Abstract
Primary Cytomegalovirus (CMV) infection is often not suspected as a cause of fever of unknown origin (FUO) in immune-competent adults. We present a case-series of symptomatic primary CMV infection in immunocompetent adults presenting as fever of unknown origin (FUO). All patients with CMV serology tested between November 2008 and June 2016 underwent chart review. Cases were defined as those between 18 and 65 years of age with documented fever and elevated serum anti-CMV IgM. Exclusion criteria were organ specific CMV disease, positive serum anti-EBV IgM, or presence of any immunocompromising condition. Sixteen patients (69% male, mean age 42.2 ± 11.7 years) met criteria. Mean duration of illness was 4.6 ± 3.3 weeks. Common symptoms other than fever included fatigue (94%), night sweats (81%), malaise (75%), myalgias (63%), and headache (56%). Eleven patients (68.8%) had contact with young children; six (35.3%) patients had children in daycare. Twelve (75%) patients had extensive testing and multiple visits or hospitalizations prior to consulting with an infectious disease specialist. Peripheral smear was done in twelve (75%) patients and all had atypical lymphocytes. Five patients (31.3%) had a leukocytosis. Peak serum transaminases were: AST 115.25 ± 50.5 IU/L and ALT 168.38 ± 92.0 IU/L. One patient had splenic infarcts. In addition, two cases of hydrops fetalis were attributed to primary CMV infection. In summary, primary CMV infection can present as FUO in immunocompetent adults. Contact with young children in daycare may be a risk factor. Heightened clinical suspicion will promote earlier diagnosis and avoid unnecessary testing.
Keywords: Cytomegalovirus, Immunocompetent, Fever of unknown origin, Mononucleosis, Atypical lymphocytosis
Background
Infection with Cytomegalovirus (CMV), a human herpes virus, is common in healthy adults and children. Seroprevelance studies have reported a prevalence range of 50%–90% in healthy adults with an increasing prevalence with advancing age [1], [2]. CMV is a common opportunistic pathogen in immunocompromised patients causing significant morbidity and mortality. CMV infection in pregnancy can result in symptomatic congenital infection in the fetus and newborn. However, primary CMV infection is often asymptomatic or only causes a mild disease in immunocompetent individuals. When symptomatic, primary infection in adults is often described as a mononucleosis with less prominent cervical lymphadenopathy than that caused by Epstein-Barr virus (EBV) [3], [4]. Most data on primary CMV infection in immunocompetent adults is reported from Europe [3], [5]. The largest series of cases in the United States (US) was published in 1986 and since then there has been no further reports from the United States [4]. This is a retrospective study on the clinical and laboratory manifestations of immunocompetent adult patients in the US who had prolonged febrile illness and were diagnosed with symptomatic primary CMV infection.
Study design
We conducted a retrospective chart review on all patients who presented to an academic tertiary care facility and who had CMV serology tested between November 2008 and December 2016. Between 2008 and 2014 patient serum was tested in a reference lab by a semi-quantitative chemiluminescent immunoassay. IgM antibody to CMV was detected at 35.0 AU/mL or greater. Since 2015, our hospital laboratory also performs a qualitative PCR CMV. This method detects CMV in a plasma sample but does not quantify viral load [6].
From these records, two reviewers (NN and UAH) identified patients who had positive CMV serology and/or CMV nucleic acids detected by polymerase chain reaction (PCR) for further review. Cases were defined as those between 18 and 65 years of age with elevated anti-CMV IgM and a history of fever. Exclusion criteria included: CMV disease affecting a single organ (e.g., CMV colitis, CMV retinitis, or CMV affecting a transplanted organ), positive serum anti-Epstein Barr Virus (EBV) IgM, or any immunocompromising medical condition such as infection with Human Immunodeficiency Virus (HIV), end stage renal disease, uncontrolled diabetes, active malignancy, or current use of immunosuppressive medications. Cases were also excluded if there was a possible alternative cause of fever or symptoms. Demographic, clinical and laboratory data were collected on all cases that satisfied both inclusion and exclusion criteria.
Results
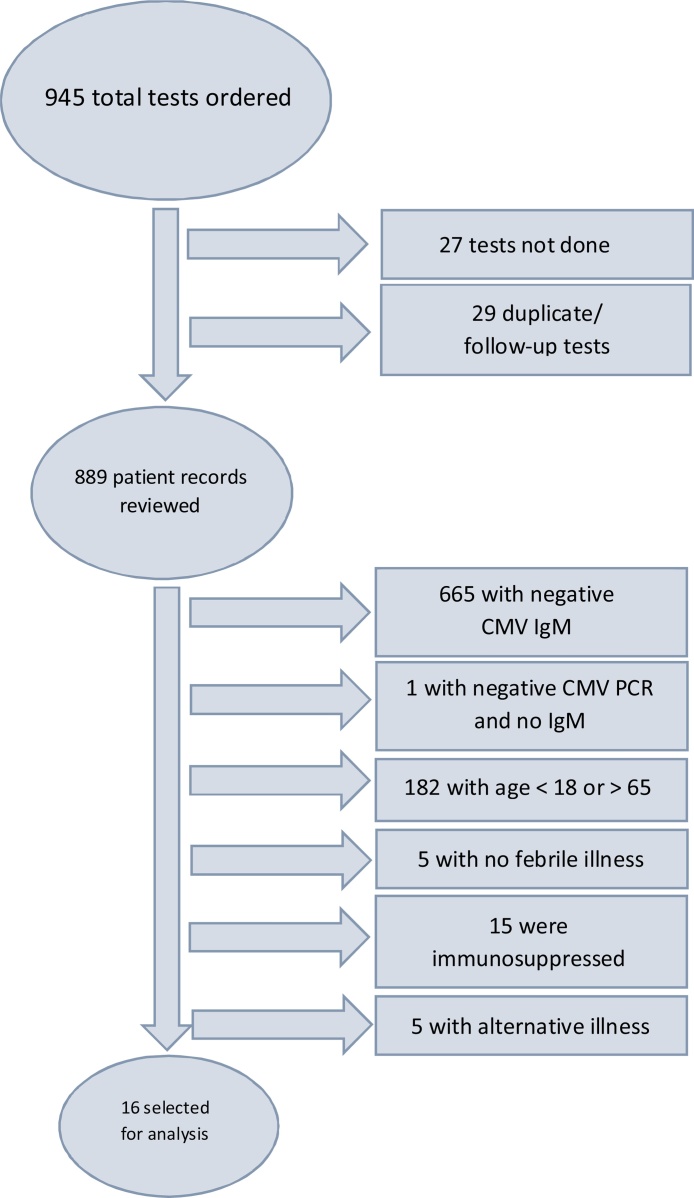
A total of 945 tests were ordered during the study period, of which sixteen patients met inclusion and exclusion criteria to be included in this analysis (Fig. 1). The mean age was 42.5 years (Median 44 years; range 23–61). Eleven (69%) were male and the median duration of illness was 4 weeks with a range of 1–16 weeks.
Fig. 1.
Selection of cases for analysis.
History of fever was part of the inclusion criteria for this series. The most common other documented symptoms were fatigue (94%), night sweats (81%), malaise (75%), myalgias (63%), and headache (56%). Approximately a quarter (25%) of patients reported abdominal pain, nausea, or decreased appetite. Rash was reported by only 3 (19%) patients. Sore throat and cough were uncommon. Table 1 lists the signs and symptoms, in decreasing order of prevalence, as reported by patients.
Table 1.
Signs and Symptoms of Patients Presenting with Primary CMV.
| Symptom | Number of Patients (N) | Percentage of Patients (%) |
|---|---|---|
| Fever | 16 | 100% |
| Fatigue | 15 | 94% |
| Night Sweats | 13 | 81% |
| Malaise | 12 | 75% |
| Myalgias | 10 | 63% |
| Headache | 9 | 56% |
| Arthralgias | 6 | 38% |
| Neck Pain | 6 | 38% |
| Anorexia | 5 | 31% |
| Abdominal Pain | 4 | 25% |
| Nausea | 4 | 25% |
| Vomiting | 3 | 19% |
| Rash | 3 | 19% |
| Weight Loss | 3 | 19% |
| Cough | 2 | 13% |
| Diarrhea | 2 | 13% |
| Sore throat | 1 | 7% |
| Back Pain | 1 | 7% |
| Jaundice | 0 | 0% |
| Sputum Productions | 0 | 0% |
The laboratory profile for CMV infection was relatively non-specific in this series (Table 2). Most had normal hemoglobin (Mean 13.3 ± 3.25 g/dL), normal platelet count (Mean 218 ± 93 × 109 platelets per liter), and normal total white cell (WBC) count (Mean 10.1 ± 6.64 × 109 cells per liter). Two patients (12.5%) had thrombocytopenia. Only five (31.3%) patients had a leukocyte count greater than 12,000 cells per microliter of blood. In regards to leukocyte differential, eleven (68.8%) patients had elevated lymphocytes defined by our institutions lab as >2.9 × 109 lymphocytes per liter. In nine patients, more than 50% of their white blood cells were lymphocytes. Peripheral blood smears were done for 12 (75%) patients and atypical lymphocytes were noted in all (100%) specimens examined.
Table 2.
Selected Laboratory Values from this Patient Set (these were all peak values).
| Patient | Age | Gender | WBC (×10[9] cells per liter) | Lymphocytes (%) | AST (U/L) | ALT (U/L) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|---|
| 1 | 49 | F | 6.59 | 54.0 | 193 | 167 | 1.1 |
| 2 | 23 | F | 3.30 | 36.6 | 47 | 31 | 1.4 |
| 3 | 55 | F | 8.40 | 45.0 | 82 | 112 | 0.4 |
| 4 | 43 | M | 17.19 | 57.0 | 105 | 183 | 2.13 |
| 5 | 31 | M | 8.10 | 65.0 | 40 | 64 | 0.5 |
| 6 | 61 | M | 8.50 | 36.0 | 79 | 99 | 0.6 |
| 7 | 49 | M | 8.00 | 31.0 | 128 | 240 | 0.6 |
| 8 | 38 | M | 12.80 | 56.7 | 56 | 63 | 0.7 |
| 9 | 36 | F | 12.36 | 54.0 | 64 | 86 | 0.8 |
| 10 | 49 | M | 15.80 | 60.0 | 163 | 389 | 0.7 |
| 11 | 31 | M | 7.90 | 31.0 | 160 | 197 | 0.4 |
| 12 | 53 | F | 3.10 | 36.7 | 166 | 200 | 0.5 |
| 13 | 46 | M | 6.60 | 34.7 | 152 | 193 | 0.6 |
| 14 | 44 | M | 9.10 | 72.8 | 110 | 157 | 1.2 |
| 15 | 28 | M | 30.01 | 67.3 | 67 | 107 | 0.4 |
| 16 | 44 | M | 4.47 | 74.0 | 232 | 406 | 1.0 |
| Reference | – | – | 3.5–10.5 | 20–40 | <40 | <50 | <1.60 |
| Mean | 42.5 | – | 10.13 | 50.74 | 115.25 | 168.37 | 0.8 |
| Median | 44 | – | 8.25 | 54 | 107.5 | 162 | 0.65 |
Elevated hepatic transaminases were present in all (100%) patients (Table 2). The average peak Aspartate Aminotransferase (AST) and Alanine Aminotransferase (AST) measured were 115.25 ± 50.5 U/L and 168.38 ± 92.0 U/L, respectively. Average peak serum bilirubin was 0.82 ± 46.2 mg/dL. Only four (25%) patients had hyperbilirubinemia, or total bilirubin value greater than 1.0 mg per deciliter.
Twelve (75%) patients were eventually referred to an infectious disease specialist after workup for fever of unknown origin and diagnosis was made by them. Seven patients (43.8%) experienced three weeks or more of workup prior to infectious disease consultation. At least one patient waited two months prior to meeting with an infectious disease specialist following which a diagnosis was made. Complications from primary CMV were rare in this series. Two patients had signs and symptoms of meningitis, both of whom had elevated white blood cells and protein in cerebral spinal fluid analysis. CMV was not tested in their CSF, however no other pathogens were identified. One patient experienced splenic infarcts. Just under a half (43.8%; N = 7) of the patients reported a contact with another person with similar illness within a few weeks preceding the onset of illness, however more than half (68.8%; N = 11) had contact with young children.
Discussion
This case series adds to the small amount of published literature on symptomatic primary CMV infections in immunocompetent adults without organ specific CMV disease in a US population [4], [7]. Our review confirms previous findings, indicating that prolonged fever, malaise, and night sweats are characteristic symptoms in adults with symptomatic primary CMV. The symptoms of primary CMV are relatively non-specific and non-focal, aside from prolonged fever, and the diagnosis is infrequently considered. Relatively few patients experienced prominent gastrointestinal or respiratory symptoms. This clinical presentation might suggest primary CMV infection rather than other common febrile illness such as Epstein-Barr Virus, Influenza, or infection with enteroviruses. Rash, which has been reported in approximately a third of patients with primary CMV infection, was relatively uncommon in our case series.
Our findings suggest that CMV is not a frequent cause of fever of unknown origin. However, many of the patients in this cohort had an extensive work up prior to serologic studies for CMV infection. Perhaps earlier testing for common viruses, including CMV, would help mitigate the costs associated with diagnosis as well as identify more cases. Further, earlier recognition would decrease unnecessary and invasive workup for patients with fever persisting more than 2 weeks, especially when associated with transaminitis. In our cases, referral to an infectious disease specialist usually occurred after 3–4 weeks of fever and an extensive workup. Three out of every four patients had their final diagnosis made by an infectious disease consultant, indicating an earlier involvement in care might be beneficial.
As reported by others, our series suggests that young children can serve as vectors for CMV infection, transmitting it to non-immune adults [8]. Previous authors have noticed this phenomenon and dubbed it the “feverish granny syndrome” [9]. This is important for two reasons. First, recognizing exposure to young children, especially those in day-care centers, should prompt consideration of primary CMV infection in an adult with prolonged fever. Second, children who are in daycare may increase the risk of congenital CMV infection if exposed to pregnant women. Two women were identified in our chart reviews, but not included in our data, who suffered stillbirth (one with hydrops fetalis) secondary to congenital CMV infection in their fetus. (These cases were not included as we did not have data from the primary maternal infection nor documentation of febrile illness.)
Obvious limitations of our study include the small number of cases and the inherent inability to retrospectively gather more complete data. Because our data were limited to other provider’s notes, missing information would prevent identification of other risk factors that may be associated with symptomatic CMV infection. This review is also limited by use of CMV IgM testing as an inclusion criteria. We suspect that many patients might have been missed as they might not have undergone CMV IgM testing. For example, Manfredi et al. found in a cohort of patients with fever of unknown origin that approximately 14% were positive for CMV infection [10]. A future direction for data gathering could include a prospective study for patients with prolonged febrile illness whereby data can be collected in a protocoled manner.
In conclusion, CMV can affect immunocompetent adults, producing an illness with prolonged fever, night sweats, fatigue, myalgias, arthralgias and transaminitis. In our patients, sore throat and cough were uncommon, helping to differentiate CMV from other common febrile illnesses (e.g., EBV, influenza, etc.). The symptoms associated with primary CMV infection can be prolonged, with fever lasting 3 weeks or more. In many of these cases there was a prolonged outpatient workup prior to CMV testing and/or referral to an infectious disease physician. Primary CMV infection should be considered in the differential diagnosis of prolonged fever as early consideration and serologic testing for CMV IgM may prevent further invasive and expensive diagnostic tests.
Conflicts of interest
None.
Conflicts of interest
None among the authors.
Author contributions
William Salzer conceived the idea behind the study. Hariharan Regunath designed the study protocol under assistance from William Salzer and obtained approval from University of Missouri Institutional Review board. Christian Rojas-Moreno made further suggestions to design and methodology. Patrick Smith reviewed the study protocol and generated the patient list. Nathanial Nolan and Aiman Halai gathered all data from the list of patients generated after applying selection criteria to patient list. Nathanial Nolan and Aiman Halai then compiled all data into an excel file and calculated descriptive statistics under guidance from Hariharan Regunath. William Salzer, Patrik Smith and Christian Rojas-Moreno then reviewed all the data. Nathanial Nolan created the first draft of the manuscript. All other authors suggested critical edits into every aspect of the manuscript. The final manuscript was approved by all co-authors before submission for publication.
Acknowledgements
These data were presented as a poster at the ACP 2016 Missouri chapter meeting in Lake of the Ozarks, MO It won third place in the research category.
References
- 1.Staras S.A., Dollard S.C., Radford K.W., Flanders W.D., Pass R.F., Cannon M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Bate S.L., Dollard S.C., Cannon M.J. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin. Infect. Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemola E., Von Essen R., Henle G., Henle W. Infectious-mononucleosis-like disease with negative heterophil agglutination test: clinical features in relation to Epstein-Barr virus and cytomegalovirus antibodies. J. Infect. Dis. 1970;121:608–614. doi: 10.1093/infdis/121.6.608. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz C.A., Henle W., Henle G., Snover D., Rudnick H., Balfour H.H., Jr. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65:124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wreghitt T.G., Teare E.L., Sule O., Devi R., Rice P. Cytomegalovirus infection in immunocompetent patients. Clin. Infect. Dis. 2003;37:1603–1606. doi: 10.1086/379711. [DOI] [PubMed] [Google Scholar]
- 6.Ross S.A., Novak Z., Pati S., Boppana S.B. Overview of the diagnosis of cytomegalovirus infection. Infect. Disord. Drug Targets. 2011 Oct;11(5):466–474. doi: 10.2174/187152611797636703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J.I., Corey G.R. Cytomegalovirus infection in the normal host. Medicine (Baltimore) 1985;64:100–114. doi: 10.1097/00005792-198503000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ford-Jones E.L., Kitai I., Davis L., Corey M., Farrell H., Petric M. Cytomegalovirus infections in Toronto child-care centers: a prospective study of viral excretion in children and seroconversion among day-care providers. Pediatr. Infect. Dis. J. 1996;15:507–514. doi: 10.1097/00006454-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wreghitt T., Behr S., Hodson J., Irwin D. Feverish granny syndrome. Lancet. 1995;346:1716. doi: 10.1016/s0140-6736(95)92889-8. [DOI] [PubMed] [Google Scholar]
- 10.Manfredi R., Calza L., Chiodo F. Primary cytomegalovirus infection in otherwise healthy adults with Fever of unknown origin: a 3-year prospective survey. Infection. 2006;34:87–90. doi: 10.1007/s15010-006-5012-0. [DOI] [PubMed] [Google Scholar]