Abstract
[Purpose] The purpose of this study was to investigate mirror therapy (MT) condition by analyzing kinematic parameters according to mirror size and angle. [Subjects and Methods] Three hemiparesis stroke patients and five healthy adults participated in this cross-sectional study. Kinematic parameters during the MT were collected over a total of 5 trials for each subject (3 mirror angles × 3 mirror sizes). Center of pressure (COP) excursion data was collected by force plate, and other kinematic parameters by infra-red cameras. [Results] The larger the size and smaller the angle, the overall dependent variables decreased in all participants. Particularly, when virtual reality reflection equipment (VRRE) was used, the value of the flexion and the lateral tilt was the closest to the midline compared to all other independent variables. Moreover, it showed tendency of moving towards the affected side. Based on the results, MT for stroke patients has a disadvantage of shifting weight and leaning towards the unaffected side during therapy. [Conclusion] Therefore, it seems to be more effective in terms of clinics to apply VRRE to make up for the weak parts and provide more elaborate visual feedback.
Key words: Stroke, Weight-bearing, Mirror neurons
INTRODUCTION
One of the most common problems stroke patients experience is upper limb paralysis1). Accordingly, 55–75% of stroke patients complain of motor impairment2) and discomfort in using the affected hand in daily life3). Various interventions were proposed for the recovery of upper extremity in stroke patients4). Such rehabilitation in hemiparesis is well established and most often encourages the use of the paralyzed part of the body to regain function5).
Typically, mirror is a tool often used to provide real-time visual feedback for therapists during therapy session. However, in mirror therapy (MT), mirror is placed between a patient’s limbs. Affected limb faces the non-reflective side, and by using the reflected image of the unaffected limb, patient recognizes the affected side. This gives an illusionary impression to the subject that he/she has two normal limbs6). MT has been used and proven to be effective in improving the upper extremity function of stroke patients7). Related studies show improvements in motor function8, 9), motor recovery8, 10), and sensory recovery10, 11).
Virtual reality reflection therapy (VRRT) is the technically developed version of MT concept. In some cases, VRRT similarly follows protocol of general MT by using camera recorder to record the unaffected upper limb movement and then displaying the reversed image on the monitor installed above the affected upper limb; or by using the same instrument to record the movement of the unaffected side in advance and play it to exercise the affected side asymmetrically12). Virtual reality reflection equipment (VRRE) allows more complete immersion into the illusion, whereas MT requires strict focus and concentration to truly perceive the illusion as real. Also, VRRE has an advantage of using only the affected side through displaying to practice single-handed tasks, which is impossible in MT13). Above all, VRRE can be used to minimize asymmetry of trunk in sitting posture, which happens to patients during MT.
It is notable that patients tilt their trunk towards the unaffected side to view the reflected image on the mirror and the size and angle of the mirrors used in MT researches were different. These factors can cause asymmetry in sitting posture and change in weight bearing14).
In formal studies, some researchers seemed to have considered a better posture during therapy sessions15). This suggests an effort to prevent asymmetry. Such attempt conveys a need for more optimal condition of the mirror used in therapy.
This study will analyze changes of kinematic parameters in patients according to mirror size and angle to suggest an optimal condition to maintain sitting posture symmetry during MT.
SUBJECTS AND METHODS
Five healthy subjects and 5 hemiparesis stroke patients were recruited. Five stroke patients were selected from the rehabilitation ward of S medical center in Seoul, who met the inclusive criteria for this study. The inclusion criteria for stroke patients were as follows: history of a stroke at least 6 months before the study; hemiparesis; able to understand and follow simple verbal instructions; a Mini-mental State Examination-Korean (MMSE-K) score of >24; dynamic sitting balance above fair level; voluntary participation and the ability to communicate effectively. The exclusion criteria included: experience of previous strokes; major hemorrhagic changes; increased intracranial pressure; hemicraniectomy or orthopedic, rheumatologic, or other diseases interfering with their ability to sit or to move either upper limb; and psychiatric disorder or dementia. In the process, 2 stroke patients failed to complete the experiment due to worsened condition. Table 1 shows general characteristics of subjects. As this study was approved by the Sahmyook University Institutional Review Board (SYUIRB 2015–092), each participant was informed about the purpose and the introduction of the research, and agreed to the procedure by signing a consent form.
Table 1. General characteristics of subjects (N=8).
| Group | Subjects | Gender | Age(years) | Height(cm) | Weight(kg) | Affected side |
|---|---|---|---|---|---|---|
| Stroke Group(n=3) | 1 | Male | 53 | 165 | 68 | Rt. |
| 2 | Female | 60 | 160 | 45 | Lt. | |
| 3 | Female | 20 | 170 | 51 | Lt. | |
| Normal Group(n=5) | 1 | Male | 31 | 178 | 78 | Lt.a |
| 2 | Female | 26 | 168 | 56 | Lt. | |
| 3 | Male | 27 | 171 | 62 | Lt. | |
| 4 | Female | 24 | 162 | 48 | Lt. | |
| 5 | Male | 24 | 179 | 69 | Lt. |
aFor healthy subjects, the affected side is referred as nondominant side.
For motion analysis during the experiment, 6 infra-red cameras (Oqus 1 series, Qualisys AB, Sweden, 2012) were used. These cameras were set at 100 frames/sec sampling rate. To track the motion with the infra-red cameras, 20 mm diameter spherical markers were glued directly onto the skin with bi-adhesive tape. Then, by taking personal comments into consideration, the set was made suitable for ‘Visual 3D’ (C-Motion, USA, 2012) analysis. In total, a set consisting of 25 markers were glued to the following spots: The head segment was defined by the markers placed right above the right and left ears (RH, LH), following up the RH and LH line which meets at the top of the head (TH), on the forehead which is transverse to the RH and LH line (AH) and the right and left zygomatic arch posterior part (RZ, LZ). The trunk segment was defined by the markers over the seventh cervical vertebra (C7), the right and left acromions (RA, LA), sagittal axis point which passes the center of the right and left should joints (RR, LL), the tenth thoracic vertebrae (T10), and the right and left sagittal axis point of T10 level (RT, LT). The pelvis segment was defined by markers placed on the right and left anterior superior iliac spines (RASIS, LASIS), the right and left posterior superior iliac spines (RPSIS, LPSIS), the right and left iliac crest point which exists on the sagittal axis (RI, LI), and the spinal process of the second sacral vertebra (S2). Also, to define femur, single markers were used on the right and left greater trochanter (RGT, LGT) and the right and left lateral epicondyles of femur (RK, LK). Furthermore, clusters of four passive-reflective markers on lateral part of the right and left thigh were attached to RGT-RK and LGT-LK in parallel. Two force plates (Type 9266AA, Kistler Instrument AG, Switzerland, 2008), sampling at 1,000 Hz, were used to collect COP excursions. Size of the big mirrors was 50 × 40 cm to reach the subject’s eye level15) and the size of the small mirrors was a conventional mirror size of 30 × 20 cm8). Screen as VRRE was the same virtual reality reflection equipment consisting of wooden box and an LCD monitor (width 54 ×length 34 ×height 21 cm), as suggested by D Lee, et al.12) (Fig. 1). ‘Qualisys Track Manager’ (Track Manager version 2.5, Qualisys, Sweden, 2012) was used for tracking the markers, and force data were integrated by connecting infra-red cameras and A/D board with internal trigger cables.
Fig. 1.
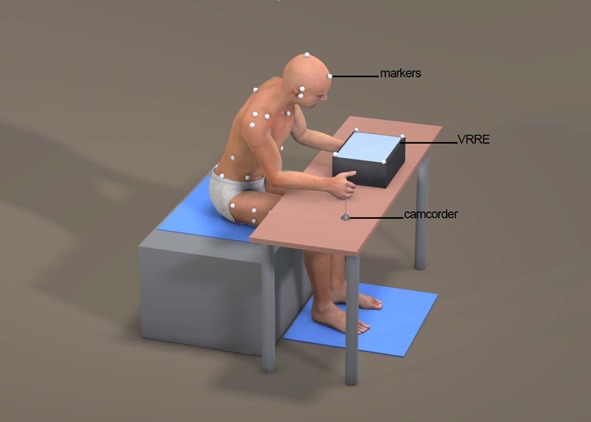
Virtual reality reflection therapy
They performed tasks according to the physiotherapist’s instruction using mirrors in the following order: big mirror 90°; big mirror 60°; small mirror 90°; small mirror 60°; and VRRE. If the patient complained of discomfort, experiment was stopped immediately. Each task was repeated three times and the average value was calculated.
To classify phases, midline of the subject motioning to perform tasks was set as event 1 and the peak point to perform the tasks was set as event 2 when obtaining data values (Fig. 2). After obtaining values, the difference between the value of event 2 and event 1 was normalized.
Fig. 2.
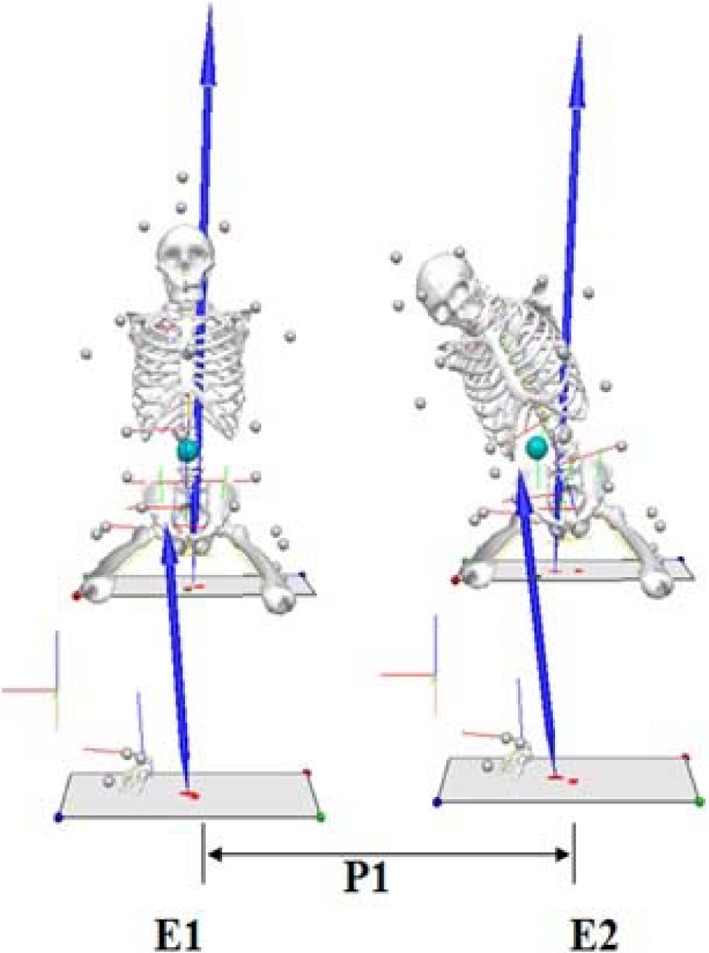
Event and phase
Kruskal-Wallis test was used to determine differences in the dependent variables of each mirror angle and size in each group. For significant differences, post hoc pairwise comparisons were performed using the Least Significant Difference (LSD) to locate the differences of each mirror angle and size in each group. SPSS version 19.0 for Windows was used to perform all analyses and p values <0.05 were regarded as significant.
RESULTS
Table 2 shows stroke group’s significant differences for all given dependent variables of mirror sizes (p<0.05), as well as mirror angles (p<0.05). Post hoc pairwise comparisons in mirror sizes revealed differences apart from trunk absolute angle on ML axis (T_ML). There were significant differences in head and trunk segment angle on AP axis (HT_AP), head and trunk segment angle on ML axis (HT_ML), and pelvis absolute angle on AP axis (P_AP) between big mirror and VRRE, and between small mirror and VRRE (p<0.05). In trunk absolute angle on AP axis (T_AP) and pelvis absolute angle on ML axis (P_ML), significant differences showed only between small mirror and VRRE (p<0.05). Post hoc pairwise comparisons in mirror angles showed differences in all given dependent variables. HT_AP and HT_ML showed significant differences (p<0.05) between 90° and 0° (VRRE), and between 60° and 0°. T_AP, P_AP, and P_ML showed significant differences (p<0.05) between 90° and 60°, and between 60° and 0°. T_ML showed significant differences (p<0.05) in all angles (between 90° and 60°, between 60° and 0°, and between 90° and 0°).
Table 2. Segment angles and absolute angles of stroke group (N=3).
| HT_AP(degree) | HT_ML(degree) | T_AP(degree) | T_ML(degree) | P_AP(degree) | P_ML(degree) | ||
|---|---|---|---|---|---|---|---|
| Size | Angle | ||||||
| Big (A) | 90 (a) | −20.86 ± 3.72 | 18.86 ± 11.77 | −2.84 ± 0.99 | 5.48 ± 1.27 | −1.95 ± 1.14 | 1.51 ± 0.76 |
| 60 (b) | −17.13 ± 7.95 | 9.88 ± 8.27 | −1.03 ± 1.15 | 1.45 ± 1.07 | −0.45 ± 0.31 | 0.28 ± 0.36 | |
| Total | −19.00 ± 5.92 | 14.37 ± 10.35 | −1.93 ± 1.38 | 3.47 ± 2.44 | −1.20 ± 1.12 | 0.89 ± 0.86 | |
| Small (B) | 90 (a) | −32.39 ± 11.53 | 23.89 ± 10.32 | −4.74 ± 2.01 | 10.13 ± 3.14 | −2.20 ± 1.26 | 2.27 ± 1.75 |
| 60 (b) | −17.89 ± 12.55 | 17.81 ± 5.47 | −1.85 ± 2.01 | 3.29 ± 1.93 | −0.69 ± 0.77 | 0.64 ± 1.06 | |
| Total | −25.14 ± 13.39 | 20.85 ± 8.10 | −3.29 ± 2.40 | 6.71 ± 4.41 | −1.45 ± 1.25 | 1.45 ± 1.57 | |
| VRRE (C) | 0 (c) | −0.07 ± 4.82 | −6.32 ± 2.69 | 0.59 ± 1.36 | −0.93 ± 0.47 | 0.69 ± 0.86 | −0.44 ± 0.12 |
| Total | 90 (a) | −26.63 ± 9.93 | 21.38 ± 10.28 | −3.79 ± 1.76 | 7.81 ± 3.33 | −2.08 ± 1.08 | 1.89 ± 1.28 |
| 60 (b) | −17.51 ± 9.41 | 13.84 ± 7.63 | −1.44 ± 1.53 | 2.37 ± 1.72 | −0.57 ± 0.54 | 0.46 ± 0.73 | |
| 0 (c) | −0.07 ± 4.82 | −6.32 ± 2.69 | 0.59 ± 1.36 | −0.93 ± 0.47 | 0.69 ± 0.86 | −0.44 ± 0.12 | |
| Total | −17.67 ± 13.07 | 12.82 ± 13.03 | −1.97 ± 2.27 | 3.88 ± 4.19 | −0.92 ± 1.35 | 0.85 ± 1.29 | |
| Size χ2 (p) | 7.167 (0.028) | 7.817 (0.020) | 6.283 (0.043) | 7.817 (0.020) | 6.125 (0.047) | 6.900 (0.032) | |
| Post-Hoc | A|C, B|C | A|C, B|C | B|C | A|C, B|C | B|C | ||
| Angle χ2 (p) | 8.100 (0.017) | 7.567 (0.023) | 8.165 (0.017) | 11.567 (0.003) | 8.625 (0.013) | 10.017 (0.007) | |
| Post-Hoc | a|c, b|c | a|c, b|c | a|b, b|c | a|b|c | a|b, b|c | a|b, b|c | |
HT_AP: head and trunk segment angle on antero-posterior axis; HT_ML: head and trunk segment angle on medio-lateral axis; T_AP: trunk absolute angle on antero-posterior axis; T_ML: trunk absolute angle on medio-lateral axis; P_AP: pelvis absolute angle on antero-posterior axis; P_ML: pelvis absolute angle on medio-lateral axis
Values are expressed as mean ± standard deviation
Table 3 shows normal group’s significant differences for all given dependent variables of mirror sizes (p<0.05), as well as mirror angles (p<0.05) apart from P_AP. Post hoc pairwise comparisons in mirror sizes revealed differences. HT_AP, HT_ML, T_ML, and P_ML showed significant differences (p<0.05) between big mirror and VRRE, and between small mirror and VRRE. T_AP showed significant differences (p<0.05) between big mirror and small mirror, and small mirror and VRRE. P_AP showed significant difference (p<0.05) only between small mirror and VRRE. Post hoc pairwise comparisons in mirror angles revealed differences in all given dependent variables except for P_AP. HT_ML showed significant differences (p<0.05) between 90° and 0° (VRRE), and between 60° and 0°. T_AP showed significant differences (p<0.05) between 90° and 60°, and between 60° and 0°. HT_AP, T_ML, and P_ML showed significant differences (p<0.05) in all angles (between 90° and 60°, between 60° and 0°, and between 90°and 0°).
Table 3. Segment angles and absolute angles of normal group (N=5).
| HT_AP(degree) | HT_ML(degree) | T_AP(degree) | T_ML(degree) | P_AP(degree) | P_ML(degree) | ||
|---|---|---|---|---|---|---|---|
| Size | Angle | ||||||
| Big (A) | 90 (a) | −21.55 ± 9.80 | 11.06 ± 9.71 | −7.06 ± 3.24 | 10.61 ± 9.14 | −2.91 ± 2.16 | 3.95 ± 2.48 |
| 60 (b) | −17.58 ± 5.19 | 7.70 ± 7.42 | −3.72 ± 0.78 | 4.24 ± 1.31 | −2.20 ± 1.01 | 1.99 ± 1.60 | |
| Total | −19.57 ± 7.68 | 9.38 ± 8.34 | −5.39 ± 2.83 | 7.43 ± 7.01 | −2.56 ± 1.63 | 2.97 ± 2.22 | |
| Small (B) | 90 (a) | −34.06 ± 10.28 | 14.42 ± 9.11 | −17.87 ± 11.15 | 15.04 ± 9.10 | −3.52 ± 1.84 | 4.73 ± 2.66 |
| 60 (b) | −19.15 ± 7.85 | 7.82 ± 6.55 | −6.10 ± 3.61 | 6.02 ± 2.81 | −3.90 ± 2.32 | 2.44 ± 2.19 | |
| Total | −26.61 ± 11.66 | 11.12 ± 8.25 | −11.98 ± 9.97 | 10.53 ± 7.93 | −3.71 ± 1.99 | 3.59 ± 2.59 | |
| VRRT (C) | 0 (c) | −4.41 ± 4.42 | −9.97 ± 5.03 | −11.85 ± 6.64 | −2.84 ± 2.84 | −1.42 ± 1.70 | −1.81 ± 1.78 |
| Total | 90 (a) | −27.81 ± 11.54 | 12.74 ± 9.05 | −15.74 ± 9.84 | 12.82 ± 8.91 | −3.22 ± 1.92 | 4.34 ± 2.46 |
| 60 (b) | −17.37 ± 6.33 | 7.76 ± 6.60 | −6.49 ± 4.44 | 5.13 ± 2.27 | −3.05 ± 1.91 | 2.22 ± 1.83 | |
| 0 (c) | −4.41 ± 4.42 | −9.97 ± 5.03 | −11.85 ± 6.64 | −2.84 ± 2.84 | −1.42 ± 1.70 | −1.81 ± 1.78 | |
| Total | −19.35 ± 12.04 | 6.21 ± 11.16 | −11.26 ± 8.30 | 6.61 ± 8.29 | −2.79 ± 1.93 | 2.26 ± 3.05 | |
| Size χ2 (p) | 12.111 (0.002) | 11.719 (0.003) | 13.749 (0.001) | 13.015 (0.001) | 3.447 (0.178) | 11.838 (0.003) | |
| Post-Hoc | A|C, B|C | A|C, B|C | A|B, B|C | A|C, B|C | A|C, B|C | ||
| Angle χ2 (p) | 12.761 (0.002) | 12.871 (0.002) | 14.101 (0.001) | 16.324 (0.000) | 2.516 (0.284) | 14.433 (0.001) | |
| Post-Hoc | a|b|c | a|c, b|c | a|b, b|c | a|b|c | a|b|c | ||
HT_AP: head and trunk segment angle on antero-posterior axis; HT_ML: head and trunk segment angle on medio-lateral axis; T_AP: trunk absolute angle on antero-posterior axis; T_ML: trunk absolute angle on medio-lateral axis; P_AP: pelvis absolute angle on antero-posterior axis; P_ML: pelvis absolute angle on medio-lateral axis
Values are expressed as mean ± standard deviation
Table 4 shows stroke group’s significant differences in pelvis COP excursion on ML axis (PCOP_ML) and COG excursion on ML axis (COG_ML) of mirror sizes (p<0.05). PCOP_ML, COG excursion on AP axis (COG_AP), and COG_ML showed significant differences in mirror angles (p<0.05). Post hoc pairwise comparisons in mirror sizes revealed differences. COG_ML showed significant differences between big mirror and VRRE, and between small mirror and VRRE (p<0.05). Pelvis COP excursion on AP axis (PCOP_AP), PCOP_ML, and COG_AP showed significant difference only between small mirror and VRRE (p<0.05). Post hoc pairwise comparisons in mirror angles revealed differences in all given dependent variables except for PCOP_AP. PCOP_ML and COG_AP showed significant differences (p<0.05) between 90° and 60°, and between 60° and 0°. COG_ML showed significant differences (p<0.05) in all angles (between 90° and 60°, between 60° and 0°, and between 90°and 0°).
Table 4. COP excursions and COG excursions of stroke group (N=3).
| PCOP_AP(cm) | PCOP_ML(cm) | COG_AP(cm) | COG_ML(cm) | ||
|---|---|---|---|---|---|
| Size | Angle | ||||
| Big (A) | 90 (a) | 9.78 ± 8.50 | 40.35 ± 34.99 | 9.93 ± 3.40 | 16.87 ± 3.66 |
| 60 (b) | 3.19 ± 3.31 | 8.93 ± 6.21 | 2.14 ± 3.90 | 5.24 ± 2.46 | |
| Total | 6.49 ± 6.80 | 24.64 ± 28.31 | 6.04 ± 5.38 | 11.06 ± 6.96 | |
| Small (B) | 90 (a) | 24.19 ± 13.03 | 81.16 ± 42.40 | 15.66 ± 8.03 | 33.60 ± 9.96 |
| 60 (b) | 6.12 ± 0.75 | 34.38 ± 28.77 | 6.91 ± 5.74 | 12.34 ± 6.63 | |
| Total | 15.16 ± 12.89 | 57.77 ± 41.31 | 11.29 ± 7.87 | 22.97 ± 13.89 | |
| VRRT (C) | 0 (c) | −0.09 ± 6.50 | −13.09 ± 5.65 | −1.20 ± 5.46 | −5.73 ± 3.06 |
| Total | 90 (a) | 16.99 ± 12.62 | 60.76 ± 41.33 | 12.80 ± 6.34 | 25.24 ± 11.36 |
| 60 (b) | 4.66 ± 2.68 | 21.66 ± 23.25 | 4.53 ± 5.11 | 8.79 ± 5.93 | |
| 0 (c) | −0.09 ± 6.50 | −13.09 ± 5.65 | −1.20 ± 5.46 | −5.73 ± 3.06 | |
| Total | 8.64 ± 10.88 | 30.35 ± 40.51 | 6.69 ± 7.70 | 12.47 ± 14.37 | |
| Size χ2 (p) | 5.017 (0.081) | 9.150 (0.010) | 5.433 (0.066) | 8.417 (0.015) | |
| Post-Hoc | B|C | A|C, B|C | |||
| Angle χ2 (p) | 5.817 (0.055) | 8.100 (0.017) | 7.833 (0.020) | 11.017 (0.004) | |
| Post-Hoc | a|b, b|c | a|b, b|c | a|b|c | ||
PCOP_AP: pelvis COP excursion on antero-posterior axis; PCOP_ML: pelvis COP excursion on medio-lateral axis; COG_AP: COG excursion on antero-posterior axis; COG_ML: COG excursion on medio-lateral axis
Values are expressed as mean ± standard deviation.
Table 5 shows normal group’s significant differences in all given dependent variables of mirror sizes (p<0.05) and mirror angles (p<0.05) except for PCOP_AP. Post hoc pairwise comparisons in mirror sizes revealed differences except for PCOP_AP. PCOP_ML and COG_ML showed significant differences between big mirror and VRRE, and between small mirror and VRRE (p<0.05). COG_AP showed significant difference only between small mirror and VRRE (p<0.05). Post hoc pairwise comparisons in mirror angles revealed differences in all given dependent variables except for PCOP_AP. COG_AP showed significant difference (p<0.05) between 60° and 0°. PCOP_ML and COG_ML showed significant differences (p<0.05) in all angles (between 90° and 60°, between 60° and 0°, and between 90°and 0°).
Table 5. COP excursions and COG excursions of normal group (N=5).
| PCOP_AP(cm) | PCOP_ML(cm) | COG_AP(cm) | COG_ML(cm) | ||
|---|---|---|---|---|---|
| Size | Angle | ||||
| Big (A) | 90 (a) | 9.59 ± 5.10 | 106.89 ± 59.61 | 22.59 ± 10.74 | 38.86 ± 21.35 |
| 60 (b) | 9.90 ± 10.01 | 56.27 ± 40.46 | 14.94 ± 6.16 | 19.75 ± 9.44 | |
| Total | 9.74 ± 7.49 | 81.58 ± 54.94 | 18.77 ± 9.19 | 29.30 ± 18.54 | |
| Small (B) | 90 (a) | 14.75 ± 10.59 | 123.11 ± 55.38 | 29.23 ± 11.43 | 52.03 ± 20.14 |
| 60 (b) | 17.11 ± 10.29 | 68.18 ± 47.24 | 25.61 ± 12.33 | 24.33 ± 11.48 | |
| Total | 15.93 ± 9.92 | 95.64 ± 56.51 | 27.42 ± 11.37 | 38.18 ± 21.26 | |
| VRRT (C) | 0 (c) | 11.26 ± 11.07 | −44.06 ± 35.10 | 9.72 ± 6.16 | −19.30 ± 12.76 |
| Total | 90 (a) | 12.17 ± 8.29 | 115.00 ± 54.92 | 25.91 ± 11.03 | 45.44 ± 20.76 |
| 60 (b) | 13.51 ± 10.30 | 62.23 ± 41.93 | 20.28 ± 10.77 | 22.04 ± 10.20 | |
| 0 (c) | 11.26 ± 11.07 | −44.06 ± 35.10 | 9.72 ± 6.16 | −19.30 ± 12.76 | |
| Total | 12.52 ± 9.31 | 62.08 ± 74.23 | 20.42 ± 11.48 | 23.13 ± 28.48 | |
| Size χ2 (p) | 2.442 (0.295) | 11.985 (0.002) | 8.027 (0.018) | 12.262 (0.002) | |
| Post-Hoc | A|C, B|C | B|C | A|C, B|C | ||
| Angle χ2 (p) | 0.116 (0.944) | 15.087 (0.001) | 6.402 (0.041) | 15.087 (0.001) | |
| Post-Hoc | a|b|c | b|c | a|b|c | ||
PCOP_AP: pelvis COP excursion on antero-posterior axis; PCOP_ML: pelvis COP excursion on medio-lateral axis; COG_AP: COG excursion on antero-posterior axis; COG_ML: COG excursion on medio-lateral axis
Values are expressed as mean ± standard deviation.
DISCUSSION
Healthy subjects showed symmetrical loading on body support interface while sitting and standing16). On the other hand, due to weaker muscles on the affected side, stroke patients showed asymmetrical posture in space and decreased weight bearing towards the contralateral part of the stroke lesion17,18,19). This causes problems in movement control and balance skills14, 20,21,22). It was suggested that the weight bearing experience would allow proprioceptive feedback for more muscle recruitment in order to meet the demand of bearing weight17, 18). Thus, for erect posture without collapse, improvements have been made to bear more weight on the affected side by shifting weight with manual guide on the hemiplegic side. However, in MT which is based on mirror neuron system (MNS) concept23), conventional rehabilitation perspective on asymmetrical posture and weight bearing is not maintained. The focus of therapy is more concentrated on stimulating MNS by instructing subjects to do task-oriented hand action or observing someone and performing similar actions, and thus enabling recruit of functionally interconnected cortical structures coupling action performance and observation through the activation of MNS24). There are many preceding researches on MT applied on upper limb of stroke patients. However, only a few studies considered details of mirror size and angle or conditions for optimal symmetrical posture. Even among the researches that mention anything about the mirror, the sizes and angles all differed in each method1, 8, 9, 25,26,27). Yet, majority of these researches set mirror angle to 90° in front of the subject, referring it as vertical or perpendicular to the subject midline. This was the most adequate angle which provides the reflected image as real as possible to the patient. However, in order to see the reflected image on the mirror, tilting of head and trunk as well as weigh bearing towards the unaffected side was observed even with naked eyes. In one case, mirror was slightly slanted for the experiment8). It is true slanted mirror lets the patient perform with more symmetrical posture and better weight bearing compared to 90° condition, however, the reflected image becomes distorted, becoming unfit for creating the right illusion for the patient to carry out tasks. Recently, there have been attempts to suggest adequate vision with correct posture for taller subjects by modifying mirror size (24 × 18 × 14 inches) during MT15). Furthermore, in a study of applying VRRE (width 54 × length 34 × height 21 cm), which shows effect of 0° in MT, anterior pelvic tilt was assumed with the flexed hip, knee, and ankle joint to avoid postural asymmetry12).
Therefore, the aim of this study was to investigate optimal MT condition by analyzing kinematic parameters according to mirror size and angle. Most of the dependent variable data of sizes and angles of each group in this study show significant differences. In segment angles and absolute angles of stroke group, all dependent variables showed significant differences in size and angles. From the kinematic parameters obtained from motion capture system, the larger the size and smaller the angle, the degree of flexion and tilting towards the lateral side decreased in both groups consistently. Particularly, when virtual reality reflection equipment (VRRE) was used, the value of the flexion decreased and the value of lateral tilt was the closest to the midline compared to all other independent variables. Moreover, it showed tendency of moving towards the affected side. Contrast to MT where the body moved towards the unaffected side, all the variables moved to the opposite direction. In the results of post hoc test comparisons on mirror sizes in stroke group, all dependent variables except T_ML showed significant differences between VRRE and small mirror (30 × 20 cm). In addition, there were differences between VRRE and big mirror (50 × 40 cm) in HT_AP, HT_ML, and P_AP. This conveys the biggest movement of head when patient flexes or tilts towards the lateral side according to the mirror size. Such result suggests connection to a study on trunk muscle activity28, 29) of existing stroke patients. Pelvic functions to provide safety to support trunk mobility. However, when there is a posterior tilt, it limits trunk mobility due to fixation. Stroke patients make posterior tilt in pelvis to compensate weakness in the abdominal muscles while sitting19). This seems to have prompted the patient to use head more than other segments due to fixation of pelvis and weakness in trunk muscles even though the mediator instructed neutral sitting position30) while the patient was performing tasks in sitting posture. Lee12) also seemed to have assumed anterior tilt in the subject’s sitting posture for the same reason. In the results of post hoc test comparisons on mirror angles in stroke group, all values between VRRE (0°) and 60° showed significant differences, as well as in normal groups. T_ML value shows correlation in all variables in post hoc test comparison in angles for both groups. It is observed that changes in mirror angle leads trunk to tilt towards lateral side. Among COP excursions and COG excursions, PCOP_ML and COG_ML in both groups showed similar degree of significance. Also, like other kinematic parameters, the larger the size and smaller the angle, the more excursions on AP and ML axes decreased. Particularly, when VRRE was used, the value of the excursion on AP axis decreased significantly and the value of tilting was the closest to the midline compared to all other variables, or showed values moving towards the affected side. Contrast to MT where the COP excursions and COG excursions moved towards the unaffected side, all the variables moved to the opposite direction. This implies that VRRE shifts movement from unaffected side to midline and takes weight bearing to the affected side. This effect, therefore, suggests a close connection to the kinematic parameters mentioned above. It is possible to evaluate this phenomenon as being the most symmetrical posture and the closest weight bearing condition expected to achieve in this study. Smaller movements and shorter COP excursions in patients compared to normal subject is similar to the result of S Messier, et al.31) study. Contrast to normal subjects, stroke patients’ use of head and upper trunk to change COG excursions in sitting posture is related to the changes in other kinematic parameters.
Limitation of this study includes lack of participants due to complex motion analysis procedure. Accordingly, it is difficult to make generalization because of small number of subjects. Further study will require larger number of sample sizes. Based on the results of the presented investigation, while mirror therapy is generally used as one of the upper extremity rehabilitation methods for stroke patients, it has a disadvantage of shifting weight and leaning towards the unaffected side during therapy. Therefore, it seems to be more effective in terms of clinics to apply VRRE method to make up for the weak points and provide more elaborate visual feedback.
REFERENCES
- 1.Samuelkamaleshkumar S, Reethajanetsureka S, Pauljebaraj P, et al. : Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Arch Phys Med Rehabil, 2014, 95: 2000–2005. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen HS, Nakayama H, Raaschou HO, et al. : Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil, 1995, 76: 406–412. [DOI] [PubMed] [Google Scholar]
- 3.Dobkin BH: Clinical practice. Rehabilitation after stroke. N Engl J Med, 2005, 352: 1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollock A, Farmer SE, Brady MC, et al. : Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev, 2014, 11: CD010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramachandran VS: Plasticity and functional recovery in neurology. Clin Med (Lond), 2005, 5: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCabe C: Mirror visual feedback therapy. A practical approach. J Hand Ther, 2011, 24: 170–178, quiz 179. [DOI] [PubMed] [Google Scholar]
- 7.Mei Toh SF, Fong KN: Systematic review on the effectiveness of mirror therapy in training upper limb hemiparesis after stroke. Hong Kong J Occup Ther, 2012, 22: 84–95. [Google Scholar]
- 8.Lee MM, Cho HY, Song CH: The mirror therapy program enhances upper-limb motor recovery and motor function in acute stroke patients. Am J Phys Med Rehabil, 2012, 91: 689–696, quiz 697–700. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Lee G, Song C: Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. J Stroke Cerebrovasc Dis, 2014, 23: 655–661. [DOI] [PubMed] [Google Scholar]
- 10.Wu CY, Huang PC, Chen YT, et al. : Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil, 2013, 94: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 11.Dohle C, Püllen J, Nakaten A, et al. : Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair, 2009, 23: 209–217. [DOI] [PubMed] [Google Scholar]
- 12.Lee D, Lee M, Lee K, et al. : Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: a randomized controlled trial. J Stroke Cerebrovasc Dis, 2014, 23: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 13.Lamont K, Chin M, Kogan M: Mirror box therapy: seeing is believing. Explore (NY), 2011, 7: 369–372. [DOI] [PubMed] [Google Scholar]
- 14.Cheng PT, Wu SH, Liaw MY, et al. : Symmetrical body-weight distribution training in stroke patients and its effect on fall prevention. Arch Phys Med Rehabil, 2001, 82: 1650–1654. [DOI] [PubMed] [Google Scholar]
- 15.Arya KN, Pandian S, Kumar D, et al. : Task-based mirror therapy augmenting motor recovery in poststroke hemiparesis: a randomized controlled trial. J Stroke Cerebrovasc Dis, 2015, 24: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 16.Drummond DS, Narechania RG, Rosenthal AN, et al. : A study of pressure distributions measured during balanced and unbalanced sitting. J Bone Joint Surg Am, 1982, 64: 1034–1039. [PubMed] [Google Scholar]
- 17.Bobath B: Adult hemiplegia: evaluation and treatment, 3rd ed. Oxford: Heinemann Medical Books, 1990. [Google Scholar]
- 18.Davies PM: Steps to follow: a guide to the treatment of adult hemiplegia: based on the concept of K. and B. Bobath. Berlin; New York: Springer-Verlag, 1985. [Google Scholar]
- 19.Davies PM: Right in the middle: selective trunk activity in the treatment of adult hemiplegia. Berlin; New York: Springer-Verlag, 1990. [Google Scholar]
- 20.Kamphuis JF, de Kam D, Geurts AC, et al. : Is weight-bearing asymmetry associated with postural instability after stroke? A systematic review. Stroke Res Treat, 2013, 2013: 692137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand AM, Bourbonnais D: Effects of upper limb unilateral isometric efforts on postural stabilization in subjects with hemiparesis. Arch Phys Med Rehabil, 2001, 82: 403–411. [DOI] [PubMed] [Google Scholar]
- 22.Pereira S, Silva CC, Ferreira S, et al. : Anticipatory postural adjustments during sitting reach movement in post-stroke subjects. J Electromyogr Kinesiol, 2014, 24: 165–171. [DOI] [PubMed] [Google Scholar]
- 23.Bhasin A, Padma Srivastava MV, Kumaran SS, et al. : Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol India, 2012, 60: 570–576. [DOI] [PubMed] [Google Scholar]
- 24.Buccino G, Binkofski F, Riggio L: The mirror neuron system and action recognition. Brain Lang, 2004, 89: 370–376. [DOI] [PubMed] [Google Scholar]
- 25.Yavuzer G, Selles R, Sezer N, et al. : Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil, 2008, 89: 393–398. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi M, Negrini S, Carda S, et al. : The value of adding mirror therapy for upper limb motor recovery of subacute stroke patients: a randomized controlled trial. Eur J Phys Rehabil Med, 2013, 49: 311–317. [PubMed] [Google Scholar]
- 27.Mirela Cristina L, Matei D, Ignat B, et al. : Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurol Belg, 2015, 115: 597–603. [DOI] [PubMed] [Google Scholar]
- 28.Bohannon RW, Walters M, Farr M: Trunk muscle performance in stroke patients. Physiother Res Int, 1997, 2: 197–199. [DOI] [PubMed] [Google Scholar]
- 29.Franchignoni FP, Tesio L, Ricupero C, et al. : Trunk control test as an early predictor of stroke rehabilitation outcome. Stroke, 1997, 28: 1382–1385. [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan K, O’Sullivan P, O’Sullivan L, et al. : What do physiotherapists consider to be the best sitting spinal posture? Man Ther, 2012, 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 31.Messier S, Bourbonnais D, Desrosiers J, et al. : Dynamic analysis of trunk flexion after stroke. Arch Phys Med Rehabil, 2004, 85: 1619–1624. [DOI] [PubMed] [Google Scholar]


