Abstract
Specific gut commensal bacteria improve host health by eliciting mutualistic regulatory T (Treg) cells responses. However, the bacteria that induce effector T (Teff) cells during inflammation are unclear. Here, we addressed this by analyzing bacterial-reactive TCR transgenic cells and TCR repertoires in a murine colitis model. Unexpectedly, we found that mucosal-associated Helicobacter species triggered both Treg responses during homeostasis and Teff responses during colitis, as suggested by an increased overlap between the Teff/Treg TCR repertoires with colitis. In fact, 4/6 Treg TCRs tested recognized mucosal-associated Helicobacter species in vitro and in vivo. By contrast, the marked expansion of luminal Bacteroides species seen during colitis did not trigger a commensurate Teff response. Unlike other Treg cell-inducing bacteria, Helicobacter species are known pathobionts and cause disease in immunodeficient mice. Thus, our study suggests a model in which mucosal bacteria elicit context-dependent Treg or effector cell responses to facilitate intestinal tolerance or inflammation.
Introduction
We cohabitate with trillions of bacteria that reside within our intestines and provide important beneficial metabolic and immunologic functions (1). However, inappropriate immune responses against these bacteria have been implicated in the pathogenesis of inflammatory bowel disease (IBD) (2–4), as germ-free mice are highly resistant to many murine colitis models. Moreover, monocolonization studies of germ-free animals revealed that IBD development may be driven by specific commensal bacterial species (5).
Tolerance to commensal bacteria is thought to depend on CD4+ regulatory T (Treg) cells (6–8), which we have recently shown can arise via peripheral Treg (pTreg) cell development from naïve T cells in response to commensal antigens during homeostasis (9). However, intestinal inflammation can result in the exposure of new antigens to the immune system. In murine models, effector T (Teff) cell generation to a commensal bacterial antigen was seen during intestinal inflammation, but not homeostasis (10). Thus, it remains unknown whether effector responses during intestinal inflammation are driven by newly exposed antigens versus the commensal antigens that normally drive Treg cell development during homeostasis.
IBD is associated with marked changes in the gut microbiota, dysbiosis (11). Dysbiosis has been hypothesized to contribute to disease pathogenesis, as it is well established that different commensal bacterial species have distinct effects on the intestinal T cell population. For example, segmented filamentous bacteria (SFB) strongly induces T helper 17 (Th17) cells in the small intestine (12), which has now been confirmed by TCR transgenic studies (13). By contrast, introduction of Clostridium clusters XIVa and IV (7, 14) or altered Schaedler flora (ASF) (15) markedly increased the frequency of colonic Foxp3+ regulatory T (Treg) cells in germ-free mice. Thus, changes in the microbiome may also affect the intestinal effector vs regulatory T cell population.
Here, we used TCR repertoire analysis coupled with in vivo studies of commensal-specific TCRs to address the influence of colitis-mediated dysbiosis on bacteria-specific Treg cell development. We also examined the interaction of T cells with luminal versus mucosal associated antigens during homeostasis and colonic inflammation. Finally, we assessed the impact of commensal-specific T cells during lymphopenic conditions. In summary, our data suggest that the mucosal-associated pathobionts, Helicobacter spp., elicit context-dependent T cell responses during homeostasis and colitis.
Results
Overlap of effector and Treg TCR antigen-specificity during colitis
To address the effect of colitis on T cell responses to commensal bacteria, we examined induced models as we wanted to compare mice that started with the same microbiota. We settled on a murine model of inflammatory colitis (Fig. 1A) (16) that incorporates 1% dextran sodium sulfate (DSS) induced mucosal injury along with anti-IL-10R antibody (αIL10R) (15, 17), which blocks an important immune regulatory pathway implicated in human IBD in genome-wide association studies (18). DSS+αIL10R treatment induced colitis as evidenced by weight loss, increased colon weight/length ratio, and a marked enhancement of Teff (CD44hiCD62Llo) and Treg cells (fig. S1). As the combination showed a greater effect on the microbiota than DSS or αIL10R alone (fig. S1E), we used DSS+aIL10R for subsequent experiments.
Fig. 1. Colonic Treg TCRs (CT2 and CT6) drive effector T cell development in inflammation.
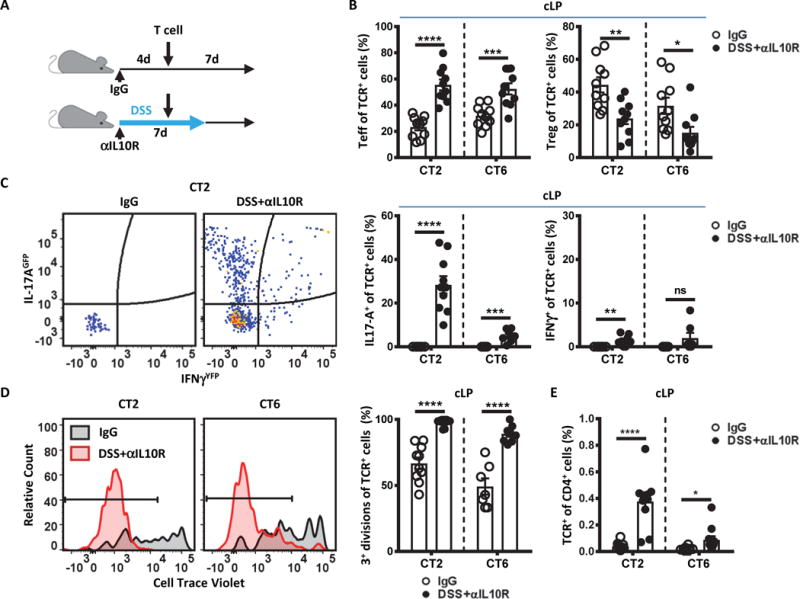
(A) Experimental model. CD45.1 4–5 week old SPF mice were administered αIL10R (1 mg/mouse) on day 0 and kept on 1% DSS water for 7 days to initiate colitis. Control mice were given isotype IgG (1 mg/mouse) on day 0. Four days after initiation of colitis, congenically marked naïve CT2/CT6 Tg cells (105) were intravenously transferred and analyzed 7 days later. (B to D) Effector cell induction and expansion with colitis. Transferred TCR Tg cells from the colon lamina propria (cLP) were analyzed by flow cytometry for (B) development of Teff (CD44hi CD62Llo Foxp3−) and Treg (Foxp3+) makers (p=0.000003; 0.0007; 0.0024; 0.0157; n=10; Student’s t-test); (C) upregulation of cytokines using IL-17AGFP and IFNγYFP (p=0.000001; 0.0007; 0.0029; n=10 for CT2; n=7 for CT6; Student’s t-test); (D) proliferation indicated by Cell Trace Violet (CTV) dilution (p=0.000002; 0.00002; n=10 for CT2; n=7, 8 for CT6; Student’s t-test); and (E) expansion indicated by the in vivo frequency amongst the host CD4+ T cells (p=0.00005; 0.0275; n=10 for CT2; n=10, 11 for CT6; Student’s t-test). Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Consistent with previous reports, naïve T cells from CT2 and CT6 TCR transgenic (Tg) lines, which express TCRs isolated from colonic Treg cells and recognize commensal antigens (8), showed substantial induction of Foxp3, the canonical Treg cell transcription factor, by 1 week after transfer into control IgG treated mice (Fig. 1A and B) (9). However, in mice undergoing DSS+αIL-10R mediated colitis, they skewed towards Teff cell generation (Fig. 1B, S2). Teff cell differentiation was primarily to the Th17 phenotype based on IL-17AGFP and IFNγYFP reporters (Fig. 1C), consistent with observations in human Crohn’s disease (19). In addition, CT2 and CT6 cells underwent extensive proliferation as assessed by Cell Trace Violet dilution and expanded as a fraction of total CD4+ cells (Fig. 1D and E). Thus, the normal tolerogenic Treg cell developmental response to commensal bacterial antigens can be re-directed to a Th17 effector response during experimental colitis.
To assess whether the responses of CT2 and CT6 TCR Tg cells were representative of the T cell population, we analyzed the effect of colitis on the TCR repertoire (fig. S3A and B, table S1) Because the great diversity in polyclonal T cells precludes experimental analysis at the individual TCR level, we utilized mice in which TCR diversity is limited by a transgenic fixed TCRβ chain (20). Although TCR diversity is diminished, this approach allows high-throughput analysis of the TCR repertoire at the individual TCR level via sequencing of the variable TCRα chains. With colitis, both Treg and Teff repertoires showed increased clonal expansion compared to controls (fig. S3C), consistent with a T cell response. Notably, we saw a marked increase in similarity between the Treg and Teff TCR repertoires within the colon lamina propria (cLP) of individual mice during colitis compared with controls (Fig. 2A, S3D). Examination of the top 25 Teff TCRs per mouse showed that many of these TCRs are also found in the Treg cell subset during colitis (Fig. 2B), consistent with our TCR transgenic studies (Fig. 1). In control mice, the relatively low degree of overlap between the Teff and Treg cell subset within individual mice (Fig. 2A and B, S3D) is consistent with our previous studies (8, 21). As before, we also observed mouse-to-mouse variability of Teff and Treg TCR repertoires between mice (Fig. 2A, S3D and E). To confirm the TCR repertoire analysis, we cloned T7-1 (Fig. 2C), the most abundant TCR found in the Teff subset of colitic mice by mean percentage (fig. S3E). As predicted by the TCR repertoire study, analysis of adoptively transferred peripheral TCli-αβ TCR Tg cells expressing T7-1 showed that this TCR facilitated Foxp3 induction during homeostasis, but skewed towards Teff cell generation with colitis (Fig. 2D, S4). Thus, these data demonstrate an increased overlap between the Teff and Treg TCRs repertoires during colitis that does not occur at homeostasis.
Fig. 2. Treg and Teff subsets show increased TCR overlap during colitis.
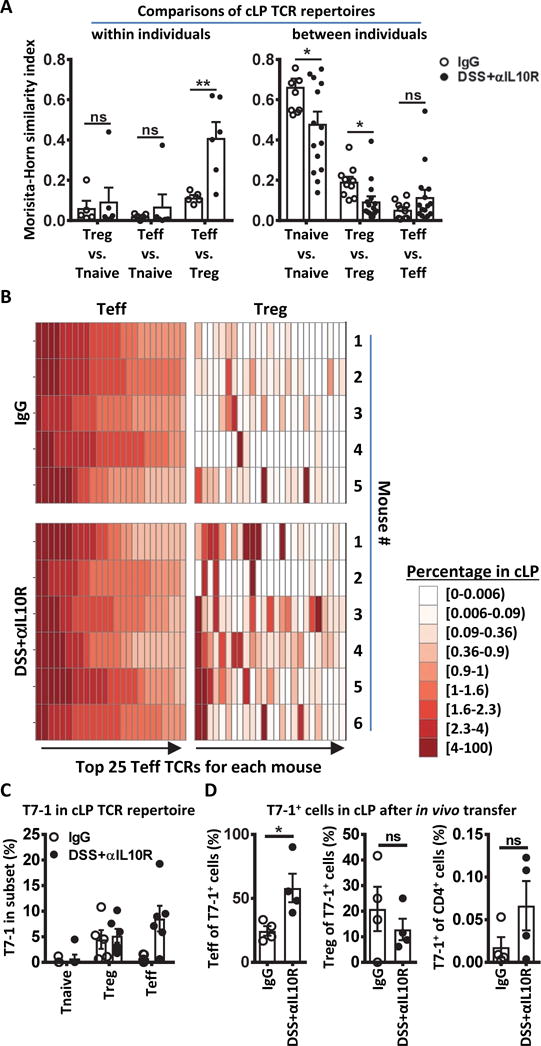
(A to C) Analysis of TCRα repertoires from Tnaive (CD44lo CD62hi), Treg (Foxp3+), and Teff (CD44hi CD62Llo) cells in the cLP of TCli TCRβ Foxp3IRES-Thy1.1 Tcra+/− mice 2 weeks after initiation of IgG or DSS+αIL10R. n=5, 6. (A) Morisita–Horn similarity comparison between two different T cell subsets within each mouse (left) or between different mice within each T cell subset (right). An index value of 1 indicates that the two samples are completely similar and an index value of 0 means they are completely dissimilar (left: p=0.009; n=5,6; right: p=0.033; 0.014; n=10,15; Student’s t-test). (B) Heatmap showing the top 25 Teff TCRs in one mouse per row and their corresponding percentage in the Treg subset. Note that each column does not represent one TCR across all mice. (C) Percentage of T7-1 TCR in repertoire of Tnaive, Treg, and Teff subsets. Each dot represents data from an individual mouse. (D) In vivo analysis of T7-1 TCR. T7-1 was retrovirally transduced into in vitro activated naïve TCliβ Rag1−/− Tg cells and 2×105 cells were transferred into IgG or DSS+αIL10R hosts as indicated in Fig. 1A. Seven days post-transfer, cells from the cLP were analyzed by flow cytometry for Teff and Treg cells, and the in vivo frequency amongst the host CD4+ T cells (p=0.029; n=4; Student’s t-test). Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Our TCR Tg data suggest that the overlap in Treg and Teff TCR usage during colitis is due to enhanced Teff generation by clones that might normally differentiate into Treg cells (Fig. 1). Alternatively, the overlap may arise from increased Treg cell generation by Teff TCRs during colitis. However, transfer of CBir1 Tg cells (22) into DSS+αIL10R hosts showed increased Teff but not Treg generation (fig. S5), consistent with a previous report in the DSS model (10). Although it remains possible that the CBir1 result in the DSS+αIL10R model is not generalizable to other model systems, in conjunction with the TCR repertoire analysis, these TCR Tg studies suggest that colitis skews T cell development such that pro-inflammatory Teff cells often use the same TCRs as anti-inflammatory Treg cells.
Helicobacter spp. are important drivers of peripheral Treg cell development during homeostasis
The observation that certain commensal antigens can activate both Treg cells during homeostasis and Teff cells during colitis suggested that these bacterial antigens are continually presented to the adaptive immune system. Notably, this pattern is different from the CBir1 commensal antigen, which is primarily presented to T cells during colitis (fig. S5) (10). To address this, we assessed the in vitro reactivity of these TCRs to fecal antigen preparations presented on CD11c+ dendritic cells (DCs) (8). Although TCR activation by fecal antigen from control mice was high with T7-1 and detectable with CT2 and CT6, the degree of enhancement in TCR activation by fecal antigens from colitic mice was limited (Fig. 3A, S6). We therefore asked whether these TCRs show more reactivity to mucosal associated (MA) antigens, which might be predicted to have greater access to the immune system via DC uptake (23), goblet associated passages (24), or outer membrane vesicles (25). Both CT2 and CT6 showed marked enhancement of TCR stimulation with MA compared with luminal antigen (Fig. 3A). We then tested additional Treg TCRs, CT1, CT7 and CT9, which we previously found to react to fecal antigens or commensal isolates in vitro (8). Amongst these 6 colonic Treg TCRs, 4 showed high level reactivity in vitro to MA compared with luminal antigen, and of these 2 were enhanced by colitis (Fig. 3A).
Fig. 3. Colonic Treg TCRs react to mucosal-associated Helicobacter species.
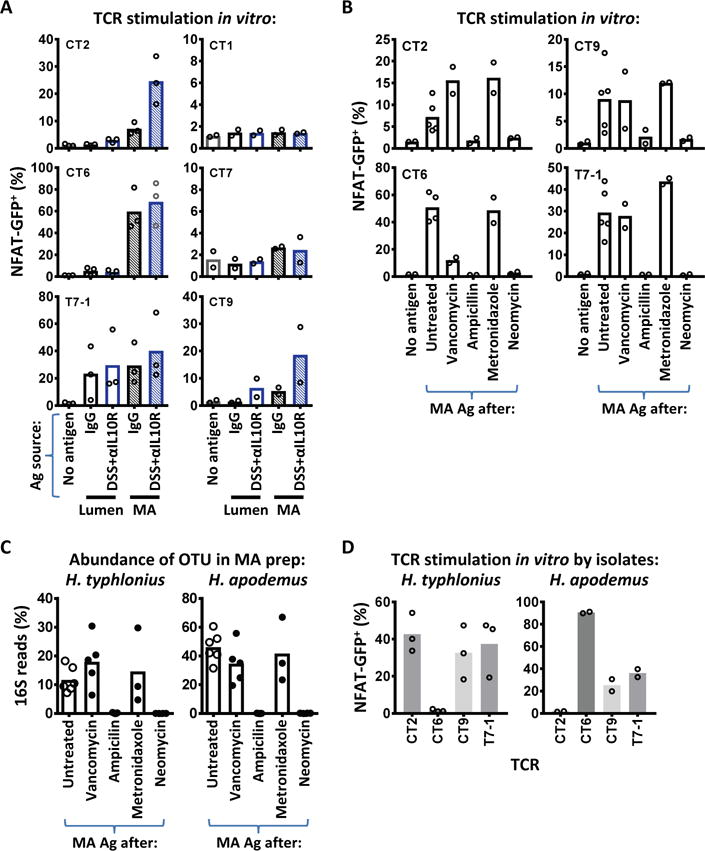
(A) Treg TCRs preferentially react to mucosal-associated antigens (Ag). Hybridoma cells expressing different TCRs were cultured with CD11c+ dendritic cells and the indicated Ag obtained 2 weeks after initiation of colitis. NFAT-GFP upregulation was assessed by flow cytometry 1.5 days later. (B) Selective elimination of MA Ag using individual antibiotics. TCRs from (A) that react to MA Ag were stimulated with colonic MA Ags isolated from antibiotic-treated or untreated mice as per (A). (C) Changes in H. typhlonius and H. apodemus OTUs correlate with in vitro reactivity to MA Ag in (B). Data shown are the percentage of 16S OTUs from the MA preparations of individual antibiotic-treated or untreated mice. (D) In vitro recognition of H. typhlonius or apodemus. Cultured isolates were tested for TCR reactivity in vitro as per (A). 2–3 independent experiments. Bars indicate mean.
To confirm that the reactivity in the MA antigen preparation was dependent on the microbiota, we treated specific-pathogen-free (SPF) mice with a broad spectrum antibiotic combination of vancomycin, ampicillin, metronidazole, and neomycin (VAMN), and found that MA antigen no longer stimulated our 4 MA antigen reactive-Treg TCR panel (CT2, CT6, CT9 and T7-1; fig. S7A). This antibiotic cocktail includes vancomycin, which has been reported to decrease Treg cell numbers and Clostridium spp. (7). We then tested the individual antibiotics to attempt to identify the bacterial spp. recognized by these TCRs by correlating in vitro TCR reactivity to MA antigen with changes in the bacteria composition.
Unexpectedly, these data suggested that Helicobacter spp., and not the predicted Clostridium spp. (7), were being recognized by the TCR panel. First, of the individual antibiotics, ampicillin and neomycin, but not vancomycin or metronidazole, markedly decreased the ability of MA antigen to stimulate the TCR panel compared with untreated controls (Fig. 3B). Second, 16S ribosomal RNA (rRNA) gene sequences from the MA antigen preparations revealed that the two most frequent Operational Taxonomic Units (OTUs) lost with ampicillin or neomycin were Helicobacter typhlonius (H. typhlonius) and H. apodemus (Fig. 3C, S7B).
To determine if Helicobacter spp. were being directly recognized by these TCRs, we tested cultured isolates that matched the 16S rRNA sequence (Fig. 3D). Notably, there were specific patterns of TCR reactivity, with T7-1 and CT9 recognizing both spp., whereas CT2 reacted only to H. typhlonius, and CT6 to H. apodemus. Recognition of Helicobacter spp. in vitro appeared to be TCR specific and not via a superantigen or other antigen-independent mechanism. First, each Helicobacter isolate stimulated only some, but not all, TCRs. Second, addition of anti-MHC II blocked TCR activation (fig. S7C). Third, testing of these TCRs against multiple bacterial isolates in vitro from different genera including Clostridium, Bifidobacterium, and Lactobacillus failed to activate our TCR panel to the same degree as Helicobacter (fig. S8). However, these TCRs could still show cross-reactivity to different bacterial species due to shared epitopes. T7-1 likely recognizes additional bacterial antigens as it reacted to luminal antigens with high efficiency (Fig. 3A). CT6 may also recognize other bacterial species as vancomycin reduced CT6 response to MA antigen without a corresponding decrease in H. apodemus frequency by 16S (Fig. 3B and C). We have also shown previously that CT6 reacted to an uncharacterized Clostridium spp. (8). While the extent of bacterial cross-reactivity remains to be defined, these data clearly demonstrate that 4 of our 6 colonic Treg TCRs recognize Helicobacter spp. in vitro.
We then asked whether these Helicobacter spp. could induce TCR-specific Treg cell responses in vivo. As expected, transfer of CT2/CT6/CT9/T7-1 TCR+ cells into ampicillin-treated mice to eliminate pre-existing Helicobacter spp. (Fig. 3C) resulted in little proliferation or differentiation (Fig. 4A). However, inoculation of ampicillin-treated mice with a single Helicobacter spp. elicited robust expansion and Foxp3 upregulation in appropriate TCR+ Tg cells (Fig. 4A). Similarly, Helicobacter-free mice obtained from Charles River Laboratories showed little ability to induce TCR-dependent proliferation or Treg cell generation, unless the appropriate Helicobacter spp. identified in vitro (Fig. 3D) was subsequently inoculated in vivo (Fig. 4B, S9). Notably, as CT2 and CT6 Tg cells were co-transferred into the same host in this experiment, it appears that these TCR clones show a high degree of specificity for individual Helicobacter strains (Fig. 4B). Thus, these data demonstrate that Helicobacter spp. are a major driver of bacterial-specific Treg cell responses during homeostasis.
Fig. 4.
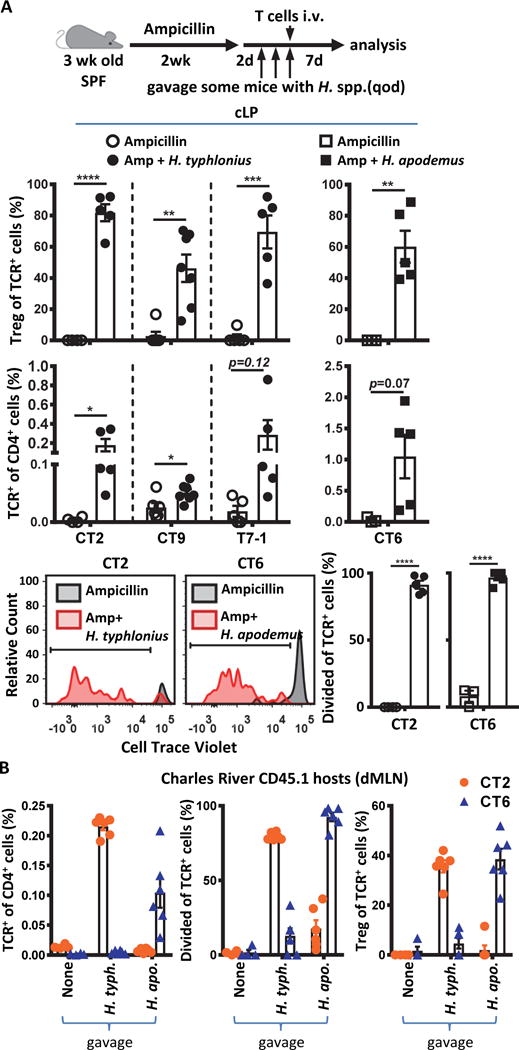
Helicobacter species induce peripheral Treg cell differentiation during homeostasis. (A) In vivo validation of TCR reactivity to Helicobacter species. 3 week old SPF mice were treated with ampicillin (amp) for 2 weeks via drinking water. Two days after the last treatment, H. typhlonius or H. apodemus were gavaged 3 times total every other day. With the last gavage, congenically marked naïve CT2/CT6 Tg cells or retrovirally expressed CT9/T7-1 cells were transferred. Seven days post transfer, cLP cells were analyzed by flow cytometry for (top) development of Treg (Foxp3+) cells (p=0.000003; 0.001; 0.0002; 0.0046; n=4, 5 for CT2; n=6, 7 for CT9; n=5 for T7-1; n=3, 5 for CT6; Student’s t-test); (middle) frequency of transferred TCR-expressing cells amongst the host CD4+ T cells (p=0.0442; 0.021; 0.0046;0.0705; n=4, 5 for CT2; n=6, 7 for CT9; n=5 for T7-1; n=3, 5 for CT6; Student’s t-test); or (bottom) CTV dilution (p=0.00000002; 0.0000007; n=4, 5 for CT2; n=3, 5 for CT6; Student’s t-test). (B) T cell response to Helicobacter in vivo is species-specific. Three week old SPF mice obtained from Charles River Laboratories were gavaged with H. typhlonius or H. apodemus (3 times total every other day). With the last gavage, congenically marked naïve CT2 and CT6 Tg cells (105 each) were co-transferred. One week post-transfer, cells from the dMLN were analyzed by flow cytometry for the frequency of transferred TCR-expressing cells amongst the host CD4+ T cells (left); CTV dilution (middle); or development of Treg (Foxp3+) cells (right) (n=4, 6, 6). Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Marked changes in bacterial composition with colitis
The observation that colitis enhanced the in vitro stimulatory ability of MA antigen (Fig. 3A), and increased the proliferation of adoptively transferred CT2 and CT6 TCR Tg cells (Fig. 1B), suggested that there might be alterations in the colonic microbiota. We therefore analyzed the changes in the MA and luminal 16S rRNA profile with colitis. Consistent with previous reports, the bacterial composition in the lumen versus mucosa is quite distinct (Fig. 5A–C) (26, 27). In the MA preparation, we noted a high frequency of Helicobacter spp. in both control and colitic mice (Fig.5B), consistent with the activation and differentiation of CT2 and CT6 Tg cells in vivo under both conditions (Fig. 1). The frequency of H. typhlonius was further increased in colitic mice (Fig. 5D and E), which may explain the increased in vitro MA antigen reactivity of CT2 and CT9 Treg TCRs with colitis (Fig. 3A). In the lumen, besides the increase in H. typhlonius (Fig. 5D and E), we noted that colitis induced a marked expansion of Bacteroides (B.) spp., particularly B. vulgatus, which represented over 20% of 16S reads in some mice (Fig 5D and F). As there was no obvious decrease in bacterial density (fig. S10), these data suggest that DSS+αIL10R colitis is associated with a major bloom of Bacteroides spp. in the lumen.
Fig. 5. DSS+αIL10R colitis is associated with differential changes of bacterial composition in the lumen and mucosa.
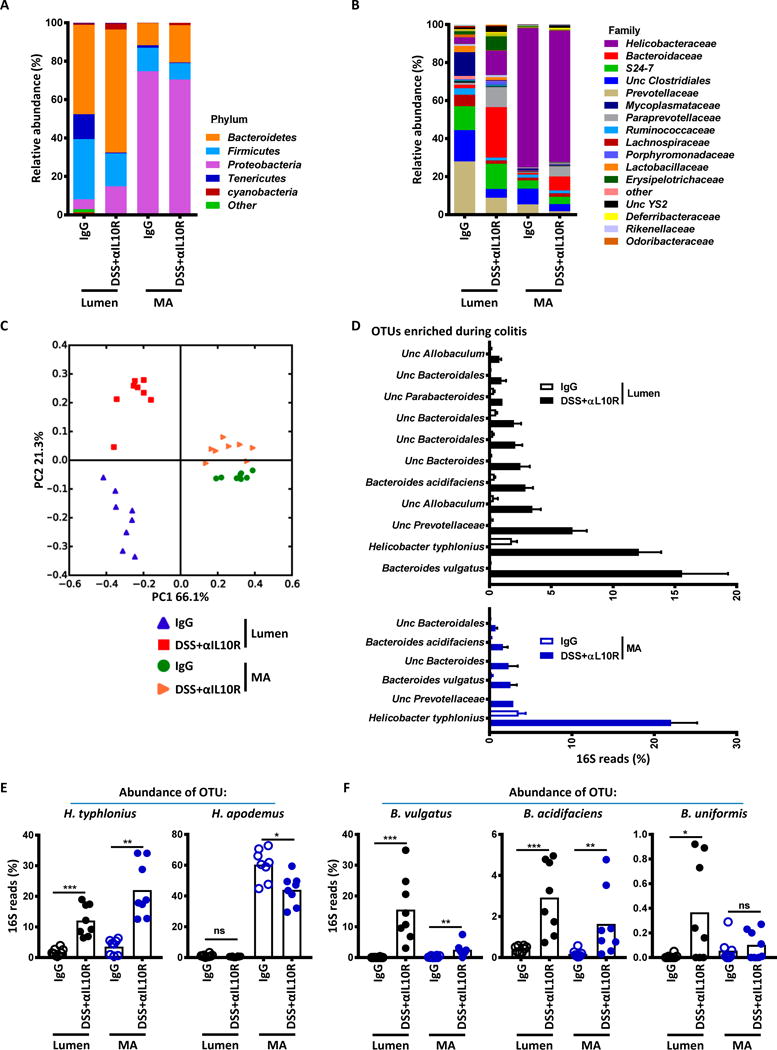
(A to D)16S rRNA sequencing of colonic lumen contents or MA preparations 2 weeks after initiation of IgG or DSS+αIL10R. n=8. Data shown are mean bacterial changes at the phyla (A) or family (B) level, and principal coordinates analysis on unweighted UniFrac distances (C). (D) Bacteria enriched during colitis. Shown are OTUs enriched in DSS+αIL10R mice with average percentage >1% and Benjamini-Hochberg adjusted p value <0.05 (Mann-Whitney U) from lumen contents or MA preparations. (E) Increase of H. typhlonius in the mucosa with colitis. Percentages of H. typhlonius and H. apodemus OTUs are shown (Benjamini-Hochberg adjusted p value: p=0.0009; 0.0023; 0.0277; n=8; Mann-Whitney U test). (F) Marked expansion of Bacteroides species in the lumen with colitis. Percentages of B. vulgatus, B. acidifaciens, and B. uniformis OTUs are shown (Benjamini-Hochberg p=0.0009; 0.0086; 0.0009; 0.0086; 0.0266; n=8; Mann-Whitney U test). Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Lack of T cell responses to the bloom of luminal Bacteroides species during colitis
As Bacteroides species have been suggested to be important triggers of intestinal inflammation in other murine models (28, 29), we asked whether the dramatic expansion of Bacteroides species in DSS+αIL10R treated mice induced strong anti-microbial effector T cell responses. We assessed the in vivo responses of several TCRs that recognize Bacteroides strains isolated from DSS+αIL10R mice in vitro, including the B. vulgatus-reactive TCR DP1 (Fig. 6A, table S2). These TCRs recognized Bacteroides strains through MHC II and did not respond in vitro to the Helicobacter strains tested (fig. S11A and B). To our surprise, adoptively transferred T cells expressing these Bacteroides-reactive TCRs did not show increased proliferation, expansion, or Teff cell development in DSS+αIL10R treated mice compared with controls (Fig. 6B and C). Consistent with the poor expansion, very few transferred cells were recovered from the colon. In fact, the percentage of the transferred TCR-expressing cells amongst total CD4+ T cells was reduced during DSS+αIL10R colitis (Fig. 6C), implying that these antigens are relatively less important during colitis despite their bloom in the lumen. We confirmed in vitro that the luminal antigens for DP1 and NT2 increased with colitis (Fig. 6D), as predicted based on 16S rRNA sequencing (Fig. 5F). By contrast, MA antigen showed lower stimulatory ability than luminal antigen for DP1 and NT2 even during colitis (Fig. 6D). We also checked that the Helicobacter spp.-reactive TCRs (Fig. 3D) did not respond to these Bacteroides strains (fig. S11C). Although it remains possible that these Bacteroides-reactive TCR clones do not compete well in the gut or are not representative of polyclonal T cell responses to Bacteroides, these data suggest that the marked expansion of luminal Bacteroides spp. in this model of colitis does not elicit a corresponding increase in antigen-specific T cell responses.
Fig. 6. Expansion of Bacteroides species during colitis does not enhance TCR-specific T cell responses.
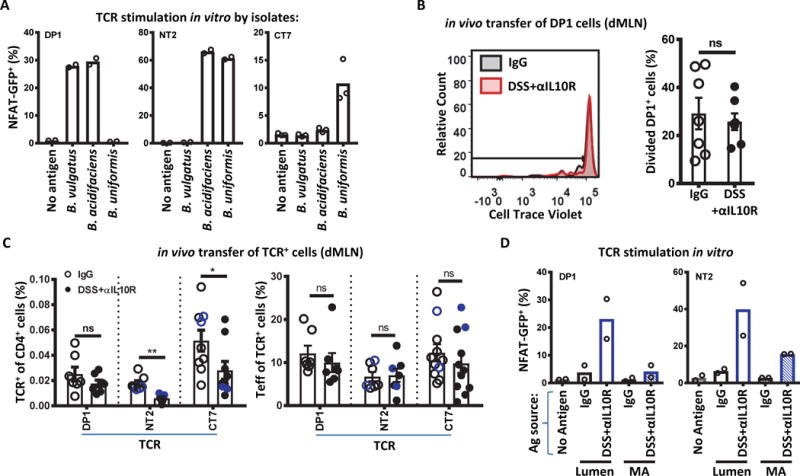
(A) Antigenic reactivity of Bacterioides-reactive TCRs used. In vitro stimulation by the Bacteroides isolates are shown as per Fig. 3A. 2–3 independent experiments. (B and C) In vivo expansion and effector cell development of Bacteroides-reactive T cells. Congenically marked naïve DP1 Tg cells (105) were transferred into CD45.1 hosts as indicated in Fig. 1A, and analyzed 7 days post-transfer by flow cytometry for (B) CTV dilution in the dMLN (n=7), (C, left) frequency amongst the host CD4+ T cells (p=0.0005; 0.047; n=7 for DP1 and NT2; n=9 for CT7; Student’s t-test), and (C, right) development of Teff (CD44hi CD62Llo Foxp3−) (n=7 for DP1 and NT2; n=11 for CT7; Student’s t-test). NT2 and CT7 (C) were retrovirally transduced into in vitro activated naïve Vα2− TCliβ Tg cells from Rag1+/− (black) or Rag1−/− (blue) mice before transfer of 2×105 cells. (D) In vitro reactivity to in vivo Ag preparations is consistent with Bacteroides expansion by 16S rRNA analysis. Colonic lumen contents or MA preparations from IgG or DSS+αIL10R mice were tested as per Fig. 3A. 2 independent experiments. Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Pathogenic potential of Helicobacter-reactive TCRs
The differentiation of colonic Treg TCRs into Teff cells during colitis (Fig. 1) suggests that the altered T cell response to bacterial antigens may contribute to colonic inflammation. To test the pathogenic potential of these Helicobacter spp.-reactive TCRs, we adoptively transferred naïve CT6 Tg cells into Rag1−/− hosts with or without H. apodemus inoculation. In contrast with wild-type hosts (Fig. 4), H. apodemus drove antigen-specific Teff generation of CT6 Tg cells (Fig. 7A, S12) in Rag1−/− hosts likely due to expansion in a lymphopenic environment. In line with a Teff response, we observed colonic inflammation in CT6-transferred hosts only in the presence of H. apodemus as evidenced by increased colon weight/length ratio and crypt dropout in histology (Fig. 7B and C). Similarly, Teff response of CT2 Tg cells to H. typhlonius lead to colonic inflammation in Rag1−/− hosts (fig. S13). We did not note major weight loss induced by these TCR Tg cells and/or Helicobacter spp. during the time frame of this experiment (fig. S13D and E), in contrast with polyclonal T cell transfer models (30). This could be due to limited systemic effects from a single TCR clone or differences in gut microbiota composition. Although Helicobacter spp. without T cell transfers have been reported to induce colitis in Rag-deficient mice (31–34), we did not observe inflammation caused by these two strains during the period of the experiment (Fig. 7, S13). Thus, these data demonstrate the potential pathological consequences of antigen-specific Teff expansion against gut bacteria.
Fig. 7. Pathogenic potential of naive Helicobacter-reactive CT6 cells in lymphopenic mice.
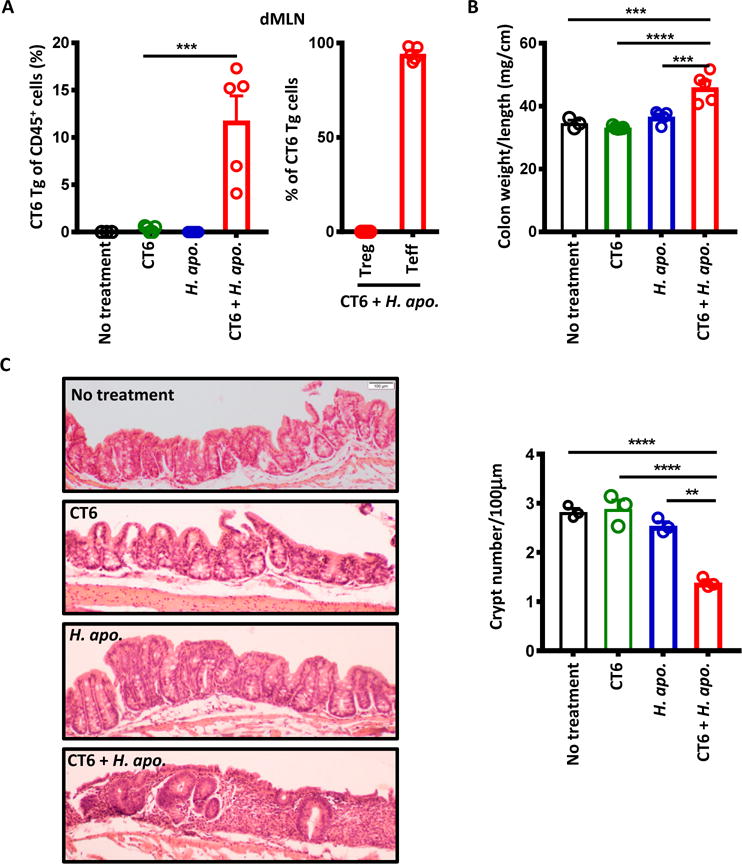
Six week old Rag1−/− mice were given H. apo. with or without naïve CT6 T cell transfer. H. apo. was gavaged 3 times total every other day. Naïve CT6 cells (105) was transferred at the time of last gavage. (A) Flow cytometry analysis for the frequency of Tg cells amongst the host CD45+ cells (one-way ANOVA, Turkey’s post-hoc test: p=0.0002 CT6 vs CT6+H. apo.; n=3, 5, 5, 5), and development of Treg (Foxp3+) and Teff (CD44hi CD62Llo Foxp3−) markers (n=5) in dMLN at 6 weeks. (B) Colon weight/length ratio at 6 weeks (one-way ANOVA with Turkey’s post-hoc test: p=0.0003 No treatment vs CT6+H. apo.; 0.00002 CT6 vs CT6+H. apo.; 0.0005 H. apo. vs CT6+H. apo.; n=3, 5, 5, 5). (C) Representative haematoxylin/eosin-stained section of the ascending colon at 6 weeks (original magnification ×10), and quantification of crypt number (one-way ANOVA with Tukey’s post-hoc test: p=0.00007 No treatment vs CT6+H. apo.; 0.00005 CT6 vs CT6+H. apo.; 0.0003 H. apo. vs CT6+H. apo.; n=3). The number of crypts observed per 100μm of ascending colon was averaged from five fields. Decreased crypt number reflect crypt dropout due to inflammation. Bars indicate mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
Finally, mucosal associated enterohepatic Helicobacter spp. in humans have been suggested to be associated with IBD (35). We have also detected the presence of Helicobacter spp. in patients with Crohn’s disease (CD) or ulcerative colitis (UC) by 16S rRNA sequencing of fecal or colonoscopy samples (fig. S14). However, it remains to be established whether these Helicobacter spp. play a similar immunomodulatory role in humans as seen in our mouse studies.
Discussion
Based on this study of T cell responses to commensal antigens during experimental colitis, we make the following observations: First, effector T cell responses during colitis are often to commensal antigens normally presented to the immune system during homeostasis, and not to antigens normally excluded by the mucosal barrier. Second, commensal bacterial species vary widely in their ability to induce T cell responses due in part to their anatomic location relative to the mucosal surfaces. Third, constitutively presented antigens appear to primarily elicit Treg cell responses during homeostasis, implying that tolerance to these antigens is important to preventing colitis. Finally, Helicobacter spp. are important inducers of T cell responses during homeostasis and colitis. Thus, these data suggest a model whereby Treg cell-mediated tolerance, and not effector cell mediated immunity, is required against commensal bacteria such as Helicobacter spp. that routinely interact with the host immune system.
Our initial goal was to study the T cell response to alterations in gut microbiota structure, a.k.a dysbiosis, that occurs with colitis and inflammatory bowel disease (IBD) and has been proposed to be involved in disease pathogenesis (4, 36, 37). However, rather than observing a dominant effector T cell response to Bacteroides spp. that bloom during colitis, the major stimulators were Helicobacter spp. that routinely interact with T cells during homeostasis. These data do not exclude the possibility that dysbiosis of Bacteroides spp. may exert non-antigen dependent effects on T cell immunity through changes in short chain fatty acids (38–40), polysaccharides (41), and aryl hydrocarbon receptor ligands (42). Furthermore, as our studies were done in specific-pathogen-free mice, variability in gut flora composition as well as strain-level differences could affect the functional outcome of specific bacteria. Nonetheless, these data suggest that dysbiosis during intestinal inflammation may not provide an important antigen-specific trigger of effector responses that contribute to colitis.
The relative inefficiency of Bacteroides spp. to induce T cell activation is correlated with their bacterial distribution, as they are preferentially found in the lumen. By contrast, mucosal associated Helicobacter spp. were potent stimulators of T cell response. This is consistent with a recent study of SFB showing that tight attachment to the intestinal epithelium plays an important role in eliciting Th17 cells (43). Thus, understanding the adaptive immune response to commensal bacteria may be facilitated by analysis of mucosal- or epithelial-associated, and not luminal, bacteria.
The recognition that Helicobacter spp. are important inducers of antigen-specific Treg cells during homeostasis is consistent with a previous study (44), and suggests that our current notion of Treg inducing bacteria as being “good bacteria” that are protective against colitis may be incomplete (7, 45). In fact, Helicobacter spp. are widely prevalent in mice colonies around the world (46), and have often been thought of as pathobionts based on their ability to induce or enhance colitis in lymphopenic mice (32–34) or mice deficient in critical immuno-regulators such as IL-10 (31, 47, 48). To reconcile these different observations regarding Helicobacter spp., our data suggest that T cell development is context-dependent, being tolerogenic during homeostasis, and inflammatory during lymphopenia or experimental colitis.
A final implication of this study is that T cell-mediated intestinal inflammation may be driven by bacteria that are always in contact with the adaptive immune system, rather than species that are mostly excluded from being presented to T cells during homeostasis. This contrasts with a previous study of CBir1 TCR Tg cells, which recognize a commensal bacteria-expressed flagellin (49) that is presented to the immune system primarily during inflammation (10). In this case, the CBir1 antigen is normally not seen by the immune system and might be considered a pathogen when seen in the context of inflammation. However, in the DSS+αIL10R model of colitis, this type of immune response to commensal antigens appears to be less relevant. Thus, our studies bring up an interesting question, that is, to what extent is the human intestinal inflammatory T cell response directed towards commensal antigens that the host is commonly in contact with, i.e. akin to “self” antigens, or commensal antigens that are only presented during injury, i.e. “foreign” antigens.
Materials and Methods
Study design
Animal experiments were performed in a specific-pathogen-free facility in accordance with the guidelines of the Institutional Animal Care and Use Committee at Washington University. Host mice were housed together and interbred to maintain microbial integrity. Gender-matched littermate host mice were used for all comparisons. Cages were randomly assigned into different treatment groups. Both male and female mice were used. All in vivo and in vitro experiments were performed independently at least two times unless otherwise stated.
Mice
CT2, CT6 (8, 9), and DP1 Tg mice were generated as described (50), and bred to Rag1−/− (Jackson Labs (JAX) #002216), Foxp3IRES-GFP (JAX #006772) or Foxp3IRES-Thy1.1 (51), and/or IL-17AIRES-GFP (JAX #18472), and IFNγIRES-YFP(GREAT) (52). TCli TCRβ Foxp3IRES-Thy1.1 TCRα+/− (53) and TCli TCRαβ (54) Foxp3IRES-Thy1.1 Rag1−/− mice were previously described. IFNγIRES-YFP mice were a gift from Richard Locksley (UCSF); Foxp3IRES-Thy1.1 mice were a gift from Alexander Rudensky (MSKCC). Experiments in Fig. 4B used Ly5.1 mice from Charles River Laboratories as hosts; experiments in fig. S6 used Ly5.2 mice inbred in our colony as hosts; all other in vivo experiments used Foxp3IRES-GFP CD45.1 (JAX #006772) mice. All mice were on a C57BL/6 genetic background.
Reagents, antibodies, and flow cytometry
Anti-IL-10R blocking antibody (BE0050) and isotype IgG1 (BE0088), anti-MHC II antibody (BE0108) were purchased from BioXCell. Fluorescently conjugated antibodies were purchased from Biolegend, eBioscience, and Becton Dickinson. Samples were analyzed using a FACSAria IIu (Becton Dickinson) and data were processed with FlowJo (Treestar).
Cell isolation from the colonic lamina propria
Cells were isolated as described (9). Colonic segments were treated with RPMI medium containing 3% FBS and 20 mM HEPES (HyClone) with DTT (Sigma) and EDTA (Thermo Fisher) for 20 mins at 37°C with constant stirring. Tissue was further digested with 28.3 μg/ml liberase TL (Roche) and 200 μg/ml DNase I (Roche), with continuous stirring at 37°C for 30 min. Digested tissue was forced through a Cellector tissue sieve (Bellco Glass) and passed through a 40 μm cell strainer.
T-cell hybridoma stimulation assay
T cell hybridoma cells expressing GFP under NFAT promoter (55) were retrovirally transduced with TCRα chains of interest as previously described (8). Hybridoma cells (1.5×104) were cultured with flt3-ligand-elicited CD11c+ dendritic cells (5×104) with and without the indicated antigen preparations (20 μg/well) in flat-bottomed 96-well plates. CD4+TCRβ+ cells were analyzed for GFP expression after 1.5 days by flow cytometry.
Antigen preparations
Total colonic lumen contents were collected from longitudinally opened colon and caecum, diluted with PBS (HyClone), vortexed, filtered through a 40 μm strainer, and autoclaved. For MA preparation, lumen contents were removed with forceps and the remaining tissue rinsed with PBS. Mucosal associated particles were released from colonic tissues using PBS with DTT (1 μM/ml) and EDTA (5 μM/ml) for 20 min at 37°C with constant stirring, followed by PBS with EDTA (2 μM/ml) for 3 times. From each of these steps, mucosal associate particles were filtered through a 40 μm strainer. Larger particles such as cells were removed by centrifugation at 1500 rpm for 10 min. The remaining particles in suspension were pelleted at 2500 rpm for 15 min, resuspended in PBS, then autoclaved. For bacterial isolates, in vitro cultures were pelleted at 2500 rpm for 15min, washed twice with PBS, resuspended in PBS, and autoclaved for 45 min.
Adoptive transfer experiments
For experiments with CT2, CT6, or DP1 Tg cells, naïve T cells were FACS purified (CD4+ Foxp3− CD25− CD44lo CD62Lhi) from peripheral lymph nodes and spleen of CD45.2 Foxp3IRES-GFP Rag1−/− or Foxp3IRES-Thy1.1 IL-17AIRES-GFP IFNγIRES-YFP Rag1−/− TCR Tg mice. 105 naïve TCR Tg were injected retro-orbitally into congenic CD45.1 Foxp3IRES-GFP mice. In some experiments, cells were first labeled with Cell Trace Violet (Thermo Fisher) before injection. For experiments using TCRs CT7, CT9, NT2, or T7-1, naïve T cells (CD4+ Foxp3− CD25− CD44lo CD62Lhi Vα2−) were FACS purified from CD45.2 Tcli TCRαβ Foxp3IRES-Thy1.1 Tg mice (Rag1+/− or Rag1−/−), and activated in vitro with soluble anti-CD3 (0.1 μg/ml; 145-2C11; Bio X Cell) and anti-CD28 (1 μg/ml; 37.51; Bio X Cell) in tissue culture plates coated with rabbit antibody to hamster IgG (127-005-099; Jackson ImmunoResearch) in the presence of anti-cytokine antibodies from Bio X Cell (Cat #): anti-TGF-β (20 μg/ml, BE0057), anti-IFN-γ (5 μg/ml, BE0054) anti-IL-4 (5 μg/ml, BE0045), and anti-IL-12 (5 μg/ml, BE0052). One day after activation, cells were retrovirally transduced with individual TCRα chain of interest (8). 2×105 total cells (both transduced and non-transduced) were transferred into congenic CD45.1 Foxp3IRES-GFP mice.
The colon lamina propria and distal mesenteric lymph node were harvested at indicated times after transfer and analyzed by flow cytometry. Transferred TCR+ cells were identified as CD4+CD45.2+CD45.1−Vβ6+Vα2+ (CT2/CT6/CT7/CT9/DP1/NT2) or CD4+CD45.2+CD45.1− Vβ6+GFP+ (T7-1).
Antibiotic treatment
Littermates were divided and treated at 3 weeks of age. Vancomycin (0.5 mg/ml), ampicillin (1 mg/ml), and neomycin (1 mg/ml) were administrated ad libitum via drinking water continuously for the time indicated. Metronidazole (1 mg/ml) was administrated by oral gavage every day for 1 week followed by gavage every other day (56).
Bacterial isolation, culture, and inoculation
Colonic lumen contents from DSS+αIL10R treated mice were homogenized and serial dilutions were plated on brain-heart-infusion (BHI, BD Difco) agar supplemented with 10% (v/v) defibrinated horse blood (Colorado Serum Co.). Plates were grown for 3 days at 37°C under anaerobic conditions (5% H2, 20% CO2, and 75% N2) in a Coy chamber. 20 colonies were picked and 16S rRNA gene was sequenced. Colonies matched to B. vulgatus, B. acidifaciens, B. uniformis were cultured in BHI medium (EMD) supplemented with 5 g/L yeast extract, 0.5 g/L L-cysteine-HCl, 1 mg/L Vitamin K3, 1.2 mg/L Hematin (all Sigma), and used for T-cell hybridoma assay. H. typhlonius (MIT 97-6810) and H. apodemus (MIT 03-7007) strains were cultured on Columbia agar (Oxoid) plate supplemented with 7% (v/v) defibrinated horse blood at 37°C under microaerobic conditions in a vented jar (Becton Dickinson) containing N2, H2 and CO2 (75:5:20) for 5 days; bacteria were collected into Brucella broth (BD Difco) and used for T-cell hybridoma assay or mice inoculation. For Helicobacter spp. inoculation studies, approximately 2×108 CFU bacteria were gavaged into indicated mice every other day for a total of 3 times.
TCR sequencing
Tnaive (CD44lo CD62hi), Treg (Foxp3+), and Teff (CD44hi CD62Llo) CD4 T cells were sorted from TCli TCRβ Foxp3IRES-Thy1.1 TCRα+/− mice using BD FACSAria IIu. TCRα cDNA synthesis from purified cells was performed as described (57). A two-step PCR was used to amplify multiple TRAV genes (58) (multiplex PCR, table S1). Amplicons were purified after each PCR reaction using the Agencourt AMPure XP magnetic purification system. The ~200–600 bp amplicons were quantified using Qubit dsDNA BR assay kit (Invitrogen) and pooled in equimolar ratios for 250 bp paired end sequencing using Illumina MiSeq at the Washington University Genome Sequencing Center. Sequences were demultiplexed and analyzed using blastn to identify the TCR alpha variable (TRAV) and TCR alpha joining (TRAJ) gene segments using the IMGT database (59). This information was then used to determine the CDR3 sequence. A unique TCR is identified by its TRAV and CDR3 amino acid sequence. Frequency filtering was applied to keep TCRs >0.1% of raw data in at least one sample. TRAVs with a frequency below 1% of the total population in multiplex PCR were excluded to limit variability from low read numbers due to low primer efficiency. The filtered TRAVs accounted for more than 76.2%±8.8% of the total TRAV repertoire. TRAV_CDR3 species frequencies were then multiplied by a correction factor determined by the ratio of TRAV sequences obtained using multiplex versus template switch PCR (fig. S3B) (60).
16S rRNA gene sequencing
Colonic lumen contents or MA preparations were processed for DNA isolation using the ISOLATE Fecal DNA kit (Bioline). For fig. S1E, terminal pellets were homogenized with PBS and prepared for Bacteria FACS sorting and 16S rRNA sequencing as in (61). The V4 region of 16S rRNA gene was PCR amplified using barcoded primer described previously (62), and sequenced using the Illumina Miseq Platform (2×250bp paired-end reads). OTU picking was performed using UPARSE (usearch v.8.0.162) (63), and taxonomy assigned using the uclust method with the Greengenes 13.8 database (QIIME v1.9 (64)).
Colon histology
Mouse colons were collected, luminal contents removed, cut open longitudinally, and pinned and fixed with 10% (vol/vol) formalin. Fixed samples were paraffin-embedded, cut into 5μm sections, and stained with Hematoxylin and Eosin (H&E) by standard procedures. Crypt height was measured from digital photographs of H&E stained colon sections taken with a Nikon ECLIPSE 50i microscope.
Statistical analysis
GraphPad Prism v7, R v3.3.0, and Qiime v1.9 were used for statistical and graphical analysis. Student’s t test, Mann-Whitney U test, one-way ANOVA, and two-way repeated-measures ANOVA were used for between-subjects analyses. Benjamini-Hochberg false discovery rate correction was used on Mann-Whitney U calculations for OTU comparisons. Morisita-Horn statistical test was used for TCR repertoire comparison. TCR Renyi diversity profiles were generated as previously indicated (53), using Renyi entropy values with α/order values ranging from 0 (natural logarithm of species richness) through 2 (natural logarithm of the inverse Simpson index). This includes α= 1, which represents the commonly used Shannon entropy.
Supplementary Material
Table S1. TRAV Multiplex primer sequences.
Table S2. TRAV designation, CDR3 amino acid (a.a.) sequences, and in vitro bacterial reactivity of indicated TCR clones.
Table S3. Excel file containing source data for all the figure panels.
Materials and Methods
Fig. S1. DSS+αIL10R treatment induces colitis and effector T cell responses.
Fig. S2. Gating strategy for TCR Tg transfer experiments in Fig. 1; Fig. 4A (CT2 and CT6); Fig. 6B and C (DP1).
Fig. S3. Effects of DSS+αIL10R-mediated colitis on the TCR repertoire.
Fig. S4. Gating strategy for T cell transfer experiments in Fig. 2D; Fig. 4A (CT9 and T7-1); Fig. 6C (NT2 and CT7).
Fig. S5. CBir1 TCR Tg cells become Teff, and not Treg, cells during DSS+αIL10R colitis.
Fig. S6. Gating strategy for T cell hybridoma experiments in Fig. 3A, B, and D; Fig. 6A and D; S7A and C; S8; S11.
Fig. S7. Colonic Treg TCRs react to mucosal-associated Helicobacter species.
Fig. S8. CT2/CT6/CT9/T7-1 TCRs do not react to other bacterial isolates tested.
Fig. S9. Gating strategy and confirmation of Helicobacter spp. inoculation in Fig. 4B.
Fig. S10. Quantification of 16S rRNA gene abundance in the lumen or MA preparation.
Fig. S11. Specificity of DP1/NT2/CT7 TCRs for Bacteroides spp.
Fig. S12. Gating strategy for Fig. 7A, S13A.
Fig. S13. Bacteria-specific induction of colitis in lymphopenic mice.
Fig. S14. Helicobacter species are detected in fecal and colonoscopy samples from IBD patients.
Fig. S15. Isotype controls for antibodies used in this study.
One-sentence summary.
Helicobacter in the intestine induce regulatory T cells during homeostasis and effector T cells during colonic inflammation.
Context is Critical in IBD.
The intestine hosts trillions of commensal microbes; however, exactly how these microbes contribute to a balanced immune response in the intestine is still being explored. Now, Chai et al. report that mucosal-associated Helicobacter species can trigger either regulatory T cell (Treg) or effector T cell (Teff) activation in mouse intestine, depending on context. T cells specific to the bacteria activated Tregs in homeostatic conditions. In contrast, in a mouse model of colitis, Helicobacter species induced Teffs. These data suggest that a pathobiont such as Helicobacter species may induce immune tolerance in homeostatic conditions, but switch to contribute to pathogenesis in the presence of inflammation.
Acknowledgments
We thank Nicole Santacruz and Chin‐Wen Lai (Wash. U) for expert technical assistance; Takeshi Egawa and Brian Kim (Wash. U) for critical reading of the manuscript and advice; Alexander Rudensky (MSKCC) and Richard Locksley (UCSF) for gifts of mice.
Funding: C.-S.H. is supported by NIH DK094995 and AI079187, Crohn’s and Colitis Foundation of America, and the Burroughs Wellcome Fund. J.G.F is supported by P30ES 002109, T32OD10978-29. A.L.K. is supported by NIH K08 AI113184 and AAAAI Foundation.
Footnotes
Author contributions
J.N.C and C.-S.H. conceived the project and designed the experiments; J.N.C, Y.P., S.R., and T.L.A. performed the experiments; J.N.C., Y.P., B.D.S., S.R., and J.S.P. analyzed the data. Z.S., K.A.K., T.T., S.N., K.H., R.D.N., C.O.E., A.L.K., D.A.P., and J.G.F provided important bacterial isolates, mice, and reagents; T.S.S. assisted in histological evaluation; J.N.C and C.-S.H wrote the manuscript.
Competing interests
The authors declare that they have no competing interests.
Data and materials availability
TCR sequencing data and 16S rRNA sequencing data are deposited at the European Nucleotide Archive.
References and Notes
- 1.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe. 2015;17:577–591. doi: 10.1016/j.chom.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 6.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, Hsieh CS. Rapid and Efficient Generation of Regulatory T Cells to Commensal Antigens in the Periphery. Cell Rep. 2016;17:206–220. doi: 10.1016/j.celrep.2016.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hand TW, Santos LM Dos, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, 3rd, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal T17 cells towards commensal bacterial antigens. Nature. 2014 doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 15.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, Yoshimura A, Kanai T. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, Vos M De, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, I. B. D. G. C. International. Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderon-Gomez E, Bassolas-Molina H, Mora-Buch R, Dotti I, Planell N, Esteller M, Gallego M, Marti M, Garcia-Martin C, Martinez-Torro C, Ordas I, Singh S, Panes J, Benitez-Ribas D, Salas A. Commensal-Specific CD4(+) Cells From Patients With Crohn’s Disease Have a T-Helper 17 Inflammatory Profile. Gastroenterology. 2016;151:489–500 e483. doi: 10.1053/j.gastro.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, Glowacki RW, Hansson GC, Allen PM, Martens EC, Stappenbeck TS. Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host Microbe. 2015;17:672–680. doi: 10.1016/j.chom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, Stolp B, Stein JV, Stecher B, Sauer U, McCoy KD, Macpherson AJ. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68:3401–3407. doi: 10.1128/AEM.68.7.3401-3407.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Jr, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 31.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullberg MC, Andersen JF, Gorelick PL, Caspar P, Suerbaum S, Fox JG, Cheever AW, Jankovic D, Sher A. Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15830–15835. doi: 10.1073/pnas.2534546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knutson CG, Mangerich A, Zeng Y, Raczynski AR, Liberman RG, Kang P, Ye W, Prestwich EG, Lu K, Wishnok JS, Korzenik JR, Wogan GN, Fox JG, Dedon PC, Tannenbaum SR. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2332–2341. doi: 10.1073/pnas.1222669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson JM, Hansen R, Berry SH, Hope ME, Murray GI, Mukhopadhya I, McLean MH, Shen Z, Fox JG, El-Omar E, Hold GL. Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS One. 2011;6:e17184. doi: 10.1371/journal.pone.0017184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut microbes. 2012;3:332–344. doi: 10.4161/gmic.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013 doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013 doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 41.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 43.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov, Sugiyama T, Nunez G, Camp JG, Hattori M, Umesaki Y, Honda K. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. Journal of Experimental Medicine. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol. 2007;45:2166–2172. doi: 10.1128/JCM.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox JG, Gorelick PL, Kullberg MC, Ge Z, Dewhirst FE, Ward JM. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;67:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG, Howard JK, Parkhill J, MacDonald TT, Lord GM. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 50.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon BD, Hsieh CS. Antigen-Specific Development of Mucosal Foxp3+RORgammat+ T Cells from Regulatory T Cell Precursors. J Immunol. 2016 doi: 10.4049/jimmunol.1601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong P, Goldrath AW, Rudensky AY. Competition for specific intrathymic ligands limits positive selection in a TCR transgenic model of CD4+ T cell development. Journal of Immunology. 2000;164:6252–6259. doi: 10.4049/jimmunol.164.12.6252. [DOI] [PubMed] [Google Scholar]
- 55.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 58.Ni PP, Solomon B, Hsieh CS, Allen PM, Morris GP. The ability to rearrange dual TCRs enhances positive selection, leading to increased Allo- and Autoreactive T cell repertoires. J Immunol. 2014;193:1778–1786. doi: 10.4049/jimmunol.1400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giudicelli V, Chaume D, Lefranc MP. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 2004;32:W435–440. doi: 10.1093/nar/gkh412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mamedov IZ, Britanova OV, Zvyagin IV, Turchaninova MA, Bolotin DA, Putintseva EV, Lebedev YB, Chudakov DM. Preparing unbiased T-cell receptor and antibody cDNA libraries for the deep next generation sequencing profiling. Frontiers in immunology. 2013;4:456. doi: 10.3389/fimmu.2013.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu TC, Stappenbeck TS, Maleta KM, Ashorn P, Dewey KG, Houpt ER, Hsieh CS, Gordon JI. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7:276ra224. doi: 10.1126/scitranslmed.aaa4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 64.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 66.Castillo M, Martin-Orue SM, Manzanilla EG, Badiola I, Martin M, Gasa J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet Microbiol. 2006;114:165–170. doi: 10.1016/j.vetmic.2005.11.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. TRAV Multiplex primer sequences.
Table S2. TRAV designation, CDR3 amino acid (a.a.) sequences, and in vitro bacterial reactivity of indicated TCR clones.
Table S3. Excel file containing source data for all the figure panels.
Materials and Methods
Fig. S1. DSS+αIL10R treatment induces colitis and effector T cell responses.
Fig. S2. Gating strategy for TCR Tg transfer experiments in Fig. 1; Fig. 4A (CT2 and CT6); Fig. 6B and C (DP1).
Fig. S3. Effects of DSS+αIL10R-mediated colitis on the TCR repertoire.
Fig. S4. Gating strategy for T cell transfer experiments in Fig. 2D; Fig. 4A (CT9 and T7-1); Fig. 6C (NT2 and CT7).
Fig. S5. CBir1 TCR Tg cells become Teff, and not Treg, cells during DSS+αIL10R colitis.
Fig. S6. Gating strategy for T cell hybridoma experiments in Fig. 3A, B, and D; Fig. 6A and D; S7A and C; S8; S11.
Fig. S7. Colonic Treg TCRs react to mucosal-associated Helicobacter species.
Fig. S8. CT2/CT6/CT9/T7-1 TCRs do not react to other bacterial isolates tested.
Fig. S9. Gating strategy and confirmation of Helicobacter spp. inoculation in Fig. 4B.
Fig. S10. Quantification of 16S rRNA gene abundance in the lumen or MA preparation.
Fig. S11. Specificity of DP1/NT2/CT7 TCRs for Bacteroides spp.
Fig. S12. Gating strategy for Fig. 7A, S13A.
Fig. S13. Bacteria-specific induction of colitis in lymphopenic mice.
Fig. S14. Helicobacter species are detected in fecal and colonoscopy samples from IBD patients.
Fig. S15. Isotype controls for antibodies used in this study.


