Abstract
Wearable mobile health (mHealth) technologies offer approaches for targeting physical activity (PA) in resource-limited, community-based interventions. We sought to explore user characteristics of PA tracking, wearable technology among a community-based population within a health and needs assessment. In 2014–2015, we conducted the Washington, D.C., Cardiovascular Health and Needs Assessment in predominantly African-American churches among communities with higher obesity rates and lower household incomes. Participants received a mHealth PA monitor and wirelessly uploaded PA data weekly to church data collection hubs. Participants (n = 99) were 59 ± 12 years, 79% female, and 99% African-American, with a mean body mass index of 33 ± 7 kg/m2. Eighty-one percent of participants uploaded PA data to the hub and were termed “PA device users.” Though PA device users were more likely to report lower household incomes, no differences existed between device users and non-users for device ownership or technology fluency. Findings suggest that mHealth systems with a wearable device and data collection hub may feasibly target PA in resource-limited communities.
Keywords: mHealth technology, Physical activity, Community-based participatory research, Obesity, African-American, Activity monitoring
BACKGROUND
Cardiovascular disease (CVD) remains the leading cause of death in the USA [1]. Vulnerable populations, including racial/ethnic minorities [2], low-income groups [3, 4], and populations living in resource-limited neighborhoods (i.e., communities with lower neighborhood-level socioeconomic status and where resources for physical activity and nutritious food options are most limited) [5, 6], are especially susceptible to CVD. Certain modifiable risk factors, including poor dietary intake, physical inactivity, and cigarette smoking, are associated with an increased incidence of CVD [7, 8]. A systematic review of studies in vulnerable populations showed that behavioral interventions targeting one or more of these modifiable risk factors can improve overall cardiovascular (CV) health, though challenges exist in changing health behavior [9].
Wearable electronic activity monitor systems, in particular, have recently been cited as a promising modality for targeting physical activity (PA) in behavioral interventions [10, 11] as they hold the promise of connecting with patients and influencing CV health behaviors in real time, when there may be no face-to-face interaction between a patient and his or her clinician [12]. As mobile devices that permit collection of real-time behavioral data and the ability to provide tailored feedback become more ubiquitous, greater evidence is needed to better understand how this technology can be used in community-based interventions [13], particularly for reaching diverse, at-risk populations in need of health interventions who appear to be adopting these devices as their primary method of connectivity [14, 15]. There is emerging evidence that combining PA tracking devices with group behavioral treatments will produce larger weight loss outcomes than either the device or group treatment alone [16]. However, less is known about adherence with wearable activity monitors within community-based behavioral interventions for targeting PA. Moreover, less is known about the level of technology fluency needed when integrating wearable devices in community-based interventions, as these devices often require additional behaviors including recharging the device and uploading and viewing data. Because wearable device users tend to be “early adopters of technology,” of a higher socioeconomic (SES), and younger than 35 years old [17], it is unlikely that reports on wearable device use and behaviors reflect those of a diverse, community-based sample.
While there have been studies reporting on adherent wearable device users within PA interventions, few studies explore differences between those who are adherent and those who are not adherent with wearables in health interventions targeting a population-based sample [18–23]. Notably, one study examined the differences between adherent device users and device non-users within a technology-based weight loss intervention, finding that device non-users were typically younger and represented racial/ethnic minorities [24]. This finding largely contradicts the premise of the “new digital divide,” where technology access disparities once most prominent along racial/ethnic and socioeconomic (SES) lines are now more pronounced between younger and older consumers [25]. As wearable technologies and wearable activity monitors evolve, they present an opportunity to reach vulnerable groups in community-based PA interventions.
One facilitator to engagement in mHealth research less explored within lower SES, community-based studies is the positive effect of monetary incentives on adherence to study requirements. Previous work in community-based preventive intervention research revealed that a positive incentive effect was stronger among prospective participants with less education and who were otherwise less likely to participate; however, it remains unknown if this holds true for studies that incorporate wearable tools [26]. Understanding differences between device users and non-users may expose barriers or facilitators to user adoption and device engagement thus providing information to help target and support non-users and to better allocate intervention resources.
The aim of the present exploratory study was to evaluate the use of mHealth technology, particularly electronic wrist-worn PA monitors, for objectively measuring PA in a predominantly African-American church population in at-risk Washington, D.C., neighborhoods. Specifically, we sought to investigate possible differences in demographic characteristics, CV health factors and behaviors, and technological fluency between those who had access to and interacted with the wrist-worn PA monitor and hub-based system and those who had access to and did not interact with the wrist-worm PA monitor and hub-based system implemented in the Washington, D.C., CV Health and Needs Assessment. We hypothesized that PA-monitoring wristbands would be a feasible tool for objectively measuring PA in resource-limited, church communities in Washington, D.C., and that a positive incentive effect on device adherence may exist among participants of a lower socioeconomic status.
METHODS
Study design
The Washington, D.C., CV Health and Needs Assessment was a community-based participatory research (CBPR)-designed observational study to evaluate CV health, psychosocial factors, cultural norms, and neighborhood environment characteristics in a predominantly African-American church population in at-risk Washington, D.C., communities. This study also evaluated the feasibility and acceptability of using mHealth technology in this community-based population for objectively measuring PA and dietary intake and for monitoring CV health markers. The Washington, D.C., CV Health and Needs assessment served as a preliminary step in the development of a community-based behavioral change intervention to improve CV health in this community.
As a CBPR study, this project involved community members at each stage of study design and coordination. To consult on the planning and implementation of the CV Health and Needs Assessment, our research team partnered with a community advisory board (CAB) comprised of a diverse group of community leaders, church leaders, and partners in research. The roles and responsibilities of the CAB, also recognized as the D.C. Cardiovascular Health and Obesity Collaborative (D.C. CHOC), have been detailed elsewhere [27].
Participant recruitment
Facilitated by the D.C. CHOC, partnerships between the research team and the targeted communities were established prior to study recruitment and enrollment. These relationships were maintained with frequent, face-to-face contact at church events, health advocacy meetings, and local health fairs. This presence allowed for the team to generate ongoing support and participation among community members. At community events and meetings, churches located in the targeted areas were informed of the study, and church pastors volunteered their churches’ participation. To participate, a church was required to be located in one of the targeted neighborhoods and have onsite internet accessibility. After a pastor volunteered a church’s participation, participants were recruited at these churches and at health-related community events in the targeted areas using posted flyers and announcements during church meetings and services. Recruitment also occurred through information given to community partners in the D.C. CHOC. To aid in recruitment and engagement of participants within the church communities, a participant from each church volunteered to serve as the church’s study leader.
To obtain a sample size of 100 individuals, no more than 150 individuals were recruited from the three targeted Washington, D.C., communities. This sample size accounted for those individuals who may be recruited for a health screening but may not show up for an appointment. Eligible individuals were between 19 and 85 years of age, were members of one of the participating churches, and possessed sufficient English language proficiency to carry out study tasks. Individuals interested in participating were screened for inclusion by an investigator prior to enrollment. All participants provided written informed consent after the study details were reviewed.
Participants were assigned to one of six data collection events, each of which could accommodate up to 30 participants. Each of the six separate data collection events were held at one of four participating churches in the target Washington, D.C., communities on a Saturday during September 2014 through February 2015. Participants rotated through six stations at the events: (1) registration and informed consent; (2) blood pressure measurement, blood sample collection, and anthropometric measures; (3) survey instrument completion; (4) mHealth device training; (5) CV risk assessment with the principal investigator; and (6) debriefing. Physical activity data collection continued for the 30-day period following the initial health and needs assessment data collection event.
PA data collection
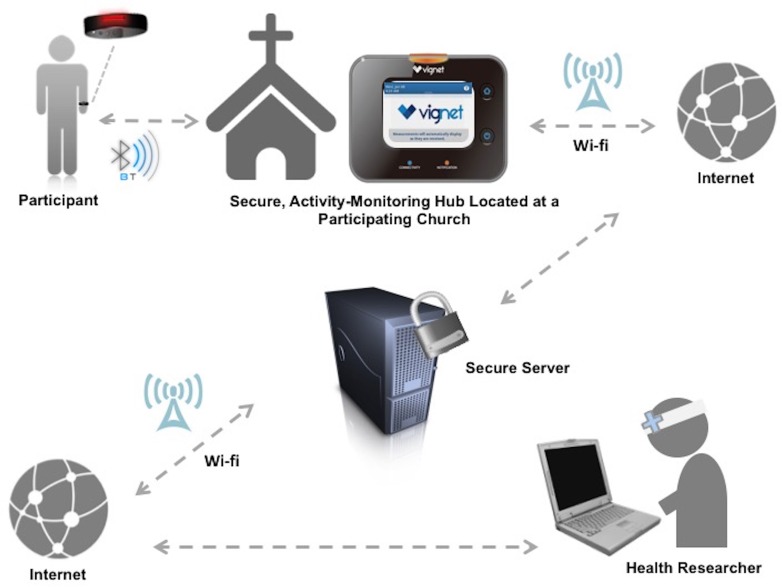
This study utilized a two-part PA-monitoring system: an electronic activity monitoring wristband (Dynamo Activity Tracker, Oregon Scientific, Tualatin, OR) with a centralized hub for data download in a community location, and a secure online account for manual tracking of CV health factors (Vignet Corp, McLean, VA). The data collection process is shown in Fig. 1. In February 2014, the proposed PA data collection system was piloted, and a post-use focus group was conducted with a similar, community-based population (n = 8) prior to implementation in the CV Health and Needs Assessment in September 2014. Changes were made to the PA-monitoring system based on findings from our mixed-methods study and have been reported previously [27]. Each participant received a PA-monitoring wristband to wear and self-monitor sleep duration, without modifying current PA and sleep routine, for 1 month during the study. Upon receipt of the device, participants were trained on use of the wristband and were provided with a written instruction manual. The training also included a video presentation embedded on the study’s publically accessible website that participants could access from a home or church-based computer. The wrist-worn PA monitor collected accelerometer-based data on PA amount and intensity (e.g., steps taken, calories burned, distance traveled, and minutes of vigorous activity). The wristband featured a pre-set goal of 30 min of vigorous activity throughout a 24-h period. The wristband used a colored-light system to communicate PA progress to the participant. Pressing the wristband button prompted the wristband light to display a specific color. The various colors indicated sleep mode, battery depletion, or progress toward the daily 30-min vigorous activity goal. Wristbands stored 14 consecutive days of PA and sleep data.
Fig 1.
Secure data collection system for electronic activity monitor data in the Washington, D.C. CV Health and Needs Assessment, (2014–2015)
A hub was installed in an easily accessible location at each church. Participants were instructed to upload recorded physical activity and sleep data wirelessly (within a 5-foot radius) from their wristband devices to the church-based centralized hub once a week during the 1-month study. Participants were able to synchronize their wristbands with the hub at any point during the study period, consistent with church hours. After successfully synchronizing their wristbands with the hub, participants had access to their recorded accelerometer-based PA data in addition to all self-logged data on the study’s website. Each church had a “super user,” a participant volunteer who communicated hub issues to investigators and helped church participants with syncing wristband data.
Participants were provided a secure account (using a de-identified username and password) on a website associated with the PA-monitoring wristband. They received training on general website use and how to log self-reported PA, weight, dietary intake, heart rate, blood pressure, and blood glucose levels, if measured. Self-reporting these measures was optional. Participants could access the study website from a personal or church-based computer. Investigators monitored usage of the wristband and website by collecting de-identified data from the website during the 1-month study.
Participants were remunerated with a US $25 gift card, compatible with time required for participation in the needs assessment. Participants who demonstrated 30 days of uploaded accelerometer-based PA data were remunerated with an additional US $25 gift card at the end of the 30-day PA data collection period.
Measures
PA device use
Adherence with using the wireless PA-monitoring system was determined by the number of days with documented PA data over the 30-day assessment period. Because syncing with the hub was required for measured PA data to be transmitted to the investigators, a participant with recorded PA data was considered adherent (i.e., synced wristband data with the hub at least once during the 30-day assessment period) and was defined as a “PA device user.” A participant with no uploaded PA data was considered non-adherent (i.e., did not sync wristband data with the hub during the 30 days) and was defined as a “PA device non-user.” A user with all 30 days of PA data was considered to be a complete user. Feasibility of the PA-monitoring system was defined a priori as adherence (i.e., uploading wristband data to data collection hub) by greater than 50% of participants.
Demographics characteristics
Participants self-reported sociodemographic characteristics on a detailed survey designed with and tailored to community input. Income was self-reported in $10,000 increments on a scale from <$10,000/year to >$100,000/year. In 2014 when the study recruitment began, the target population was Washington, D.C., neighborhoods with median annual household incomes significantly lower than the average median annual household income across Washington, D.C., which, at that time was $66,583/year [28]. For analysis, income categories were grouped into three groups according to this framework: those with a median household income less than the median household income across Washington, D.C., (<$60,000/year), those who fall into the highest bracket on our survey (≥$100,000/year), and those who fall between the two groups ($60,000–$99,999).
Cardiovascular health measurements
Participant CV health factors (e.g., blood pressure, fasting blood glucose, cholesterol, hemoglobin A1c, fasting plasma lipids, body mass index) were collected on the day of the health and needs assessment data collection event. Blood pressure was measured using the protocol established by the JNC-VII guidelines [29]. The average of up to three blood pressure measurements was taken using a recently calibrated automatic blood pressure cuff (Welch-Allyn Inc., Skaneateles Falls, NY).
Participants fasted for 12 h prior to their participation in the data collection event. A finger stick capillary blood sample was collected from each participant. The blood sample was used for analyses of fasting plasma lipids and blood glucose using a Cholestech LDX point-of-care analyzer (Alere, Inc., Waltham, MA) and for hemoglobin A1c (HgbA1c) using a DCA Vantage Analyzer (Siemens, Inc.–Laboratory Diagnostics, Tarrytown, NY).
Height was measured using a stadiometer (Perspective Enterprises, Portage, MI). Weight was measured using a digital scale (Doran Scales, Inc., Batavia, IL). Body mass index (BMI) was calculated on the basis of height and weight recorded by study investigators [30].
CV health behaviors were self-reported and assessed using a culturally tailored survey instrument. CV health behaviors (i.e., PA, dietary habits, tobacco use, and alcohol consumption) were evaluated using questions extracted from the 2011 Behavioral Risk Factor Surveillance System (BRFSS) [31] and the 2009–2010 National Health and Nutrition Examination Survey (NHANES) dietary screener questionnaire [32]. To assess fruit and vegetable (F/V) consumption, study participants recorded the number of times per day, per week, or per month they consumed a particular F/V group (e.g., fruit juice, fruit, green leafy salad, orange-colored vegetables, other vegetables, beans). To calculate consumption in times per day, daily frequencies were maintained, weekly frequencies were divided by seven, and monthly frequencies were divided by 30. Total daily F/V consumption (times/day) was the sum of each F/V group frequency.
PA and sedentary behavior, which were both captured using questions abstracted from the NHANES, were also assessed. Participants self-reported time spent performing different types of PA in a typical week in addition to time spent sitting and/or reclining in a typical week. Weekly totals were divided by seven to represent the average amount of time spent per day doing the activity (hour-day-1). “Moderate-intensity activities” were defined as activities that require moderate physical effort and cause small increases in breathing or heart rate, while “vigorous-intensity activities” were defined as activities that require hard physical effort and cause large increases in breathing or heart rate. Leisure-time and work-related activity were assessed separately.
Regarding tobacco use, current smokers were defined as those who had smoked at least 100 cigarettes (i.e., 5 packs) during their lifetimes and, at the time of the survey reported smoking every day or some days. Former smokers were defined as those who reported smoking at least 100 cigarettes during their lifetime though, at the time of the survey, did not smoke.
Technology fluency
Utilization of internet and mobile phone technology was evaluated with questions abstracted from the Pew Research Center Mobile Technology survey. Fluency with the internet and mobile phone technology was evaluated with questions abstracted from a validated self-report instrument, the computer-email-web (CEW) fluency scale [33]. We included 17 items from the CEW fluency scale. Each item measured skill levels from 1 (no fluency) to 5 (high fluency) across 3 subscales (i.e., computer skills, email skills, web skills). Participants were considered to have some level of fluency on a skill if they recorded a response other than “Not at All.”
Data analysis
Objective PA data
Accelerometer-based PA data (i.e., steps, distance, calories, minutes) were analyzed across the 30-day period following device receipt. Wristband utilization frequency was measured by the number of days with wristband-measured activity. Days with no wristband-measured activity (i.e., days with zero steps) were included when calculating number of adherent days, but were not included when calculating average PA steps per day. Days with no recorded PA data were likely the product of a depleted battery or a participant not wearing the wristband. PA data were divided into sedentary or active categories based on the participants’ step count as measured by the wristband in accordance with the indices set forth for healthy adults; sedentary activity was classified as <5000 steps/day while active was classified as ≥10,000 steps/day as per convention [34].
Statistical methods
Descriptive baseline demographic characteristics of the sample were presented as means ± SD or as percentages. Categorical variables (e.g., sex, education, income, employment, marital status, health insurance, smoking status, objectively measured sedentary behavior) were assessed through Fisher’s exact tests. Continuous variables (e.g., age, F/V intake, self-reported PA and sedentary behavior, BMI, blood pressure measurements, fasting blood glucose, total cholesterol, technology fluency) were analyzed utilizing independent t tests.
Two analyses were performed to explore a potential incentive effect. A logistic regression analysis was performed to determine the association between household income and adherence (i.e., using the PA system). We hypothesized that those participants with a lower annual household income would be more motivated by the incentive and thus more likely to be a device user. In this analysis, adherence with the PA-monitoring device was evaluated as a binary variable based on whether participants synchronized their device with the hub during the 30-day study period (adherent/device user: uploaded data at least once; non-adherent/device non-user: did not upload data). The second analysis was a logistic regression analysis to determine if annual household income was associated with the number of days adherent to device use (among users). We hypothesized that those with a lower annual household income would be more motivated by the incentive and therefore more likely to provide more days of data. Among users of the PA device, days of adherence were examined as an ordinal variable (number of days = 1–30). To assess adherence behavior at baseline compared to 30 days, the McNemar’s test was used. Statistical analyses were conducted using SAS version 9.2. (SAS Institute, Inc. Cary, NC). The significance level was set as p ≤ 0.05.
RESULTS
Ninety-nine participants completed all baseline measurements (i.e., survey, anthropometric measures, etc.). Incomplete survey and clinical measure components resulted in omission of one individual from analysis. The final study sample (n = 99) was 59 ± 12 years, 79% female, and 99% African-American, and had a mean BMI of 33 ± 7 kg/m2. Within the sample, 89% were either overweight or obese. Across the 30-day study period, 81% of participants uploaded PA wristband data to a centralized hub at least once. The 81% with uploaded data were defined as users (mean age = 60 ± 12, 78% female), while the remaining 19% were non-users (mean age = 57 ± 13, 83% female). Baseline characteristics for the users and non-users are presented in Table 1. Significant sociodemographic differences between the user and non-user groups were observed for annual household income, with users being more likely to report an income lower than the median annual household income across Washington, D.C. (i.e., <$60,000/year, p = 0.02).
Table 1.
Participant (n = 99) sociodemographic characteristics in the Washington, D.C. CV Health and Needs Assessment (2014–2015)
| Total n = 99) |
Users (n = 81) |
Non-users (n = 18) |
p* | |
|---|---|---|---|---|
| Female, N (%) | 77 (79) | 62 (78) | 15 (83) | 0.5 |
| Age, yearsa | 59.1 (12) | 60 (12) | 57 (13) | 0.4 |
| Education, N (%) | 0.3 | |||
| <High school | 9 (9) | 6 (7.5) | 3 (17) | |
| High school | 10 (10) | 10 (12.5) | 0 (0) | |
| Some college | 34 (35) | 27 (34) | 7 (39) | |
| College+ | 45 (46) | 37 (46) | 8 (44) | |
| Annual household incomeb, N (%) | 0.02 | |||
| <$60,000 | 40 (47) | 36 (51) | 4 (27) | |
| $60,000–99,999 | 28 (33) | 20 (29) | 8 (53) | |
| $100,000+ | 17 (20) | 14 (20) | 3 (20) | |
| Employed, N (%) | 45 (50) | 35 (47) | 10 (63) | 0.3 |
| Marital status, N (%) | 0.2 | |||
| Single | 53 (56) | 41 (53) | 12 (71) | |
| Married | 43 (56) | 36 (47) | 5 (29) | |
| Health insurance, N (%) | 97 (98) | 79 (98) | 18 (100) | 0.5 |
*p-value of ≤0.05 (represented in Italics) considered statistically significant
aMean (standard deviation)
bAnnual Household Income was surveyed in $10 K/year increments (i.e., <$60,000 includes 0–$9999/year, $10,000–$19,999/year, $20,000–$29,999/year, $30,000–$39,999/year, $40,000–$49,999/year, $50,000–$59,999). The reported p value reflects the difference across the $10,000 increment categories
Table 2 displays CV health behaviors and factors for both users and non-users. CV health factors (e.g., BMI, SBP, DBP, fasting blood glucose, total cholesterol) were measured by health care professionals, and all CV health behaviors except those captured by the activity monitor (e.g., step count, sedentary behavior <5000 steps) were self-reported on the survey. Significant differences between the user and non-user groups were observed for smoking status and moderate leisure-time PA, with users being more likely to report never being a smoker (p = 0.05), and to report engaging in higher levels of moderate leisure-time PA (p = 0.05). No significant differences in reported daily vigorous leisure-time PA, moderate employment-time PA, vigorous employment-time PA, sedentary activity, or TV-watching hours were observed between users and non-users. Among users, mean steps/day was 8710 ± 4324 with 23% of users registering <5000 steps/day and 39% registering ≥10,000 steps/day. Users logging ≥10,000 steps/day were younger (p = 0.04) and more likely to report participation in moderate (p = 0.03) and vigorous activity (p = 0.002) when compared to users logging <5000 steps/day (data not shown). No significant differences in CV health factors were found between the two groups.
Table 2.
CV health behaviors and factors among participants (n = 99) in the Washington, D.C. CV Health and Needs Assessment (2014–2015)
| Variable | Total (n = 99) | Users (n = 81) | Non-users (n = 18) | p* |
|---|---|---|---|---|
| CV health behaviors | ||||
| Never smoker, N (%) | 70 (73) | 61 (78) | 9 (50) | 0.05 |
| Fruit/vegetable intake, servings/daya | 3.2 (2.7) | 3.3 (2.6) | 2.7 (3.2) | 0.2 |
| Physical activitya, b | ||||
| Mod leisure-time PA, min/week | 79 (99) | 85 (104) | 33 (35) | 0.05 |
| Vig leisure-time PA, mins/week | 52 (74) | 53 (71) | 46 (96) | 0.3 |
| Mod employment-time PA, min/day | 122.3 (166) | 105.9 (169) | 169.3 (160) | 0.1 |
| Vig employment-time PA, min/day | 53.8 (106) | 49.1 (107) | 69.6 (111) | 0.5 |
| Steps: activity monitor, strides/day | – | 8710 (4324) | – | – |
| Sedentary Behaviora | ||||
| Sedentary activity, hours/daya | 6.64 (3.8) | 6.35 (3.4) | 7.85 (5.3) | 0.4 |
| TV-watching, hours/daya | 3.37 (1.5) | 3.42 (1.5) | 3.12 (1.5) | 0.5 |
| < 5000 steps: activity monitor, N (%) | – | 19 (23) | – | – |
| CV Health Factorsa | ||||
| BMI, kg/m2 | 33 (7) | 32 (7) | 35 (7) | 0.08 |
| SBP, mmHg | 133 (15) | 133 (15) | 133 (15) | 0.8 |
| DBP, mmHg | 80 (12) | 80 (11) | 81 (14) | 0.9 |
| Fasting blood glucose, mg/dL | 95 (28) | 95 (26) | 96 (37) | 0.5 |
| Total cholesterol, mg/dL | 195 (46) | 196 (48) | 191 (28) | 0.8 |
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure
*p-value of ≤0.05 considered statistically significant
aMean (standard deviation)
bModerate (Mod) PA: activities that require moderate physical effort and cause small increases in breathing or heart rate; Vigorous (Vig) PA: activities that require hard physical effort and cause large increases in breathing or heart rate
No differences between the user and non-user groups were noted for computer access (93.8 vs. 94.4% respectively, p = 0.2) or cell phone ownership (87.7 vs. 88.9% respectively, p = 0.2). As shown in Table 3, technology fluency was similar, with all non-users and most users (95%) reporting some level of technology fluency (i.e., some level of technology fluency = a response other than “Not at All” on the CEW) for the 17 surveyed skills.
Table 3.
Technology fluency among participants (n = 99) in the Washington, D.C. CV Health and Needs Assessment (2014–2015)
| Users (n = 81) |
Non-users (n = 18) |
p* | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Computer skills | |||||
| a. I can switch a computer on. | 3.96 | 0.51 | 4.06 | 0.24 | 0.5 |
| b. I can restart a computer. | 3.89 | 0.64 | 4.17 | 0.38 | 0.05 |
| c. I can begin typing a new document. | 3.80 | 0.72 | 4.00 | 0.59 | 0.2 |
| d. I can open a previously saved file from any directory. | 3.76 | 0.85 | 3.89 | 0.58 | 0.7 |
| e. I can use “save as” when appropriate. | 3.81 | 0.78 | 3.89 | 0.58 | 0.8 |
| f. I can print a document. | 3.89 | 0.71 | 3.94 | 0.54 | 0.8 |
| Total subscale | 3.85 | 0.60 | 3.99 | 0.35 | 0.3 |
| Email skills | |||||
| g. I can open an email program. | 3.86 | 0.73 | 4.00 | 0.59 | 0.4 |
| h. I can read new email messages. | 3.92 | 0.66 | 4.06 | 0.42 | 0.6 |
| i. I can open a file attached to an email. | 3.80 | 0.83 | 3.88 | 0.60 | 1.0 |
| j. I can delete read email messages. | 3.88 | 0.72 | 4.00 | 0.35 | 0.8 |
| k. I can send an email message. | 3.82 | 0.80 | 4.06 | 0.43 | 0.3 |
| l. I can use the “reply’ and “forward” features for email. | 3.86 | 0.81 | 3.94 | 0.56 | 0.9 |
| Total subscale | 3.88 | 0.69 | 3.99 | 0.42 | 0.5 |
| Web navigation skills | |||||
| m. I can use a browser such as Internet Explorer, Firefox, or Google Chrome to navigate the World Wide Web. | 3.75 | 0.86 | 3.94 | 0.43 | 0.7 |
| n. I can open a web address directly. | 3.69 | 0.94 | 3.89 | 0.58 | 0.6 |
| o. I can identify the host server from the web address. | 3.54 | 1.17 | 3.72 | 0.83 | 0.7 |
| p. I can use “back” and “forward” to move between web pages. | 3.80 | 0.86 | 3.72 | 0.67 | 0.4 |
| q. I can use search engines such as Yahoo and Google. | 3.80 | 0.81 | 3.67 | 0.77 | 0.4 |
| Total subscale | 3.70 | 0.85 | 3.78 | 0.56 | 0.9 |
| Cumulative score | 3.81 | 0.69 | 3.92 | 0.36 | 0.6 |
*p value of ≤0.05 (represented in Italics) considered statistically significant
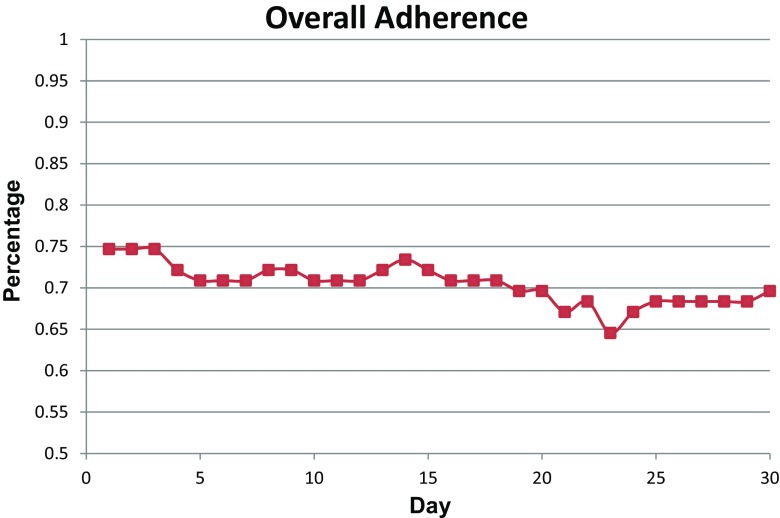
As depicted in Fig. 2, overall adherence (i.e., days with proof of device use) with using the hub-based PA-monitoring system among users in the study slightly decreased over the study period from 75% in the beginning of the 30-day assessment period to 70% by the end of the assessment period with minimum participation reaching as low as 65% at day 23. The change in adherence over time was not statistically significant (p = 0.08). To examine the effect of incentive distribution on use of the PA devices among device users, we conducted a logistic regression analysis to determine the association between household income and number of days of adherence (since participants received a monetary incentive if they provided a full dataset consisting of 30 consecutive days of wristband captured PA data). There was no statistically significant relationship between annual household income and days of adherence (p > 0.5).
Fig 2.
Adherence rates among PA device users (n = 81) during a 30-day period for the Washington, D.C. CV Health and Needs Assessment, (2014–2015)
DISCUSSION
This study sheds light on the adherent device users of a mHealth PA-monitoring wristband in a predominantly African-American population recruited from churches from at-risk Washington, D.C., neighborhoods. Our exploratory analysis investigating differences in demographic characteristics, cardiovascular health factors and behaviors, and technological fluency of users and non-users of the proposed PA-monitoring system revealed that individuals of a lower socioeconomic status, particularly those with a lower annual household income, were more likely to use the mHealth PA-monitoring system for objectively measuring PA over a 30-day period.
This is one of the first studies to investigate whether demographic characteristics, cardiovascular health factors and behaviors, and technological fluency may facilitate or impede user adoption of wearable mHealth technology in resource-limited communities (i.e., communities with lower neighborhood-level socioeconomic status and where resources for physical activity and nutritious food options are most limited). Our findings suggest that lower SES individuals may be more likely than higher SES participants to interact with the hub-based mHealth PA-monitoring system in this specific resource-limited community. Additionally, device non-users’ lower self-reported moderate leisure-time activity and lower likelihood of being a non-smoker point to the need for novel engagement strategies when optimizing technologies to increase leisure-time PA levels, reduce sedentary time, and provide smoking cessation within this population. Despite lower socioeconomic status among users, lower technology fluency does not appear to impede adoption of this mHealth PA-monitoring system. These results suggest that user adoption of a mHealth PA-monitoring system with a data collection hub may be independent of technology fluency.
This study is among the first to investigate user adoption of wearable PA-monitoring devices among individuals from a primarily African-American, resource-limited community. mHealth approaches show promise for extending the reach of behavioral interventions to diverse and historically underserved populations. Despite such promise, few prior studies have observed user adoption and continued use of mHealth PA-monitoring devices within a resource-limited community-based population, a group disproportionately burdened with obesity and obesity-related chronic diseases [18–23]. Recently, there has been a resounding call for rigorous testing of wearable PA-monitoring consumer devices among diverse populations, a gap that our study aimed to fill [35].
Our findings suggest that lower SES individuals may be more likely than higher SES participants within this community-based population to interact with the study’s particular mHealth-based PA-monitoring system with a centralized hub. This finding is consistent with the premise of the “new digital divide,” where technology access disparities may no longer be most prominent along racial/ethnic and socioeconomic lines [25]. With recent data supporting the inverse association of CVD rates and adult SES, especially wealth, among African-Americans, our findings highlight a critical opportunity to gather objective measures and potentially intervene on CV-related health behaviors, particularly PA, among at-risk individuals in a resource-limited, community-based setting [36].
Our findings also suggest that those who engaged with the PA-monitoring device may be more likely to have already healthy behaviors. We also observed that individuals who engaged with the PA-monitoring system were more likely to participate in PA and to not smoke. This finding may suggest that individuals who have already-established healthy behaviors may already have the motivation for healthy behaviors and desire a trigger or a means of receiving positive reinforcement to maintain or improve upon already-established healthy behaviors; thus, they were more likely to engage with the PA-monitoring device. Furthermore, social support may play a role in an individual’s engagement with the device. A recent study on activity trackers confirmed that social support, delivered/obtained via social networking, increased users’ adherence and engagement with activity trackers which in turn reinforced social support in shaping PA behaviors and habits [37].
Despite such promise, our results also highlight the difficulties in reaching one of the groups most at-risk for cardiovascular risk: past and present smokers. Reviews have called for a better understanding of recruiting, engaging and intervening on this population, as traditional recruitment methods (e.g., face-to-face recruitment, fliers placed in high-traffic areas) may not be as successful with hard to reach smokers as with other demographics for research studies [38]. Facebook recruitment appears to be one efficacious and cost-effective mechanism, providing a wider reach at a lower cost than traditional methods while providing both privacy and flexibility for participants [39]. In terms of engagement, Burke and colleagues asserted that engaging current smokers in mHealth-based smoking cessation interventions is an inadequately researched area [3]. Qualitative studies on mHealth tools for smoking cessation have pointed to social networking components, the inclusion of more interactive features, and allowing for greater control by participants of program output as ways to improve engagement among smokers [40].
Not surprising, Burke et al. ranked “identifying strategies that promote sustained user engagement” as a key priority moving forward for mHealth-based CVD prevention efforts [41]. Findings from mHealth-based behavioral weight loss intervention studies have suggested that continuous use of a technology-based system results in greater weight loss when compared with intermittent use [16, 24]. Though our study was not designed to assess the impact of continuous and intermittent device use on step count, the study’s measure of adherence gauged daily usage. A unique feature of our study was the assessment of daily adherence with a wearable mHealth PA monitor in a resource-limited, community-based setting. We observed that our most adherent participants (i.e., the users) self-reported higher levels of leisure-time PA than the non-adherent (i.e., non-users), indicating that non-users were more likely to be those with sub-optimal PA levels. Reaching these disengaged participants is a promising strength of mHealth PA-monitoring devices—lending interventionists the opportunity to capture objective measurements indicative of engagement, which could allow for early identification of less engaged participants. Optimizing mHealth tools to re-engage participants may result in more sustainable, personalized PA interventions capable of extending beyond the health care setting.
When integrating mHealth technology in community-based interventions in resource-limited settings, limited technology access, fluency, and usage must be understood to reduce potential disparities. This is particularly true when targeting groups that are more likely to have decreased access to the internet (i.e., those of lower socioeconomic status, minority racial/ethnic groups, older age, and poorer health) [42–46]. Unlike prior studies which have used a smartphone, mobile phone, or website as the primary mode of delivery [35], this study was designed to assess usage of a wearable PA-monitoring device, particularly as it relates to technology fluency. Our study demonstrated that lower technology fluency does not appear to impede adoption of this mHealth PA-monitoring system, despite lower socioeconomic status among users. This relationship, likely attributed to users’ and non-users’ similar access to technology and rates of mobile device ownership, is consistent with the growing body of work on widespread mobile device adoption across demographic and racial/ethnic groups [47–49]. The study’s findings suggest that a mHealth PA-monitoring system incorporating a data collection hub may facilitate a future community-based PA intervention, independent of technology fluency of the targeted group. Though transmission of device data required syncing with a church-based hub rather than with an at-home computer or device, it should be noted that the incorporation of a centralized hub had the added benefit of community-wide accessibility. The hub made uploading and viewing PA data possible for all participants regardless of computer, mobile device, or internet access. Addressing these well-documented access barriers by incorporating a centralized hub ensures that the PA technology is equally accessible to all participants, regardless of technology access, usage, and fluency.
Strengths and limitations
Our study answers the recent call for prioritized mobile health research among populations with the highest rates of obesity and obesity-related chronic disease: racial/ethnic minorities and the socioeconomically disadvantaged [35]. However, by targeting this population, we limit the generalizability of our findings to church-going, predominantly African-American individuals in resource-limited, urban settings. Despite targeting individuals from churches in Washington, D.C.’s lowest income and most resource-limited communities, most participants were college graduates or had some college education and over half reported annual household incomes greater than $60,000/year. As a result, these findings may not necessarily extend to individuals with a lesser socioeconomic status within these particular resource-limited communities. Furthermore, the study design and statistical analyses used in this study did not allow for control of confounding variables, which may limit the generalizability of our findings. Future research could include more advanced statistical methods to adjust for potentially confounding effects. Our study was also limited in its ability to capture long-term adherence, engagement, retention, and attrition of participants. Future work would benefit from extension of this study to a larger and more diverse sample and a longer study period to gauge user engagement factors. Our study did not evaluate the effectiveness of the mHealth PA monitor to modify behaviors or improve health outcomes (e.g., weight reduction, increase in PA). However, such preliminary user data enables behavioral interventionists to identify potential barriers and evaluate feasibility of mHealth devices within a particular context before implementing an intervention. Moreover, in a recent review, Kumanyika et al. [50] highlighted the potential of mHealth interventions in addressing obesity in racial/ethnic minority adults, and called for evidence to inform the development of mHealth-based interventions. The traction of popular, commercially available PA-monitoring devices (e.g., Fitbit®, Jawbone®) suggest that competition within the market may lead to more accessible and affordable options that support widespread implementation across diverse, low-resource communities. This study’s authors recognize that cost is often a prohibitive factor to device ownership especially among lower SES communities, so the higher engagement among lower SES participants should not be anticipated in related studies if a cost was associated with the device. Understanding the role of additional devices for syncing wearable device data, particularly through personal devices and smartphones, should be a critical consideration in future feasibility studies. This study is limited in its capacity to establish causality and address the complex dynamics of technology use within a resource-limited community-based setting. Findings from this particular hub-based system can exist as preliminary steps and inform future mHealth-based interventions in resource-limited communities in Washington, D.C.
CONCLUSIONS
A mHealth PA-monitoring system using wearable technology and a wireless, community-based hub was an effective modality for objectively measuring PA in resource-limited, church communities in Washington, D.C. Our findings suggest that lower SES individuals, particularly those with a lower annual household income, may be more likely to interact with the hub-based PA-monitoring system than higher SES participants. Additionally, lower technology fluency does not appear to impede adoption of this mHealth PA-monitoring system, a relationship likely explained by similar access to technology among users and non-users. This study provides evidence to support further evaluating PA-monitoring technologies as part of future PA interventions in resource-limited, community-based settings in Washington, D.C. However, further work is needed to evaluate the effectiveness and sustainability of this mHealth PA-monitoring system.
BMI body mass index, BRFSS Behavioral Risk Factor Surveillance System, CAB community advisory board, CBPR community-based participatory research, CEW computer-email-web, CV cardiovascular, CVD cardiovascular disease, D.C. CHOC Washington, D.C., Cardiovascular Health and Obesity Collaborative, F/V fruit and vegetables, HgbA1c hemoglobin A1c, mHealth mobile health, NHANES National Health And Nutrition Examination Survey, PA physical activity, SES socioeconomic status
Acknowledgements
We would like to acknowledge the participating church communities for warmly welcoming our research team and providing feedback from preliminary stages. Additionally, we acknowledge the D.C. CHOC for their contribution to the study design and their insightful recommendations. We would like to acknowledge Ms. Darlene Allen for her work with blood testing for the study.
We would also like to acknowledge the work on this project by Mr. Praduman Jain and colleagues from Vignet Corporation through use of their Precision Medicine Initiative (PMI) toolkit under contract #HHSN268201400023P. The PMI toolkit enables custom mHealth programs for data collection, population surveillance, interactive informed consent, assessments, remote monitoring, CBPR, consumer engagement, interventions, motivation, and behavior change.
Compliance with ethical standards
Disclosure of potential conflict of interest and funding sources
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. The authors declare that they have no conflict of interest.
This study was funded by grant HL006168. Funding for TP-W, LY, and VM is provided through the Division of Intramural Research of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). Funding for CA is provided through a professional services contract (contract #HHSN268201300173P) through the Division of Intramural Research of NHLBI at NIH. Funding for ST and JA-B is provided through the Office of Intramural Training and Education of the NIH. Funding for GW and AB is provided through the Clinical Center, NIH. Funding for MP-L is provided through the Division of Intramural Research of NHLBI at NIH.
Research involving human participants
Statement of human rights: All procedures involving human participants performed in the Washington, D.C. CV Health and Needs Assessment study were in accordance with the ethical standards of the National Heart, Lung, and Blood Institute (NHLBI) Institutional Review Board study and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Footnotes
Trial registration: NCT01927783
Implications
Practice: Community-based behavioral interventions targeting cardiometabolic health in resource-limited communities should consider incorporation of wearable mHealth technology.
Policy: Efforts to reduce barriers to using mHealth technology in resource-limited settings may aid in decreasing cardiometabolic health disparities in at-risk populations.
Research: Future research is needed to determine how wearable mHealth technology can be leveraged to promote increased PA and improve cardiometabolic health in resource-limited communities.
References
- 1.Kochanek, K.D., et al., Mortality in the United States, 2013. NCHS Data Brief, 2014(178): p. 1–8. [PubMed]
- 2.Smedley BD, S.A., Nelson AR, editors, Unequal treatment: confronting racial and ethnic disparities in health care. Washington DC: 2002 by the National Academy of Sciences; 2003. [PubMed]
- 3.Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(25):3137–3147. doi: 10.1161/01.CIR.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 4.Mensah GA M.A., Ford ES, Greenlund KJ, Croft JB, State of disparities in cardiovascular health in the United States. Circulation 2005;111(10):1233–1241. doi: 10.1161/01.cir.0000158136.76824.04. [DOI] [PubMed]
- 5.Diez-Roux AV, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146(1):48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- 6.Diez Roux AV, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 7.Elosua R, et al. Dose-response association of physical activity with acute myocardial infarction: do amount and intensity matter? Prev Med. 2013;57(5):567–572. doi: 10.1016/j.ypmed.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Haskell WL, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 9.Walton-Moss B, et al. Community based cardiovascular health interventions in vulnerable populations: a systematic review. The Journal of cardiovascular nursing. 2014;29(4):293–307. doi: 10.1097/JCN.0b013e31828e2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Case MA, et al. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313(6):625–626. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 11.Thorndike AN, et al. Activity monitor intervention to promote physical activity of physicians-in-training: randomized controlled trial. PLoS One. 2014;9(6):e100251. doi: 10.1371/journal.pone.0100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asch DA, Muller RW, Volpp KG. Automated hovering in health care—watching over the 5000 hours. N Engl J Med. 2012;367(1):1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 13.Crossing the Quality Chasm: The IOM Health Care Quality Initiative. 2001, Institute of Medicine: Washington, DC.
- 14.Smith, J.C. and B.R. Schatz. Feasibility of mobile phone-based management of chronic illness. in AMIA annual symposium proceedings. 2010. American Medical Informatics Association. [PMC free article] [PubMed]
- 15.Pew Research Internet Project: cell phone and smartphone ownership demographics, http://www.pewinternet.org/data-trend/mobile/cellphone-and-smartphone-ownership-demographics/, Accessed October 23, 2014.
- 16.Shuger SL, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313(5):459–460. doi: 10.1001/jama.2014.14781. [DOI] [PubMed] [Google Scholar]
- 18.Fitzsimons, C. F., et al. (2013). Using an individualised consultation and activPAL feedback to reduce sedentary time in older Scottish adults: results of a feasibility and pilot study. Prev Med, 57(5). [DOI] [PubMed]
- 19.Hurling R, et al. Using internet and mobile phone technology to deliver an automated physical activity program: randomized controlled trial. J Med Internet Res. 2007;9(2):e7. doi: 10.2196/jmir.9.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellegrini CA, et al. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity (Silver Spring) 2012;20(2):356–363. doi: 10.1038/oby.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reijonsaari K, et al. The effectiveness of physical activity monitoring and distance counseling in an occupational setting—results from a randomized controlled trial (CoAct) BMC Public Health. 2012;12:344. doi: 10.1186/1471-2458-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slootmaker SM, et al. Feasibility and effectiveness of online physical activity advice based on a personal activity monitor: randomized controlled trial. J Med Internet Res. 2009;11(3):e27. doi: 10.2196/jmir.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabak M, op den Akker H, Hermens H. Motivational cues as real-time feedback for changing daily activity behavior of patients with COPD. Patient Educ Couns. 2014;94(3):372–378. doi: 10.1016/j.pec.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Polzien KM, et al. The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity (Silver Spring) 2007;15(4):825–830. doi: 10.1038/oby.2007.584. [DOI] [PubMed] [Google Scholar]
- 25.Atienza AA, Patrick K. Mobile health: the killer app for cyberinfrastructure and consumer health. Am J Prev Med. 2011;40(5 Suppl 2):S151–S153. doi: 10.1016/j.amepre.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Guyll M, Spoth R, Redmond C. The effects of incentives and research requirements on participation rates for a community-based preventive intervention research study. J Prim Prev. 2003;24(1):25–41. doi: 10.1023/A:1025023600517. [DOI] [Google Scholar]
- 27.Yingling LR, et al. Community engagement to optimize the use of web-based and wearable technology in a Cardiovascular Health and Needs Assessment Study: a mixed methods approach. JMIR mHealth uHealth. 2016;4(2):e38. doi: 10.2196/mhealth.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United States Census Bureau: www.census.gov. Accessed 10 April 2015.
- 29.Chobanian AV, B.G., Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, Committee tNHBPEPC., Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003. [DOI] [PubMed]
- 30.National Heart, L., and Blood Institute Expert Panel. [Accessed April 3, 2011];Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Available at: http://www.nhlbi.nih.gov/guidelines/obesity.
- 31.Mercer K, et al. Behavior change techniques present in wearable activity trackers: a critical analysis. JMIR Mhealth Uhealth. 2016;4(2):e40. doi: 10.2196/mhealth.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arsand E, et al. Performance of the first combined smartwatch and smartphone diabetes diary application study. J Diabetes Sci Technol. 2015;9(3):556–563. doi: 10.1177/1932296814567708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunz U. The computer-email-web (CEW) fluency scale-development and validation. International journal of human-computer interaction. 2004;17(4):479–506. doi: 10.1207/s15327590ijhc1704_3. [DOI] [Google Scholar]
- 34.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Burke LE, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015;132(12):1157–1213. doi: 10.1161/CIR.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebreab SY, et al. The impact of lifecourse socioeconomic position on cardiovascular disease events in African Americans: the Jackson heart study. J Am Heart Assoc. 2015;4(6):e001553. doi: 10.1161/JAHA.114.001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang RC-S, et al. Reciprocal reinforcement between wearable activity trackers and social network services in influencing physical activity behaviors. JMIR mHealth and uHealth. 2016;4:e84. doi: 10.2196/mhealth.5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramo DE, et al. Facebook recruitment of young adult smokers for a cessation trial: methods, metrics, and lessons learned. Internet Interventions. 2014;1(2):58–64. doi: 10.1016/j.invent.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter-Harris L, et al. Beyond traditional newspaper advertisement: leveraging Facebook-targeted advertisement to recruit long-term smokers for research. Journal of Medical Internet Research. 2016;18(6):e117. doi: 10.2196/jmir.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock B, et al. A text message delivered smoking cessation intervention: the initial trial of TXT-2-Quit: randomized controlled trial. JMIR mHealth and uHealth. 2013;1(2):e17. doi: 10.2196/mhealth.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke LE, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med. 2012;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kontos EZ, Bennett GG, Viswanath K. Barriers and facilitators to home computer and internet use among urban novice computer users of low socioeconomic position. J Med Internet Res. 2007;9(4):e31. doi: 10.2196/jmir.9.4.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontos EZ, et al. Communication inequalities and public health implications of adult social networking site use in the United States. J Health Commun. 2010;15(Suppl 3):216–235. doi: 10.1080/10810730.2010.522689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou WY, et al. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11(4):e48. doi: 10.2196/jmir.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson LA, et al. Race, gender, and information technology use: the new digital divide. Cyberpsychol Behav. 2008;11(4):437–442. doi: 10.1089/cpb.2007.0157. [DOI] [PubMed] [Google Scholar]
- 46.Wang JY, Bennett K, Probst J. Subdividing the digital divide: differences in internet access and use among rural residents with medical limitations. J Med Internet Res. 2011;13(1):e25. doi: 10.2196/jmir.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, A., Mobile Access 2010. Pew Internet and American Life Project, July 7 (2010) Available from: http://pewinternet.org.ezproxy.nihlibrary.nih.gov/Reports/2010/Mobile-Access-2010.aspx.
- 48.Leena K, Tomi L, Arja RR. Intensity of mobile phone use and health compromising behaviours—how is information and communication technology connected to health-related lifestyle in adolescence? J Adolesc. 2005;28(1):35–47. doi: 10.1016/j.adolescence.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Coughlin SS, Smith SA. A review of community-based participatory research studies to promote physical activity among African Americans. Journal of the Georgia Public Health Association. 2016;5(3):220–227. [PMC free article] [PubMed] [Google Scholar]
- 50.Kumanyika SK, Whitt-Glover MC, Haire-Joshu D. What works for obesity prevention and treatment in black Americans? Research directions. Obes Rev. 2014;15(Suppl 4):204–212. doi: 10.1111/obr.12213. [DOI] [PubMed] [Google Scholar]