonly considered the most aggressive histologic subtype of thyroid cancer with the worst mortality [5], most thyroid cancer deaths, however, are due to advanced stage RAIR DTCs [3]. An analogous contrast in prognosis by stage is present in MTC as well where 5-year survival for locoregional disease (Stages I to III) is 93% compared with 28% for distant stage IV disease [3].
The notably poor survival in late stage MTC and RAIR DTC compared with earlier stages reflects the lack of effective durable systemic treatment options for advanced disease [13]. Until 2011, the standard of care for systemic therapy for such patients was doxorubicin, which was approved in 1974 for advanced thyroid cancer [14–16,104]. Since then, multiple small studies have demonstrated limited efficacy with doxorubicin used either alone or in combination with other cytotoxic chemotherapeutic agents [13–16]. Based on this lack of efficacy and the promising results of newer TKIs, traditional cytotoxic chemotherapy is no longer recommended as first-line therapy in either MTC or RAIR DTC [3,6,8].
2. Approved TKIs in Advanced Thyroid Cancer
Landmark preclinical research implicating tyrosine kinase receptors (TKRs) and their downstream signaling cascades as drivers in the proliferation of MTC and DTC led to the development of numerous small molecule competitive inhibitors, the tyrosine kinase inhibitors (TKIs) [11,17,18]. Four of these agents are now approved by regulatory agencies for use in advanced thyroid cancer (Table 1) [20]. Some of the TKIs inhibit specific TKRs associated with known genetic lesions in thyroid cancer, whereas most are multi-targeted, affecting a variety of TKRs [10,11]. Rather than exhibiting a direct cytotoxic action, the effect of TKR blockade is to inhibit further growth and proliferation [10,19]. In addition, a significant portion of the anti-tumor effect exhibited by multi-targeted TKIs may be due to anti-angiogenesis mediated by vascular endothelial growth factor receptor (VEGFR) inhibition, with resulting tumor blood supply deprivation [11,19].
Table 1A.
In 2011, vandetanib, a multi-targeted inhibitor of several tyrosine kinase receptors including RET, VEGFR2&3, and EGFR, became the first TKI to garner regulatory approval for MTC [20,98–100]. In the randomized controlled phase 3 ZETA trial [21] involving 331 patients with locally advanced or metastatic MTC, vandetanib demonstrated prolonged projected median progression-free survival (PFS) of 30.5 months (m) compared to 19.3 m with placebo (Hazard Ratio 0.46; 95% Confidence Interval (CI), 0.31 to 0.69; P < .001). Overall response rate (ORR) in patients treated with vandetanib was 45%, compared with 13% in patients receiving placebo. Moreover, nearly all responses in patients randomized to placebo occurred after these patients crossed over into the open-label treatment phase of the study. Statistically significant decreases in calcitonin and carcinoembryonic antigen (CEA) levels were also seen in treated patients. The most common severe adverse effects (AE) were diarrhea (11%), hypertension (9%), and QTc prolongation (8%), which prompted the FDA to issue a “black box warning” upon vandetanib’s approval. Overall, this agent was well-tolerated, with only a 12% rate of treatment discontinuation over a median treatment duration of 21 m. More recently, a randomized placebo-controlled ‘registration’ trial of vandetanib in DTC (VERIFY trial) has now also been completed, but final results had not been published at the time of this review.
In 2012, cabozantinib, a multitargeted TKI affecting (RE-arranged during Transfection) RET kinase, VEGFR1&2, and the tyrosine-protein kinase Met (MET), attained regulatory approval for MTC [20,101]. This approval was predicated by favorable results from the randomized, controlled Phase III EXAM trial [22] involving 330 patients with unresectable locally advanced or metastatic progressive MTC randomized to receive either cabozantinib or placebo. In this trial, cabozantinib-treated patients demonstrated median PFS of 11.2 m compared to 4.0 m in the placebo group (HR 0.28; 95% CI, 0.19 to 0.40; P < .001). Progression-free survival on cabozantinib at 1 year was 47% while only 7.2% for the placebo group. The ORR for the cabozantinib group was 28% compared to 0% in the placebo group (P < .001). Calcitonin and CEA levels displayed significant decreases in the cabozantinib-treated group, compared to increases with placebo. The most common severe AEs related to treatment with cabozantinib were diarrhea (16%), Hand-Foot Skin Reaction, or HFSR (13%), fatigue (10%), and hypertension (8.4%). GI perforations and fistulas were reported in 3% and 1% of patients respectively, prompting the FDA to issue a “black box warning” upon cabozantinib’s approval [93]. Dose interruption or modification was necessary in 79% of patients, and 16% withdrew from treatment due to AEs.
In 2013, sorafenib, another multitargeted TKI affecting VEGFR1–3, platelet derived growth factor receptor (PDGFR), RET, and the Rapidly Accelerated Fibrosarcoma kinase (RAF), became the first TKI approved by regulatory agencies for RAIR DTC [20,102]. Sorafenib had previously been approved by the US FDA for treatment of renal cell carcinoma and hepatocellular carcinoma [20], and was the first targeted systemic agent to show promising results in RAIR DTC. In the DECISION trial [23], a phase 3 RCT in which 417 patients with RAIR, progressive DTC were randomized to undergo treatment with sorafenib or placebo, patients treated with sorafenib demonstrated median progression-free survival (PFS) of 10.8 m versus 5.8 m with placebo, with a 41% decrease in risk of disease progression or death (HR 0.59; 95% CI, 0.45 – 0.76; p < 0.0001). ORR was 12.2% with sorafenib, compared with 0.5% with placebo (p<0.0001). Median thyroglobulin level demonstrated changes in tandem with treatment response to sorafenib, compared to an increase from baseline in the placebo arm. Subgroup analyses of patients with BRAF mutations and Rat Sarcoma protein (RAS) mutations identified differences in PFS associated with overall prognosis, but the presence of either mutation did not materially affect the degree of improvement in PFS seen with treatment. Dose reduction due to AEs was necessary in 64% of patients treated with sorafenib, and the most common reasons for dose modifications were HFSR and diarrhea. Other severe AEs were hypertension (9.7%), weight loss (5.8%), fatigue (5.8%), and hypocalcemia (9.2%), which required hospitalization in one patient. Serum thyroid stimulating hormone (TSH) increase occurred in 33% of patients. AEs leading to treatment withdrawal were reported in 18.8% of patients. Secondary malignancy (most commonly squamous cell carcinoma of the skin) occurred in 4.3% of patients in the treatment group, compared with 1.9% of those treated with placebo. A more recent evaluation of quality of life (QoL) effects of sorafenib treatment showed slightly worse QoL in patients in the sorafenib-treated arm versus those in the placebo-treated arm. [140]
In 2015, Lenvatinib became the latest TKI to obtain regulatory approval for use in RAIR DTC [20,96]. This approval stems from the promising results reported in the SELECT trial [27]. Lenvatinib is a multitargeted TKI affecting VEGFR1–3, fibroblast growth factor receptor(FGFR), PDGFR, RET, and KIT. In the phase 3 randomized controlled SELECT trial, 392 patients with progressive RAIR DTC were randomized to receive either lenvatinib or placebo. In this study, 24% of randomized patients had previously undergone treatment with a different TKI regimen, most commonly sorafenib. Median PFS in patients treated with lenvatinib was 18.3 m compared with 3.6 m with placebo (HR 0.21; 99% CI, 0.14 to 0.31, p<0.001). At 18-months, 51.1% of patients treated with lenvatinib demonstrated no disease progression compared with 3.8% of those treated with placebo. Analogous to results seen with sorafenib, neither BRAF nor RAS mutation status seemed to affect the degree of increase in PFS in patients treated with lenvatinib. Response rate in patients treated with lenvatinib was 64.8% compared with 1.5% in the placebo arm (OR 28.9; 95% CI, 12.46 to 66.9, p<0.001). Four of the patients treated with lenvatinib demonstrated a complete response, which was sustained through the final data collection time point. A subgroup analysis of patients previously treated with another TKI showed preserved improvements in PFS of 15.2 m with lenvatinib vs. 3.6 m with placebo (HR 0.22, 95% CI 0.12–0.41) and ORR of 62.1% vs. 3.7%, respectively. Severe treatment-related AEs were reported in 75.9% of patients treated with lenvatinib, of which 10.1% were fatal. Severe AEs included hypertension (43%), proteinuria (10%), and thromboembolic events (6.5%) including fatal PE, and hemorrhagic stroke. Other AEs included prolonged QTc (8%), renal failure (4.2%), gastrointestinal fistula (1.5%), hepatic failure (0.4%), and posterior reversible encephalopathy (0.4%). Of patients treated with lenvatinib, 82.4% required dose interruption and 67.8% required dose reduction, beginning at a median of 3.0 m (95% CI, 2.7 to 3.7) after initiation of treatment. Most AEs responded to standard clinical management or dose modification, but 14.2% of patients required discontinuation of lenvantinib for dose-limiting toxicities. Despite the longer median overall survival, a 2–3% increase in the rate of deaths attributed to therapy was observed in the lenvatinib group over that of the placebo group.
3. Adopting TKIs into Treatment Algorithms for Thyroid Cancer
The four trials described above represent the foundational evidence supporting the introduction of TKIs as single-agent therapy into clinical practice for advanced thyroid cancers. Inclusion criteria in each trial limited the study population to patients with anatomically-defined progressive disease, either metastatic or unresectable, for which alternative therapies were not available [21–23,27]. These indications are appropriately mirrored in treatment algorithms proposed by the American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) in their recently updated guidelines [3,6,8]. No data are yet available to support TKI use in other contexts, including neoadjuvant treatment before surgery or as adjuvant treatment following R0 or R1 resection (unlike RAI, for instance), in response to elevated tumor markers without evidence of structural disease, or in combination with any other therapies. Given the morbidity and treatment-related mortality associated with TKIs, these agents should be initiated judiciously, only for indications supported by robust evidence, or administered in the setting of a controlled clinical trial [3,6,8,9,28].
For patients with advanced DTC, the first question that must be answered is whether the disease is RAIR [9,28,29]. Inclusion criteria for the SELECT and DECISION trials defined RAIR disease by either: a) the presence of at least one lesion without uptake on 131I scan, b) at least one lesion progressing after treatment with RAI, or c) cumulative administered 131I dose > 600 mCi [23,27]. In patients with RAI-responsive unresectable locoregional recurrence or metastasis who are candidates for systemic therapy, the first-line treatment modality still should be RAI [3,6,9,28]. There is currently no reliable evidence to support the use of TKIs in patients with RAI-responsive DTC. A more recent update on the definition of RAIR tumors was published by Schlumberger in 2014 noting that in addition to previous criteria, it is controversial whether patients receiving more than 600 mCi of radioactive iodine should be considered as RAIR, and whether radioactive iodine treatment should be abandoned [141]. The decision whether to continue radioactive iodine treatment in such patients should be based on their response to previous treatment courses, persistence of a RAI uptake during the previous treatment course, low FDG uptake in tumor foci, and an absence of significant side-effects from RAI treatment. [141] A recent study evaluated RAIR and survival in 153 cases of metastatic DTC [142]. Here, 91 patients (59%) met criteria for RAIR: that is, 60% (n = 55) had at least 1 metastasis without 131I uptake; 21% (n = 19) had progressive disease (PD) despite 131I; 19% (n = 17) had persistent disease despite a cumulative activity of 131I of ≥600 mCi. After the diagnosis of RAIR, median OS was 8.9 years (95% confidence interval [CI]); median cause-specific survival was 9.6 years (95% CI). In multivariate analyses, PD despite 131I as one of the criteria for RAIR and the time from initial diagnosis of DTC to diagnosis of RAIR <3 years were the only independent prognostic factors for poor overall survival. The conclusion of the study was that identification of prognostic factors for decreased survival in RAIR-DTC may improve the selection of patients for targeted agents[142].
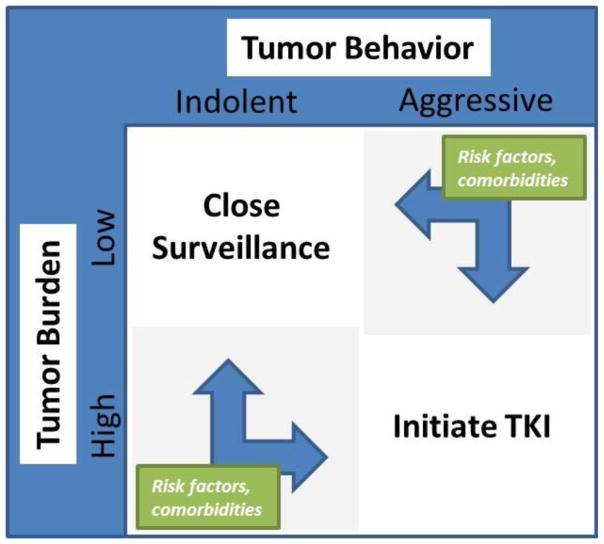
The target population for TKI use in thyroid cancer is inherently diverse, owing to differences in biological behavior among individuals with advanced disease. Classification of patients by tumor burden and pace of disease progression aids decision-making (Figure 1) [28]. In patients with localized recurrence, the preferred initial treatment remains surgical resection where feasible [3,6,8,105]. Similarly, in those with oligometastatic disease, metastasectomy for anatomically amenable lesions can be considered in patients with good performance status. In such situations, it is advisable to demonstrate an indolent pace of disease progression, which may be achieved with short interval active surveillance (3–12 m) prior to finalizing the treatment recommendation, if the behavior of the tumor is not already known. In this manner, higher morbidity procedural interventions for isolated lesions in patients that will rapidly develop recurrence or disseminated metastases can be avoided. In patients with oligometastatic disease not meeting criteria for surgery, local ablative therapies such as thermal ablation (radiofrequency ablation [RFA], or cryoablation), ethanol ablation, or external-beam radiation therapy (EBRT) can be considered in select cases for lesions in accessible locations [3,6,8,28].
Figure 1.
Systemic Therapy Decision-Making Matrix
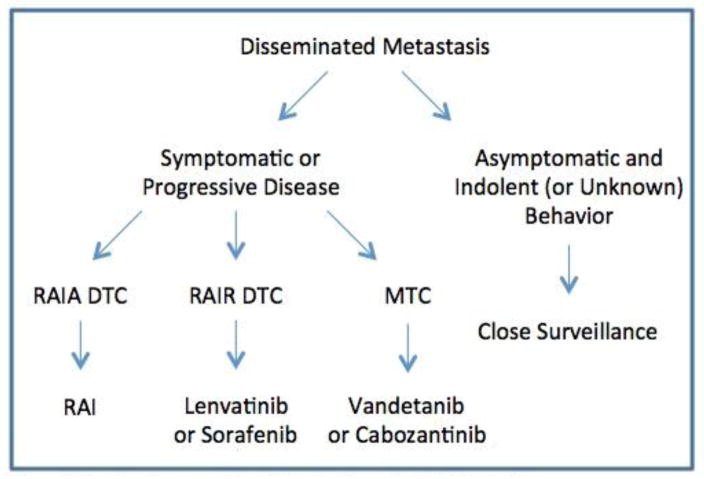
Appreciation for disease stability or pace of progression is crucial for appropriate direction of systemic therapy in advanced thyroid cancer [9,28]. Patients with stable asymptomatic metastatic disease may be best served by an active surveillance program with TSH suppression [3,6,8,30]. For patients who have symptomatic, progressive metastatic disease not amenable to RAI, TKIs have now replaced single-agent or combinatorial cytotoxic chemotherapeutic regimens as first-line treatment (Figure 2) [3,6,8]. Symptoms in patients with advanced disease may include focal pain or result from compression or impingement on nearby anatomic structures from tumor mass effects. Patients with progressive, hormonally active metastasis from MTC may have worsening diarrhea or glucocorticoid excess due to ectopic ACTH production [3,8]. The decision to initiate systemic therapy with TKIs should not be made solely on the basis of rising tumor markers such as calcitonin and CEA in MTC, or thyroglobulin in DTC [3,6,8,28]. Rather, evidence of structural disease progression is necessary, and objective criteria such as Response Evaluation Criteria in Solid Tumors (RECIST) should be used to quantify progression [9,28]. Prior to initiating treatment with TKIs, patient comorbidities with respect to known AEs should be carefully considered. Alternative agents may be more appropriate in patients at higher risk for particular treatment-related complications, and risk profile may play a role in decision-making regarding initial or subsequent agent selection (Table 2). To date no robust comparative evidence has established the superiority of any specific TKI over any other for either DTC or MTC, therefore standardized recommendations for initial agent selection cannot be made. Both cabozantinib and vandetanib are supported by phase 3 trials in advanced MTC, while sorafenib and lenvatinib are each supported by phase 3 trials in advanced RAIR DTC. As such, these four agents have attained regulatory approval and are logical choices for initial treatment in accordance with guidelines published by the ATA and NCCN [3,6,8]. The remarkable responses to lenvatinib in both the first-line and second-line setting reported in the SELECT trial suggest that this agent be given particular consideration, especially for patients with severe disease burden or symptoms, or for those failing prior TKI treatment, although the frequency and severity of AEs must be considered as well [27,97]. In recent years, Phase 2 clinical studies of several other agents in both MTC and DTC have been completed, several of which have shown promising results and could support the off-label use of alternative commercially-available TKIs in select patients with contraindications to approved agents, or as second line therapies to patients with poor tolerance or lack of response to standard first line agents [31–45]. Practical considerations such as institutional or regional availability, practice preferences, clinical familiarity, and cost may also be factored into treatment decisions [28].
Figure 2.
First-line Treatment Algorithm for Disseminated Metastasis
Table 2.
| TSH Increase |
| Hand Foot Skin Reaction (HFSR) |
| Fatigue |
| Diarrhea |
| Hypertension |
| QTc Prolongation* |
| Electrolyte Disturbance |
| Weight Loss |
| Enteriitis/Colitis/Sepsis+ |
| Gastrointestinal Fistula |
| Gastrointestinal Hemorrhage |
| Proteinuria |
| Thromboembolic Events |
| Cytopenias |
Significant for vandetanib with FDA Black box warning
Black box warning for cabozantinib
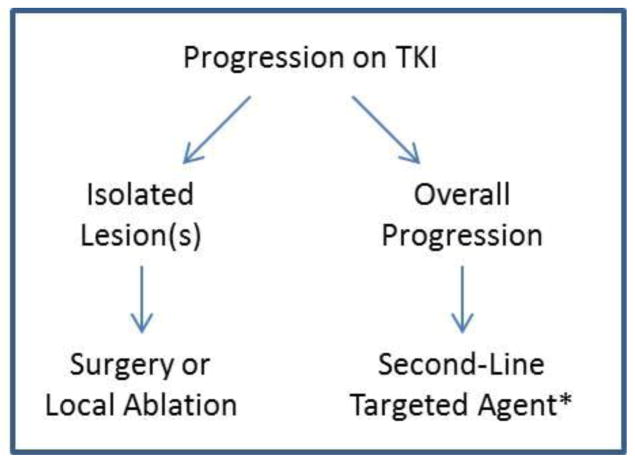
Defining standard intervals for surveillance and criteria for treatment response remains an area lacking uniform guidelines and should therefore be individualized [3,6,8]. Patients initiated on TKI treatment require close surveillance for tumor response or progression as well as development of any AEs [9]. This should be accomplished with frequent office visits, imaging, and tumor biomarker evaluation [3,6,8]. Patients demonstrating uniform response or stability may be continued on their treatment regimen as long as the benefits of therapy outweigh any AEs experienced [9]. Patients demonstrating a heterogeneous response profile with global improvement or stability but isolated lesions with progression may benefit from local treatments for accessible lesions. If widespread disease progression continues despite treatment, transition to a second-line agent should be considered. Consideration should be given for second-line targeted therapy following first-line treatment failure based on reports of possible incremental benefit and minimal cross-resistance between agents despite their similar mechanisms of action [27,38,42–45,50,56,97]. However, there is a paucity of robust data to guide choice of agent or to conclusively support improved outcomes with second-line therapy in most thyroid cancers, and it is therefore recommended that eligibility for enrollment in available clinical trials be investigated in these circumstances [3,6,8].
Surveillance of patients undergoing treatment with TKIs is also crucial for identification and management of AEs [9,97]. In the seminal phase 3 trials described above, severe AEs lead to dose modification in 64–82% of patients, and agent discontinuation in 12–19% of patients [21–23,27]. Commonly reported AEs associated with TKI use are hypertension, QTc prolongation (especially significant with vandetanib which was given a blackbox warning from the FDA), diarrhea, weight loss, anorexia, fatigue, HFSR, and rash (Table 2) [21–23,27,31–37,46,47,57,58]. In addition, elevation in TSH requiring adjustment in thyroid hormone replacement during treatment is a frequently observed phenomenon. Early identification of AEs and aggressive symptom management are necessary for preventing unnecessary dose modifications or treatment withdrawals [9]. Several AEs have proven life-threatening and garnered special regulatory attention. Significant increases in QTc, as well as torsades de pointes and sudden cardiac death have been reported with vandetanib, leading the US FDA to approve a Risk Evaluation and Mitigation Strategy (REMS) protocol for this agent, requiring that providers and pharmacies obtain special training and certification before adopting its use in clinical practice [10]. Hemorrhage from the gastrointestinal tract and skin lesions have led to mortality in patients undergoing treatment with a number of agents, and lethal cases of abdominal sepsis from severe enteritis and colitis with perforation have been reported as well [21–23,27,46,47].
4. Other TKIs Studied in Thyroid Cancer
Each of the approved agents discussed above has also been evaluated for use in other thyroid cancer histotypes in earlier phase clinical studies. Most of these studies are small and open-label in design, preventing robust conclusions and comparisons, but may still support the off-label use of these agents in the appropriate clinical context. Vandetanib was studied for efficacy in RAIR DTC in a large randomized phase 2 study involving 145 patients [48]. In this study, median PFS for patients treated with vandetanib was 11.1 m vs. 5.9 m for placebo (HR 0.63, 60% CI 0.54 – 0.74). In a subgroup analysis, patients with PTC undergoing treatment with vandetanib demonstrated median PFS of 16.2 m compared to 5.9 m with placebo, whereas those with FTC or poorly differentiated thyroid cancer demonstrated a median PFS of 7.7 m with vandetanib vs. 5.6 m with placebo, raising the question of whether vandetanib may be more appropriate for patients with PTC specifically, rather than FTC or poorly differentiated tumors. It should also be noted, however, that no statistically significant difference was found in ORR between patients treated with vandetanib (8%) compared with placebo (5%) and there was no significant reduction in thyroglobulin levels in treated patients. These observations have led some experts to question whether vandetanib is appropriate for use against RAIR DTC [12]. An on-going phase 3 RCT of vandetanib in patients with RAIR DTC (the VERIFY trial, NCT01876784) is due to conclude in 2017 and may help answer this question definitively [49]. Early outcomes data were recently presented at the European Thyroid Association meeting in 2016 and have yet to be published but are promising for stabilization of disease with treatment.
Cabozantinib, now approved for MTC, has also been studied in the setting of RAIR DTC, albeit preliminarily. In a phase 1 trial performed in 15 patients with RAIR DTC, a partial response (PR) was demonstrated in 53% (CI 27–79%) of treated patients [50]. Notably, 73% of patients enrolled in this study had previously undergone treatment with another VEGF pathway inhibitor, underscoring the potential for clinical benefit of second-line TKI therapy. Two on-going phase 2 studies are more closely examining the efficacy of cabozantinib in patients with RAIR DTC (NCT02041260, NCT01811212) [51,52].
Sorafenib, approved for RAIR DTC, has been studied in a phase 2 trial in 16 patients with sporadic MTC, and demonstrated some degree of tumor shrinkage in all patients, however only 1 patient (6%) met RECIST criteria for a PR [53]. Another phase 2 study of sorafenib in 35 patients with a variety of thyroid cancer histotypes included 15 patients with MTC; in this subgroup, a response rate of 25% was demonstrated at 1 year [54]. A third phase 2 study of sorafenib in advanced MTC is currently on-going, with completion expected in 2017 (NCT00390325) [55].
Finally, lenvatinib, approved in RAIR DTC, was studied in a single phase 2 trial involving 59 patients with advanced MTC: an ORR of 36% (CI 24–49%) was reported, with a median PFS of 9 m [56]. Of patients enrolled in this study, 44% had previously undergone treatment with another anti-VEGFR agent, of which cabozantinib and sorafenib were the most common. Remarkably, there was no difference in ORR between patients who had previously undergone treatment with another anti-VEGFR agent compared with those who did not.
Other multi-targeted TKIs have also been evaluated for their efficacy in thyroid cancer in a variety of phase 2 studies [31–37,57,58]. Although not yet approved by the FDA for use in thyroid cancer, given early positive preliminary results in this disease and similar mechanisms of action, consideration may be given for off-label use in carefully selected patients [3,6,8]. Among the most promising and well-studied agents are axitinib, pazopanib, and sunitinib [31–38]. In a phase 2 study of axitinib in 60 patients, including 45 with RAIR DTC and 11 with MTC, an ORR of 30% (95%CI 19–43) was reported, all of which were PRs, with a 21 m median duration of response (95%CI 13–46) [34]. In another phase 2 study of 52 patients including 45 with RAIR DTC and 6 with MTC, an ORR of 35% (95% CI 22–49) was reported with axitinib, with a median duration of response of 17 m (95% CI 14–26) [35]. A phase 2 study of 37 patients with RAIR DTC treated with pazopanib demonstrated an ORR of 49% (95% CI 35–68), which were all PRs with a median PFS of 11.7 m (1 to > 23 m) [32], and an additional phase 2 trial is pending (NCT01813136) [138]. Pazopanib has also been studied in 35 patients with MTC, demonstrating a PR rate of 14% (90% CI 5.8–28), with a median PFS of 9.4 m [36]. Results of a phase 2 trial of sunitinib in 23 patients with RAIR DTC were recently reported [37]. In this study, ORR was 26% with no complete responses and median PFS was 8.0 m (3.8 to 17.2). An earlier phase 2 trial of sunitinib in a mixed group of 35 patients (27 with DTC and 7 with MTC), demonstrated an ORR of 31% (95%CI 16–47) [33], including one complete response. ORR was 28% for DTC and 50% for MTC. Finally, in a retrospective analysis of 57 patients with RAIR thyroid cancer undergoing treatment with sunitinib, a PR rate of 35% was observed [38]. The study included 25 patients in whom sunitinib was given as a second-line agent, and ORR in these patients was 20%, compared with 47% in the patients getting the drug as first-line therapy. An additional phase 2 study of sunitinib in patients with either RAIR DTC or MTC is currently ongoing and due to conclude in late 2016 (NCT00381641) [59]. Treatment-related toxicities observed in these studies were typical of the TKI class (Table 2) [31–37,57,58].
5. Additional Targets and Strategies in Late-Stage Development
Each of the agents described above has demonstrated efficacy in clinical trials of patients with RAIR DTC and MTC within the limitations inherent to their early phase study designs. Several other multi-targeted TKIs have also been evaluated for use in thyroid cancer, including imatinib and motesanib, however, early reported outcomes with these agents have not been promising [57,58,60,61,119]. A deeper understanding of the genetic alterations in thyroid cancer has led to an appreciation for the limited number of oncogenic mutations involved in many cases of thyroid cancer [10]. This revelation has motivated increased interest in selective inhibition of pathogenically altered receptors such as RET and ALK and downstream effectors of the signaling cascade, such as RAS, MEK, and BRAF [11,12]. Most agents selective for these targets remain in early phases of development, but inhibitors directed at several specific targets are reaching maturity [10–12]. The BRAF V600E mutation, which is present in a significant proportion of cases of PTC [62], is the target of the small molecule inhibitors vemurafenib and dabrafenib [39–41], which are both agents approved by the FDA for treatment of advanced melanoma harboring this mutation [20].
The efficacy of vemurafenib in thyroid cancer was demonstrated in a recently published phase 2 study in advanced RAIR DTC patients who were either naïve (cohort 1, N=26) or had prior exposure to a VEGFR inhibitor (cohort 2, N=25) [35]. ORR in cohort 1 was 38.5% (95%CI 20–59), with all 10 of 26 patients having PRs. Severe AEs were noted in 65% of cohort 1 and 68% of cohort 2 with up to 27% of these being squamous cell carcinomas of the skin. Two patients died on study from AEs. Currently, a phase 2 study of vemurafenib for use as neoadjuvant treatment for locally advanced PTC (T3 or T4 with lymph node involvement) is recruiting (NCT01709292) [63]. This study is also notable because its target population consists of patients with potentially resectable tumors, who are earlier in their course of disease compared with those studied in most other trials of targeted agents.
Dabrafenib was studied in a phase 1 trial primarily focused on melanoma, which included 14 patients with BRAF V600E mutated thyroid carcinoma (12 DTC, 2 poorly differentiated tumors) [41]. An ORR was noted in 29% (95%CI 8–58), which were all PRs. No severe treatment-related AEs occurred. Cutaneous AEs included skin papilloma (57%), hyperkeratosis (36%), seborrheic keratosis (14%), and skin hypertrophy (14%). Remarkably, in another study of 10 patients with BRAF V600E-mutated PTC who underwent treatment with dabrafenib, development of new RAI uptake was demonstrated in 6 patients, 2 of whom achieved PR after subsequent treatment with RAI, for an ORR of 20% following these serial treatments [40]. No patients required dose modification due to AEs. New skin lesions or changes occurred in 80%, including one patient with SCC.
In addition to dabrafenib, several other agents are undergoing evaluation for the potential to reintroduce RAI uptake into RAIR tumors. Sorafenib was specifically evaluated for this purpose in an earlier phase 2 study of patients with RAIR DTC, and although PRs were promising (a clinically important effect later to be confirmed in the DECISION trial described above), sorafenib was not found to be effective for reintroduction of RAI avidity [64]. However, selumetinib, a specific inhibitor of MEK1/2 (a downstream target of RAF in the MAPKinase cellular signaling cascade), has demonstrated more promising results in this domain [65–67]. Despite a disappointing anti-tumor effect reported in a phase 2 trial of 39 patients with RAIR PTC (PR only 3%) [66], a later study of 24 patients having a mix of PTC (65%) and poorly differentiated thyroid cancer (35%), demonstrated RAI uptake increase in 60% of patients treated with selumetinib [67]. Of these, 67% went on to receive RAI treatment with PR reported in 62%. Tumor genotype appeared to correlate with changes in RAI avidity as all 5 patients with NRAS-mutant tumors had increases in uptake, and 80% had a PR following RAI treatment, whereas results for BRAF-mutant or tumors harboring RET/PTC translocations were less consistent. The utility of selumetinib as an adjuvant to RAI for patients with recurrent or metastatic RAI-avid (RAIA) thyroid cancer is currently being evaluated in a phase 2 trial that is still recruiting participants (NCT02393690) [68]. In addition, results of a phase 3 trial in non-metastatic DTC comparing the efficacy of RAI in combination with selumetinib versus RAI alone are due to report soon (ASTRA trial, NCT01843062) [69]. Demonstrated efficacy in these settings could drive treatment algorithm changes to incorporate selumetinib as an RAI-sensitizing agent in the future, however more clinical evidence is necessary before recommendations for current practice can be made.
Another downstream molecular target that has generated interest is mTOR, for which two inhibitors have been studied in thyroid cancer: everolimus, and temsirolimus. Everolimus was evaluated in a phase 2 study of patients with locally advanced or metastatic thyroid cancer as single-agent treatment [71]. The study included 40 patients with variety of histotypes including DTC (60%), and MTC (22%). PR was seen in only 8% of patients with DTC, and no responses meeting RECIST criteria for PR were noted in the other histotypes. An improved understanding of the cross-talk between signaling cascades involving mTOR and other effectors discussed above has led investigators to hypothesize that early adaptive resistance can be induced by single-agent treatment, and such an escape phenomenon has been proposed to explain resistance observed in other studies of targeted agents as well [10,11,19,70,108]. Investigators are therefore evaluating combinatorial treatment strategies in an effort to thwart the development of resistance by this mechanism. Several on-going phase 2 trials pair mTOR inhibition with other targeted agents (NCT02143726, NCT01141309, NCT01263951, NCT01025453, NCT01625520) [72–77], and others combine agents targeted to different receptor/effector pairs [78,82,84,85,88–92]. Additionally, early ongoing clinical studies are evaluating combinations of targeted agents or a targeted agent paired with traditional cytotoxic chemotherapeutics [79–92]. These combinatorial approaches are the main focus of several on-going studies in patients with anaplastic cancers [80,83,89,124], for which effective treatment options remain elusive despite on-going investigation with both novel and traditional therapies [5,106,107]. Finally, a number of other exciting agents with various mechanisms of action are undergoing earlier-phase translational study, including other MAPK-pathway inhibitors, additional TKIs, histone deacetylase inhibitors, PPAR-γ-directed agents, proteosome inhibitors, immunotherapy strategies, farnesyl transferase inhibitors, somatostatin receptor radionuclide therapy, and gene therapy techniques. To date, no randomized controlled clinical evidence exists to support their use in thyroid cancer, but a role for these agents may develop as additional data becomes available [10,11,80,82,85,108–118,119–138].
6. Summary
An improved understanding of thyroid cancer pathogenesis has lead to a remarkably rapid change in the landscape for available systemic therapy over the last decade. Four targeted agents (vandetanib, cabozantinib, sorafenib, and lenvatinib) have attained regulatory approval for advanced thyroid cancer, and several other related agents are now commonly used off-label as first- and second-line treatments for selected patients. The recommended indications for targeted agents remain limited to patients with advanced, refractory disease. Given the cost and potential serious toxicities of these agents, treatment decisions should be made judiciously by experienced clinicians, and patients should undergo close surveillance for progression and adverse events. Newer approaches to RAI resensitization and combination strategies may prove beneficial in the years to come as the role of targeted agents in this disease continues to evolve and several ongoing clinical studies with neoadjuvant treatments and drug combinations to target multiple pathways report their findings.
Figure 3.
Algorithm for Disease Progression on TKI *Or clinical trial
Table 1B.
Unapproved Tyrosine Kinase Inhibitors with Late-Phase Clinical Evidence in RAIR DTC
| Agent | Indication | Trial | Number of Patients | ORR | Reference |
|---|---|---|---|---|---|
| Vandetanib | RAIR DTC | Phase 2 RCT | 145 | 8% vs. 5% | Leboulleux et al 2012 [48,49] |
| Cabozantinib | RAIR DTC | Phase 1 | 15 | 53% | Cabanillas et al 2014 [50] |
| Phase 2 pending (NCT02041260, NCT01811212) [51,52] | |||||
| Sorafenib | MTC | Phase 2 | 16 | 6% | Lam et al 2010[53] |
| MTC | Phase 2 | 15 | 25% | Ahmed et al 2010 [54] | |
| MTC | Phase 2 pending (NCT00390325) [55] | ||||
| Lenvatinib | MTC | Phase 2 | 59 | 36% | Schlumberger et al 2009 [56] |
| Axitinib | Mixed | Phase 2 | 45 RAIR DTC, 11 MTC | 30% | Locati et al 2008 [34] |
| Mixed | Phase 2 | 45 RAIR DTC, 6 MTC | 35% | Cohen et al 2014 [35] | |
| Pazopanib | RAIR DTC | Phase 2 | 37 | 49% | Bible et al 2010 [32] |
| MTC | Phase 2 | 35 | 14% | Bible et al 2014 [36] | |
| RAIR DTC | Phase 2 pending (NCT01813136) [138] | ||||
| Sunitinib | RAIR DTC | Phase 2 | 23 | 26% | Bikas et al 2016 [37] |
| Mixed | Phase 2 | 27 RAIR DTC, 7 MTC | 31% | Carr et al 2010 [33] | |
| Mixed | Phase 2 pending (NCT00381641) [59] | ||||
Complete response in one patient
KEY POINTS.
Four targeted agents have attained regulatory approval for advanced thyroid cancers and several newer agents are being used off-label in this disease.
These targeted treatments have cost and toxicity issues that need to be factored into patient treatment decisions, which should be made by clinicians experienced with these drugs.
Acknowledgments
Funding
No direct funding was paid for the writing of this article however support for research and effort for MSC and FW was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA046592. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support effort for MSC and RL was through the Department of Surgery at the University of Michigan.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD,: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) [Accessed 8/1/2016];Clinical Practice Guidelines in Oncology, Thyroid Carcinoma. 2016 Available From: https://www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf.
- 4.Aschebrook-kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21(2):125–34. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad RI, Lydiatt WM, Ball DW, et al. Anaplastic Thyroid Carcinoma, Version 2.2015. J Natl Compr Canc Netw. 2015;13(9):1140–50. doi: 10.6004/jnccn.2015.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tufano RP, Clayman G, Heller KS, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015;25(1):15–27. doi: 10.1089/thy.2014.0098. [DOI] [PubMed] [Google Scholar]
- 8.Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worden F. Treatment strategies for radioactive iodine-refractory differentiated thyroid cancer. Ther Adv Med Oncol. 2014;6(6):267–79. doi: 10.1177/1758834014548188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bible KC, Ryder M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol. 2016;13(7):403–16. doi: 10.1038/nrclinonc.2016.19. [DOI] [PubMed] [Google Scholar]
- 11.Rajhbeharrysingh U, Taylor M, Milas M. Medical therapy for advanced forms of thyroid cancer. Surg Clin North Am. 2014;94(3):541–71. doi: 10.1016/j.suc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan S, Colevas AD. Current Standards in Treatment of Radioiodine Refractory Thyroid Cancer. Curr Treat Options Oncol. 2016;17(6):30. doi: 10.1007/s11864-016-0404-6. [DOI] [PubMed] [Google Scholar]
- 13.Massoll N, Mazzaferri EL. Diagnosis and management of medullary thyroid carcinoma. Clin Lab Med. 2004;24(1):49–83. doi: 10.1016/j.cll.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Martins RG, Rajendran JG, Capell P, Byrd DR, Mankoff DA. Medullary thyroid cancer: options for systemic therapy of metastatic disease? J Clin Oncol. 2006;24(11):1653–5. doi: 10.1200/JCO.2005.05.4106. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22(6):464–8. doi: 10.1016/j.clon.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb JA, Hill CS. Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med. 1974;290(4):193–7. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 17.White PT, Cohen MS. The discovery and development of sorafenib for the treatment of thyroid cancer. Expert Opin Drug Discov. 2015;10(4):427–39. doi: 10.1517/17460441.2015.1006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott LJ. Lenvatinib: first global approval. Drugs. 2015;75(5):553–60. doi: 10.1007/s40265-015-0383-0. [DOI] [PubMed] [Google Scholar]
- 19.Viola D, Valerio L, Molinaro E, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23(4):R185–205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- 20.US FDA Hematology/Oncology (Cancer) [Accessed Sept 2016];Approvals & Safety Notifications. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm.
- 21.Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta-abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26(29):4714–9. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27(10):1675–84. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brose MS, Nutting CM, Sherman SI, et al. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. doi: 10.1186/1471-2407-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 28.Brose MS, Smit J, Capdevila J, et al. Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev Anticancer Ther. 2012;12(9):1137–47. doi: 10.1586/era.12.96. [DOI] [PubMed] [Google Scholar]
- 29.Na’ara S, Amit M, Fridman E, Gil Z. Contemporary Management of Recurrent Nodal Disease in Differentiated Thyroid Carcinoma. Rambam Maimonides Med J. 2016;7(1) doi: 10.5041/RMMJ.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong N, Marqusee E, Gordon MS, et al. Long-term, treatment-free survival in select patients with distant metastatic papillary thyroid cancer. Endocr Connect. 2014;3(4):207–14. doi: 10.1530/EC-14-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26(29):4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11(10):962–72. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16(21):5260–8. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locati LD, Licitra L, Agate L, et al. Treatment of advanced thyroid cancer with axitinib: Phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer. 2014;120(17):2694–703. doi: 10.1002/cncr.28766. [DOI] [PubMed] [Google Scholar]
- 35.Cohen EE, Tortorici M, Kim S, Ingrosso A, Pithavala YK, Bycott P. A Phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother Pharmacol. 2014;74(6):1261–70. doi: 10.1007/s00280-014-2604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bible KC, Suman VJ, Molina JR, et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab. 2014;99(5):1687–93. doi: 10.1210/jc.2013-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bikas A, Kundra P, Desale S, et al. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur J Endocrinol. 2016;174(3):373–80. doi: 10.1530/EJE-15-0930. [DOI] [PubMed] [Google Scholar]
- 38.Atallah V, Hocquelet A, Do cao C, et al. Activity and Safety of Sunitinib in Patients with Advanced Radioiodine Refractory Thyroid Carcinoma: A Retrospective Analysis of 57 Patients. Thyroid. 2016;26(8):1085–92. doi: 10.1089/thy.2015.0648. [DOI] [PubMed] [Google Scholar]
- 39.Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272–82. doi: 10.1016/S1470-2045(16)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothenberg SM, Mcfadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028–35. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 41.Falchook GS, Millward M, Hong D, et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. 2015;25(1):71–7. doi: 10.1089/thy.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owonikoko TK, Chowdry RP, Chen Z, et al. Clinical efficacy of targeted biologic agents as second-line therapy of advanced thyroid cancer. Oncologist. 2013;18(12):1262–9. doi: 10.1634/theoncologist.2013-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23(5):600–4. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dadu R, Devine C, Hernandez M, et al. Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab. 2014;99(6):2086–94. doi: 10.1210/jc.2013-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massicotte MH, Brassard M, Claude-desroches M, et al. Tyrosine kinase inhibitor treatments in patients with metastatic thyroid carcinomas: a retrospective study of the TUTHYREF network. Eur J Endocrinol. 2014;170(4):575–82. doi: 10.1530/EJE-13-0825. [DOI] [PubMed] [Google Scholar]
- 46.Giacchero D, Ramacciotti C, Arnault JP, et al. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch Dermatol. 2012;148(12):1418–20. doi: 10.1001/2013.jamadermatol.192. [DOI] [PubMed] [Google Scholar]
- 47.Worden F, Fassnacht M, Shi Y, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer. 2015;22(6):877–87. doi: 10.1530/ERC-15-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13(9):897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 49. [Accessed Sept 2016];Evaluation of Efficacy, Safety of Vandetanib in Patients With Differentiated Thyroid Cancer (VERIFY) https://clinicaltrials.gov/ct2/show/NCT01876784.
- 50.Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24(10):1508–14. doi: 10.1089/thy.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. [Accessed Sept 2016];A Phase II Trial of Cabozantinib for the Treatment of Radioiodine (RAI)-Refractory Differentiated Thyroid Carcinoma (DTC) in the First-line Setting. https://clinicaltrials.gov/ct2/show/NCT02041260.
- 52. [Accessed Sept 2016];Cabozantinib-S-Malate in Treating Patients With Refractory Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01811212.
- 53.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28(14):2323–30. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165(2):315–22. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 55. [Accessed Sept 2016];Sorafenib Tosylate in Treating Patients With Metastatic, Locally Advanced, or Recurrent Medullary Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT00390325.
- 56.Schlumberger M, Jarzab B, Cabanillas ME, et al. A Phase II Trial of the Multitargeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Advanced Medullary Thyroid Cancer. Clin Cancer Res. 2016;22(1):44–53. doi: 10.1158/1078-0432.CCR-15-1127. [DOI] [PubMed] [Google Scholar]
- 57.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 58.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27(23):3794–801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 59. [Accessed Sept 2016];Sunitinib in Treating Patients With Thyroid Cancer That Did Not Respond to Iodine I 131 and Cannot Be Removed by Surgery. https://clinicaltrials.gov/ct2/show/NCT00381641.
- 60.Frank-raue K, Fabel M, Delorme S, Haberkorn U, Raue F. Efficacy of imatinib mesylate in advanced medullary thyroid carcinoma. Eur J Endocrinol. 2007;157(2):215–20. doi: 10.1530/EJE-06-0695. [DOI] [PubMed] [Google Scholar]
- 61.De groot JW, Zonnenberg BA, Van ufford-mannesse PQ, et al. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(9):3466–9. doi: 10.1210/jc.2007-0649. [DOI] [PubMed] [Google Scholar]
- 62.Daliri M, Abbaszadegan MR, Bahar MM, et al. The role of BRAF V600E mutation as a potential marker for prognostic stratification of papillary thyroid carcinoma: a long-term follow-up study. Endocr Res. 2014;39(4):189–93. doi: 10.3109/07435800.2013.879169. [DOI] [PubMed] [Google Scholar]
- 63. [Accessed Sept 2016];Vemurafenib Neoadjuvant Trial in Locally Advanced Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01709292.
- 64.Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161(6):923–31. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 65.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26(13):2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18(7):2056–65. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623–32. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. [Accessed Sept 2016];Iodine I-131 With or Without Selumetinib in Treating Patients With Recurrent or Metastatic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02393690.
- 69. [Accessed Sept 2016];Study Comparing Complete Remission After Treatment With Selumetinib/Placebo in Patient With DifferentiatedThyroid Cancer. (ASTRA) https://clinicaltrials.gov/ct2/show/NCT01843062.
- 70.Carracedo A, Ma L, Teruya-feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim SM, Chang H, Yoon MJ, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24(12):3089–94. doi: 10.1093/annonc/mdt379. [DOI] [PubMed] [Google Scholar]
- 72. [Accessed Sept 2016];Sorafenib Tosylate With or Without Everolimus in Treating Patients With Advanced, Radioactive Iodine Refractory Hurthle Cell Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02143726.
- 73. [Accessed Sept 2016];Study of Everolimus and Sorafenib in Patients With Advanced Thyroid Cancer Who Progressed on Sorafenib Alone. https://clinicaltrials.gov/ct2/show/NCT01263951.
- 74. [Accessed Sept 2016];Combination of Temsirolimus and Sorafenib in the Treatment of Radioactive Iodine Refractory Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01025453.
- 75. [Accessed Sept 2016];Evaluating the Combination of Everolimus and Sorafenib in the Treatment of Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01141309.
- 76. [Accessed Sept 2016];SOM230 Alone or in Combination With RAD001 in Patients With Medullary Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01625520.
- 77. [Accessed Sept 2016];Everolimus and Vatalanib in Treating Patients With Advanced Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT00655655.
- 78. [Accessed Sept 2016];Phase 1 Study of Pazopanib With GSK1120212 in Advanced Solid Tumors, Enriched With Patients With Differentiated Thyroid Cancer, Soft-tissue Sarcoma, and Cholangiocarcinoma. https://clinicaltrials.gov/ct2/show/NCT01438554.
- 79. [Accessed Sept 2016];Lenvatinib and Capecitabine in Patients With Advanced Malignancies. https://clinicaltrials.gov/ct2/show/NCT02915172.
- 80. [Accessed Sept 2016];Efatutazone With Paclitaxel Versus Paclitaxel Alone in Treating Patients With Advanced Anaplastic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02152137.
- 81. [Accessed Sept 2016];Gemcitabine - Oxaliplatin for Advanced Refractory Thyroid Cancer Patients: a Phase II Study (THYGEMOX) https://clinicaltrials.gov/ct2/show/NCT02472080.
- 82. [Accessed Sept 2016];Enhancing Radioiodine Incorporation Into BRAF Mutant Thyroid Cancers With the Combination of Vemurafenib andKTN3379. https://clinicaltrials.gov/ct2/show/NCT02456701.
- 83. [Accessed Sept 2016];A Phase I/II Trial of Crolibulin (EPC2407) Plus Cisplatin in Adults With Solid Tumors With a Focus on Anaplastic Thyroid Cancer (ATC) https://clinicaltrials.gov/ct2/show/NCT01240590.
- 84. [Accessed Sept 2016];Pasireotide & Everolimus in Adult Patients With Radioiodine-Refractory Differentiated & Medullary ThyroidCancer. https://clinicaltrials.gov/ct2/show/NCT01270321.
- 85. [Accessed Sept 2016];Cediranib Maleate With or Without Lenalidomide in Treating Patients With Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01208051.
- 86. [Accessed Sept 2016];Paclitaxel and Bortezomib in Treating Patients With Metastatic or Unresectable Malignant Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT00667641.
- 87. [Accessed Sept 2016];Imatinib in Combination With Dacarbazine and Capecitabine in Medullary Thyroid Carcinoma. https://clinicaltrials.gov/ct2/show/NCT00354523.
- 88. [Accessed Sept 2016];Temsirolimus and Vinorelbine Ditartrate in Treating Patients With Unresectable or Metastatic Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT01155258.
- 89. [Accessed Sept 2016];Intensity-Modulated Radiation Therapy and Paclitaxel With or Without Pazopanib Hydrochloride in Treating Patients With Anaplastic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01236547.
- 90. [Accessed Sept 2016];Dabrafenib With or Without Trametinib in Treating Patients With Recurrent Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01723202.
- 91. [Accessed Sept 2016];Dabrafenib and Lapatinib Ditosylate in Treating Patients With Refractory Thyroid Cancer That Cannot Be Removed by Surgery. https://clinicaltrials.gov/ct2/show/NCT01947023.
- 92. [Accessed Sept 2016];Efficacy and Safety of the Combination Therapy of Dabrafenib and Trametinib in Subjects With BRAF V600E- Mutated Rare Cancers. https://clinicaltrials.gov/ct2/show/NCT02034110.
- 93. [Accessed Oct 2016];US FDA Full Prescribing Information Data Sheet: Cabozantinib. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203756lbl.pdf.
- 94.Su DH, Chang SH, Chang TC. The impact of locoregional recurrences and distant metastases on the survival of patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2015;82(2):286–94. doi: 10.1111/cen.12511. [DOI] [PubMed] [Google Scholar]
- 95.Shi X, Liu R, Basolo F, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. J Clin Endocrinol Metab. 2016;101(1):264–74. doi: 10.1210/jc.2015-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scott LJ. Lenvatinib: first global approval. Drugs. 2015;75(5):553–60. doi: 10.1007/s40265-015-0383-0. [DOI] [PubMed] [Google Scholar]
- 97.Costa R, Carneiro BA, Chandra S, et al. Spotlight on lenvatinib in the treatment of thyroid cancer: patient selection and perspectives. Drug Des Devel Ther. 2016;10:873–84. doi: 10.2147/DDDT.S93459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thornton K, Kim G, Maher VE, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2012;18(14):3722–30. doi: 10.1158/1078-0432.CCR-12-0411. [DOI] [PubMed] [Google Scholar]
- 99.Robinson BG, Paz-ares L, Krebs A, et al. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2664–71. doi: 10.1210/jc.2009-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wells SA, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28(5):767–72. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–6. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen L, Shen Y, Luo Q, Yu Y, Lu H, Zhu R. Response to sorafenib at a low dose in patients with radioiodine-refractory pulmonary metastases from papillary thyroid carcinoma. Thyroid. 2011;21(2):119–24. doi: 10.1089/thy.2010.0199. [DOI] [PubMed] [Google Scholar]
- 103.Ali SM, He J, Carson W, et al. Extended Antitumor Response of a BRAF V600E Papillary Thyroid Carcinoma to Vemurafenib. Case Rep Oncol. 2014;7(2):343–8. doi: 10.1159/000363377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matuszczyk A, Petersenn S, Bockisch A, et al. Chemotherapy with doxorubicin in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res. 2008;40(3):210–3. doi: 10.1055/s-2008-1046781. [DOI] [PubMed] [Google Scholar]
- 105.Kwon J, Wu HG, Youn YK, Lee KE, Kim KH, Park DJ. Role of adjuvant postoperative external beam radiotherapy for well differentiated thyroid cancer. Radiat Oncol J. 2013;31(3):162–70. doi: 10.3857/roj.2013.31.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bible KC, Suman VJ, Menefee ME, et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97(9):3179–84. doi: 10.1210/jc.2012-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373(8):726–36. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montero-conde C, Ruiz-llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–33. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reddi HV, Madde P, Cohen YC, et al. Antitumor Activity of VB-111, a Novel Antiangiogenic Virotherapeutic, in Thyroid Cancer Xenograft Mouse Models. Genes Cancer. 2011;2(10):993–5. doi: 10.1177/1947601912437933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. [Accessed Sept 2016];Safety and Efficacy of VB-111 in Subjects With Advanced Differentiated Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01229865.
- 111. [Accessed Sept 2016];Ceritinib in Mutation and Oncogene Directed Therapy in Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02289144.
- 112. [Accessed Sept 2016];A Drug-Drug Interaction Study of the Effects of XL184 (Cabozantinib) on Rosiglitazone in Subjects With Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT01100619.
- 113.Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid. 2009;19(9):953–6. doi: 10.1089/thy.2008.0371. [DOI] [PubMed] [Google Scholar]
- 114. [Accessed Sept 2016];Phase II Study of Tipifarnib in Squamous Head and Neck Cancer With HRAS Mutations. https://clinicaltrials.gov/ct2/show/NCT02383927.
- 115. [Accessed Sept 2016];Trial of LBH589 in Metastatic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01013597.
- 116.Sherman EJ, Su YB, Lyall A, et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013;23(5):593–9. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. [Accessed Sept 2016];Romidepsin in Treating Patients With Recurrent and/or Metastatic Thyroid Cancer That Has Not Responded to Radioactive Iodine. https://clinicaltrials.gov/ct2/show/NCT00098813.
- 118. [Accessed Sept 2016];A Phase II Trial of Valproic Acid in Patients With Advanced Thyroid Cancers of Follicular Cell Origin. https://clinicaltrials.gov/ct2/show/NCT01182285.
- 119.Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18(3):317–23. doi: 10.1089/thy.2007.0120. [DOI] [PubMed] [Google Scholar]
- 120.Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94(1):164–70. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harvey RD, Owonikoko TK, Lewis CM, et al. A phase 1 Bayesian dose selection study of bortezomib and sunitinib in patients with refractory solid tumor malignancies. Br J Cancer. 2013;108(4):762–5. doi: 10.1038/bjc.2012.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. [Accessed Sept 2016];Bortezomib in Treating Patients With Metastatic Thyroid Cancer That Did Not Respond to Radioactive Iodine Therapy. https://clinicaltrials.gov/ct2/show/NCT00104871.
- 123. [Accessed Sept 2016];REVLIMID® (Lenalidomide) for Therapy of Radioiodine-Unresponsive Papillary and Follicular ThyroidCarcinomas. https://clinicaltrials.gov/ct2/show/NCT00287287.
- 124.Sosa JA, Elisei R, Jarzab B, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid. 2014;24(2):232–40. doi: 10.1089/thy.2013.0078. [DOI] [PubMed] [Google Scholar]
- 125. [Accessed Sept 2016];Trametinib in Increasing Tumoral Iodine Incorporation in Patients With Recurrent or Metastatic ThyroidCancer. https://clinicaltrials.gov/ct2/show/NCT02152995.
- 126.Lim SM, Chung WY, Nam KH, et al. An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer. Eur J Cancer. 2015;51(12):1588–95. doi: 10.1016/j.ejca.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 127.Dickson MA, Gordon MS, Edelman G, et al. Phase I study of XL281 (BMS-908662), a potent oral RAF kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2015;33(2):349–56. doi: 10.1007/s10637-014-0191-5. [DOI] [PubMed] [Google Scholar]
- 128. [Accessed Sept 2016];A Phase 1 Study to Evaluate the Safety and Pharmacokinetics of KTN3379 in Adult Subjects With Advanced Tumors. https://clinicaltrials.gov/ct2/show/NCT02014909.
- 129. [Accessed Sept 2016];MEK162 for Patients With RAS/RAF/MEK Activated Tumors (SIGNATURE) https://clinicaltrials.gov/ct2/show/NCT01885195.
- 130. [Accessed Sept 2016];A Phase II Study of Anlotinib in MTC Patients (ALTN/MTC) https://clinicaltrials.gov/ct2/show/NCT01874873.
- 131. [Accessed Sept 2016];Study of Anlotinib in Patients With Differentiated Thyroid Cancer(ALTER01032) https://clinicaltrials.gov/ct2/show/NCT02586337.
- 132. [Accessed Sept 2016];Study of Anlotinib in Patients With Medullary Thyroid Carcinoma(ALTER01031) https://clinicaltrials.gov/ct2/show/NCT02586350.
- 133. [Accessed Sept 2016];Study of Oral Lucitanib (E-3810), a Dual VEGFR-FGFR Tyrosine Kinase Inhibitor, in Patients With Solid Tumors. https://clinicaltrials.gov/ct2/show/NCT01283945.
- 134. [Accessed Sept 2016];Nintedanib(BIBF1120) in Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT01788982.
- 135. [Accessed Sept 2016];Pembrolizumab in Anaplastic/Undifferentiated Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02688608.
- 136. [Accessed Sept 2016];A Phase II Study of MLN0128 in Metastatic Anaplastic Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02244463.
- 137. [Accessed Sept 2016];A Study Using Regorafenib as Second or Third Line Therapy in Metastatic Medullary Thyroid Cancer. https://clinicaltrials.gov/ct2/show/NCT02657551.
- 138.Czepczyński R, Matysiak-Grześ M, Gryczyńska M, et al. Peptide Receptor Radionuclide Therapy of Differentiated Thyroid Cancer: Efficacy and Toxicity. Arch Immunol Ther Exp (Warsz) 2015;63(2):147–154. doi: 10.1007/s00005-014-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ibrahimpasic T, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014;99:1245–52. doi: 10.1210/jc.2013-3842. [DOI] [PubMed] [Google Scholar]
- 140.Schlumberger M, et al. Randomized, double-blinded, placebo controlled trial of sorafenib in locally advanced or metastatic patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) — exploratory analyses of patient-reported outcomes [abstract 100]. Presented at the 83rd Annual Meeting of the American Thyroid Association; 2013. [Google Scholar]
- 141.Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2(5):356–8. doi: 10.1016/S2213-8587(13)70215-8. [DOI] [PubMed] [Google Scholar]
- 142.Wassermann J, Bernier MO, Spano JP, et al. Outcomes and Prognostic Factors in Radioiodine Refractory Differentiated Thyroid Carcinomas. Oncologist. 2016 Jan;21(1):50–8. doi: 10.1634/theoncologist.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]