Abstract
Accurate identification of smoking behaviour is crucial to monitor the smoking rate. This study used urinary cotinine (UC) as a biomarker to verify the effectiveness of self-reported smoking behaviour among conscripts during recruit training. The influence of second-hand smoke (SHS) on the UC concentration was also analysed. A cross-sectional study was conducted from July 2014 to December 2014. The participants comprised a total of 621 military service and basic military training conscripts. A self-administered questionnaire survey and a urine test were performed to verify the participants’ smoking behaviour. The UC concentration of 100 ng/mL was adopted as the baseline to identify smokers. A high level of consistency was observed between the conscripts’ self-reported results and the results validated by the UC concentrations (the overall kappa coefficient was 0.918). Moreover, the overall sensitivity and specificity were 92.9% and 98.1%, respectively. The sensitivity for the military service conscripts was significantly lower than that for the basic military training conscripts (86.1% vs. 97.5%, P-value = 0.002). For the self-reported nonsmokers among the military service conscripts, SHS exposure was related to their UC concentrations. The method of self-reporting through a questionnaire survey can serve as a tool to assess conscripts’ smoking behaviour.
Introduction
Tobacco has been proven to be harmful to human health. Research on tobacco hazards has reported that smoking induces numerous diseases such as chronic lung disease and lung cancer1. The general smoking status of various groups should be understood before the implementation of tobacco hazard prevention strategies. Thus, the smoking rate is a crucial indicator for tobacco hazard prevention, which can be determined through the self-reporting of smokers or using biomarker concentrations in those smokers. Cotinine is a major metabolite of nicotine and can be detected in blood, saliva, and urine samples2,3. In particular, urinary cotinine (UC) concentration is an easily obtainable and highly effective biological indicator for smoking4.
Previous review study have reported that the method of self-reporting, compared with biomarker approaches, may underestimate the smoking rate, and the extent of underestimation varies according to the research participants’ attributes5. In Canada, a large, nationwide survey revealed that the self-reported smoking rate of the country was 18.8%; in comparison, the smoking rate determined through the UC concentrations was reported to be 19.1%. Although, the self-reported smoking rate was underestimated, the two results were highly consistent. The sensitivity and specificity of the self-reported smoking rate was 91.6% and 98.3%, respectively6. However, studies on specific groups, such as pregnant women and patients with chronic obstructive pulmonary disease, showed that the self-reported smoking rate was significantly lower than that derived using the UC concentrations7–10. This might be attributable to the ways in which these groups are prompted to conceal their smoking behaviour under social pressure and expectations, which leads to inaccurate self-reported smoking assessment results.
Throughout the Taiwanese military, a tobacco hazard prevention program, called the National Army Tobacco and Betel Nut Hazard Prevention Project, was initiated jointly by the Ministry of Defense and the Health Promotion Administration in 2003. Specifically, to monitor the smoking rate of the army and the effectiveness of tobacco hazard prevention policies, questionnaire surveys were conducted to estimate smoking prevalence using self-reporting approaches. However, whether such methods are effective in identifying smokers remains to be verified. Furthermore, the effectiveness of tobacco hazard prevention policies can also be monitored by understanding nonsmokers’ level of exposure to second-hand smoke (SHS), and the urinary nicotine metabolite concentrations can be used as an indicator for SHS exposure11,12. In Taiwan, conscripts are divided into military service and basic military training conscripts. Military service conscripts are assigned to various units after undergoing two months of recruit training. By comparison, basic military training conscripts are only required to undergo two months of recruit training and two months of specialty training and are not required to continue their military service. In other words, the service time for military service conscripts was longer than that for basic military training conscripts. Conscripts are at the lowest level in the military hierarchy. To meet superiors’ expectations and prevent subsequent controls concerning smoking, conscripts may conceal their smoking behaviour and therefore miss the opportunity to receive adequate assistance in smoking cessation in the military. Few studies have investigated the consistency between the smoking status self-reported by the conscripts and that obtained by measuring the UC concentrations. Therefore, this study aimed to evaluate the prevalence of cigarette smoking and SHS among conscripts in Taiwan and compare the result of a questionnaire survey with the conscripts’ urine test result. Additionally, the effect of SHS on the UC concentration was explored.
Methods
Study Population and Design
A cross-sectional study was adopted to examine conscripts who were receiving training in recruit training centres. Two recruit training centres were targeted through purposive sampling, from which two companies were selected using random sampling. In this study, we included male conscripts who were aged at least 18 years old and were being trained at a new training regiment in Taichung city, Taiwan. Two participants were excluded because they were receiving nicotine replacement therapy (NRT), and eight participants with incomplete data were excluded from the final analysis. In total, 621 conscripts were included in the data analysis. After the conscripts were fully informed of the research content, objectives, and the rights of participants, a total of 629 willing participants were recruited (comprising 324 military service conscripts and 305 basic military training conscripts). Figure 1 shows that of the 629 enrolled participants, three did not provide a urine sample and five did not complete the questionnaire. The response rate was 98.7%. Written informed consent was obtained from all participants. The study design and protocols were reviewed and approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center and performed according to the guidelines of the Declaration of Helsinki (2–103–05–012).
Figure 1.
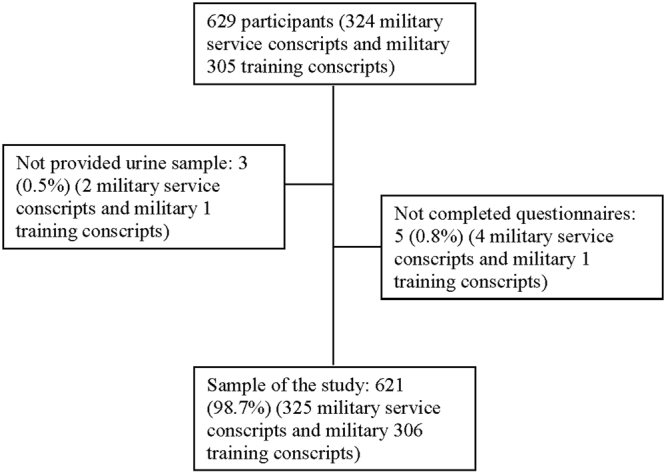
Flow chart of the study.
Procedure
The data were collected from July 2014 to December 2014 by administering both the questionnaire survey and urine test on the penultimate day of the recruit training. The conscripts were asked to first complete a self-reporting questionnaire and then a urine test.
The urine specimens were analysed within one week after collection using enzyme-linked immunosorbent assay (ELISA) kits (Calbiotech Co., Spring Valley, CA, USA). Cotinine concentrations were measured following the manufacturer’s instructions13. The urine samples were stored at −20 °C. Before analysing, urine specimens were brought to room temperature (18–26 °C). In this assay, 10 μl of standards, controls, and specimens were added to the selected wells in duplicate. One hundred microliters of the enzyme conjugation solution was added into each well. The plates were shaken for 10–30 seconds to ensure proper mixing. After 60 minutes of incubation at room temperature (18–26 °C) in the dark, the wells were washed 6 times with 300 μl of distilled water. Then, the wells were inverted and vigorously slapped dry on absorbent paper and 100 μl of substrate reagents were added. After incubating for 30 minutes at room temperature, 100 μl of stop solution was added to each well, and the plate was gently shaken to mix the solution. The limit of detection (LOD) of this assay was 1.0 ng/ml. The coefficient of variation of the assay was within 10% during the period of sample analysis.
Measurement
The questionnaire used in this study, which was comprised information regarding demographics, smoking status, smoking quantity, nicotine dependence level, and SHS exposure, was designed according to previous investigations6–10,14. The question for smoking status was “Do you currently have the habit of smoking?” with the answers of yes and no. Those who answered yes were regarded as smokers, while those who answered no were nonsmokers. The question for smoking quantity was “On average, how many cigarettes have you smoked per day in the past 30 days?” For SHS exposure, the question was “Has anyone smoked near you in the past 30 days?”14 with the answers of yes and no. The nicotine dependence level was measured using the Chinese version of the Fagerstrom Test for Nicotine Dependence (FTND), which has six questions15.
The UC concentration was adopted as the biomarker. The rate of nicotine metabolism varies among people of different races. This study referred to previous studies that have been conducted in South Korea, and smokers were identified as those with UC levels of ≥100 ng/mL10,16.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 22.0 software. The results from the military service participants and the basic military training participants were compared. The variables of age, educational degree, smoking quantity, and nicotine dependence level were described by numerical values, namely frequency, percentage, mean, and standard deviation. In addition, the chi-squared (χ2) test and independent t-test were performed to compare the demographic data of the military service conscripts with those of the basic military training conscripts. The conscripts’ self-reported smoking behaviours and urine test results were compared using the kappa coefficient, sensitivity, and specificity. Additionally, generalized estimating equations (GEE) analysis was used to compare the concordance of self-reported and cotinine-validated smoking status and to adjust for covariates. In the equation, the dependent variable was smoking status (smoker and nonsmoker). Independent variables were age, educational attainment level, and SHS exposure. The relationship between the SHS exposure level and UC concentration for the military service conscripts was compared with that for the basic military training conscripts by the Mann-Whitney U test.
Results
Characteristics of Conscripts
The conscripts in this study consisted of 318 military service conscripts and 303 basic military training conscripts (Table 1). All the conscripts were male. The conscripts averaged 20.9 years of age, with the military service conscripts being significantly older than the basic military training conscripts (22.5 and 19.1 years old, respectively; P-value < 0.001). The educational attainment levels of the two groups differed significantly. Approximately 91% of the military service conscripts graduated from a junior college or above, whereas 94.4% of the basic military training conscripts graduated from a senior high school or below. The self-reported smoking rates of the conscripts was 30.9%, which was significantly higher in the basic military training conscripts (39.3%) than in the military service conscripts (23.0%). In addition, the basic military training conscripts were exposed to a significantly higher level of SHS than were the military service conscripts (47.9% and 11.3%, respectively; P-value < 0.001). The FTND scores for the two groups differed non-significantly.
Table 1.
Characteristics of conscripts.
| Type of military services | Overall N = 621 | Military service conscript n = 318 | Basic military training conscript n = 303 | P-valuea |
|---|---|---|---|---|
| Age (Mean ± SD) | 20.9 ± 1.9 | 22.5 ± 1.0 | 19.1 ± 0.8 | <0.001 |
| Educational attainment level | <0.001 | |||
| Senior high school and below | 281 (50.1) | 27 (9.2) | 254 (94.4) | |
| Junior college and above | 280 (49.9) | 265 (90.8) | 15 (5.6) | |
| Self-reported smoking status | <0.001 | |||
| Yes | 192 (30.9) | 73 (23.0) | 119 (39.3) | |
| No | 429 (69.1) | 245 (77.0) | 184 (60.7) | |
| SHS exposure | <0.001 | |||
| Yes | 181 (29.1) | 36(11.3) | 145(47.9) | |
| No | 440 (70.9) | 282(88.7) | 158(52.1) | |
| Smoking quantity (Mean ± SD)b | 10.1 ± 5.8 | 10.8 ± 6.3 | 9.8 ± 5.4 | 0.275 |
| Score of nicotine dependence (Mean ± SD)bc | 3.0 ± 2.2 | 2.6 ± 2.1 | 3.2 ± 2.2 | 0.101 |
aThe results of military service and basic military training conscripts were compared by conducting the χ2 test and independent t-test.
bOnly self-reported smokers were analysed.
cNicotine dependence was measured using the FTND.
Concordance of Self-reported and Cotinine-validated Smoking Status among Conscripts
Among all the conscripts, 29.6% (n = 184) were self-reported smokers whose UC concentrations were ≥100 ng/mL, and 66.8% (n = 415) were self-reported nonsmokers whose UC concentrations were <100 ng/mL. The overall agreement rate was 96.4%, and the kappa coefficient was 0.918 (P-value < 0.001). A total of 302 military service conscripts demonstrated consistent self-reported and cotinine-validated smoking statuses. The agreement rate was 94.9%, and the kappa coefficient was 0.862 (P-value < 0.001). Among the basic military training conscripts, 297 participants (98.0%) had consistent self-reported and cotinine-validated smoking statuses, with a kappa coefficient value of 0.958 (P-value < 0.001; Table 2). Results from the GEE model (Table 3) further confirmed that after the covariates were controlled for, the frequency of smoking behavior between self-reported and cotinine-validated smoking behavior did not differ significantly among all the conscripts (P-value = 0.107), military service conscripts (P-value = 0.655), and basic military training conscripts (P-value = 0.108).
Table 2.
Concordance of self-reported and cotinine-validated smoking status among conscripts.
| Type of military services | Self-reported Smoking Status | Cotinine-validated Smoking Status | ||
|---|---|---|---|---|
| ≥100 ng/mL n (%) | <100 ng/mL n (%) | Total | ||
| Overall | Smoker | 184 (29.6) | 8 (1.3) | 192 (30.9) |
| Nonsmoker | 14 (2.3) | 415 (66.8) | 429 (69.1) | |
| Total | 198 (31.9) | 423 (68.1) | 621 (100.0) | |
| Kappa coefficient = 0.918*** | ||||
| Military service conscript | Smoker | 68 (21.4) | 5 (1.6) | 73 (23.0) |
| Nonsmoker | 11 (3.5) | 234 (73.5) | 245 (77.0) | |
| Total | 79 (24.9) | 239 (75.1) | 318 (100.0) | |
| Kappa coefficient = 0.862*** | ||||
| Basic military training conscript | Smoker | 116 (38.3) | 3 (1.0) | 119 (39.3) |
| Nonsmoker | 3 (1.0) | 181 (59.7) | 184 (60.7) | |
| Total | 119(39.3) | 184 (60.7) | 303 (100.0) | |
| Kappa coefficient = 0.958*** | ||||
*** P-value < 0.001.
Table 3.
Results of GEE for self-reported and cotinine-validated smoking status.
| Parameter | Overall | Military service conscript | Basic military training conscript | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Smoking status | 0.107 | 0.655 | 0.108 | |||
| Cotinine-validated | 1 | 1 | 1 | |||
| Self-reported | 0.931 (0.854~1.016) | 0.979 (0.890~1.076) | 0.883 (0.758~1.028) | |||
| Age | 0.766 (0.640~0.917) | 0.004 | 0.459 (0.307~0.686) | <0.001 | 0.691 (0.489~0.976) | 0.036 |
| Educational attainment level | 0.143 | 0.442 | 0.188 | |||
| Senior high school and below | 1 | 1 | 1 | |||
| Junior college and above | 1.678 (0.840~3.354) | 1.737 (0.425~7.106) | 0.592 (0.272~1.292) | |||
| SHS exposure | <0.001 | <0.001 | 0.004 | |||
| No | 1 | 1 | 1 | |||
| Yes | 5.466 (3.606~8.285) | 9.066 (5.117~16.062) | 3.111 (1.443~6.707) | |||
GEE = generalized estimating equations.
The sensitivity of self-reported smoking status for all the conscripts was 92.9%, indicating that 92.9% of the conscripts who were identified as smokers by the urine test also reported themselves as smokers. The sensitivity of the self-reported smoking status of the basic military training conscripts was higher than that of the military service conscripts (97.5% and 86.1%, respectively; P-value = 0.002). The overall specificity for the conscripts was 98.1%. Although the specificity for the military training soldiers was slightly higher than that for the military service conscripts (98.4% and 97.9%, respectively), the difference was nonsignificant (Table 4).
Table 4.
Sensitivity and specificity of self-reported smoking status.
| Overall | Military service conscript | Basic military training conscript | ||||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Sensitivity | 92.9 | 88.5~95.7 | 86.1 | 76.8~92.0 | 97.5 | 92.9~99.1 |
| Specificity | 98.1 | 96.3~99.0 | 97.9 | 95.2~99.1 | 98.4 | 95.3~99.4 |
CI = confidence interval.
Effect of SHS Exposure on UC Levels
Regardless of the type of military service, the UC levels of the self-reported smokers were higher than those of the self-reported nonsmokers. For the self-reported smokers, the SHS exposure did not affect their UC levels. However, among the self-reported nonsmokers, the military service conscripts who had been exposed to SHS attained higher UC median levels than did those who had not been exposed to SHS (4.4 ng/mL and 2.5 ng/mL, respectively, P-value = 0.023) (Table 5).
Table 5.
The UC levels of conscripts who self-reported their smoking behaviour and SHS exposurea.
| Type of military services | Self-reported Smoker | Self-reported Nonsmoker | P-valuec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | SHS Exposure | SHS Unexposed | P-valueb | Total | SHS Exposure | SHS Unexposed | P-valueb | ||
| Overall | 407.2 | 408.8 | 399.6 | 0.318 | 2.6 | 2.7 | 2.6 | 0.874 | <0.001 |
| Military service conscript | 418.9 | 424.7 | 418.9 | 0.700 | 2.6 | 4.4 | 2.5 | 0.023 | <0.001 |
| Basic military training conscript | 396.5 | 407.2 | 306.9 | 0.063 | 2.7 | 2.6 | 2.8 | 0.215 | <0.001 |
aMedian level of urinary cotinine ng/mL; b p-value was examined by the Mann-Whitney U test; ccompared self-reported smoker and nonsmoker by the Mann-Whitney U test; SHS = second-hand smoke.
Discussion
This study was the first in Taiwan to verify the self-reported smoking behaviour of conscripts. The results showed that, although self-reporting surveys typically underestimate the smoking rate of the respondents, such survey outcomes are still highly consistent with those determined through a urinary test. Thus, self-reporting surveys can serve as an effective tool for assessing smoking behaviour. In this study, the self-reported smoking rate of the conscripts was 30.9%, which was lower than the smoking rate determined using the UC concentration test (i.e., 31.9%). Additionally, for the military service conscripts, the self-reported smoking rate was lower than that determined using the UC concentration test (23.0% and 24.9%, respectively). However, for the basic military training conscripts, both the self-reporting survey and UC concentration test yielded the same smoking rate (39.3%). Some studies on conscripts in Taiwan found that the prevalence of smoking was 51.3% to 52.6%17–19. The smoking rate was higher than that noted in our study. This may be due to the different distribution of education level among conscripts. The percentage of conscripts graduating from a senior high school or below in our study was 50%, which was lower than that in prior studies (74%)17–19. However, the smoking rate of conscripts was higher than that of males of similar age (16.1%) in the 2014 Adult Smoking Behaviour Survey in Taiwan20. The systematic literature review conducted by previous studies has reported that self-reporting data may underestimate smoking rates5. A Canadian study targeting the general public found that the self-reported smoking rate was 0.3% lower than the smoking rate determined using a UC concentration test; however, the extent of underestimation was nonsignificant, indicating the effectiveness of self-reporting assessments6. The US National Health and Nutrition Examination Survey (NHANES) also asserted that self-reporting methods can be used to accurately measure smoking behavior21,22. We can conclude that using a UC concentration test to estimate smoking behaviour is relatively objective compared with a self-reporting survey. The present study confirmed that the conscripts’ self-reported results and cotinine-validated results demonstrated a high consistency level (i.e., the overall kappa coefficient value was 0.918; the kappa coefficient values for the military service and basic military training conscripts were 0.862 and 0.958, respectively). This indicates that a low-cost questionnaire survey which is applicable to large samples can effectively determine the smoking status of conscripts.
The sensitivity and specificity of the self-reported smoking status of all the conscripts were 92.9% and 98.1%, respectively, which aligns with the results of the Canadian study (in which the sensitivity and specificity were 91.6% and 98.3%, respectively)6. Furthermore, the sensitivity of the military service conscripts was significantly lower than that of the basic military training conscripts (86.1% and 97.5%, respectively), which might be attributable to the fact that the data collection occurred on the penultimate day of recruit training. After recruit training, basic military training conscripts can leave the military, while military service conscripts are reassigned to various military units to continue their service. Therefore, military service conscripts may conceal their smoking behaviour to meet the military officials’ expectations. Although no previous studies on the military can be compared with this study, similar results have been reported in studies on patients who had smoking-related diseases. Those patients also concealed their smoking behaviour to meet the physicians’ expectations9,23–25.
In our study, the prevalence of SHS exposure among conscripts was 29.1%. Moreover, the basic military training conscripts had a higher prevalence of SHS exposure than the military service conscripts (39.3% vs. 23.0%). A study executed by Seo et al.26 found that the self-reported prevalence of SHS exposure in 7 days among college students was 45%. The 2014 Adult Smoking Behaviour Survey in Taiwan revealed that 64.6% of men aged 18 to 24 experienced SHS20. The rate of SHS exposure among conscripts in our study was lower than that among college students and similar age groups. This may be due to the fact that the data were collected on the penultimate day of the recruit training in our study, and conscripts were exposed to more smoking-restricted environments than college students and other young males. The present study further analysed the effect of SHS exposure on the UC concentrations for the two participant groups. Among the self-reported nonsmokers, the military service conscripts exposed to SHS demonstrated significantly higher UC concentrations than did those who had not been exposed to SHS (4.4 ng/mL and 2.5 ng/mL, respectively; p = 0.023). Aurrekoetxea et al.6 also reported that the UC median levels of pregnant nonsmokers who had been exposed to SHS was higher than that of those who had not been exposed to SHS (7.6 ng/mL and <4 ng/mL, respectively). SHS exposure increased UC concentrations by 1.9 ng/mL among military service conscripts who did not smoke. Previous studies have suggested that a UC concentration of >20 ng/mL can be a baseline for assessing SHS exposure25. However, in this study, the UC median levels of the self-reported nonsmokers did not reach the baseline. This may be because the Taiwanese military has implemented smoking prohibition policies, which only permit soldiers to smoke in the smoking zone during the recruit training period. Thus, the effect of SHS exposure on the nonsmokers was minimal.
Although this study verified the accuracy of the conscripts’ self-reported smoking behaviour, several research limitations remained. First, human genes affect the rate of nicotine metabolism and further influence the UC concentrations27–29. Therefore, the baseline of UC level affects the accuracy of smoking status assessment. Although previous studies have defined the baseline UC level for smokers as 50 ng/mL6,30, the present study considered the effect of genes and adopted 100 ng/mL as the baseline by referring to Korean studies. Second, diet also influences UC concentrations in a range from 0.6 to 6.2 ng/mL31. The present study did not collect data related to diet; however, during the training period, the meals were all prepared by the military. Thus, differences in diet may have exerted minimal influence on the UC concentrations of the conscripts in this study. Third, this study conducted a questionnaire survey to assess the SHS exposure level over a period of 30 days. However, the half-life of nicotine metabolism was 16 to 20 hours; hence, the observed UC concentrations may be unable to reflect the influence of SHS exposure. Nonetheless, because all the conscripts experienced the same environment during recruit training, using the UC concentrations to represent the SHS exposure level was still feasible. Finally, this study is limited because our sample consists only of male conscripts who were being trained at a new training regiment; hence, the accuracy of self-reporting of smoking behaviour in the study may not be representative of that of the entire military population. In addition, because the study did not include a control group of the general population, the results should be carefully generalized to soldiers in other service types. Future studies should extend assessments to the entire military population.
Despite the above-mentioned limitations, this study was the first in Taiwan to objectively verify conscripts’ self-reported smoking behaviour using UC concentrations as a biomarker. In addition, because the questionnaire survey and urine test were performed on the same day, the error of incorrect categorization of smoking behaviour that arises from different data collection periods can be reduced.
Conclusion
This study found that the smoking behaviour self-reported by the participants in the questionnaire survey was highly consistent with that determined using the UC concentration test. Subsequent studies can also conduct questionnaire surveys to monitor the smoking behaviour of conscripts. Moreover, future scholars are recommended to investigate the smoking behaviour of soldiers at various hierarchical positions to confirm the accuracy of their self-reported smoking behaviour. Furthermore, future studies are recommended to obtain further information on the factors associated with inaccurate self-reported of smoking status, such as sociodemographic background, knowledge of tobacco hazards, and tobacco cessation history.
Acknowledgements
This research was supported by Ministry of National Defense-Medical Affairs Bureau, Taiwan, R.O.C. The study was supported by grants from the Health Promotion Administration, Ministry of Health and Welfare, Taiwan, R.O.C.
Author Contributions
Authors Senyeong Kao, Fu-Gong Lin, Ching-Huang Lai, and Shu-Ling Hwang designed the study and wrote the protocol. Authors Yu-Lung Chiu, Shan-Ru Li, and Ya-Mei Tzeng conducted literature searches and provided summaries of previous research studies. Authors Yu-Lung Chiu, Shu-Jia Huang, Chung-Chi Huang, and Shiang-Huei Jiang conducted the statistical analysis. Author Yu-Lung Chiu wrote the first draft of the manuscript, and all authors contributed to and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fu-Gong Lin, Email: fugong@mail.ndmctsgh.edu.tw.
Senyeong Kao, Email: kao@ndmctsgh.edu.tw.
References
- 1.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avila-Tang E, et al. Assessing secondhand smoke using biological markers. Tob Control. 2012;22:164–171. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarvis MJ, Primatesta P, Erens B, Feyerabend C, Bryant A. Measuring nicotine intake in population surveys: comparability of saliva cotinine and plasma cotinine estimates. Nicotine Tob Res. 2013;5:349–355. doi: 10.1080/1462220031000094213. [DOI] [PubMed] [Google Scholar]
- 4.Nagano T, et al. Biomonitoring of urinary cotinine concentrations associated with plasma levels of nicotine metabolites after daily cigarette smoking in a male Japanese population. Int J Environ Res Public Health. 2012;7:2953–2964. doi: 10.3390/ijerph7072953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 6.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23:47–53. [PubMed] [Google Scholar]
- 7.Aurrekoetxea JJ, et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ Open. 2013;3:e002034. doi: 10.1136/bmjopen-2012-002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton GR, Brinthaupt J, Stehle JM, James GD. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. J Obstet Gynecol Neonatal Nurs. 2004;33:306–311. doi: 10.1177/0884217504264866. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan C, et al. Assessing the accuracy of self-reported smoking status and impact of passive smoke exposure among pregnant Aboriginal and Torres Strait Islander women using cotinine biochemical validation. Drug Alcohol Rev. 2010;29:35–40. doi: 10.1111/j.1465-3362.2009.00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilberink SR, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med. 2011;4:85–90. doi: 10.2147/IJGM.S15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jhun HJ, et al. Self-reported smoking and urinary cotinine levels among pregnant women in Korea and factors associated with smoking during pregnancy. J Korean Med Sci. 2010;25:752–757. doi: 10.3346/jkms.2010.25.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielinska-Danch W, Wardas W, Sobczak A, Szoltysek-Boldy I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12:484–496. doi: 10.1080/13547500701421341. [DOI] [PubMed] [Google Scholar]
- 13.Calbiotech & Farhat, Y: Cotinine ELISA Protocol (Calbiotech). Available at: https://www.dropbox.com/s/0fxilrm2f8fxvik/2013_0419_Cotinine%20ELISA%20Protocol_Calbiotech.pdf (Accessed: 15th Sep 2016) (2013)
- 14.Global Tobacco Surveillance System (GTSS). Global Adult Tobacco Survey (GATS): Indicator Guidelines: Definition and Syntax. Available at: http://www.who.int/tobacco/surveillance/en_tfi_gats_indicator_guidelines.pdf (Accessed: 15th December 2016) (2009).
- 15.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 16.Kang YH, et al. Usefulness of urinary cotinine test to distinguish smokers from nonsmokers. Korean J Lab Med. 2003;23:92–97. [Google Scholar]
- 17.Chu NF, Wu DM, Shen MH, Lin YS. Prevalence of adverse behaviors among young military conscripts in Taiwan. Mil Med. 2006;171:301–305. doi: 10.7205/MILMED.171.4.301. [DOI] [PubMed] [Google Scholar]
- 18.Lin YS, et al. Factors associated with cigarette smoking among young military conscripts in taiwan. J Chin Med Assoc. 2008;71:559–565. doi: 10.1016/S1726-4901(08)70169-2. [DOI] [PubMed] [Google Scholar]
- 19.Wu DM, Chu NF, Lin YS, Lai HR. Aggregation of adverse behaviors and its affecting factors among young military conscripts in Taiwan. Addict Behav. 2007;32:1302–1308. doi: 10.1016/j.addbeh.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Health Promotion Administration, Ministry of Health and Welfare. Adult Smoking Behavior Survey, 2014. Available at: https://olap.hpa.gov.tw/index.aspx. (Accessed: 15th December 2016) (2014).
- 21.Arheart KL, et al. Accuracy of self-reported smoking and secondhand smoke exposure in the US workforce: the National Health and Nutrition Examination Surveys. J Occup Environ Med. 2008;50:1414–1420. doi: 10.1097/JOM.0b013e318188b90a. [DOI] [PubMed] [Google Scholar]
- 22.Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination Survey. Med Care. 2010;48:1128–1132. doi: 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]
- 23.Gerritsen M, et al. Self-Reporting of Smoking Cessation in Cardiac Patients: How Reliable Is It and Is Reliability Associated With Patient Characteristics? J Addict Med. 2015;9:308–316. doi: 10.1097/ADM.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 24.Morales NA, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren GW, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo DC, Torabi MR, Kim N, Lee CG, Choe S. Smoking among East Asian college students: prevalence and correlates. Am J Health Behav. 2013;37:199–207. doi: 10.5993/AJHB.37.2.7. [DOI] [PubMed] [Google Scholar]
- 27.Moyer TP, et al. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. 2002;48:1460–1471. [PubMed] [Google Scholar]
- 28.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 29.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 30.Balhara YP, Jain R, Sundar AS, Sagar R. Use of cotinine urinalysis to verify self-reported tobacco use among male psychiatric out-patients. Lung India. 2012;29:217–220. doi: 10.4103/0970-2113.99102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis RA, Stiles MF, deBethizy JD, Reynolds JH. Dietary nicotine: a source of urinary cotinine. Food Chem Toxicol. 1991;29:821–827. doi: 10.1016/0278-6915(91)90109-K. [DOI] [PubMed] [Google Scholar]


