Abstract
Although regular physical activity (PA) is a cornerstone of treatment for type 2 diabetes (T2D), most adults with T2D are sedentary. Randomized controlled trials (RCTs) have proven the effectiveness of PA behavioral interventions for adults with T2D but have rarely been conducted in healthcare settings. We sought to identify PA interventions that are effective and practical to implement in clinical practice settings. Our first aim was to use the valid Pragmatic-Explanatory Continuum Indicator Summary 2 (PRECIS-2) tool to assess the potential for future implementation of PA interventions in clinical practice settings. Our second aim was to identify interventions that effectively increased PA and glycemic control among the interventions in the top tertile of PRECIS-2 scores. We searched PubMed MEDLINE from January 1980 through May 2015 for RCTs of behavioral PA interventions coordinated by clinical practices for patients with T2D. Dual investigators assessed pragmatism by PRECIS-2 scores, and study effectiveness was extracted from original RCT publications. The PRECIS-2 scores of the 46 behavioral interventions (n = 13,575 participants) ranged from 3.0 to 4.8, where 5 is the most pragmatic score. In the most pragmatic tertile of interventions (n = 16) by PRECIS-2 scores, 30.8 and 31.3% of interventions improved PA outcomes and hemoglobin A1c, respectively. A minority of published evidence-based PA interventions for adults with T2D were both effective and pragmatic for clinical implementation. These should be tested for dissemination using implementation trial designs.
Keywords: Diabetes, Physical activity, Implementation science, Dissemination, Pragmatic, PRECIS-2
INTRODUCTION
Between 1980 and 2014, the prevalence of diabetes mellitus almost quadrupled [1], and recent estimates suggest that one third of all Americans born after the year 2000 will develop diabetes [2]. Among adults, type 2 diabetes (T2D) accounts for over 90% of diabetes diagnoses [3]. T2D more than doubles the risk of cardiovascular disease, including myocardial infarction and stroke [4–7]. Even if people with T2D do not develop cardiovascular disease or microvascular complications of T2D, they remain at increased risk for early mortality and morbidity, including disability and other functional problems [8, 9].
To mitigate these serious risks and successfully manage T2D, a healthy lifestyle is important. In particular, regular physical activity (PA) is a cornerstone of T2D management due to its major health benefits [10]. Observational data have linked regular PA to improved all-cause mortality, lower rates of cardiovascular disease, lower rates of breast and colon cancer, improved symptoms of depression and anxiety, and better functional outcomes in people with and without T2D [11]. Data from randomized controlled trials (RCTs) of lifestyle interventions that included both PA and weight loss have also yielded better health outcomes, such as lower incidence of stroke, improved fitness, and improved mobility [12–14]. Based on observational data, it also appears that healthcare costs are lower for people with T2D who report regular PA [15, 16]. For example, among patients with T2D whose health insurance provided a health club membership, those who attended health clubs at least twice weekly over 12 months had $1252 lower mean healthcare costs than patients with T2D who attended PA classes less than once weekly (P < 0.001) [15]. The potential cost savings of regular PA are particularly important given that the annual medical costs of T2D in the US were recently estimated at $176 billion in direct medical costs and $69 billion in reduced productivity [17].
Based on the substantial benefits of PA, the American Diabetes Association, the American College of Sports Medicine, and the US Physical Activity Guidelines all recommend that people with diabetes engage in at least 150 min of moderate to vigorous intensity PA, such as brisk walking, as well as two to three bouts of resistance training each week [11, 18]. However, most patients with T2D do not meet these standards [19, 20]. In fact, people with T2D are less likely to engage in regular PA than their peers without diabetes, even though the majority of people with T2D recognize that regular PA is important [19, 20]. This disconnect between knowledge and action highlights the potential for implementing evidence-based interventions in clinical care settings to increase reach and address personal barriers to PA for patients with T2D.
Currently, delivery of evidence-based PA interventions for people with T2D is not common practice in most clinical care settings [21, 22]. One possible explanation for this is that large-scale interventions which improved PA were not fully integrated into clinical practice settings; thus, they are not easy to translate into real-world settings [12, 14]. Studying interventions which are clinically integrated is critically important because limited clinic resources pose challenges to the translation of effective PA interventions into primary care [23, 24]. Difficulties in bringing evidence-based interventions to clinical practice are not limited to diabetes and PA behavior—the field of implementation science has developed rapidly to address concerns that <10% of evidence-based interventions are implemented in real-world settings [25].
There are valuable real-world alternatives to integrating interventions into clinical practice: community-based programs are able to deliver evidence-based interventions, often tailored to specific community needs. Community-based interventions have been translated broadly for the Diabetes Prevention Program, and economic analyses suggest that these programs are highly cost-effective [26]. In contrast to diabetes prevention, there are more limited examples of community-based programs independently delivering diabetes self-management separately from clinical practices [27–29]. The National Diabetes Education Program has sought to clarify the key roles that communities can play to support patients with diabetes in their self-management [30], and a recent review summarizes how communities and clinical practices may collaborate to improve diabetes self-management [29]. While the relevance of community-based programs for diabetes self-management should not be ignored, the focus of this review will be on the delivery of evidence-based physical activity counseling programs into clinical practice. The rationale for focusing on programs integrated within clinical practices is because this provides the benefit of utilizing clinician’s judgment to identify patients who may participate safely; clinics also have the potential to receive reimbursement for these programs through health insurance.
To address the limited translation of effective physical activity programs into clinical practices, stakeholders with experience in clinical care, healthcare financing, and clinical trial design developed a tool to help clinical trial designers plan RCTs to test the delivery of evidence-based interventions in pragmatic real-world settings, as compared with planning RCTs of interventions to test efficacy that should be delivered in more idealized research settings [31]. This measurement tool was named the PRagmatic Explanatory Continuum Indicator Summary (PRECIS) model. In 2015, >80 international trialists, clinicians, and policy-makers revised and validated an updated version: the PRECIS-2 model [32]. The PRECIS-2 model may serve two purposes—to inform future trial design and to allow researchers to assess the “real-world applicability” of published studies [33].
There is a lack of published information regarding the potential for clinical translation of effective PA interventions for patients with T2D [10]. To address this gap, the goal of this review is to identify existing effective and pragmatic PA interventions for patients with T2D that may be translated into clinical practice. Our specific aims are (1) to use the PRECIS-2 tool to assess the pragmatism for implementation of PA interventions into clinical practice settings and (2) to identify interventions that effectively increased PA and glycemic control, respectively, among interventions ranked in the highest tertile by PRECIS-2 for pragmatism. By addressing these aims, we hope to spur further study of the benefits and costs of implementing pragmatic and effective PA interventions for patients with T2D into diverse clinical care settings.
METHODS
Data sources and searches
Our study team developed an a priori set of relevant inclusion and exclusion criteria (Appendix Table 2). Generally, these criteria led us to include RCTs that tested an intervention which included PA counseling for patients with T2D who were cared for in a clinical care practice. We required that PA counseling was conducted consistently for all patients in the intervention group. However, in keeping with real-world practice needs, we also included interventions that targeted additional T2D self-management behaviors, such as diet, medication adherence, and smoking cessation. Study interventions needed to be clinically integrated, which we defined a priori as regular bidirectional communication between the research and clinical care teams. Furthermore, for studies in which the intervention was delivered by a research team member rather than a clinical care team member, we required that the research team interventionists must be healthcare professionals (e.g., physicians, nurses, pharmacists, social workers, psychologists, or behavioral therapists) and that any face-to-face intervention visits must take place in a longitudinal clinical care setting, in order to ensure that this intervention had the potential for future translation into a clinical practice setting.
Table 2.
Inclusion and exclusion criteria for inclusion in systematic review
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Topic area | Physical activity counseling OR exercise prescription | Supervised exercise training Acute effects of exercise Not in humans Breathing/stretching exercises Type 1 diabetes Gestational diabetes Metabolic syndrome |
| Setting | Ambulatory (outpatient) Healthcare/clinical Integration of intervention with ambulatory clinical setting |
Community-based Based in school, workplace, home, or inpatient (hospital) settings Advertising in community |
| Study design | Clinical trial | Case series/case control Cohort Qualitative Pilot study not powered for outcome Small sample size (N < 20) Pretest-posttest design |
| Target audience | Adults with type 2 diabetes | Type 1 diabetes Pre-diabetes Gestational diabetes Children |
| Language | Article in English | Article not in English |
Study selection
Working with our library informatics staff, we developed a search strategy for behavioral interventions with a PA component targeted to patients with T2D. The PubMed MEDLINE database was considered to be a comprehensive source for our needs, given our interest in trials of behavioral interventions that were integrated into clinical care. Our complete literature search strategy included terms for behavioral interventions, PA and T2D (Appendix Fig. 4).
Fig. 4.
Terms used in literature search strategy
One author (KAL) preliminarily screened the search results by reviewing the manuscript title and abstract. For this initial screen, we applied our eligibility criteria conservatively to select RCTs conducted among adults with T2D which utilized a self-management intervention that might possibly contain PA, including diet and weight loss interventions. After this initial screen, the remaining articles were reviewed independently in a secondary screen by dual raters (KAL, AGH, and/or IML). For this secondary screen, raters independently applied the study eligibility criteria (Appendix Table 2) to the full-text articles to identify qualifying RCTs. In instances of rater disagreement on whether a study met inclusion/exclusion criteria, these disagreements were resolved through discussion and arbitration by another co-author, when necessary. When both raters felt that there was insufficient information to determine eligibility, we contacted the RCT authors for clarification.
Data extraction and quality assessment
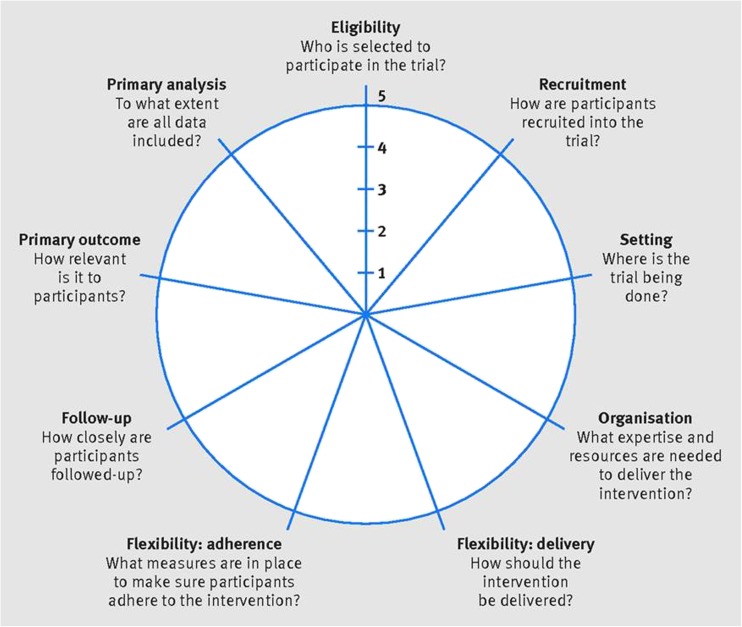
In order to rank interventions by their “real-world” applicability [33, 34], we rated each study in terms of pragmatism, as measured by PRECIS-2. PRECIS-2 contains nine domains (Fig. 1): participant eligibility criteria, participant recruitment, trial setting, organization of intervention delivery, flexibility of delivery, flexibility of adherence, participant follow-up, primary analysis, and relevance of primary outcome [32]. The details of the PRECIS-2 rating scale, including pragmatic and explanatory extremes of each domain, have been described extensively elsewhere [32]. We adapted an existing rating tool that was used to assess PRECIS domains in a prior review article [33]. Our rationale was that highly pragmatic clinical trials more closely mimic real-world circumstances in terms of design. As mentioned earlier, the PRECIS-2 model is a revised version of the original PRECIS tool designed by Thorpe et al. to provide a means of evaluating a study design in terms of pragmatism [31].
Fig. 1.
Factors measured by PRECIS-2 wheel. PRECIS-2 Pragmatic-Explanatory Continuum Indicator Summary 2. Reproduced with permission from BMJ Publishing Group Ltd. from “The PRECIS-2 tool: designing trials that are fit for purpose”; K Loudon, S Treweek, F Sullivan, P Donnan, KE Thorpe, M Zwarenstein; 350:h2147; 2015
Because the PRECIS-2 tool is not the only tool to assess the translation potential for clinical trials, we also assessed other pragmatic factors based on the widely used Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) framework. RE-AIM was designed with the intention of increasing reporting on the robustness, translatability, and public health impact of healthcare-related trials [33, 34]. We identified important practical feasibility factors derived from the RE-AIM framework that are not represented in the PRECIS-2 domains. These practical feasibility factors that we identified include reports of participant engagement, adaptation/change of intervention, program sustainability, unintended effects of intervention, and monetary costs of intervention [32–34]. We adapted an existing RE-AIM rating tool that was used to assess these practical feasibility factors in a prior review article [33]. Scoring for both RE-AIM and PRECIS-2 factors was assessed from the perspective of a patient-centered medical home (PCMH), rather than a traditional primary care clinic, as the PCMH model of care is rapidly spreading [35] and provides a model for population health teams to deliver behavior change interventions more optimally than traditional primary care practices [36, 37].
We also extracted study data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards of patient population and study outcomes [38]. One author (KAL or IML) extracted the key contextual components of each study. To ensure uniformity, the same author extracted the information from all studies for each PRISMA criterion. In addition to the PRISMA standards, we further extracted data regarding other external validity factors that may relate to future implementation decisions, such as the level of training of interventionists, the clinical setting, whether PA tracking was incorporated, the use of electronic health/mobile health resources, and whether interventions effectively improved PA outcomes and HbA1c outcomes, respectively.
Data synthesis and analysis
For the studies that met our inclusion/exclusion criteria, each was scored independently for the PRECIS-2 domains and RE-AIM practical feasibility factors by at least two raters (KAL, IML, ALN, JCM, AGH). To ensure clarity of the scoring rubric and calibration of raters, the five raters performed two iterative cycles of review of other RCTs by the PRECIS-2 and RE-AIM practical feasibility factors. During the iterative cycles of review, any areas of discrepancy were resolved through team discussions with senior co-authors (JGR, ALD, REG). In addition, when dual rater scores differed by >1 point on any given PRECIS-2 domain/RE-AIM factor, the raters reassessed that domain/factor together and came to consensus on scores that differed by no more than 1 point. A third rater arbitrated any ratings that still differed by >1 point.
For each intervention (n = 46), we averaged the raters’ scores to calculate mean numerical scores for each domain/factor. We averaged the mean score of all nine PRECIS-2 domains to create a composite PRECIS-2 score that we used to rank the interventions by tertiles. Across all interventions, we also assessed the prevalence of reporting on practical feasibility factors and the mean score averaged across all practical feasibility factors.
We defined effective interventions as those that led to a statistically significant increase in a valid PA behavior outcome over the intervention period in the intervention group, as compared to the control group (P < 0.05). We also defined separately the interventions that improved HbA1c in the intervention vs. control groups (P < 0.05). A single author extracted the information on intervention effectiveness from the relevant publication, and a second author confirmed this information (KAL, IML).
We used logistic regression (SAS software version 9.4) to conduct a post hoc analysis of the relationship between PRECIS-2 scores and PA outcomes across all RCTs, considering the PRECIS-2 score as the independent variable and the PA outcome measure as the dichotomous dependent variable (yes/no for statistically significant change in PA). We used the identical method in a separate regression model to compare the relationship between PRECIS-2 and HbA1c outcomes across all RCTs.
RESULTS
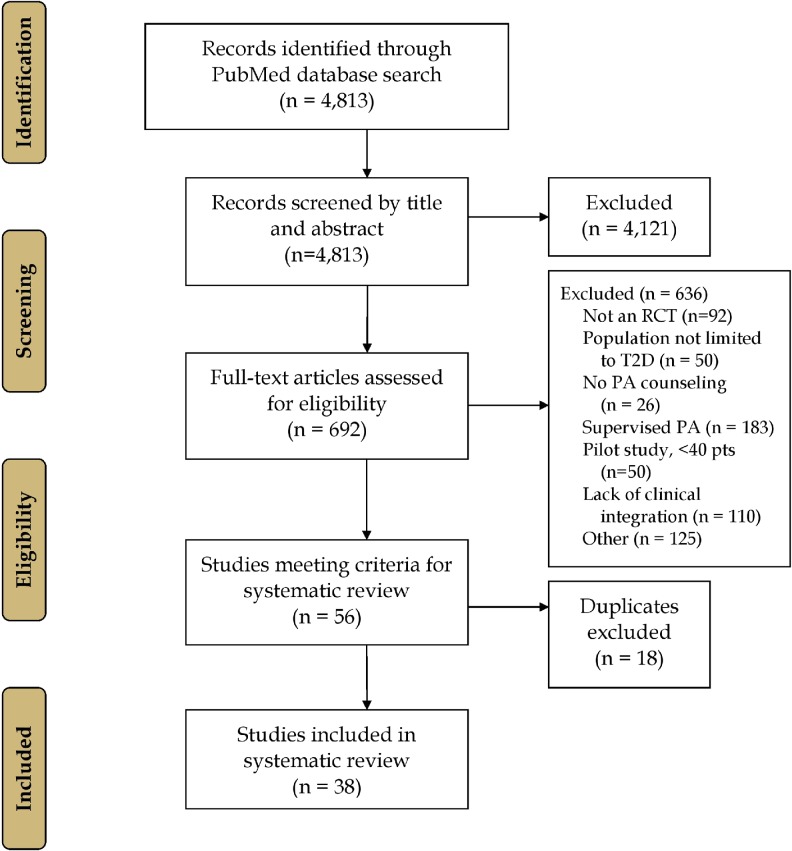
The PubMed literature search revealed 4813 citations (Fig. 2). After screening by title and abstract, 4121 articles were excluded, leaving 692 full-text articles that were considered for inclusion. Of these, 651 did not meet the study inclusion criteria. The most common reasons for exclusion were supervised PA (183), lack of clinical integration (110), and study design other than RCT (92). The final RCTs selected (n = 38) represented 46 independent interventions, as some trials tested >1 intervention [14, 39–72]. The final RCTs selected were published between 1996 and 2015, as we did not identify any articles meeting our inclusion criteria that were published from 1980 to 1995.
Fig. 2.
Study flow diagram
PRECIS-2 scores
The composite PRECIS-2 score for each of the 46 interventions ranged from 3.0 to 4.8. The top tertile of interventions (n = 16) had composite PRECIS-2 scores ≥4.08. Mean scores varied across the nine PRECIS-2 domains: we found less pragmatic scores for PRECIS-2 domains of organization (3.12 [0.82]) and setting (3.24 [1.18]) and higher PRECIS-2 domain scores for flexibility of adherence (4.62 [0.50]) and primary analysis (4.83 [0.63]), with values expressed as (mean [SD]).
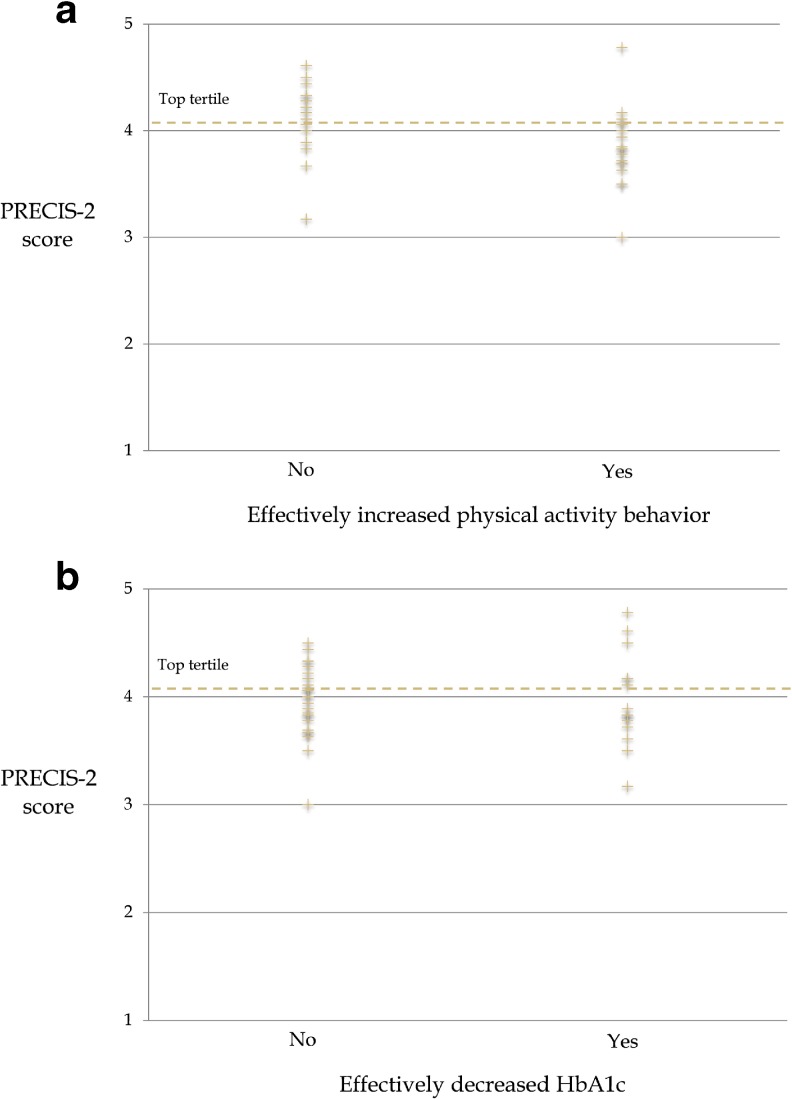
The majority of interventions studied (52.9%) reported effective improvements in PA outcomes. However, in the top tertile of interventions (n = 16) ranked by PRECIS-2 score, only 30.8% of interventions reported effective improvements in PA outcomes (Fig. 3a). As illustrated in Fig. 3a, our post hoc analysis suggested a possible inverse relationship between level of pragmatism by PRECIS-2 and PA effectiveness outcomes, albeit not statistically significant (P = 0.06). Just over one third of interventions studied (37.5%) effectively decreased HbA1c. In the top PRECIS-2 tertile, 31.3% of interventions effectively decreased HbA1c (Fig. 3b). The relationship between PRECIS-2 score and HbA1c effectiveness was not significant (P = 0.92).
Fig. 3.
PRECIS-2 score is plotted separately with regard to the RCT intervention effectiveness for physical activity (a) and hemoglobin A1c (b), respectively. PRECIS-2 Pragmatic-Explanatory Continuum Indicator Summary 2
Practical feasibility scores derived from RE-AIM
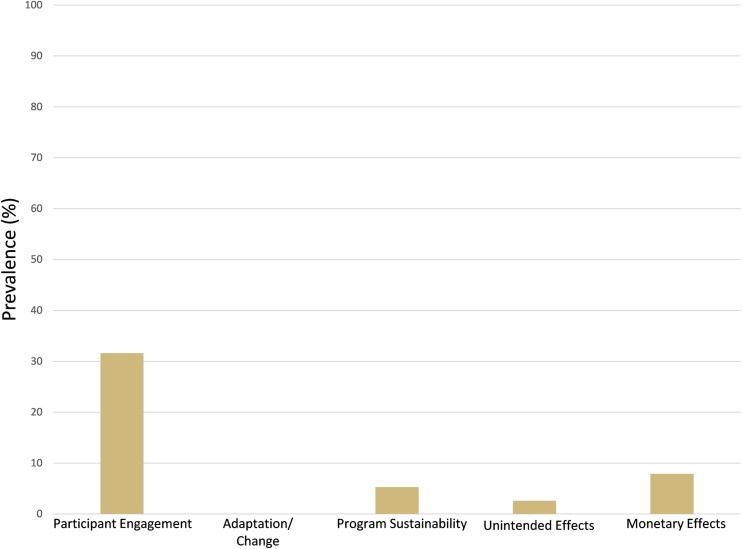
The majority of studies (62%) did not report on any of the practical feasibility factors that we assessed and thus received the lowest score of 1. Of the 38% of studies that reported on at least one practical feasibility domain, rates of reporting were highest for participant engagement (32%) and monetary costs of intervention (8%) (Appendix Fig. 5). No study authors reported on adaptation/change of the intervention to accommodate to clinical site needs or on unintended effects of the intervention. The composite practical feasibility for each intervention (n = 46) ranged from 1 to 2.2 on a possible five-point scale.
Fig. 5.
Prevalence of studies reporting on RE-AIM practical feasibility factors
Intervention characteristics
Given this review’s focus on identifying RCTs of therapies that may be translated into clinical practice, we report on both the typical PRISMA intervention characteristics as well as additional factors related to external validity (see Table 1 for top tertile and Appendix Table 3 for bottom two tertiles). Of the 46 interventions included, 38% used PA tracking and/or feedback methods as an intervention strategy. The PA tracking/feedback methods used were diverse, ranging from web-based communication to one-on-one visits with study personnel to review PA diaries. Participants were given pedometers, accelerometers, or other unspecified “activity monitors” to use for PA tracking in 21% of interventions. Only 11% of interventions utilized electronic health (eHealth) modalities such as the internet or mobile phones as part of the intervention. Studies took place in more than 10 different countries between 1990 and 2015, and the study duration ranged from 2.8 months to 8 years.
Table 1.
Characteristics related to external validity for trials in PRECIS-2 top tertile
| First author, year, location | Participants (n), age, females (%), non-white (%), HbA1c (mean) | Clinical setting | Intervention duration, intervention frequency, follow-up | Intervention staff type, level of training | |
| Sperl-Hillen (IE) et al., 2011, USA | I: n = 246, 61.6 yr., 49.4% f, 37.3% n-w, A1c—NR C: n = 134, 63.3 yr., 46.3% f, 32.8% n-w, A1c—NR |
2 large medical groups in NM and MN | Duration: 3.8 mo. Frequency: Three monthly 1-h sessions Follow-up: 6.8 mo. |
CDEs (RNs and RDs) | |
| Nielsen et al., 2006, Denmark | I: n = 459, 65.5 yr., 47.6% f, NR n-w, A1c—10.2% C: n = 415, 65.3 yr., 46.9% f, NR n-w, A1c—10.2% |
187 solo practices and 124 group practices | Duration: 6 yrs. Frequency: consultations every 3 mo. Follow-up: 6 yrs. |
GPs | |
| Sperl-Hillen (GE) et al., 2011, USAa | I: n = 243, 61.2 yr., 51.0% f, 33.5% n-w, A1c—NR C: n = 134, 63.3 yr., 46.3% f, 32.8% n-w, A1c—NR |
2 large medical groups in NM and MN | Duration: 2.8 mo. Frequency: Four 2-h sessions at 1-wk. intervals Follow-up: 6.8 mo. |
CDEs (RNs and RDs) | |
| Adachi et al., 2013, Japan | I: n = 100, 60.4 yr., 55% f, NR n-w, A1c—NR C: n = 93, 62.3 yr., 58% f, NR n-w, A1c—NR |
Multiple clinics in Kanagawa prefecture, Japan | Duration: 6 mo. Frequency: 3–4 sessions Follow-up: 6 mo. |
Trained RDs | |
| Babamoto (CM) et al., 2009, USA | I: n = 60, 50.0 yr., 52% f, NR n-w, A1c—8.5% C: n = 54, 50.0 yr., 78% f, NR n-w, A1c—9.5% |
3 inner city family health centers in Los Angeles, CA | Duration: 6 mo. Frequency: “usually seen on a monthly basis,” follow-up calls “as determined by the case manager” Follow-up: 6 mo. |
Two linguistically competent and culturally sensitive RNs | |
| Davies et al., 2008, England and Scotland | I: n = 437, 59.0 yr., 47% f, 6% n-w, A1c—8.3% C: n = 387, 60.0 yr., 43% f, 6% n-w, A1c—7.9% |
13 primary care sites | Duration: 12 mo. Frequency: One 6-h session delivered once at diagnosis Follow-up: 12 mo. |
Registered healthcare professionals | |
| Mash et al., 2014, South Africa | I: n = 710, 55.8 yr., 71.5% f, NR n-w, A1c—8.9% C: n = 860, 56.4 yr., 75.7% f, NR n-w, A1c—9.3% |
34 community health centers in the working class areas of Cape Town Metropole, South Africa | Duration: approximately 6 mo. Frequency: Four 60-min sessions Follow-up: 12 mo. |
Health promoters recruited from district health services and trained over 6 days | |
| Shibayama et al., 2007, Japan | I: n = 67, 61.0 yr., 34.8% f, NR n-w, A1c—7.3% C: n = 67, 62.0 yr., 34.8% f, NR n-w, A1c—7.4% |
OP clinic, Department of Diabetes and Metabolism, University of Tokyo Hospital | Duration: 1 yr. Frequency: monthly counseling Follow-up: 1 yr. |
CEN in diabetes nursing | |
| Irwig et al., 2012, USA | I: n = 52, 54.8 yr., 50% f, 86.5% n-w, A1c—10.2% C: n = 51, 57.2 yr., 56.9% f, 74.5% n-w, A1c—9.7% |
Single academic multi-specialty clinic | Duration: 9 mo. Frequency: every 3 mo. (total of 4 visits) Follow-up: 9 mo. |
MDs (2 endocrinologists and 3 internists) | |
| Jansink et al., 2013, Netherlands | I: n = 229, 64.1 yr., 44.1% f, NR n-w, A1c—7.8% C: n = 292, 63.9 yr., 46.0% f, NR n-w, A1c—7.7% |
58 general practices | Duration: 14 mo. Frequency: Four half-day training sessions spread over 6 mo; also received quarterly phone calls Follow-up: 14 mo. |
Primary care nurses | |
| De Greef et al. (GP), 2011, Belgium | I: n = 22, 66.6 yr., 22.7% f, NR n-w, A1c—7.23% C: n = 24, 66.0 yr., 29.2% f, NR n-w, A1c—7.0% | Three Belgian general practices | Duration: 12 wks. Frequency: Three individual 15-min face-to-face consultations Follow-up: 12 wks. |
GPs | |
| Christian et al., 2008, USA | I: n = 155, 53.0 yr., 65% f, 100% n-w, A1c—8.08% C: n = 155, 53.4 yr., 68% f, 100% n-w, A1c—8.29% |
2 large urban community-based health centers in CO | Duration: 12 mo. Frequency: visits every 3 mo. Follow-up: 12 mo. |
MDs | |
| Trento et al., 2010, Italy | I: n = 421, 69.0 yr., 52.0% f, NR n-w, A1c—7.75% C: n = 394, 69.6 yr., 46.4% f, NR n-w, A1c—7.81% |
13 hospital-based diabetes clinics | Duration: 4 yrs. Frequency: 7 1-h group care sessions every 3 months over 2 yr. and repeated; individual consultations at least yearly Follow-up: 4 yrs. |
MDs and an educationist (M.T.) | |
| Lee et al., 2011, Hong Kong | I: n = 84, NR yr., 60.9% f, NR n-w, A1c—8.18% C: n = 73, NR yr., 63.1% f, NR n-w, A1c—8.04% |
General OP clinic, Hospital Authority New Territory East Cluster of Hong Kong | Duration: 6 wks. Frequency: weekly 2.5-h DM self-management course Follow-up: 28 wks. | Social worker accredited as a trainer for the self-management program | |
| DiLoreto et al., 2003, Italy | I: n = 182, 62.0 yr., 51.6% f, NR n-w, A1c—7.6% C: n = 158, 61.6 yr., 53.8% f, NR n-w, A1c—7.7% | OP diabetes clinic | Duration: 2 yrs. Frequency: initial 30-min counseling session, home phone call 1 mo. later; 15-min appointments in clinic every 3 mo. Follow-up: 2 yrs. |
MDs | |
| Glasgow (CASM) et al., 2012, USA | I: n = 169, 58.7 yr., 44.6% f, 25.9% n-w, A1c—NR C: n = 132, 58.7 yr., 51.5% f, 29.4% n-w, A1c—8.16% |
Five primary care clinics within Kaiser Permanente Colorado | Duration: 12 mo. Frequency: initial website orientation; action plan at 6 wks; periodic motivational calls Follow-up: 12 mo. |
Research staff member | |
| First author, year, location | PA tracking method/feedback provided | eHealth | Control group | Effectiveness (PA), effectiveness (HbA1c) | PRECIS-2 score |
| Sperl-Hillen (IE) et al., 2011, USA | N/A | N/A | Usual care | PA: yes HbA1c: yes | 4.78 |
| Nielsen et al., 2006, Denmark | N/A | N/A | Usual care | PA: no HbA1c: yes | 4.61 |
| Sperl-Hillen (GE) et al., 2011, USAa | N/A | N/A | Usual care | PA: no HbA1c: no | 4.50 |
| Adachi et al., 2013, Japan | N/A | N/A | General advice from RDs | PA: NR HbA1c: yes | 4.50 |
| Babamoto (CM) et al., 2009, USA | N/A | N/A | Usual care | PA: no HbA1c: no | 4.44 |
| Davies et al., 2008, England and Scotland | N/A | N/A | Enhanced standard care | PA: no HbA1c: no | 4.33 |
| Mash et al., 2014, South Africa | N/A | N/A | Usual care | PA: no HbA1c: no | 4.33 |
| Shibayama et al., 2007, Japan | Feedback provided at monthly sessions | N/A | Usual care | PA: NR HbA1c: no | 4.28 |
| Irwig et al., 2012, USA | N/A | N/A | Completed questionnaires only | PA: no HbA1c: no | 4.28 |
| Jansink et al., 2013, Netherlands | N/A | N/A | Usual care | PA: no HbA1c: no | 4.22 |
| De Greef et al. (GP), 2011, Belgium | Pedometers used to track progress and to encourage discussions with the GP | N/A | Usual care | PA: no HbA1c: no | 4.17 |
| Christian et al., 2008, USA | Results from computer-based assessment used to tailor intervention | “Computer expert system” | Usual care | PA: yes HbA1c: no | 4.17 |
| Trento et al., 2010, Italy | N/A | N/A | Usual care | PA: NR HbA1c: yes | 4.17 |
| Lee et al., 2011, Hong Kong | N/A | N/A | Usual care | PA: no HbA1c: no | 4.11 |
| DiLoreto et al., 2003, Italy | Phone calls at 1 month to determine level of PA and reinforce instructions | N/A | Usual care | PA: yes HbA1c: yes | 4.11 |
| Glasgow (CASM) et al., 2012, USA | Participants recorded progress on daily goals and received feedback using website | “My Path toHealthy Life” (Mi Camino a La Vida Sana) website | Enhanced usual care | PA: yes HbA1c: no | 4.08 |
C control group, CDE(s) certified diabetes educator(s), hr./hrs. hour/hours, I intervention group, MD(s) doctor(s), mo. month/months, N/A not applicable, NR not reported, OP outpatient, PA physical activity, PCP(s)/GP(s) primary care physician(s)/general practitioner(s), RD(s) registered dietitian(s), RN(s) registered nurse(s), wk./wks. week/weeks, yr./yrs. year/years
aA secondary intervention from an already noted study
Table 3.
Characteristics related to external validity for trials in PRECIS-2 bottom two tertiles
| First author, year, location | Participants (n), age, females (%), non-white (%), HbA1c (mean) | Clinical setting | Intervention duration, intervention frequency, follow-up |
| Middle tertile | |||
| Babamoto (CHW) et al., 2009, USAa | I: n = 75, 51 yr., 64% f, NR n-w, A1c—8.6% C: n = 54, 50 yr., 78% f, NR n-w, A1c—9.5% |
3 inner city family health centers in Los Angeles, CA | Duration: 6 mo. Frequency: 11.3 sessions (in person and telephone contact) Follow-up: 6 mo. |
| De Greef (Group) et al., 2011, Belgiuma | I: n = 21, 70.0 yr., 38.1% f, NR n-w, A1c—7.12% C: n = 24, 66.0 yr., 29.2% f, NR n-w, A1c—7.00% |
Three Belgian general practices | Duration: 12 wks. Frequency: three 90-min group counseling sessions Follow-up: 12 wks. |
| Van der Weegen (SSP w/ tool) et al., 2015, Netherlands | I: n = 65, 57.5 yr., 52.3% f, NR n-w, A1c—NR C: n = 68, 59.2 yr., 54.4% f, NR n-w, A1c—NR |
24 general practices in the south of the Netherlands | Duration: 6 mo. Frequency: 4 consultations with practice nurse occurring during first wk., after 2 wks., after 8–12 wks., and after 16–24 wks. Follow-up: measured at baseline after the intervention (4–6 mo.), and 3 mo. thereafter |
| Keyserling (Group B) et al., 2002, USA | I: n = 66, 59.8 yr., 100% f, 100% n-w, A1c—11.1% C: n = 67, 59.2 yr., 100% f, 100% n-w, A1c—11.3% |
Primary care practices in central NC (5 community health centers, 1 staff model health maintenance organization, 1 general medicine clinic at an academic health center) | Duration: 6 mo. Frequency: 4 monthly visits Follow-up: 12 mo. |
| Van der Weegen (SSP) et al., 2015, Netherlandsa | I: n = 66, 56.9 yr., 47.0% f, NR n-w, A1c—NR C: n = 68, 59.2 yr., 54.4% f, NR n-w, A1c—NR |
24 general practices in the south of the Netherlands | Duration: 6 mo. Frequency: 4 consultations with practice nurse occurring during first wk., after 2 wks., after 8–12 wks., and after 16–24 wks. Follow-up: measured at baseline after the intervention (4–6 mo.), and 3 mo. thereafter |
| Edelman et al., 2015, USA | I: n = 193, 57.8 yr., 54.4% f, 50.8% n-w, A1c—9.2% C: n = 184, 59.6 yr., 54.9% f, 49.5% n-w, A1c—9.0% |
9 primary care practices in the Duke Clinical Research Institute Primary Care Research Consortium | Duration: 2 yrs. Frequency: phone calls every 2 mo. (12 total) Follow-up: 2 yrs. |
| Naik et al., 2011, USA | I: n = 45, 63.82 yr., NR f, 33.3% n-w, A1c—8.86% C: n = 42, 63.45 yr., NR f, 28.6% n-w, A1c—8.74% |
Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas | Duration: 3 mo. Frequency: 4 group sessions every 3 wks. Follow-up: 3 mo. |
| Glasgow (CASM+) et al., 2012, USAa | I: n = 162, 58.7 yr., 53.7% f, 29.3% n-w, A1c—NR C: n = 132, 58.7 yr., 51.5% f, 29.4% n-w, A1c—8.16% |
Five primary care clinics within Kaiser Permanente Colorado | Duration: 12 mo. Frequency: three 120-min group sessions after week 6; follow-up telephone calls at wks. 2 and 8; periodic motivational calls Follow-up: 12 mo. |
| Francosi et al., 2011, Italy | I: n = 46, 48.9 yr., 30.4% f, NR n-w, A1c—7.9% C: n = 16, 48.7 yr., 12.5% f, NR n-w, A1c—7.9% |
3 OP diabetes clinics | Duration: 6 mo. Frequency: Face-to-face encounter every 3 mo. with diabetes nurses and additional telephone contact monthly Follow-up: 6 mo. |
| Maindal et al., 2014, UK | I: n = 322, 62.0 yr., 47.2% f, NR n-w, A1c—6.3% C: n = 187, 62.0 yr., 46.0% f, NR n-w, A1c—6.2% |
33 general practices in a large Danish county | Duration: 3 mo. Frequency: 2 individual counseling interviews and 8 group sessions, totaling 18 h. Follow-up: 3 yrs. |
| Jarab et al., 2012, Jordan | I: n = 85, 63.4 yr., 42.4% f, NR n-w, A1c—8.5% C: n = 86, 65.3 yr., 44.2% f, NR n-w, A1c—8.4% |
OP diabetes clinic at the 762-bed Royal Medical Services Hospital | Duration: 8 wks. Frequency: baseline face-to-face objective directed education and weekly telephone follow-up calls Follow-up: 6 mo. |
| Schillinger (ATSM) et al., 2009, USA | I: n = 112, 55.9 yr., 58.0% f, 87.5% n-w, A1c—9.3% C: n = 114, 55.8 yr., 55.3% f, 93.8% n-w, A1c—9.8% |
9 clinics in the San Francisco Department of Public Health’s Community Health Network (CHNSF) | Duration: 9 mo. Frequency: weekly automated phone calls w/ follow-up calls from care manager if necessary Follow-up: 1 yr. |
| Trento et al., 2008, Italy | I: n = 25, 64.6 yr., 48.0% f, NR n-w, A1c—8.0% C: n = 24. 68.1 yr., 33.3% f, NR n-w, A1c—8.0% |
Department of Internal Medicine, University of Turin | Duration: 2 yrs. Frequency: 40–50 min. Group sessions very 3–4 mo. Follow-up: 2 yrs. |
| Trento et al., 2002, Italy | I: n = 56, 62.0 yr., 51.8% f, NR n-w, A1c—7.4%’ C: n = 56, 61.0 yr., 39.3% f, NR n-w, A1c—7.4% |
Diabetes clinic, Department of Internal Medicine, University of Turin | Duration: 4 yrs. Frequency: group educational sessions once every 3 mo. for 2 yrs.; 7 sessions spread over yrs. 3–4 Follow-up: 4 yrs. |
| Barratt et al., 2008, UK | I: n = 27, NR yr., NR f, NR n-w, A1c—9.6% C: n = 26, NR yr., NR f, NR n-w, A1c—9.7% |
Two tertiary hospitals in southeast England | Duration: 6 mo. Frequency: one 90-min. session and five 30-min. sessions Follow-up: 6 mo. |
| Bottom tertile | |||
| Kim (PM) et al., 2006, South Korea | I: n = 22, NR yr., NR f, NR n-w, A1c—7.51% C: n = 23, NR yr., NR f, NR n-w, A1c—7.87% |
OP diabetes clinic at a large university hospital in South Korea | Duration: 12 wks. Frequency: 2× in first 2 wks., again at 6 wks. Follow-up: 12 wks. |
| Kim (WB) et al., 2006, South Koreaa | I: n = 28, NR yr., NR f, NR n-w, A1c—7.99% C: n = 23, NR yr., NR f, NR n-w, A1c—7.87% |
OP diabetes clinic at a large university hospital in South Korea | Duration: 12 wks. Frequency: 2× in first 2 wks., again at 6 wks. Follow-up: 12 wks. |
| Keyserling (Group A) et al., 2002, USAa | I: n = 67, 58.5 yr., 100% f, 100% n-w, A1c—10.8% C: n = 67, 59.2 yr., 100% f, 100% n-w, A1c—11.3% |
Primary care practices in central NC (5 community health centers, 1 staff model health maintenance organization, 1 general medicine clinic at an academic health center) | Duration: 12 mo. Frequency: 4 monthly visits, 3 group sessions, and 4 monthly phone calls Follow-up: 12 mo. |
| Schillinger (GMV) et al., 2009, USAa | I: n = 113, 56.5 yr., 63.7% f, 91.1% n-w, A1c—9.4% C: n = 114, 55.8 yr., 55.3% f, 93.8% n-w, A1c—9.8% |
9 clinics in the San Francisco Department of Public Health’s Community Health Network (CHNSF) | Duration: 9 mo. Frequency: monthly group meetings (90 min. each) Follow-up: 1 yr. |
| Anderson et al., 2010, USA | I: n = 146, NR yr., 58.9% f, 72.6% n-w, A1c—7.6% C: n = 149, NR yr., 57.1% f, 73.8% n-w, A1c—8.4% |
2 largest Community Health Center, Inc. locations in CT | Duration: 1 yr. Frequency: weekly/bi-weekly/monthly (depending on risk stratification) Follow-up: 1 yr. |
| Lim et al., 2015, South Korea | I: n = 50, 64.3 yr., 20% f, NR n-w, A1c—8.1% C: n = 50, 65.8 yr., 30% f, NR n-w, A1c—7.9% |
OP clinic, Bundang Hospital, Seoul National University | Duration: 6 mo. Frequency: 1-h diet and exercise counseling at baseline, 3, and 6-month visits Follow-up: 6 mo. |
| Thoolen et al., 2009, Netherlands | I: n = 78, 62.0 yr., 36.0% f, NR n-w, A1c—NR C: n = 102, 61.9 yr., 45.0% f, NR n-w, A1c—NR |
General practices in southwest Netherlands | Duration: 12 wks. Frequency: two individual and four group sessions (2 h./session) with nurse Follow-up: 3 and 12 mo. |
| Taylor et al., 2003, USA | I: n = 84, 55.5 yr., 50% f, 33.3% n-w, A1c—9.5% C: n = 85, 54.8 yr., 44.7% f, 43.5% n-w, A1c—9.5% |
Kaiser Permanente Medical Center in Santa Clara, CA | Duration: 1 yr. Frequency: 90 min. RN consultation; weekly 1- to 2-h group classes for 4 wks; telephone follow-up before 4th group session and at wks. 5, 8, 12, 16, 20, 28, 36, and 44 (∼15 mins./call) Follow-up: 1 yr. |
| Moriyama et al., 2009, Japan | I: n = 42, 66.4 yr., 59.5% f, NR n-w, A1c—7.5% C: n = 23, 65.2 yr., 43.5% f, NR n-w, A1c—7.4% |
2 hospitals with less than 200 beds in a Japanese city | Duration: 1 yr. Frequency: monthly ∼30-min. sessions; phone calls from nurse educator every 2 wks. Follow-up: 1 yr. |
| Van Dyck et al., 2011, Belgium | I: n = 60, 62.37 yr., NR f, NR n-w, A1c—NR C: n = 32, 60.59 yr., NR f, NR n-w, A1c—NR |
Endocrinology Department of the Ghent University Hospital | Duration: 24 wks. Frequency: face-to-face 30-min. session with psychologist; phone call every 2 wks. For first month, every 4 wks. For next 20 wks. (7 calls, ∼20 mins. each) Follow-up: 1 yr. |
| Kim et al., 2011, South Korea | I: n = 21, 56.62 yr., 47.6% f, 100% n-w, A1c—7.40% C: n = 22, 54.68 yr., 31.8% f, 100% n-w, A1c—7.41% |
OP diabetic center at a large university hospital in South Korea | Duration: 16 wks. Frequency: 60–90-min. initial counseling session; 30–40-min. follow-up counseling session every 2 mo; 10–30-min. weekly telephone calls Follow-up: 16 wks. |
| Sone et al., 2010, Japan | I: n = 1017, 58.5 yr., 46.0% f, NR n-w, A1c—7.8% C: n = 1016, 58.6 yr., 47.0% f, NR n-w, A1c—7.9% |
59 university and general hospitals that specialize in diabetes care | Duration: 8 yrs. Frequency: 15-min. telephone counseling sessions at least once every 2 wks; 5–10-min. extra during each clinic visit Follow-up: 7.8 yrs. |
| Uusitupa et al., 1996, Finland | I: n = 38, NR yr., 44.7% f, NR n-w, A1c—8.4% C: n = 40, NR yr., 40.0% f, NR n-w, A1c—9.0% |
OP clinic of Department of Medicine, Kuopio University Hospital | Duration: 1 yr. Frequency: six visits to OP clinic at 2-mo. intervals Follow-up: 1 yr. |
| Gaede et al., 1999, Denmark | I: n = 80, 54.9 yr., 21.3% f, NR n-w, A1c—8.4% C: n = 80, 55.2 yr., 30.0% f, NR n-w, A1c—8.8% |
Steno Diabetes Center | Duration: approximately 4–5 yrs., or when study endpoint was reached Frequency: every 3-mo. follow-up: At 2 and 4 yrs. |
| Huffman et al., 2010, USA | I: n = 38, NR yr., 0% f, NR n-w, A1c—NR C: n = 46, NR yr., 0% f, NR n-w, A1c—NR |
Durham Veterans Affairs Medical Center in NC | Duration: 1 yr. Frequency: baseline counseling session; 3 biweekly phone calls over first 6 wks., monthly phone calls for study duration Follow-up: 1 yr. |
| First author, year, location | Intervention staff type, level of training | PA tracking method/feedback provided | eHealth |
| Middle tertile | |||
| Babamoto (CHW) et al., 2009, USAa | CHWs | N/A | N/A |
| De Greef (Group) et al., 2011, Belgiuma | Clinical psychologist with a background in behavior change strategies | Pedometers used to track progress and to encourage discussions with the behavioral expert | N/A |
| Van der Weegen (SSP w/ tool) et al., 2015, Netherlands | Practice nurse trained in delivery of the intervention | Participants used activity monitors to track activity on mobile phone and web app. Personal activity goals set based on dialog sessions and activity results | It’s LiFe! monitoring and feedback tool consisting of a three-dimensional activity monitor, a mobile phone app, and a web app |
| Keyserling (Group B) et al., 2002, USA | Nutritionists | N/A | N/A |
| Van der Weegen (SSP) et al., 2015, Netherlandsa | Practice nurse trained in delivery of the intervention | Participants recorded PA in diaries. They discussed their progress and made individual goals during sessions with practice nurses. | N/A |
| Edelman et al., 2015, USA | RN with extensive experience in case management | N/A | N/A |
| Naik et al., 2011, USA | PCPs trained in goal setting and action planning methodology | Participants received feedback on specific goals during one-on-one sessions with PCP | N/A |
| Glasgow (CASM+) et al., 2012, USAa | “Research project staff member,” diabetes care coordinator, nutritionist, bilingual family MD | Participants recorded progress on daily goals and received feedback using website. Patients also received follow-up calls at 2 and 8 weeks to discuss the their action plans | “My Path to Healthy Life” (Mi Camino a La Vida Sana) website |
| Francosi et al., 2011, Italy | Diabetes RNs trained in diabetes care during a 1-day session | Feedback provided during monthly phone calls | N/A |
| Maindal et al., 2014, UK | RNs, RDs, physiotherapists, and GPs who received formal training in autonomy support, participant-centered communication and action plan support | N/A | N/A |
| Jarab et al., 2012, Jordan | MDs and pharmacists | N/A | N/A |
| Schillinger (ATSM) et al., 2009, USA | Nurse care manager | Participant responses to automated phone calls triggered automated health education messages and/or nurse phone follow-up | N/A |
| Trento et al., 2008, Italy | RD and RNs trained in management of T2D | N/A | N/A |
| Trento et al., 2002, Italy | MDs and an educationist | N/A | N/A |
| Barratt et al., 2008, UK | RDs | Progress on personal goals discussed at each appointment | N/A |
| Bottom tertile | |||
| Kim (PM) et al., 2006, South Korea | Research RNs | PA frequency, intensity, and duration monitored weekly by diaries and kcal-pedometer. Research nurses provided feedback at clinic visits or with phone calls | N/A |
| Kim (WB) et al., 2006, South Koreaa | Research RNs | PA frequency, intensity, and duration monitored weekly by diaries and kcal-pedometer. Research nurses provided feedback at clinic visits or with phone calls. Web-based psychological and physical readiness questionnaires used to assess current stages of PA | Web site included stage-based personalized sections on goal setting, activity planning, determining target heart rates, and questionnaires |
| Keyserling (Group A) et al., 2002, USAa | Community diabetes advisors and nutritionists | N/A | N/A |
| Schillinger (GMV) et al., 2009, USAa | PCP and health educator | N/A | N/A |
| Anderson et al., 2010, USA | RNs trained in intervention delivery | N/A | N/A |
| Lim et al., 2015, South Korea | RD, exercise specialist, exercise physiologist | PA monitor results linked to the main server to provide tailored service | Dedicated website containing a glucose control section, diet control section, physical activity section, and an integrated widget. PA monitors and glucometers linked with the u-healthcare system |
| Thoolen et al., 2009, Netherlands | RN | N/A | N/A |
| Taylor et al., 2003, USA | RN | N/A | N/A |
| Moriyama et al., 2009, Japan | Nurse educators | Nurse educators contacted participants every 2 weeks to monitor exercise goals | N/A |
| Van Dyck et al., 2011, Belgium | Psychologists | Participants asked to keep a pedometer diary to track progress and encourage discussion with psychologist | N/A |
| Kim et al., 2011, South Korea | RN/MD Researchers | Weekly exercise and dietary logs indicated frequency, duration, and kilocalories for energy expenditure by an accelerometer | N/A |
| Sone et al., 2010, Japan | MDs, RNs, RDs, psychotherapists, and other co-medical staff | Pedometers used for objective assessment of PA | N/A |
| Uusitupa et al., 1996, Finland | MDs, RNs, and nutritionists | PA monitored by daily activity records | N/A |
| Gaede et al., 1999, Denmark | MD, RN, and RD | N/A | N/A |
| Huffman et al., 2010, USA | Health counselors and PCPs | Pedometers used to track PA. Regular phone calls to check in with participants and adjust PA goals as needed. | N/A |
| First author, year, location | Control group | Effectiveness (PA); effectiveness (HbA1c) | PRECIS-2 score |
| Middle tertile | |||
| Babamoto (CHW) et al., 2009, USAa | Usual care | PA: no HbA1c: no | 4.06 |
| De Greef (Group) et al., 2011, Belgiuma | Usual care | PA: yes HbA1c: no | 4.06 |
| Van der Weegen (SSP w/ tool) et al., 2015, Netherlands | Usual care | PA: yes HbA1c: NR | 4.05 |
| Keyserling (Group B) et al., 2002, USA | Educational pamphlets sent by mail | PA: yes HbA1c: no | 4.00 |
| Van der Weegen (SSP) et al., 2015, Netherlandsa | Usual care | PA: no HbA1c: NR | 4.00 |
| Edelman et al., 2015, USA | Non-tailored phone calls | PA: no HbA1c: no | 3.89 |
| Naik et al., 2011, USA | “Traditional education intervention” | PA: NR HbA1c: yes | 3.89 |
| Glasgow (CASM+) et al., 2012, USAa | Enhanced usual care, including additional tailored web-based feedback on health behaviors | PA: yes HbA1c: no | 3.85 |
| Francosi et al., 2011, Italy | Standard counseling and routine follow-up | PA: NR HbA1c: yes | 3.83 |
| Maindal et al., 2014, UK | Usual care | PA: no HbA1c: no | 3.83 |
| Jarab et al., 2012, Jordan | Usual care | PA: yes HbA1c: yes | 3.83 |
| Schillinger (ATSM) et al., 2009, USA | Usual care | PA: yes HbA1c: no | 3.83 |
| Trento et al., 2008, Italy | Usual care | PA: NR HbA1c: yes | 3.83 |
| Trento et al., 2002, Italy | Usual care | PA: NR HbA1c: no | 3.81 |
| Barratt et al., 2008, UK | Usual care | PA: NR HbA1c: no | 3.78 |
| Bottom tertile | |||
| Kim (PM) et al., 2006, South Korea | Usual care | PA: yes HbA1c: yes | 3.78 |
| Kim (WB) et al., 2006, South Koreaa | General information sheet from the clinic. Participants visited the clinic once at the beginning of the intervention period | PA: yes HbA1c: yes | 3.72 |
| Keyserling (Group A) et al., 2002, USAa | Educational pamphlets sent by mail | PA: yes HbA1c: no | 3.69 |
| Schillinger (GMV) et al., 2009, USAa | Usual care | PA: no HbA1c: no | 3.67 |
| Anderson et al., 2010, USA | Usual care | PA: NR HbA1c: no | 3.67 |
| Lim et al., 2015, South Korea | Dedicated website containing a glucose control section and diet control section | PA: yes HbA1c: yes | 3.67 |
| Thoolen et al., 2009, Netherlands | Brochure on diabetes self-management | PA: yes HbA1c: NR | 3.63 |
| Taylor et al., 2003, USA | Educational pamphlets and instructions to continue regular care with PCP | PA: NR HbA1c: yes | 3.61 |
| Moriyama et al., 2009, Japan | Educational textbook and asked to consult their physician as usual | PA: NR HbA1c: yes | 3.56 |
| Van Dyck et al., 2011, Belgium | Usual care | PA: yes HbA1c: NR | 3.56 |
| Kim et al., 2011, South Korea | Usual care | PA: yes HbA1c: no | 3.50 |
| Sone et al., 2010, Japan | Usual care | PA: yes HbA1c: no | 3.46 |
| Uusitupa et al., 1996, Finland | Usual care | PA: no HbA1c: no | 3.29 |
| Gaede et al., 1999, Denmark | Usual care | PA: no HbA1c: yes | 3.17 |
| Huffman et al., 2010, USA | Usual care | PA: yes HbA1c: NR | 3.00 |
C control group, CHW(s) community health worker(s), hr./hrs. hour/hours, I intervention group, MD(s) doctor(s), min minute/minutes, mo. month/months, N/A not applicable, NR not reported, OP outpatient, PA physical activity, PCP(s)/GP(s) primary care physician(s)/general practitioner(s), RD(s) registered dietitian(s), RN(s) registered nurse(s), wk./wks. week/weeks, yr./yrs. year/years
aA secondary intervention from an already noted study
Risk of bias
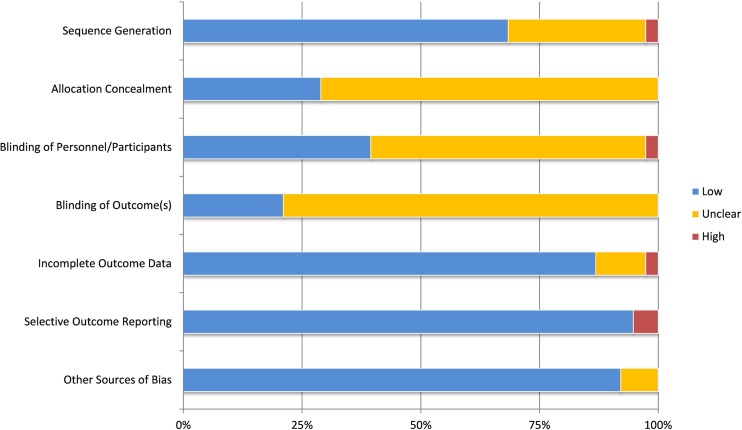
For each of the seven areas within the Cochrane risk of bias tool [73], two authors (IML and AGH) independently assessed the risk of bias for each included study. We resolved differences in reviewer ratings through discussion. In each of the seven categories, less than 6% of studies had a high risk of bias (Appendix Fig. 6). As no studies had a high risk of bias on all seven categories, we did not exclude any based on these bias risk assessments.
Fig. 6.
Prevalence of Cochrane risk of bias categories
CONCLUSIONS
This systematic review identified behavioral interventions that are feasible to implement in clinical practice settings and that effectively increased PA for people with T2D. The range of PRECIS-2 scores from 3.0 to 4.8 across the 46 interventions studied demonstrates a range of pragmatism from modestly pragmatic to highly pragmatic. It is of possible concern that the level of study pragmatism tended to be inversely related to improvements in PA, albeit of borderline statistical significance (P = 0.06). This suggests that PA interventions conducted in more real-world settings may be less effective when compared to studies in more idealized and heavily resourced contexts. Nevertheless, among the behavioral interventions that we ranked in the most pragmatic tertile by composite PRECIS-2 scores, four interventions by Sperl-Hillen et al. [39], Christian et al. [50], Di Loreto et al. [53], and Glasgow et al. [54] effectively improved PA outcomes—two of these four effective PA interventions also improved HbA1c outcomes [31, 45].
A key question that emerges from this review is how do we identify the moderators and key components of successful interventions that are highly pragmatic and translational? The existing literature on predictors of effective PA interventions has already identified several key factors that predict improved PA outcomes, including tracking/self-monitoring of PA, setting PA goals, increasing self-efficacy for PA, enlisting social support to be active, and utilizing behavioral theory-informed intervention techniques [74–76]. What remains unclear is how these evidence-based techniques may be optimally implemented with pragmatic approaches in clinical care. This review was not a meta-analysis and cannot statistically dissect the factors that moderated success among the four interventions in the top tertile by PRECIS-2 scores that also improved PA outcomes. Nevertheless, we did note some common themes among the four highly pragmatic and effective interventions that may be instructive. The majority of these interventions used PA tracking that allowed PA intervention content to be tailored to individual activity levels; interventions also tended to be delivered over a duration of 12 months or longer. Other than the regularity of PA tracking/feedback, the intervention approaches were fairly diverse, but commonly used simple tools to help participants and healthcare providers jointly identify and track behavior patterns and behavioral predictors of PA levels. These tools included a simplified form of in-person motivational interviewing [53], a computerized self-assessment that automatically generated individualized and tailored feedback [50, 54], or the use of a “conversation map” of diabetes self-management challenges [39].
While we already know much about what factors are necessary for effective interventions, we need to better understand how to ensure that these techniques are effective when delivered pragmatically in clinical settings. For example, both the effectiveness on PA/HbA1c outcomes and the pragmatism of Sperl-Hillen’s intervention were superior when delivered 1:1 rather than using a group visit delivery format [39]. This finding stands in stark contrast to the existing literature that suggests that group and individual diabetes self-management education are equivalent [77] and suggests that further pragmatic trials to assess the comparative effectiveness of individual and group self-management education of T2D in usual practice may be warranted. Sperl-Hillen’s findings from their pragmatic trial may suggest that individual interventions to improve T2D self-management are especially important when individual motivators and barriers are more heterogeneous, but another possibility is that this was a chance finding.
As another example, in the study conducted by De Greef et al., the effects of the same behavioral intervention were significantly different based on who delivered the intervention and the dose of intervention—a trained behavioral expert providing counseling over three separate 90-min sessions effectively increased PA but a primary care clinician providing the same counseling content over three separate 15-min clinic visits did not increase PA [49]. Other review articles have lamented the lack of data on fidelity of intervention delivery in RCTs and have noted this as a limitation to identifying key moderators of PA interventions [76]. The highly pragmatic and effective intervention by Di Loreto et al. that we studied in the present review balanced the competing clinical demands and fidelity to intervention techniques by condensing several effective behavioral change techniques into a simple yet tailored counseling checklist which identified enjoyable and appropriate PA options at the individual level and encouraged patients to continue with these programs through the use of social support, problem-solving, and PA tracking [45]. Another important component of this intervention that used the counseling checklist was to deliver the intervention directly after a 30-min clinical in-person assessment of T2D that allowed the opportunity to address competing clinical concerns immediately prior to delivering the behavioral counseling [53].
In recent years, the proliferation of mobile phone users coupled with the increasing popularity of PA tracking devices that sync with phones/computers have provided novel opportunities for eHealth applications. Diabetes researchers have also been encouraged by the promise of eHealth to enhance the reach and effectiveness of T2D self-management interventions, including PA interventions [78, 79]. Thus, we were intrigued to notice that only 6 of the 46 interventions that we studied utilized any measures related to eHealth [50, 54, 55, 64, 80]. However, it is important to note that most of the interventions we studied were conducted before the recent explosion of smartphone and wearable technology for tracking PA [81, 82]; in the coming years, these percentages may change considerably as more researchers begin to embrace eHealth. Our review covered a broad range of publication dates (1996–2015), which introduces a concern for any secular PA trends over that timeframe. PA guidelines for patients with type 2 diabetes over that time span did remain relatively unchanged, promoting PA of 30 min of moderate intensity on most days of the week (≥120 min/week) in 1996 and recommendations of 150 min of weekly moderate intensity activity in 2015. Perhaps more importantly, the increase in PA tracking use over the past decade may have allowed patients’ greater ability to simply self-monitor their PA levels. While they represented the minority of interventions that we studied, 100 and 40% of the aforementioned eHealth interventions improved PA and HbA1c outcomes, respectively. Although the eHealth interventions we studied uniformly improved PA outcomes, it is interesting to note that only two of the six eHealth interventions that we studied were in the top tertile by PRECIS-2 scores. To improve the pragmatism of future PA interventions using eHealth methods, one opportunity may be to enhance the linkage of PA tracking to existing electronic medical records [83]. This linking would allow clinicians and patients to more readily monitor physical activity levels and communicate regarding new activity goals. As wearable PA tracking devices and technology use continuously expand [81, 82], this is an area that will see growth and constant adaptations year after year.
To enhance the translation of effective studies into real-world settings, it is also important for researchers to report on outcomes other than effectiveness that will influence future implementation. The PRECIS-2 tool incorporates many outcomes that predict the future implementation of interventions, but we also assessed certain additional practical feasibility factors derived from RE-AIM, such as costs and program sustainability, that are not measured by the PRECIS-2 tool. It was concerning that almost no studies reported on these dimensions important to potential adopters of PA interventions in clinical settings. As in this review, the other literature assessing the feasibility to translate interventions into clinical practice found that authors severely underreport important measures from RE-AIM and PRECIS-2, such as intervention costs, “Reach” in RE-AIM that corresponds to the PRECIS-2 domains of eligibility and recruitment, as well as the organizational burden of the staff, training, resources, and infrastructure required to deliver an intervention [33, 84–87]. Without transparent reporting on the cost, adaptations made, and sustainability of PA interventions, it will be impossible for health systems to determine the value of these interventions for their clinical populations with T2D. Recently, the Standards for Reporting Implementation Studies (StaRI) initiative developed a checklist of items to report on for implementation science research [88]; with the future adaptation of these standards by researchers and journal editors, transparent reporting on factors relevant to stakeholders should allow health systems to more readily identify feasible programs for adoption.
This systematic review is innovative in its approach to categorize effective PA-related interventions for individuals with T2D by their level of pragmatism. One limitation, however, is that the variability in the number of participants in each study may have led us to underestimate the number of studies that were effective for PA or HbA1c outcomes. To mitigate this limitation, our a priori criteria were set to exclude pilot studies with <40 participants or that reported <80% power to address the primary RCT outcome. Also, because this is not a meta-analysis, we could not determine the moderators of effectiveness in the interventions that were both highly pragmatic and improved PA outcomes. Regarding our assessment of the PRECIS-2 criteria, we conducted scoring from the perspective of a PCMH rather than a standard primary care clinic. This is a limitation, as it would be more challenging for clinics without a population health team to implement interventions that rely on staff contact outside of face-to-face clinician visits. However, two of the effective interventions in the top tier of pragmatism would be pragmatic to deliver in standard primary care clinics, as well as PCMH models, as they did not rely on a population health team for intervention delivery [50, 53]. Finally, we only assessed interventions that were clinically integrated; a prior review has identified important strategies for partnership between clinics and community organizations to improve diabetes self-management [29], and future research should identify pragmatic and effective interventions that can be delivered in partnership with community organizations.
If models of healthcare continue to shift from fee-for-service to patient-centered medical homes and accountable care organizations [37, 89], the emphasis to deliver high-value care that improves important health outcomes should continue to grow. An additional trend that may further the translation of PA and other behavioral interventions into clinical practice is the recent Medicare approval of Chronic Care Management funding codes in 2015 to reimburse clinical counseling and coordination of care that is delivered outside of face-to-face clinic visits [90]. Health system administrators may consider implementing the interventions that we identified as effective and pragmatic when the costs are deemed reasonable and the characteristics of the trials are a good match with their health systems. To enhance implementation further, the interventions that we identified as both pragmatic and effective merit further study in diverse real-world settings. In addition to reporting effectiveness, such future RCTs of PA interventions in real-world settings should report on intervention fidelity and adaptation, in order to inform the assessment of intervention moderators. Perhaps most importantly, such future RCTs of PA interventions in real-world settings must report on key practical feasibility factors that have been typically ignored, including intervention costs and sustainability.
Appendix
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the studies. No IRB approval was required for the completion of this review study.
Findings reported here have not been previously published, and this manuscript is under no consideration at other journals. Data here is original and has not been previously published. All authors had full control of all primary data and agree to allow Translational Behavioral Medicine to review this data, if requested. This study was primarily funded by the National Heart, Lung, and Blood Institute (5K23HL118133).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Kelsey A. Luoma and Ian M. Leavitt contributed equally to this manuscript.
Implications
Practice: Health system administrators may consider implementing the interventions that we identified as effective and pragmatic when the costs are deemed reasonable and the characteristics of the trials are a good match with their health systems.
Policy: Future RCTs of PA interventions in real-world settings must report on key practical feasibility factors that have been typically ignored, including intervention costs and sustainability.
Research: Future research should focus on implementing the four identified highly pragmatic and effective trials in diverse real-world settings.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA. 2014.
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: general information and national estimates on diabetes in the United States, 2011. 2012; http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 4.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. American Journal of Public Health. 1991;81(9):1158–1162. doi: 10.2105/ajph.81.9.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huebschmann AG, Kohrt WM, Regensteiner JG. Exercise attenuates the premature cardiovascular aging effects of type 2 diabetes mellitus. Vascular Medicine. 2011;16(5):378–390. doi: 10.1177/1358863X11419996. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Annals of Internal Medicine. 2007;147(3):149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. Journal of the American Medical Association. 2004;292(20):2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 8.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23(9):1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 9.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Experimental Gerontology. 2013;48(9):888–897. doi: 10.1016/j.exger.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colberg SR, Albright AL, Blissmer BJ, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2010;42(12):2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 11.Physical Activity Guidelines Committee . Physical activity guidelines committee report, 2008. Washington, D.C: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 12.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England Journal of Medicine. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. The New England Journal of Medicine. 2012;366(13):1209–1217. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sone H, Tanaka S, Iimuro S, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study) Diabetologia. 2010;53(3):419–428. doi: 10.1007/s00125-009-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen HQ, Maciejewski ML, Gao S, Lin E, Williams B, Logerfo JP. Health care use and costs associated with use of a health club membership benefit in older adults with diabetes. Diabetes Care. 2008;31(8):1562–1567. doi: 10.2337/dc08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotnikoff RC, Karunamuni ND, Johnson JA, Kotovych M, Svenson LW. Health-related behaviours in adults with diabetes: associations with health care utilization and costs. Canadian Journal of Public Health. 2008;99(3):227–231. doi: 10.1007/BF03405479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 20.Zhao G, Ford ES, Li C, Mokdad AH. Compliance with physical activity recommendations in US adults with diabetes. Diabetic Medicine. 2008;25(2):221–227. doi: 10.1111/j.1464-5491.2007.02332.x. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PM, Schoenborn CA. Trends in adults receiving a recommendation for exercise or other physical activity from a physician or other health professional. NCHS Data Brief. 2012;86:1–8. [PubMed] [Google Scholar]
- 22.Joy EL, Blair SN, McBride P, Sallis R. Physical activity counselling in sports medicine: a call to action. British Journal of Sports Medicine. 2013;47(1):49–53. doi: 10.1136/bjsports-2012-091620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagoto S. The current state of lifestyle intervention implementation research: where do we go next? Translational Behavioral Medicine. 2011;1(3):401–405. doi: 10.1007/s13142-011-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillam S, Siriwardena AN. Evidence-based healthcare and quality improvement. Quality in primary care. 2014;22(3):125–132. [PubMed] [Google Scholar]
- 25.Green LW, Ottoson JM, Garcia C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annual Review of Public Health. 2009;30:151–174. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo X, Zhang P, Gregg EW, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff. (Millwood) Jan. 2012;31(1):50–60. doi: 10.1377/hlthaff.2011.1115. [DOI] [PubMed] [Google Scholar]
- 27.Philis-Tsimikas A, Gallo LC. Implementing community-based diabetes programs: The scripps whittier diabetes institute experience. Current diabetes reports. 2014;14(2):462. doi: 10.1007/s11892-013-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krukowski RA, Hare ME, Talcott GW, et al. Dissemination of the look AHEAD intensive lifestyle intervention in the United States Air Force: study rationale, design and methods. Contemporary Clinical Trials. 2015;40:232–239. doi: 10.1016/j.cct.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portillo MC, Regaira E, Pumar-Mendez MJ, et al. Voluntary organizations and community groups as new partners in diabetes self-management and education: a critical interpretative synthesis. The Diabetes Educator. 2015;41(5):550–568. doi: 10.1177/0145721715594026. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National Diabetes Education Program - Working in Communities. 2016; https://www.cdc.gov/diabetes/ndep/communities/index.html Accessed April 13, 2017.
- 31.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ. 2009;180(10):E47–E57. doi: 10.1503/cmaj.090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez MA, Rabin BA, Gaglio B, et al. A systematic review of eHealth cancer prevention and control interventions: new technology, same methods and designs? Translational Behavioral Medicine. 2013;3(4):392–401. doi: 10.1007/s13142-013-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. American Journal of Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bresnick J. Patient-Centered Medical Home keeps spending low, quality high. 2016; http://healthitanalytics.com/news/patient-centered-medical-home-keeps-spending-low-quality-high.
- 36.Stange KC, Nutting PA, Miller WL, et al. Defining and measuring the patient-centered medical home. Journal of General Internal Medicine. 2010;25(6):601–612. doi: 10.1007/s11606-010-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinley EL, Gabbay RA. The impact of new payment models on quality of diabetes care and outcomes. Current diabetes reports. 2016;16(6):51. doi: 10.1007/s11892-016-0743-5. [DOI] [PubMed] [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Archives of Internal Medicine. 2011;171(22):2001–2010. doi: 10.1001/archinternmed.2011.507. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen AB, de Fine ON, Gannik D, Hindsberger C, Hollnagel H. Structured personal diabetes care in primary health care affects only women’s HbA1c. Diabetes Care. 2006;29(5):963–969. doi: 10.2337/diacare.295963. [DOI] [PubMed] [Google Scholar]
- 41.Adachi M, Yamaoka K, Watanabe M, et al. Effects of lifestyle education program for type 2 diabetes patients in clinics: a cluster randomized controlled trial. BMC Public Health. 2013;13:467. doi: 10.1186/1471-2458-13-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babamoto KS, Sey KA, Camilleri AJ, Karlan VJ, Catalasan J, Morisky DE. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Education & Behavior. 2009;36(1):113–126. doi: 10.1177/1090198108325911. [DOI] [PubMed] [Google Scholar]
- 43.Davies MJ, Heller S, Skinner TC, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ [British Medical Journal] 2008;336(7642):491–495. doi: 10.1136/bmj.39474.922025.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khunti K, Gray LJ, Skinner T, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ. 2012;344:e2333. doi: 10.1136/bmj.e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mash RJ, Rhode H, Zwarenstein M, et al. Effectiveness of a group diabetes education programme in under-served communities in South Africa: a pragmatic cluster randomized controlled trial. Diabetic Medicine. 2014;31(8):987–993. doi: 10.1111/dme.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibayama T, Kobayashi K, Takano A, Kadowaki T, Kazuma K. Effectiveness of lifestyle counseling by certified expert nurse of Japan for non-insulin-treated diabetic outpatients: a 1-year randomized controlled trial. Diabetes Research and Clinical Practice. 2007;76(2):265–268. doi: 10.1016/j.diabres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Irwig MS, Sood P, Ni D, et al. A diabetes scorecard does not improve HbA(1c), blood pressure, lipids, aspirin usage, exercise and diabetes knowledge over 9 months: a randomized controlled trial. Diabetic Medicine. 2012;29(9):1206–1212. doi: 10.1111/j.1464-5491.2012.03610.x. [DOI] [PubMed] [Google Scholar]
- 48.Jansink R, Braspenning J, Keizer E, van der Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scandinavian Journal of Primary Health Care. 2013;31(2):119–127. doi: 10.3109/02813432.2013.797178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Greef K, Deforche B, Tudor-Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three-arm randomized controlled trial. International journal of behavioral medicine. 2011;18(3):188–198. doi: 10.1007/s12529-010-9124-7. [DOI] [PubMed] [Google Scholar]
- 50.Christian JG, Bessesen DH, Byers TE, Christian KK, Goldstein MG, Bock BC. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Archives of Internal Medicine. 2008;168(2):141–146. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- 51.Trento M, Gamba S, Gentile L, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care. 2010;33(4):745–747. doi: 10.2337/dc09-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee A, Siu CF, Leung KT, Lau LC, Chan CC, Wong KK. General practice and social service partnership for better clinical outcomes, patient self efficacy and lifestyle behaviours of diabetic care: randomised control trial of a chronic care model. Postgraduate Medical Journal. 2011;87(1032):688–693. doi: 10.1136/pgmj.2011.118885. [DOI] [PubMed] [Google Scholar]
- 53.Di Loreto C, Fanelli C, Lucidi P, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care. 2003;26(2):404–408. doi: 10.2337/diacare.26.2.404. [DOI] [PubMed] [Google Scholar]
- 54.Glasgow RE, Kurz D, King D, et al. Twelve-month outcomes of an Internet-based diabetes self-management support program. Patient Education and Counseling. 2012;87(1):81–92. doi: 10.1016/j.pec.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Weegen S, Verwey R, Spreeuwenberg M, Tange H, van der Weijden T, de Witte L. It’s LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. Journal of medical Internet research. 2015;17(7):e184. doi: 10.2196/jmir.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keyserling TC, Samuel-Hodge CD, Ammerman AS, et al. A randomized trial of an intervention to improve self-care behaviors of African-American women with type 2 diabetes: impact on physical activity. Diabetes Care. 2002;25(9):1576–1583. doi: 10.2337/diacare.25.9.1576. [DOI] [PubMed] [Google Scholar]
- 57.Edelman D, Dolor RJ, Coffman CJ, et al. Nurse-led behavioral management of diabetes and hypertension in community practices: a randomized trial. Journal of General Internal Medicine. 2015;30(5):626–633. doi: 10.1007/s11606-014-3154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Archives of Internal Medicine. 2011;171(5):453–459. doi: 10.1001/archinternmed.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabetic Medicine. 2011;28(7):789–796. doi: 10.1111/j.1464-5491.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 60.Maindal HT, Carlsen AH, Lauritzen T, Sandbaek A, Simmons RK. Effect of a participant-driven health education programme in primary care for people with hyperglycaemia detected by screening: 3-year results from the ready to act randomized controlled trial (nested within the ADDITION-Denmark study) Diabetic Medicine. 2014;31(8):976–986. doi: 10.1111/dme.12440. [DOI] [PubMed] [Google Scholar]
- 61.Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. Journal of managed care pharmacy : JMCP. 2012;18(7):516–526. doi: 10.18553/jmcp.2012.18.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schillinger D, Handley M, Wang F, Hammer H. Effects of self-management support on structure, process, and outcomes among vulnerable patients with diabetes: a three-arm practical clinical trial. Diabetes Care. 2009;32(4):559–566. doi: 10.2337/dc08-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson DR, Christison-Lagay J, Villagra V, Liu H, Dziura J. Managing the space between visits: a randomized trial of disease management for diabetes in a community health center. Journal of General Internal Medicine. 2010;25(10):1116–1122. doi: 10.1007/s11606-010-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim S, Kang SM, Kim KM, et al. Multifactorial intervention in diabetes care using real-time monitoring and tailored feedback in type 2 diabetes. Acta Diabetologica. 2016;53(2):189–198. doi: 10.1007/s00592-015-0754-8. [DOI] [PubMed] [Google Scholar]
- 65.Thoolen BJ, de Ridder D, Bensing J, Gorter K, Rutten G. Beyond good intentions: the role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychology & health. 2009;24(3):237–254. doi: 10.1080/08870440701864504. [DOI] [PubMed] [Google Scholar]
- 66.Taylor CB, Miller NH, Reilly KR, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care. 2003;26(4):1058–1063. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- 67.Moriyama M, Nakano M, Kuroe Y, Nin K, Niitani M, Nakaya T. Efficacy of a self-management education program for people with type 2 diabetes: results of a 12 month trial. Japan journal of nursing science : JJNS. 2009;6(1):51–63. doi: 10.1111/j.1742-7924.2009.00120.x. [DOI] [PubMed] [Google Scholar]
- 68.Van Dyck D, De Greef K, Deforche B, et al. Mediators of physical activity change in a behavioral modification program for type 2 diabetes patients. The international journal of behavioral nutrition and physical activity. 2011;8:105. doi: 10.1186/1479-5868-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim CJ, Kim DJ, Park HR. Effects of a cardiovascular risk reduction intervention with psychobehavioral strategies for Korean adults with type 2 diabetes and metabolic syndrome. The Journal of Cardiovascular Nursing. 2011;26(2):117–128. doi: 10.1097/JCN.0b013e3181ec02ae. [DOI] [PubMed] [Google Scholar]
- 70.Uusitupa MI. Early lifestyle intervention in patients with non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Annals of Medicine. 1996;28(5):445–449. doi: 10.3109/07853899608999106. [DOI] [PubMed] [Google Scholar]
- 71.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the steno type 2 randomised study. Lancet. 1999;353(9153):617–622. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 72.Huffman KM, Sloane R, Peterson MJ, et al. The impact of self-reported arthritis and diabetes on response to a home-based physical activity counselling intervention. Scandinavian Journal of Rheumatology. 2010;39(3):233–239. doi: 10.3109/03009740903348973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychology. 2000;19(1 Suppl):32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 75.Qiu SH, Sun ZL, Cai X, Liu L, Yang B. Improving patients’ adherence to physical activity in diabetes mellitus: a review. Diabetes & metabolism journal. 2012;36(1):1–5. doi: 10.4093/dmj.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care. 2012;35(12):2681–2689. doi: 10.2337/dc11-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dalmau Llorca MR, Garcia Bernal G, Aguilar Martin C, Palau GA. Group versus individual education for type-2 diabetes patients. Atencion Primaria. 2003;32(1):36–41. doi: 10.1016/S0212-6567(03)78854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arsand E, Froisland DH, Skrovseth SO, et al. Mobile health applications to assist patients with diabetes: lessons learned and design implications. Journal of diabetes science and technology. 2012;6(5):1197–1206. doi: 10.1177/193229681200600525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sieverdes JC, Treiber F, Jenkins C. Improving diabetes management with mobile health technology. The American Journal of the Medical Sciences. 2013;345(4):289–295. doi: 10.1097/MAJ.0b013e3182896cee. [DOI] [PubMed] [Google Scholar]
- 80.Kim CJ, Kang DH. Utility of a web-based intervention for individuals with type 2 diabetes: the impact on physical activity levels and glycemic control. Computers, informatics, nursing : CIN. 2006;24(6):337–345. doi: 10.1097/00024665-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Anderson M. Technology Device Ownership: 2015. October 2015; http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015/. Accessed 10/03/2016.
- 82.Kim E. Fitbit’s growth has been astounding. 2015; http://www.businessinsider.com/fitbit-astounding-growth-in-paid-active-users-and-devices-sold-2015-5.
- 83.Connelly J, Kirk A, Masthoff J, MacRury S. The use of technology to promote physical activity in type 2 diabetes management: a systematic review. Diabetic Medicine. 2013;30(12):1420–1432. doi: 10.1111/dme.12289. [DOI] [PubMed] [Google Scholar]
- 84.Harden SM, Gaglio B, Shoup JA, et al. Fidelity to and comparative results across behavioral interventions evaluated through the RE-AIM framework: a systematic review. Systematic reviews. 2015;4:155. doi: 10.1186/s13643-015-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang M, Chao A, Whittemore R. Evaluating intervention programs targeting parents to manage childhood overweight and obesity: a systematic review using the RE-AIM framework. Journal of Pediatric Nursing. 2015;30(6):877–887. doi: 10.1016/j.pedn.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Compernolle S, De Cocker K, Lakerveld J, et al. A RE-AIM evaluation of evidence-based multi-level interventions to improve obesity-related behaviours in adults: a systematic review (the SPOTLIGHT project) The international journal of behavioral nutrition and physical activity. 2014;11:147. doi: 10.1186/s12966-014-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGoey T, Root Z, Bruner MW, Law B. Evaluation of physical activity interventions in children via the reach, efficacy/effectiveness, adoption, implementation, and maintenance (RE-AIM) framework: a systematic review of randomized and non-randomized trials. Preventive Medicine. 2016;82:8–19. doi: 10.1016/j.ypmed.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Pinnock H, Barwick M, Carpenter CR, et al. Standards for reporting implementation studies (StaRI) statement. BMJ. 2017;356:i6795. doi: 10.1136/bmj.i6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herzlinger RE, Schleicher SM, Mullangi S. Health care delivery innovations that integrate care? Yes!: but integrating what? JAMA. 2016;315(11):1109–1110. doi: 10.1001/jama.2016.0505. [DOI] [PubMed] [Google Scholar]
- 90.Hebert ET, Caughy MO, Shuval K. Primary care providers’ perceptions of physical activity counselling in a clinical setting: a systematic review. British Journal of Sports Medicine. 2012;46(9):625–631. doi: 10.1136/bjsports-2011-090734. [DOI] [PubMed] [Google Scholar]