Abstract
According to expansion of dialysis-dependent population, more than half of patients with critical ischemic limbs are dialysis-dependent in Japan. Although patients with end-staged renal disease are well-known as poor life prognosis, well-managed dialysis patients in Japan can survive much longer compared to dialysis patients in the United States and Europe. Therefore, some dialysis patients can enjoy the long-term benefits of bypass surgery. To decide the indication of bypass surgery, patient’s general condition, nutrition status, and vein availability are more important rather than arterial disease anatomy. Ultrasound guided nerve block anesthesia blocking both sciatic and femoral nerve is contributing greatly to quick postoperative recovery of high risk patients. Preoperative ultrasound examination also contribute to not only vein mapping but also find out the graftable segment of artery. The selection of distal target should be decided based on the degree of arterial disease (luminal surface as well as wall calcification), and arterial run-off. Several tips regarding anastomosis to heavily calcified artery have been established including how to create bloodless operative field without arterial clamps. Adequate wound management after bypass surgery is also important. Detection of deep infection such as osteomyelitis and the adequate treatment may avoid major amputation of salvageable limbs. In the era of endovascular treatment, the evidences guiding how to select dialysis patients suitable for bypass surgery are awaiting. (This is a translation of Jpn J Vasc Surg 2017; 26: 33–39.)
Keywords: dialysis-dependent renal failure, critical limb ischemia, peripheral arterial disease, distal bypass surgery, limb salvage
Introduction
The opinion that bypass, endovascular treatment, or non-revascularization should be selected for patients with critical limb ischemia (CLI), considering the general condition and life expectancy, in addition to the states of artery lesions and ischemic limbs, is commonly accepted.
The PREVENT III score was proposed1) as a parameter of general condition or surgery-related risks, but most patients are classified as extremely high risk only due to the risk of dialysis-dependent renal failure; revascularization may not be indicated for most patients according to the classifications prepared in Europe and the United States. Under these circumstances, approximately 50% of patients with CLI in Japan are receiving dialysis.2,3) In Japan, the number of dialysis patients is markedly higher than in other countries based on the background data from patients enrolled in clinical studies of CLI (Table 1).4–6) In addition, bypass strategies for extremely-serious-status patients have also been established in Japan, contributing to the management of such patients.7,8) According to an annual National Clinical Database-based report on the number of patients who underwent vascular surgery published by the Database Management Committee of the Japanese Society for Vascular Surgery, 34% of patients who underwent infrapopliteal bypass surgery in Japan in 2011 had received dialysis, and 50% of patients who underwent endovascular treatment (EVT) for the infrapopliteal artery by vascular surgeons had received dialysis.9) According to the Japanese Critical Limb Ischemia Database (JCLIMB) in 2014, 761 patients with CLI were registered, and dialysis patients accounted for 79% (n=600).10)
Table 1 Demographic differences of patients with CLI.
| Study name Country (Year) | BASIL trial UK (2005) | PREVENT III USA (2005) | OLIVE registry Japan (2013) | CRITSCH registry Germany (2015) |
|---|---|---|---|---|
| Type of study | multicenter | multicenter | multicenter | multicenter |
| RCT | RCT | registry | registry | |
| Revascularization | Bypass vs EVT | Bypass | EVT | Bypass vs EVT |
| No. Pts | 452 | 1404 | 314 | 1200 |
| Comorbidities | ||||
| Hypertention | 58% | 82% | 79% | |
| Diabetes | 42% | 64% | 71% | 47% |
| Coronary artery disease | 36% | 48% | 46% | 45% |
| Cerebrovascular disease | 21% | 20% | 21% | 12% |
| ESRD* on dialysis | 0% | 12% | 52% | 9% |
*ESRD: end-staged renal disease
The reason why revascularization is commonly performed for dialysis patients in Japan is that their lifespan is 3.8- and 2.8-times longer than in the United States and Europe, respectively,11) as indicated in the Dialysis Outcomes and Practice Pattern Study (DOPPS), which was published in 2004. According to recent data from the DOPPS, the mortality rate of dialysis patients in Japan is markedly lower than in other countries.12) Prolongation of lifespan of dialysis-dependent patients results in high incidence of advanced arterial lesions that increase the incidence of CLI requiring revascularization, and medical service should respond the social obligation to provide a specific level of quality of life (QOL) during their lifespan. These backgrounds may be the reasons why the percentage of dialysis patients is high in those undergoing revascularization.
In this article, we introduce the bypass procedures for dialysis patients based on the above background specific to Japan. Adequate case selection, revascularization strategies, and perioperative management may contribute to further advances.
Preparation and Technical Tips for Bypass Surgery
Preoperative preparation for bypass
General condition and main organ assessment
To evaluate whether patients can undergo surgery and predict the prognosis, functional assessment of the main organs is performed. Even if there is no time to conduct detailed preoperative examination due to serious ischemia or infection, echocardiography is essential, and coronary artery assessment (coronary computed tomography (CT), pharmacological stress myocardial perfusion scintigraphy, or coronary angiography) should also be performed if possible. In patients receiving dialysis, stenosis of the aortic valve is frequently observed, making perioperative management difficult; therefore, it is important to review the presence or absence of aortic valve stenosis before surgery in cooperation with anesthesiologists. As an anesthetic method, ultrasound-guided nerve block anesthesia has commonly been used, contributing to early general-condition recovery after surgery in high-risk patients. A reduction in anesthetic invasiveness is one of the recent advances.13)
Vein assessment
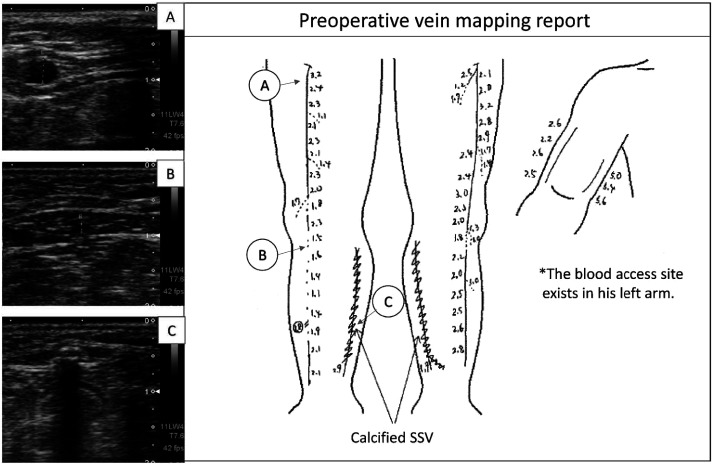
Decision making which autologous vein should be harvested for bypass is the most important for preparing revascularization strategies. Superficial vein assessment by ultrasonography is essential (Fig. 1). When ultrasonography of the superficial veins is performed immediately after dialysis, venous collapse may lead to the underestimation of vein quality; for dialysis patients, ultrasonography should be conducted on non-dialysis days.
Fig. 1 Preoperative ultrasound examination in a CLI case with end-staged renal failure on hemodialysis for 13 years. Infrapopliteal segment of right GSV looks poor quality (panel B) and both SSV walls are calcified with acoustic shadow (panel C). Both limbs underwent popliteo-pedal bypasses using upper half of GSVs as non-reversed fashion. CLI: critical limb ischemia; GSV: great saphenous vein; SSV: small saphenous vein.
Evaluation of foot lesions
It is important to accurately evaluate the degree of ischemia, presence or absence of tissue defects, their sites/extents/depth, and presence or absence of infection. Briefly, the wound, ischemia, and foot infection (WIfI) classification should be routinely recorded.14)
Dialysis patients, especially those with diabetes, are compromised in terms of resisting to bacteria. However, when ischemia is marked, infection-related signs (flare, swelling, pain) may not be apparent. It is important to identify pathogenic bacteria before surgery, considering postoperative infection. Culture of tissue specimens is recommended (not wiping culture). Bacteria identification is also important to prevent nosocomial infection, and it is ideal to conduct a culture test in a primary-care hospital or at an outpatient clinic.
Plain X-ray of the lower limbs
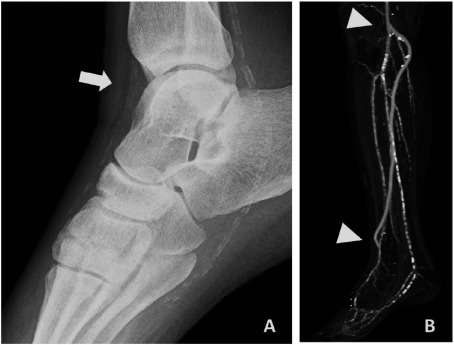
When indicating revascularization for dialysis arterial patients, calcification must be considered. To select the adequate site of anastomosis and an artery-blocking method during anastomosis, plain X-ray films are necessary (Figs. 2 and 3).
Fig. 2 Foot plain X-ray film (panel A) and CT angiogram after bypass surgery (panel B). X-ray film shows heavy calcification in his infrapopliteal arterial tree, but there are some less calcium segments especially around ankle joint (arrow in panel A). If the calcium-free space can be found like this case, the anastomosis will not be difficult. The arrowheads indicate each anastomotic site (panel B).
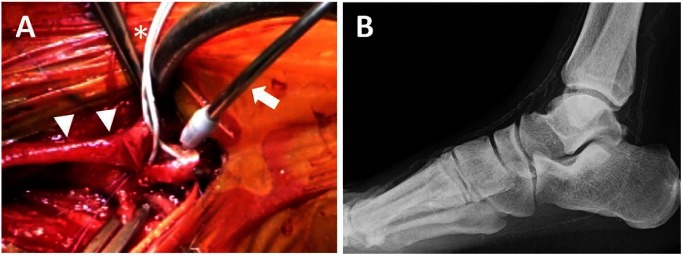
Fig. 3 Operative view of a distal anastomosis to dorsalis pedis artery of dialysis-dependent patient (panel A). Two balloon catheters (*) are using to block arterial flow. A CO2 blower (arrow) is also useful to blow away blood and create bloodless field. Arrow heads indicate vein graft. Panel B shows the plain X-ray of the foot underwent the bypass surgery introduced in panel A.
Selection of a proximal anastomotic site
In addition to the extent of an arterial lesion, the available length of vein material is important for selecting a proximal anastomotic site.
If there is no femoral artery lesion, the popliteal artery should be selected as a proximal anastomotic site. The popliteal artery, especially the mid-popliteal artery, shows less calcified in most cases, therefore, there may be no technical difficulties by medial approach (Fig. 2). However, in some patients with marked arterial calcification, only a portion at the center of the popliteal fossa is available as a favorable inflow route. In such cases, bypass from popliteal artery to the peroneal, posterior tibial, or dorsal foot arteries using the small saphenous vein through an approach to the mid-popliteal artery in a prone position is possible.
When stenosis of the superficial femoral artery is present, it depends on the available length of a vein whether the femoral artery proximal to the stenotic lesion is selected as the site of central anastomosis or whether the popliteal artery is selected as the site of proximal anastomosis after EVT for superficial femoral artery stenosis.
Recently, we have often encountered dialysis patients with marked stenosis and calcification of the common femoral artery. Stenosis of the common femoral artery leads to ischemia involving the deep femoral artery region, causing severe ischemia; therefore, stenosis of the common femoral artery must be removed. In this case, endarterectomy/plasty of the common femoral artery should be performed, and its portion may be established as the site of proximal anastomosis for distal bypass.
Selection of a distal anastomotic site
In dialysis patients, a culprit lesion is localized more periphery in comparison with non-dialysis patients; therefore, anastomosis to more distal arteries is more frequently required, and the frequency of anastomosis to foot arteries is significantly higher (Table 2).
Table 2 Infrainguinal bypass using vein graft for CLI (10 years experiences in Asahikawa Medical Univ.).
| Dialysis cases | Non-dialysis cases | |
|---|---|---|
| No. of bypasses | 205 | 237 |
| Distal anastomotic site | ||
| Popliteal | 27 (13.1%) | 61 (25.7%) |
| Above-knee | 4 | 14 |
| Below-knee | 23 | 47 |
| Crural | 82 (40.0%) | 121 (51.1%) |
| Anterior tibial | 23 | 25 |
| Posterior tibial | 49 | 76 |
| Peroneal | 10 | 20 |
| Pedal | 96 (46.8%) | 55 (23.2%) |
| Dorsalis pedis | 82 | 37 |
| Plantar | 10 | 14 |
| Other branch | 4 | 4 |
| Vein graft source | 204 | 224 |
| Single vein graft | 131 (64.2%) | 159 (71.0%) |
| Ipsilateral GSV | 129 | 154 |
| Contralateral GSV | 1 | 2 |
| Other vein materials | 1 | 3 |
| Spliced vein graft | 73 (35.8%) | 65 (29.0%) |
| GSV only | 28 | 16 |
| Including SSV | 12 | 13 |
| Including arm vein | 28 | 24 |
GSV: great saphenous vein; SSV: short saphenous vein
If the affected limb exhibits Rutherford 4 ischemia, even incomplete revascularization may result in the disappearance of symptoms. However, in patients with Rutherford 5 or higher grade, complete paramalleolar or inframalleolar revascularization should be performed to achieve the complete healing of foot ulcers. It is not rare that distal target artery cannot be contrast-enhanced due to upstream advanced arterial lesions despite the patency of peripheral arteries, but duplex scan is useful for detecting such target arterial segment invisible by angiography. A colored foot artery detected on duplex scan provides evidence for its patency, reflecting that anastomosis is possible due to ultrasonic-permeable slight calcification.
In the presence of tissue defects, it is controversial whether an angiosome-based distal target should be selected to achieve wound healing or whether a good artery should be selected regardless of the angiosome concept to obtain long-term patency. Few studies have examined ulcer healing in regard of angiosome after bypass in dialysis patients. However, blood flow through bypass graft is directly perfusing into a foot artery at a blood pressure similar to the central pressure; therefore, perfusion may be achieved beyond the angiosome, differing from EVT. Based on our experience, there were no marked differences in the ulcer healing or major amputation rates between angiosome-indirect and -direct revascularization procedures. However, in many dialysis patients, lesions involve an arterial network connecting the dorsal side of the foot with the plantar side, such as the pedal arch, therefore, caution is needed.7) In our hospital, we usually select a good artery regardless of the angiosome concept to achieve long-term patency. When ulcer healing was protracted, additional two-stage bypass or EVT was conducted later to increase blood flow in an affected area. In patients in whom blood flow to the ulcer site may be restricted, it is useful to predict ulcer healing using intraoperative digital subtraction angiography (DSA) findings, such as wound blushes, after the completion of bypass and intraoperative evaluation procedures, such as living angiosome staining with indocyanine green, which sometimes provide useful information for postoperative ulcer management.15) If ulcer healing at a single peripheral target is considered difficult based on the results of evaluation, dual bypass is rarely performed.
Selection of graft materials and graft preparation
As materials for bypass to below the knees, autologous veins should be basically used, adopting the great/small saphenous, arm vein, or deep vein of lower limb as graft materials. There were no marked differences in the autologous vein or ipsilateral great saphenous vein utilization rates between dialysis and non-dialysis patients (Table 2).
Preoperative venous assessment should be performed. Based on its results, additional venous mapping in the surgical field must be conducted after anesthesia induction, and, finally, graft availability should be evaluated based on the venous diameter and distensibility under direct vision during surgery.
As described above, paramalleolar or inframalleolar artery bypass is frequently selected for dialysis patients; therefore, it is not easy to anastomose the proximal end of the great saphenous vein, with a large orifice diameter and thick wall, to such a small caliber artery. The distal end of an autologous vein graft prepared in non-reversed fashion or the in-situ fashion, well fit to paramalleolar or pedal artery in terms of the diameter and wall thickness.16)
The patency of a spliced vein graft, involving the connection of several vein materials, is less marked than that of a single vein graft. If the great saphenous vein of the ipsilateral limb is unfavorable, strategies to achieve bypass by minimizing the number of vein materials to be connected, such as the utilization of the small saphenous vein effectively or shortening of the bypass distance by use of EVT. At that case, the results of preoperative vein mapping provides useful information to consider the vein use strategy.
There has been no artificial prosthesis available for revascularization below the knees. However, new release of heparin-binding artificial graft has facilitated long-term patency in the presence of 1 or more infrapopliteal arteries as favorable out-flow arteries.17) In some patients without suitable veins for bypass material, such artificial graft are available, but the risk of artificial graft-related infection must be considered when adopting them for compromised patients such as those receiving dialysis.
Distal anastomosis
A calcification-free area is appropriate for anastomosis, but most dialysis patients have marked calcification in entire arterial tree. An arterial segment with relatively free from luminal lesions as well as less-calcification should be selected as a candidate site of distal anastomosis by comparing preoperative plain X-ray films with intraoperative DSA findings (Fig. 2). After confirming favorable blood flow on ultrasonography immediately before surgery, a small skin incision should be performed. While observing the artery under direct vision, the arterial rigidity should be evaluated. The anastomotic orifice must be designed so that the most appropriate segment is located at the toe of the anastomotic site. If luminal lesions are marked, it may be necessary to perform patch angioplasty with a piece of vein and place the distal anastomosis of a bypass graft on the patch.
Arterial flow blocking methods include simple blockage with a microclip, the Esmarch-air tourniquet method, balloon catheter interpolation, and insertion of an internal shunt usually used for the coronary artery bypass surgery. The first and second methods are maybe useless for dialysis patients with marked calcification, as shown in Fig. 3. With the fourth method, it is difficult to match the size on the irregular surface of the calcified lumen. Therefore, the third method may be the most reliable due to its availability and adaptability for various lesions. Technically, a balloon should not be inserted into too deep, and a carbon dioxide blower should be used to manage bloodless operative field (Fig. 3).
Concerning suture methods, strategies may have been established in each hospital. In our hospital, the thickness of the needle is determined in accordance with density of calcification. Regarding the handling of needle during sewing is based on the following strategies: heel, parachute suturing; toe, 3 to 5 needles of node; and side wall, running suture. Recently, the performance of needles has markedly improved, and tungsten needles for sutures became commercially available, reducing surgeon’s stress related with the management of calcified arteries.
After the completion of anastomosis, graft blood flow must be measured to check the presence or absence of problems affecting graft blood flow between the site of proximal anastomosis and graft/peripheral vascular bed. Completion angiography is useful for evaluating the state of the run-off vascular bed, predicting wound healing, and reviewing options if the wound healing is protracted.
Postoperative management of foot lesions
In dialysis patients with ischemic ulcers/gangrene, healing at the site of tissue defects is delayed even if revascularization is successful. Negative-pressure wound therapy shortens the ulcer healing time (UHT), but the UHT is 2-times longer than in non-dialysis patients. The median UHT in non-dialysis and dialysis patients with Rutherford 5 lesions was 47 and 73 days, respectively. In those with Rutherford 6 lesions, it was 115 and 237 days, respectively.7) As dialysis patients are compromised, infection is often advanced before ulcer healing, requiring major amputation in some cases. It is necessary to resect the site of necrosis at the time of revascularization and perform adequate wound management until complete wound healing while carefully examining the presence or absence of infectious signs. The administration of antibiotics in accordance with the susceptibility of cultured bacteria, pressure relief of tissue defects, edema control, and adequate wound protection should be conducted. If ulcer healing is protracted, it may be important to check the presence or absence of circulatory abnormalities, or deep infection in the process of healing.
In patients with extensive tissue defects in whom deep infection may be present, the incidences of residual deep abscess and osteomyelitis are high, and the presence or absence of bone destruction must be confirmed based on X-ray films. If possible, magnetic resonance imaging (MRI) should be performed.
Results of Surgery
Bypass for CLI in dialysis patients was initially reported by many investigators in the United States, but the number of such patients undergoing this procedure decreased thereafter. Recently, reports from Japan have been increasingly published.7,8,18) Many studies indicated that the patency of bypass grafts was similar between dialysis and non-dialysis patients, whereas the limb salvage and ulcer healing rates in dialysis patients were significantly lower than in non-dialysis patients. In particular, infection led to major lower-limb amputation in many patients. Risk factors for unfavorable outcomes include hypoalbuminemia and preoperative non-ambulatory status. In patients with such factors, the limb salvage and ulcer healing rates are low,7,8) and the prognosis is extremely poor,18) suggesting the importance of patient selection (Fig. 4). However, these are the results of retrospective, single-center studies.
Fig. 4 Cumulative amputation-free survival rate in CLI patients with tissue loss. Patient background has great impact on the amputation-free survival. Non-ESRD: patients without ESRD; ESRD norAlb: ESRD patients without severely low albuminemia; ESRDlowAlb: ESRD patients with severe low albuminemia (<3.0 g/dL). There was a significant difference among groups; p<0.001 by long-rank test [Data from Reference No. 7].
In the guidelines prepared by the American Heart Association based on the results of the BASIL trial, first-choice EVT is recommended for CLI patients with a life expectancy of ≤2 years. Most dialysis patients with CLI correspond with this group. Under such circumstances, it is important to adequately select patients requiring bypass. A prospective, observational study of CLI, the SPINACH study, involving dialysis patients (≥50%), may provide useful information.19)
Conclusion
As a criterion for selecting bypass or EVT, whether general anesthesia is possible has been reviewed based on clinical experience. However, recent advances in block anesthesia have enabled to provide bypass surgery without general anesthesia. Even for dialysis patients, bypass can be indicated and may be completed in 3 hours under the following conditions: heart failure is absent; gait function recovery is expected after revascularization, and good-quality superficial veins are present. Thus, complete ulcer healing or favorable QOL may be achieved. As bypass procedures for dialysis patients have been established, strategies to select patients based on accurate preoperative patient/prognosis assessment should be designed in the future.
Acknowledgments
We thank Dr. Akasaka, Department of Laboratory Medicine, Asahikawa Medical University and clinical vascular technicians (Ms. Nakamori, Mr. Higuchi, and Ms. Osada).
Disclosure Statement
There is no conflict of interest.
Additional Note
An abstract of this study was presented at the 44th educational seminar held by the Japanese Society for Vascular Surgery, and the Editorial Committee requested us to write it for Jpn J Vasc Surg.
This is a translation of Jpn J Vasc Surg 2017; 26: 33–39.
References
- 1).Schanzer A, Mega J, Meadows J, et al. Risk stratification in critical limb ischemia: deviation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 2008; 48: 1464-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Iida O, Nakamura M, Yamauchi Y, et al. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE Registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv 2013; 6: 68-76. [DOI] [PubMed] [Google Scholar]
- 3).Takahara M, Iida O, Soga Y, et al. Absence of preceding intermittent claudication and its associated clinical freatures in patients with critical limb ischemia. J Atheroscler Thromb 2015; 22: 718-25. [DOI] [PubMed] [Google Scholar]
- 4).Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of leg (BASIL): multicenter, randomised controlled trial. Lancet 2005; 366: 1925-34. [DOI] [PubMed] [Google Scholar]
- 5).Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006; 43: 742-51. [DOI] [PubMed] [Google Scholar]
- 6).Bisdas T, Borowski M, Torsello G, et al. Current practice of first-line treatment strategies in patients with critical limb ischemia. J Vasc Surg 2015; 62: 965-73. [DOI] [PubMed] [Google Scholar]
- 7).Azuma N, Uchida H, Kokubo T, et al. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg 2012; 43: 322-8. [DOI] [PubMed] [Google Scholar]
- 8).Kodama A, Sugimoto M, Kuma S, et al. Clinical outcomes after infrainguinal bypass grafting for critical limb ischaemia in patients with dialysis-dependent end-stage renal failure. Eur J Vasc Endovasc Surg 2014; 48: 695-702. [DOI] [PubMed] [Google Scholar]
- 9).Japanese Society for Vascular Surgery Database Management Committee Member. NCD vascular surgery data analysis team. Vascular Surgery in Japan: 2011 Annual Report by the Japanese Society for Vascular Surgery. Jpn J Vasc Surg 2017, in press. [Google Scholar]
- 10).The Japanese Society for Vascular Surgery JCLIMB committee. NCD JCLIMB analysis team. 2014 JAPAN Critical Limb Ischemia Database (JCLIMB) annual report. Ann Vasc Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 2004; 14: 3270-7. [DOI] [PubMed] [Google Scholar]
- 12).Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yazigi A, Madi-Gebara S, Haddad F, et al. Combined sciatic and femoral nerve blocks for infrainguinal arterial bypass surgery: a case series. J Cardiothorac Vasc Anesth 2005; 19: 220-1. [DOI] [PubMed] [Google Scholar]
- 14).Mills JL Sr, Conte MS, Armstrong DG, et al. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014; 59: 220-34.e2. [DOI] [PubMed] [Google Scholar]
- 15).Azuma N, Koya A, Uchida H, et al. Ulcer healing after peripheral intervention: can we predict it before revascularization? Circ J 2014; 78: 1791-800. [DOI] [PubMed] [Google Scholar]
- 16).Connolly JE. In situ saphenous vein bypass: forty years later. World J Surg 2005; 29 Suppl 1: S35-8. [DOI] [PubMed] [Google Scholar]
- 17).Daenens K, Schepers S, Fourneau I, et al. Heparin-bonded ePTFE grafts compared with vein grafts in femoropopliteal and femorocrural bypasses: 1- and 2-year results. J Vasc Surg 2009; 49: 1210-6. [DOI] [PubMed] [Google Scholar]
- 18).Orimoto Y, Ohta T, Ishibashi H, et al. The prognosis of patients of hemodialysis with foot lesions. J Vasc Surg 2013; 58: 1291-9. [DOI] [PubMed] [Google Scholar]
- 19).Azuma N, Iida O, Takahara M, et al. Surgical reconstruction versus peripheral intervention in patients with critical limb ischemia: a prospective multicenter registry in Japan: The SPINACH study design and rationale. Vascular 2014; 22: 411-20. [DOI] [PubMed] [Google Scholar]



![Fig. 4 Cumulative amputation-free survival rate in CLI patients with tissue loss. Patient background has great impact on the amputation-free survival. Non-ESRD: patients without ESRD; ESRD norAlb: ESRD patients without severely low albuminemia; ESRDlowAlb: ESRD patients with severe low albuminemia (<3.0 g/dL). There was a significant difference among groups; p<0.001 by long-rank test [Data from Reference No. 7].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/54ed/5684170/818761e417a1/avd-10-3-ra.17-00076-figure04.jpg)