Abstract
Background
A significant proportion of patients develop heart failure (HF) after acute myocardial infarction (MI). Predicting this development with novel biomarkers would allow tailoring healthcare to each individual. We recently identified a circular RNA called MICRA which was associated with HF development after MI. Here, we tested whether MICRA was able to risk stratify MI patients.
Methods
MICRA was assessed in whole blood samples collected at reperfusion in 472 patients with acute MI. Left ventricular ejection fraction (EF) was evaluated by echocardiography at 4 months. Multivariable analyses with ordinal regression were conducted to determine the ability of MICRA to classify patients into 3 EF groups: reduced EF (≤ 40%), mid-range EF (4149%) and preserved EF (≥ 50%).
Results
Eighty seven patients (18%) had a reduced EF, 106 (22%) had a mid-range EF and 279 (59%) had a preserved EF at 4 months. MICRA classified patients into EF groups with an adjusted odds ratio [95% confidence interval] of 0.78 [0.64–0.95]. MICRA improved the predictive value of a multivariable clinical model as attested by a decrease of the Akaike Information Criteria (p = 0.012). Bootstrap internal validation confirmed the incremental prognostic value of MICRA.
Conclusion
We report that the circRNA MICRA improves risk classification after MI, supporting the added value of this novel biomarker in future prognostication strategies.
Keywords: Myocardial infarction, Heart failure, Prognosis, Biomarkers, Non-coding RNAs, Circular RNAs
1. Introduction
Acute myocardial infarction (MI) is a widespread event worldwide with a 30-day mortality rate close to 10% in most European and North American countries [1], [2]. Despite modern reperfusion strategies, MI survivors are not exempted of further difficulties, among which left ventricular (LV) remodelling leading to heart failure (HF) could complicate the prognosis in approximately one fifth of the patients. Identification of patients at high risk of developing HF after MI would allow tailoring healthcare to each individual and reduce the associated socio-economic burden. However, currently available prognostication strategies lack accuracy and would benefit from novel biomarkers. Indeed, markers of myocardial injury (creatine phosphokinase, CPK; cardiac troponin, cTn) or markers of stress (brain natriuretic peptide, BNP), suffer some limitations to predict outcome after MI due to low specificity or fluctuations of circulating levels in the post MI setting [3], [4].
While most of current biomarkers are circulating proteins, recent investigations suggest that the blood transcriptome may also represent an invaluable, yet poorly explored, source of novel biomarkers [5]. Among the different types of protein-coding RNAs or non-coding RNAs, circular RNAs (circRNAs) are particularly attractive biomarkers due to their resistance to degradation by exoribonuclease. It appeared that circRNAs may have some potential biomarker value for HF [6], [7]. We identified a circRNA, named MICRA for Myocardial Infarction-associated Circular RnA, which was associated with outcome after MI [8]. In this previous study, patient outcome was determined using the ejection fraction (EF) at 4-month follow-up. Patients were classified as having LV dysfunction (4-month EF ≤ 40%) or no LV dysfunction (4-month EF > 40%) and the ability of MICRA to classify patients between these 2 groups was determined.
The last guidelines of the European Society of Cardiology [9] presented a novel classification of HF patients in three groups: EF below 40% named reduced EF (rEF), 40–49% named mid-range EF (mrEF) and 50% and higher as preserved EF (pEF). In the present study, we aimed to determine whether MICRA was able to risk stratify patients after MI using this 3-group classification.
2. Methods
2.1. Patients
We enrolled 472 acute MI patients (78% ST-elevation MI and 22% non-ST-elevation MI) of the Luxembourg Acute Myocardial Infarction Registry [10]. At the time of reperfusion after percutaneous intervention, whole blood samples were withdrawn via an arterial catheter into PAXgene™ RNA tubes (BD Biosciences, Erembodegem, Belgium). Left ventricular EF was determined after 4 months using echocardiography and patients were classified according to the 3-group classification of the ESC guidelines for HF patients (rEF ≤ 40%; mrEF 41–49%; pEF ≥ 50%) [9]. The protocol has been approved by the ethics committee of Luxembourg and all patients signed an informed consent.
2.2. MICRA measurement
Total RNA was extracted from PAXgene™ RNA tubes with the PAXgene™ blood RNA kit (Qiagen, Venlo, Netherlands) as described previously [11]. MICRA was measured using quantitative RT-PCR with divergent primers (forward: GCTAAAGAAAGTCAAGTC; reverse: TCAAGGACATCTTAGAGT) allowing for amplification of circRNA. SF3a1 was used as housekeeping gene for normalization of PCR results.
2.3. Statistical analysis
One-way analysis of variance on Ranks followed by all pairwise multiple comparison procedures using the Holm-Sidak method was used to compare continuous variables between the 3 EF groups. Chi-square test was used for qualitative data. A P value < 0.05 was considered significant. Multivariable analysis with ordinal regression was used to assess the ability of MICRA in combination with various demographic and clinical parameters to classify patients into EF groups. All right skewed continuous data were log2 transformed and then all continuous data were scaled before analysis. Missing values were filled using 100-fold multiple imputation. To address the incremental predictive value of MICRA to a multi-parameter clinical model, the Akaike Information Criteria (AIC) was calculated. Contrarily to the area under the curve, the AIC is adjusted for the multiplication of variables into the model, avoiding for overfitting. A lower AIC indicates a better model fit. The Wald chi-square test was used to measure the significance of models and the likelihood ratio test was used to compare the models with and without MICRA. Bootstrap internal validation was used to estimate the robustness of the models with MICRA. All prediction analyses were performed on the R version 2.14.2 statistical platform using the packages Hmisc, PredictABEL, bootStepAIC and rms.
3. Results
Clinical characteristics of the 472 patients enrolled in the present study are displayed in Table 1. 87 patients (18%) had a rEF 4 months after MI, 106 (22%) had a mrEF and 279 (59%) had a pEF. Patients within the 3 groups were similar in terms of age, gender ratio, and other cardiovascular risk factors. Patients with decreased EF had higher blood cell counts, CPK, cTnT and Nt-proBNP levels as compared to patients with preserved EF. Expression levels of MICRA were lower in patients with rEF compared to patients with mrEF or pEF, and were comparable between patients with mrEF and pEF (Fig. 1A).
Table 1.
Clinical parameters and biomarker levels.
| rEF n = 87 |
mrEF n = 106 |
pEF n = 279 |
p value | |
|---|---|---|---|---|
| Age, yrs | 65 (37–89) | 60 (30–88) | 60 (30–91) | 0.09 |
| Sex, male | 65 (75%) | 85 (80%) | 206 (74%) | 0.43 |
| BMI, kg/m2 | 28 (19–51) | 27 (18–37) | 27 (19–47) | 0.33 |
| Diabetes | 26 (30%) | 22 (21%) | 52 (19%) | 0.07 |
| Hypertension | 43 (51%) | 46 (44%) | 135 (49%) | 0.61 |
| Hypercholesterolemia | 37 (45%) | 41 (39%) | 126 (46%) | 0.53 |
| Smoking | 37 (44%) | 53 (50%) | 125 (45%) | 0.59 |
| WBC, × 109 cells/L | 11.9 (3.7–29.0) | 11.1 (5.0–22.8) | 10.4 (3.1–29.0) | 0.001 |
| Previous MI | 11 (13%) | 16 (15%) | 23 (8%) | 0.11 |
| Ischemic time (1 = 24 h) | 0.15 (0.04–0.62) | 0.15 (0.03–0.94) | 0.14 (0.03–0.94) | 0.42 |
| CPK, U/L | 3109 (34–12,096) | 2167 (119–13,038) | 1172 (76–9574) | < 0.001 |
| cTnT, μg/L | 6.94 (0.03–26.93) | 5.17 (0.03–21.97) | 2.71 (0.01–25.66) | < 0.001 |
| NT-proBNP, pg/mL | 578 (5–13,977) | 170 (19–35,000) | 170 (10–15,679) | < 0.001 |
Data are expressed as median (range) for continuous variables and as number of patients (percentage) for qualitative variables.
rEF: reduced ejection fraction (≤ 40%); mrEF: mid-range EF (41–49%); pEF: preserved EF (≥ 50%).
BMI: Body Mass Index; WBC: White Blood Count; ischemic time: delay in hours between chest pain onset and revascularization; CPK: Creatine PhosphoKinase; cTnT: cardiac Troponin T; NT-pro-BNP: N-Terminal pro-Brain Natriuretic Peptide.
P values < 0.05 are shown in bold.
Fig. 1.
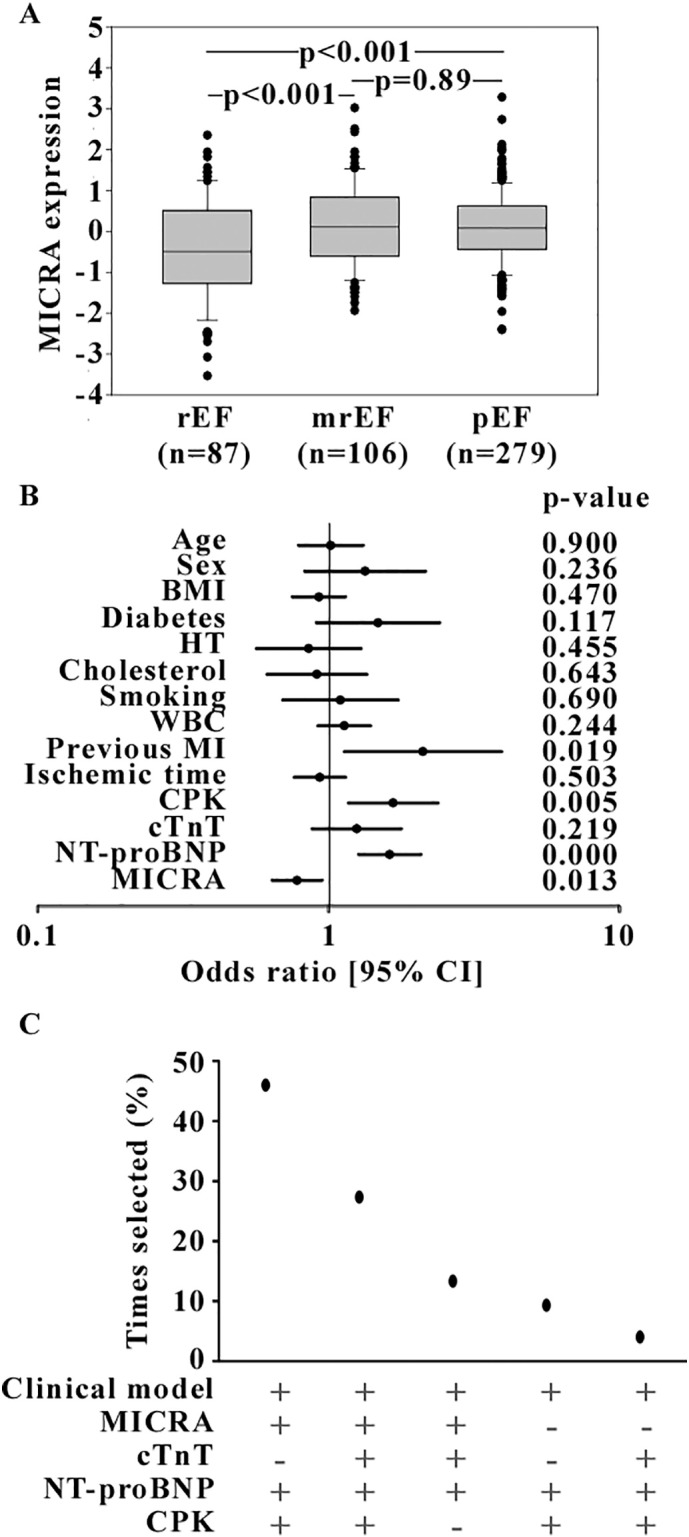
A. Expression levels of MICRA in patients with reduced EF (rEF), mid-range EF (mrEF) and preserved EF (pEF) 4 months after acute MI. Log2-transformed and scaled values are shown. B. Multivariable analyses. Displayed forest plot shows the odds ratios with 95% confidence intervals (CI) for the 3-group classification. Cholesterol = hypercholesterolemia; HT = hypertension. C. Representation of the bootstrap internal validation showing the number of times (in percentage of the 150 bootstrap samples) a model containing the clinical parameters and the indicated biomarkers is selected.
Multivariable analyses were conducted with ordinal regression to assess the ability of MICRA to classify patients into the 3 EF groups. All demographic and clinical parameters and biomarkers displayed in Table 1 were included in the models. We observed that patients with an antecedent of MI (odds ratios (OR) [95% confidence intervals (CI)] 2.11 [1.13–3.93]), elevated levels of CPK (1.67[1.17–2.38]) or NTpro-BNP (1.62[1.27–2.07]) were at high risk of decreased EF (Fig. 1B). Patients with lower levels of MICRA were also at high risk of decreased EF (0.78[0.64–0.95]). The AIC of the model including all clinical parameters and biomarkers was 849.34. Adding MICRA to this model leads to a significant improvement of the AIC (845.07, p = 0.012). To identify the optimal predictive biomarkers, we conducted a bootstrap internal validation and we observed that MICRA was selected in 86% of the models (Fig. 1C), being present in the top 3 selected models. Nt-proBNP was selected in all models. The model selected the highest number of times out of the 150 bootstrap samples (46% of times) was a model including the clinical parameters, Nt-proBNP, CPK and MICRA.
4. Discussion
We report that the circRNA MICRA is associated with the extent of LV dysfunction after MI.
In our past study, MICRA was identified for its value to predict the development of HF as assessed by a 4-month EF ≤ 40%. In the present study, we extend this finding and show that MICRA is able to risk stratify patients after MI. Although the 3-group classification recommended by the 2016 ESC guidelines [9] relates to the severity of HF in HF patients, we chose to apply this classification to test the risk stratification value of MICRA after acute MI. Our data confirm the association between MICRA and outcome after MI, as well as the inverse relationship between MICRA and the EF. A functional association between MICRA and LV remodelling remains hypothetical at this stage and may involve a sponge effect of microRNA-150 which has been shown to be associated with the development of HF after AMI [12], [13].
We observed that MICRA was absent in plasma and serum samples and was mostly expressed by peripheral blood cells (data not shown). In the present cohort, white blood cells count was higher in patients with a decreased EF compared with patients with a preserved EF, although this parameter was not associated with outcome in multivariable analyses. Since MICRA was less expressed in patients with a decreased EF, the association between MICRA and LV dysfunction may not be a simple mirror of the activation of inflammation that occurs within the few hours after MI and which is a sign of poor outcome. This is consistent with the belief that transcriptomic signatures of blood cells can inform about prognosis [5] and most importantly can provide an incremental predictive value to classical markers.
To address this added value, we conducted specific statistical approaches developed and validated in previous studies [14], [15]. These analyses were weighted to avoid model overfitting due to the multiplication of predictive variables. In the present study, we used ordinal regression to classify patients into the 3 EF groups, when logistic regression is suitable for dichotomous outcomes. Together with bootstrap internal validation, ordinal regression analysis attested for the incremental value of MICRA to risk stratify MI patients. This result is clinically relevant since, in the era of personalized medicine, identifying patients at risk of developing HF shortly after MI may help adapting healthcare. However, currently available markers suffer from important limitations which preclude their clinical application.
Other types of long non-coding RNAs than circRNAs are associated with HF development after MI [11], [16]. As compared to linear long non-coding RNAs, circRNAs are abundant, conserved and resistant to degradation by exoribonucleases, which confers them with significant advantages for their potential future as biomarkers [6], [17], [18]. The development of novel techniques to measure gene expression such as digital droplet PCR may help refining the use of MICRA towards clinical application. This study is limited by the choice of the 3-group classification of the ESC which was designed for HF patients. However, our data suggest that biomarkers (MICRA and Nt-proBNP) may help in fine tuning risk stratification after MI in addition to predicting whether or not a patient will develop LV dysfunction. It has to be noted that in our study patients with an EF equal to 40% were included in the rEF group. Considering the estimated inaccuracy above 6% of the echocardiography used to assess EF [19], the boundaries between groups are difficult to define. In our patient population, the inclusion of patients with an EF of 40% in the mrEF subgroup leads to an increase of the odds ratio of MICRA in the multivariable analyses (0.83[0.69–1.01]; p = 0.066), reflecting a loss of statistical power due to a very small group of rEF patients (n = 41). Another limitation resides in the lack of EF determination shortly after AMI which reflects infarct size and influences the extent of LV remodelling and the development of HF. However, cardiac injury markers CPK and cTnT measured at admission were entered in multivariable models to account for the effect of infarct size. In this study, MICRA was measured at the time of reperfusion. Further studies are required to determine the time-point allowing the best predictive value, to characterize the day-to-day variability, and to evaluate the reproducibility of MICRA measurement.
In conclusion, we show that MICRA provides an incremental value to risk stratify patients after MI. Further prospective studies are warranted to confirm this predictive value and the clinical usefulness of this finding.
Acknowledgements of grant support
This work was funded by the Ministry of Higher Education and Research of Luxembourg and the Society for Research on Cardiovascular Diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
The authors are grateful to Dr. Daniel Wagner, head of cardiology at Luxembourg Hospital (LU) and Lausanne Hospital (CH) who initiated the patient cohort used in this study. We thank Loredana Jacobs for sample collection, and Christelle Nicolas, Bernadette Leners and Angela Tavares-Furtado for expert technical assistance.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur. Heart J. 2016;37(42):3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe A.S., Vasile V.C., Milone M., Saenger A.K., Olson K.N., Apple F.S. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J. Am. Coll. Cardiol. 2011;58(17):1819–1824. doi: 10.1016/j.jacc.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Talwar S., Squire I.B., Downie P.F., McCullough A.M., Campton M.C., Davies J.E. Profile of plasma N-terminal proBNP following acute myocardial infarction; correlation with left ventricular systolic dysfunction. Eur. Heart J. 2000;21(18):1514–1521. doi: 10.1053/euhj.1999.2045. [DOI] [PubMed] [Google Scholar]
- 5.Devaux Y. Transcriptome of blood cells as a reservoir of cardiovascular biomarkers. Biochim. Biophys. Acta, Mol. Cell Res. 2017;1864(1):209–216. doi: 10.1016/j.bbamcr.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Devaux Y., Creemers E.E., Boon R.A., Werfel S., Thum T., Engelhardt S. Circular RNAs in heart failure. Eur. J. Heart Fail. 2017;19(6):701–709. doi: 10.1002/ejhf.801. [DOI] [PubMed] [Google Scholar]
- 7.Elia L., Quintavalle M., Condorelli G. Circular RNAs and heart failure: new players for an old disease. Cardiovasc. Res. 2017;113(3):254–255. doi: 10.1093/cvr/cvx007. [DOI] [PubMed] [Google Scholar]
- 8.Vausort M., Salgado-Somoza A., Zhang L., Leszek P., Scholz M., Teren A. Myocardial infarction-associated circular RNA predicting left ventricular dysfunction. J. Am. Coll. Cardiol. 2016;68(11):1247–1248. doi: 10.1016/j.jacc.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. 2016. [DOI] [PubMed] [Google Scholar]
- 10.Wagner D.R., Devaux Y., Collignon O. Door-to-balloon time and mortality. N. Engl. J. Med. 2014;370(2):180–181. doi: 10.1056/NEJMc1313113. [DOI] [PubMed] [Google Scholar]
- 11.Vausort M., Wagner D.R., Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ. Res. 2014;115(7):668–677. doi: 10.1161/CIRCRESAHA.115.303836. [DOI] [PubMed] [Google Scholar]
- 12.Devaux Y., Vausort M., McCann G.P., Zangrando J., Kelly D., Razvi N. MicroRNA-150: a novel marker of left ventricular remodeling after acute myocardial infarction. Circ. Cardiovasc. Genet. 2013;6(3):290–298. doi: 10.1161/CIRCGENETICS.113.000077. [DOI] [PubMed] [Google Scholar]
- 13.Goretti E., Vausort M., Wagner D.R., Devaux Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int. J. Cardiol. 2013;168(4):4548–4550. doi: 10.1016/j.ijcard.2013.06.092. [DOI] [PubMed] [Google Scholar]
- 14.Devaux Y., Salgado-Somoza A., Dankiewicz J., Boileau A., Stammet P., Schritz A. Incremental value of circulating MiR-122-5p to predict outcome after out of hospital cardiac arrest. Theranostics. 2017;7(10):2555–2564. doi: 10.7150/thno.19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaux Y., Vausort M., McCann G.P., Kelly D., Collignon O., Ng L.L. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumarswamy R., Bauters C., Volkmann I., Maury F., Fetisch J., Holzmann A. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014;114(10):1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 17.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58(5):870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Lam C.S., Solomon S.D. Fussing over the middle child: heart failure with mid-range ejection fraction. Circulation. 2017;135(14):1279–1280. doi: 10.1161/CIRCULATIONAHA.117.027324. [DOI] [PubMed] [Google Scholar]


