Abstract
The speciation of Ra2+ and Ba2+ with EDTA was investigated at 25 °C in aqueous alkaline NaCl media as a function of ionic strength (0.2–2.5 mol·L−1) in two pH regions where the EDTA4− and HEDTA3− species dominate. The stability constants for the formation of the [BaEDTA]2− and [RaEDTA]2− complexes were determined using an ion exchange method. Barium-133 and radium-226 were used as radiotracers and their concentrations in the aqueous phase were measured using liquid scintillation counting and gamma spectrometry, respectively. The specific ion interaction theory (SIT) was used to account for [NaEDTA]3− and [NaHEDTA]2− complex formation, and used to extrapolate the logarithms of the apparent stability constants (log10 K) to zero ionic strength (BaEDTA2−: 9.86 ± 0.09; RaEDTA2−: 9.13 ± 0.07) and obtain the Ba2+ and Ra2+ ion interaction parameters: [ε(Na+, BaEDTA2−) = − (0.03 ± 0.11); ε(Na+, RaEDTA2−) = − (0.10 ± 0.11)]. It was found that in the pH region where HEDTA3− dominates, the reaction of Ba2+ or Ra2+ with the HEDTA3− ligand also results in the formation of the BaEDTA2− and RaEDTA2− complexes (as it does in the region where the EDTA4− ligand dominates) with the release of a proton. Comparison of the ion interaction parameters of Ba2+ and Ra2+ strongly indicates that both metal ions and their EDTA complexes have similar activity coefficients and undergo similar short-range interactions in aqueous NaCl media.
Keywords: Alkaline-earth metal, EDTA, Complex formation, Activity coefficient, Specific ion interaction theory, Infinite dilution
Introduction
Barium and radium are members of the alkaline-earth metal group. While barium is an abundant element in the earth’s crustal rocks (340 mg·kg−1), radium occurs in nature only in trace amounts (0.1 ng·kg−1) [1]. Radium has no stable isotopes and the most abundant radium isotope is 226Ra with a half-life of 1600 years. Radium-226 is part of the 238U decay chain and decays to the short lived (t 1/2 = 3.4 d) α-emitting gas 222Rn.
Both 226Ra and 222Rn are among the most radiotoxic elements present in the environment [2]. As a consequence of some anthropogenic processes, 226Ra is concentrated in waste streams. For example, in uranium mining, uranium is usually leached from milled uranium ore or leached in situ using sulfuric acid. After leaching, the tailings (solid and liquid residues) are usually neutralized and disposed in surface ponds in the form of a slurry [3, 4]. Predominantly, radium is rapidly dissolved in leaching and co-precipitates in the form of Ba(Ra)SO4 [5]. The concentration of 226Ra in such tailings is higher than in the natural uranium ore and can reach up to 43.4 kBq·kg−1 (1186.7 ng·kg−1) [6]. The background radiation levels are also increased, mostly because of radium and its decay products, for example, from 0.1 to 0.2 μSv·h−1 in reference areas such as the tailings storage facility up to 10–20 μSv·h−1 on the top of waste dumps [6]. Radium-226 concentrations up to 200 Bq·L−1 (0.2 nmol·L−1) also occur in water produced from the petroleum industry, which is above limits for industrial effluents [7]. Radium-226 is usually removed by addition of sulfate salts which allow it to co-precipitate in the form of Ba(Ra)SO4. Therefore, co-precipitation of radium with barite (BaSO4), mostly via an inclusion (lattice replacement) process [7], is the main mechanism controlling radium behavior in the waste streams and its migration in the environment [5, 8]. To decontaminate uranium tailings or solid residues from, e.g., the petroleum industry, it is necessary to dissolve Ba(Ra)SO4.
Pure radium and barium sulfate salts and their co-precipitates are, in principle, insoluble in water and aqueous solutions of mineral acids and alkali at room temperature [9] (the recommended values for the decadic logarithm of the BaSO4 and RaSO4 solubility products at zero ionic strength and 25 °C are −9.95 and −10.21, respectively [10, 11]). At room temperature, Ba(Ra)SO4 can be dissolved using chelating agents. The most commercially available chelating agent for Ba(Ra)SO4 dissolution is ethylenediaminetetraacetic acid (EDTA) and its derivatives. Aqueous alkaline EDTA solutions have been found to be effective in the dissolution of Ba(Ra)SO4 and in the extraction of 226Ra from uranium tailings [12]. Approximately 80–85% of 226Ra was extracted from uranium tailings using a 0.04 mol·L−1 aqueous alkaline EDTA solution at Elliot Lake, Ontario, Canada [13]. Moreover, alkaline EDTA solutions have been used for dissolution of irradiated 226RaSO4 targets and the preparation of 227Ac/223Ra radiopharmaceutical generators [14]. One of the reasons for the high Ba(Ra)SO4 solubility in alkaline EDTA solutions is the formation of a strong complex between Ba2+ or Ra2+ and EDTA. Therefore, it is necessary to know accurately the stability constants of the BaEDTA2− and RaEDTA2− complexes to model the Ba(Ra)SO4 dissolution equilibrium in alkaline EDTA systems including decontamination using EDTA.
Experimental studies of Ba2+ and Ra2+ complex formation are also important on a fundamental level. Radium and barium have similar solution chemistry and one of the main reasons for this is the similarity of the effective ionic radii, which are equal to 1.42 Å for Ba2+ and 1.48 Å for Ra2+ (in 8-fold coordination) [15]. Due to the high radiotoxicity of radium and its daughters, experimental thermodynamic data for radium are limited. For example, to the best of our knowledge, the experimental determination of radium activity coefficients or ion interaction parameters have never been reported in the literature. Due to the lack of experimental data, extrapolation of the ion interaction parameters for radium from values of the other alkaline-earth metals using ionic radii or using interaction parameters of barium directly are the methods used to calculate radium activity coefficients [5, 16, 17]. All approaches for modelling activity coefficients are semi-empirical, with one or more fitted parameters, thus the obtained ion interaction parameters can be brought into question. Therefore, an experimental study of Ba2+ and Ra2+ complex formation using a background electrolyte would be beneficial on both applied and fundamental levels.
The objective of this work was to study the complex formation of Ra2+, as well as Ba2+, with EDTA as a function of ionic strength using NaCl as an ionic medium. Sodium chloride is an inert ionic electrolyte which is also omnipresent in the environment. Due to the high radiotoxicity of radium, the complex formation was studied via an ion exchange method which only requires trace amounts of radium. The specific ion interaction theory (SIT) was used to extrapolate the apparent stability constants of the studied complexes to zero ionic strength, and for determining the ion interaction parameters of the species involved in the complex formation.
Experimental Section
Sample Preparation
The complexation of Ba2+ and Ra2+ with EDTA was studied as a function of NaCl ionic strength (0.22, 0.5, 1.0, 2.0 and 2.5 mol·L−1) via an ion exchange method with batch and radiotracer techniques. The method is based on the different distribution of metal ions (133Ba2+ or 226Ra2+) and negatively charged metal–EDTA complexes using a strong cation exchange resin. Distribution experiments were performed in polypropylene tubes with aqueous phase volumes of 10 mL in the case of Ba2+, and 1 mL in the case of Ra2+, with 0.5 g (Ba2+) and 0.05 g (Ra2+) of ion exchange resin added to each tube. The ionic strength in the aqueous phase was adjusted using concentrated NaCl stock solutions. Different doses of Na2EDTA stock solution were added to each sample and its concentration was varied throughout the sample series, ranging between 0 and 6.67 × 10−5 mol·L−1. The apparent EDTA dissociation constants at various NaCl ionic strengths were determined using the SIT methodology and the H+ concentration was adjusted using potentiometric titrations to maximize the molar fractions of EDTA4− (−log10 [H+] = 12.4; more than 99% EDTA4−) or HEDTA3− (−log10 [H+] = 7.9–8.3 depending on the ionic strength; always more than 98% HEDTA3−). Samples without the ion exchange resin and EDTA were prepared to measure the total radioactivity of 133Ba2+ or 226Ra2+ in the samples. Preliminary kinetic studies confirmed that the metal–EDTA equilibria were achieved within 24 h under the experimental conditions used. The experiments were performed in duplicate where each series contained 11 samples per ionic strength. All samples were kept at 25 ± 1 °C.
Chemicals Used
All aqueous solutions were prepared using MQ water with 18.2 MΩ·cm resistivity at 25 °C and a total organic content of less than 5 mg·L−1. The barium stock solution was in the form of 133Ba with a specific activity of 37 kBq·µL−1 in 0.1 mol·L−1 HCl with an additional 10 µg·mL−1 of BaCl2 carrier (Eckert and Ziegler Isotope Products radionuclide purity > 99%). Radium carbonate was synthesized from RaSO4 powder as previously described [9]. The synthesized RaCO3 was dissolved in 0.1 mol·L−1 HCl (Sigma–Aldrich 99.999% trace metals basis) to obtain 14 mL of radium stock solution with a 226Ra specific activity of (2.5 ± 0.1) × 104 Bq·µL−1. The purity of the synthesized radium stock solution was measured previously and it was found that the mass fraction of stable barium and lead was 0.2 and 0.003, respectively [18]. The cation exchange resin was in sodium form (Biorad AG 50W-X8 200–400 mesh molecular biology grade). EDTA stock solutions were prepared from solid Na2EDTA·2H2O (Sigma p.a. ≥ 99.0%). The ionic strength and −log10 [H+] were adjusted using a NaCl stock solution prepared from solid NaCl (Sigma–Aldrich ACS reagent p.a. ≥ 99.0%) and standard NaOH and HCl solutions (Fixanal, Sigma-Aldrich).
Apparatus
All solid chemicals were weighed on a standard analytical balance (Sartorius Quintix125D-1S) and samples were kept at a constant temperature of 25 ± 1 °C in a shaking water bath (Julabo SW23). Potentiometric measurements were performed using two pH meters coupled with combined glass electrodes (827 pH laboratory Metrohm coupled with Metrohm Primatrode electrode and Radiometer MeterLab PHM240 coupled with A Radiometer PHC3006-9 electrode). Both electrodes were filled with a 3 mol·L−1 NaCl reference electrolyte and calibrated using the activity scale with standard buffer solutions (NIST and SRM traceable, Certipur, Merck), and were subsequently calibrated in the concentration scale using a potentiometric titration with negligible volume change [19]. The radioactivity of 133Ba was measured using liquid scintillation counting (LSC) (Perkin Elmer Guardian 1414) and aqueous 133Ba samples were subsequently mixed with an Emulsifier safe LSC cocktail. The radioactivity of 226Ra was measured using two High Purity Germanium detectors (HPGe) (Canberra GEM23195 closed-end coaxial HPGe detector coupled with digital spectrum analyzer Canberra-2000/A and Ortec GEM-C5060 coaxial HPGe coupled with digital spectrum analyzer Ortec DSPEC50). Both detectors were calibrated using a mixed radionuclide reference solution (NIST traceable, Eckert and Ziegler). Nuclide half-lives, gamma emission energies and photon emission probabilities were taken from the Decay Data Evaluation Project [20].
The Model
The speciation of a metal ion (M2+) with various forms of EDTA can be described by the reaction:
| 1 |
where 0 ≤ r ≤ 6.
The stability constant for reaction 1 at zero ionic strength is defined as:
| 2 |
The SIT model developed by Brønsted [21, 22], Scatchard [23], Guggenheim and Turgeon [24] can be used to express the activity coefficients γ i of an ion i at ionic strengths below about 3.5 mol·kg−1:
| 3 |
where z i is the charge of the ion i, ε(i,j,I m ) is the interaction parameter of ion i with all oppositely charged ions j, I m is ionic strength in mol·kg−1, m j is molal concentration of ion j and D H is the Debye–Hückel term which is defined as:
| 4 |
where A is a temperature dependent constant equal to 0.5090 and 0.5047 kg1/2·mol−1/2 at 25 °C and 20 °C, respectively, for aqueous solutions [25]. The value 1.5 is the product of B (a constant dependent on temperature and the solvent relative permittivity) and a (distance of closest approach or effective Debye–Hückel ionic radius). In the SIT, this product is usually taken to be 1.5 to minimize the effect of ionic strength on the ion interaction parameters. In this work, each ionic strength of NaCl was recalculated to the molal scale (from molar) using the relevant conversion factors [25]. Substituting the activity coefficients calculated using Eq. 3 into Eq. 2 yields:
| 5 |
From Eq. 5 it can be concluded that plotting the difference between the determined decadic logarithm of the apparent stability constants and ∆z 2·D H against ionic strength of the same background electrolyte will result in an intercept which is the decadic logarithm of the stability constant at zero ionic strength and a slope which is the ion interaction parameter term.
Measurement of the metal ion radioactivity in the aqueous phase allows for calculation of the distribution ratio between the solid phase and the aqueous phase according to:
| 6 |
where A total is the total radioactivity of the metal ion in the sample, A aq is the radioactivity of the metal ion in the aqueous phase after the distribution equilibrium has been reached, V is the solution volume (mL) and m is the mass of the ion exchange resin (g).
The distribution ratio can also be expressed through the apparent stability constant:
| 7 |
where λ is the distribution ratio without the ligand (mL·g−1) and K is the apparent stability constant for the MHrEDTA(r−2) complex.
The apparent dissociation constants of the HrEDTA(r−4) complexes can be computed via the SIT (Eq. 3) using the EDTA dissociation constants at zero ionic strength and their ion interaction parameters given in the literature [26]. The constants calculated in this manner have been used in this work. Molar fractions of the different EDTA species can be computed as a function of hydrogen ion concentration using the calculated apparent dissociation constants of HrEDTA(r−4). The concentration of H+ at which the molar fractions of EDTA4− and HEDTA3− are maximized were calculated for all studied ionic strengths, and −log10 [H+] was adjusted according to these calculations.
The hydrolysis of Ba2+ and Ra2+ at a −log10 [H+] of 12.4 (the highest −log10 [H+] used in this work) can be neglected [27] compared to the metals strong complexation with EDTA. Polynuclear complexes are also not formed when a metal ion is at radiotracer levels, therefore the M2+ concentration terms in Eq. 7 cancel. Only one form of HrEDTA(r−4) is dominant under each of the two experimental conditions studied. As a result, Eq. 7 can be simplified to:
| 8 |
Thus, the apparent stability constants of the MHrEDTA(r−2) complexes can be determined using linear regression.
The [HrEDTA(r−4)] term in Eq. 8 refers to the free concentration of the ligand. However, EDTA also forms strong complexes with Na+, which was used as part of the ionic medium. The Na+ concentration was considerably higher than the M2+ concentration under all experimental conditions. As a result, the concentration of free EDTA was adjusted by the EDTA complex formation with Na+. The effect of complex formation between EDTA4− or HEDTA3− and Na+ has been found to be important [28] and can be described by the following reactions:
| 9 |
| 10 |
As a result, the free EDTA4− or HEDTA3− concentration in Eq. 8 can be expressed as:
| 11 |
| 12 |
where K HEDTA refers to the protonation constant of EDTA4− and K NaEDTA or K NaHEDTA refer to the stability constants for reactions 9 and 10, respectively.
Results and Discussion
Sodium Speciation with EDTA
The dissociation constant of EDTA and stability constant for reaction 9 have been experimentally studied by many researchers and a comprehensive review is available [26]. The values of the protonation constants and the NaEDTA3− stability constant at zero ionic strength were taken from Hummel and co-workers [25] and are listed in Table 1. The SIT ion interaction parameters and associated uncertainties were derived from all available experimental data of NaEDTA3− and EDTA4− protonation in NaCl media at 25 °C listed in the review [26]. Subsequently, the apparent stability constants were calculated using the derived SIT ion interaction parameters. The apparent EDTA4− protonation constants and NaEDTA3− stability constants obtained were used to calculate the Ba2+ and Ra2+ stability constants (see Table 5) and free EDTA4− concentration (Eq. 11), respectively. All these stability constants are listed in Table 1.
Table 1.
Stability constants and SIT ion interaction parameters at 25 °C used in this work
| Equilibrium reaction | I m (mol·kg−1) | Stability constant log10 K | Specific ion interaction parameters (NaCl) ∆ε (mol·kg−1) |
|---|---|---|---|
| H+ + HEDTA3− ⇌ H2EDTA2− | 0 | 6.80 ± 0.02 | 0.40 ± 0.03 |
| H+ + EDTA4− ⇌ HEDTA3− | 0 | 11.24 ± 0.03 | 0.55 ± 0.04 |
| 0.22 | 10.24 ± 0.03 | ||
| 0.51 | 10.12 ± 0.03 | ||
| 1.02 | 10.21 ± 0.04 | ||
| 2.09 | 10.51 ± 0.06 | ||
| 2.64 | 10.74 ± 0.08 | ||
| Na+ + EDTA4− ⇌ NaEDTA3− | 0 | 2.80 ± 0.20 | 0.27 ± 0.33 |
| 0.22 | 1.74 ± 0.22 | ||
| 0.51 | 1.54 ± 0.31 | ||
| 1.02 | 1.44 ± 0.52 | ||
| 2.09 | 1.51 ± 1.0 | ||
| 2.64 | 1.59 ± 1.3 |
Table 5.
Comparison of reported stability constants for the formation of BaEDTA2− and RaEDTA2−
| Method | Ionic medium | Temperature (°C) | Reported log10 K | Extrapolated to zero ionic strength log10 K° | Reference |
|---|---|---|---|---|---|
| Ion exchange | 0.2; 0.5; 1.0; 2.0; 2.5 mol·L−1 (NaCl) | 25 | Tables 3 and 4 | 9.86 ± 0.09 | This work |
| Review | 0.1 mol·L−1 | 25 | 7.86 ± 0.08 | 9.64 | Smith and Martell [38] |
| pH | 0.1 mol·L−1 (KCl) | 20 | 7.76 | 9.54 | Schwarzenbach and Ackermann [36] |
| pH | 0.1 mol·L−1 a | 25 | 7.73 | 9.51 | Carini and Martell [41] |
| pH | 0.1 mol·L−1 | 25 | 7.9 | 9.68 | Schmid and Reilley [42] |
| Ion exchange | 0 | 25 | 9.92 | 9.92 | Astakhov and Fomenko [43] |
| pH | 0.1 mol·L−1 (KNO3) | 25 | 7.63 | 9.41 | Bohigian and Martell [44] |
| Paper electrophoresis | 0.1 mol·L−1 (KNO3) | 20 | 8 | 9.78 | Jokl and Majer [45] |
| pH | 0.1 mol·L−1 (KNO3 or (CH3)4N(NO3)) | 25 | 7.8 | 9.58 | Delgado and Da Silva [46] |
| Ion exchange | 0.2; 0.5; 1; 2; 2.5 mol·L−1 (NaCl) | 25 | Table 3 | 9.13 ± 0.07 | This work |
| Ion exchange | 0.1 mol·L−1 a | 20a | 7.12 | 8.9 | Nikolsky et al. [47] |
| Ion exchange | 0.1 mol·L−1 b (sodium salt) | 20 | 7.07b ± 0.06 | 9.22b | Baetsle and Bengsch [48] |
| Solvent extraction | 0.1 mol·L−1 (NaClO4) | 25 | 7.7 | 9.29 | Sekine et al. [49] |
| Estimated | 0.1 mol·L−1 | 25 | 7.4 | 9.2 | Nelson et al. [50] |
aIonic strength and temperature were assumed
bContribution of the 0.01 mol·L−1 EDTA to the total ionic strength has been considered
Only a few experimental data for the formation of the NaHEDTA2− complex (Eq. 10) are available in the literature and the reported log10 K° values vary significantly from 0 to 1.5 [29–32]. The main reason for the log10 K° data discrepancies is that the NaHEDTA2− complex is quite weak. In the case of weak complex formation, it is usually impossible to separate the weak complex formation effect from potential activity coefficient changes. This and other challenges associated with the determination of the stability constants of weak complexes have been previously discussed in detail [33, 34]. Perhaps, the most reasonable value for the stability constant of the NaHEDTA2− complex was reported by Palaty [31]. The author used ion selective electrodes to study the proton dissociation reactions of EDTA and the sodium–EDTA equilibrium and the obtained stability constant values are in good agreement with the values listed in Table 1 (11.34, 6.81 and 2.61, respectively [31]). Tetramethylammonium chloride was used as the background electrolyte with a total ionic strength of 0.12 mol·L−1. The temperature was not given by the author [31] but based on all the obtained values it can be assumed that the reported equilibria were studied at 25 °C. The reported value for the log10 K° value of the NaHEDTA2− complex was −0.03. The value is subject to some uncertainty and it is assumed that the actual log10 K° value at zero ionic strength lies in the range from −0.5 to 0.5 (i.e., log10 K = 0 ± 0.5). Most probably, the assignment of such a high, but reasonable, uncertainty for the stability constant of a weak complex is the only way to overcome the lack of reliable data. The proposed log10 K° value of 0 ± 0.5 is in accord with the statement made by Marcus and Hefter in relation to log10 K° values less than 1, where substantial care needs to be taken in obtaining the exact magnitude of such constants by either experiment or theory [34].
To be able to extrapolate the log10 K° value of 0 ± 0.5 for the NaHEDTA2− complex at the ionic strengths used in this work, it is necessary to know the following SIT interaction parameters: ε(Na+, Cl−), ε(Na+, HEDTA3−) and ε(Na+, NaHEDTA2−). The first two parameters, with their associated uncertainties, are available in the literature [25, 26] and to the best of our knowledge the last parameter has never been reported. A comparison of the sodium SIT ion interactions with many different negatively charged ligands shows that this parameter usually varies from −0.3 to 0.1 [25] (the sodium ion with a divalent anion). Moreover, the sodium SIT ion interaction with ligands similar to H2EDTA2− is −0.37 [26]. Consequently, based on these values, the ε(Na+, NaHEDTA2−) SIT parameter has been estimated as −(0.2 ± 0.3) kg·mol−1. All the parameters associated with the NaHEDTA2− complex (Eq. 10) used in this work are listed in Table 2.
Table 2.
Stability constants and SIT ion interaction parameters for the NaHEDTA2− complex formation (Eq. 2) at 25 °C
| Parameter | Value | References |
|---|---|---|
| log10 K° | 0 ± 0.5 | Estimated in this work, based on available experimental data from Palaty [31] |
| ε(Na+, Cl−) | 0.03 ± 0.01 (kg·mol−1) | Guillaumont et al. [25] |
| ε(Na+, HEDTA3−) | −(0.1 ± 0.14) (kg·mol−1) | Hummel et al. [26] |
| ε(Na+, NaHEDTA2−) | −(0.2 ± 0.3) (kg·mol−1) | Estimated in this work |
Stability Constants for the Complex Formation of Ba2+ and Ra2+ with EDTA
The apparent stability constants for the BaEDTA2− and RaEDTA2− complexes were obtained from distribution coefficients (from experiments conducted at a −log10 [H+] of 12.4) using a weighted linear regression (ω i = σ i) with a zero intercept (Eq. 8). The free EDTA4− concentrations were obtained by correcting for the formation of the NaEDTA3− complex (Eq. 9) using Eq. 11 and the values which are listed in Table 1. The standard deviations of the free EDTA4− concentrations were propagated from the standard deviation of the apparent NaEDTA3− stability constants, also listed in Table 1. The standard deviations of the distribution ratio without the ligand (λ) and the distribution ratio with the ligand (D) were calculated based on duplicate series (biased standard deviation with (n − 1) in the denominator) and were propagated to the standard deviations of (λ/D − 1). Standard uncertainty propagation was used in the both cases.
The uncertainties in the linear fitting were obtained using the method of Allard and Ekberg [35]. After obtaining the uncertainties in both the (λ/D − 1) term and the free EDTA concentration, 30 points were sampled from each uncertainty space using a normal distribution with the mean and standard deviation obtained. Thus, the obtained simulated data points covered the entire standard deviation region in both x and y forming confidence ellipses for each point. Negative simulated values of the free EDTA4− concentrations were discarded. All these points were then used for the linear regression and the estimation of the associated uncertainty analysis.
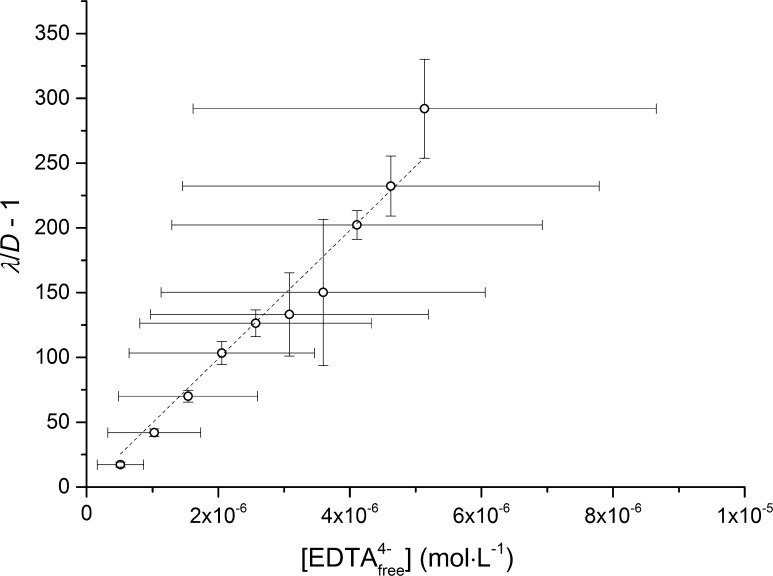
Figure 1 shows a representative dataset for the linear regression of the BaEDTA2− (reaction 1) apparent stability constant in 0.22 mol·kg−1 NaCl.
Fig. 1.
Determination of BaEDTA2− apparent stability constants using linear regression (0.22 mol·kg−1 NaCl, reaction 1, Eq. 8)
As can be observed from Fig. 1, the standard deviations of the free EDTA4− concentrations are large and increase with an increase in ionic strength (NaCl). These large standard deviations are a consequence of the error propagation that results principally from the large uncertainties in the NaEDTA3− stability constants (Table 1).
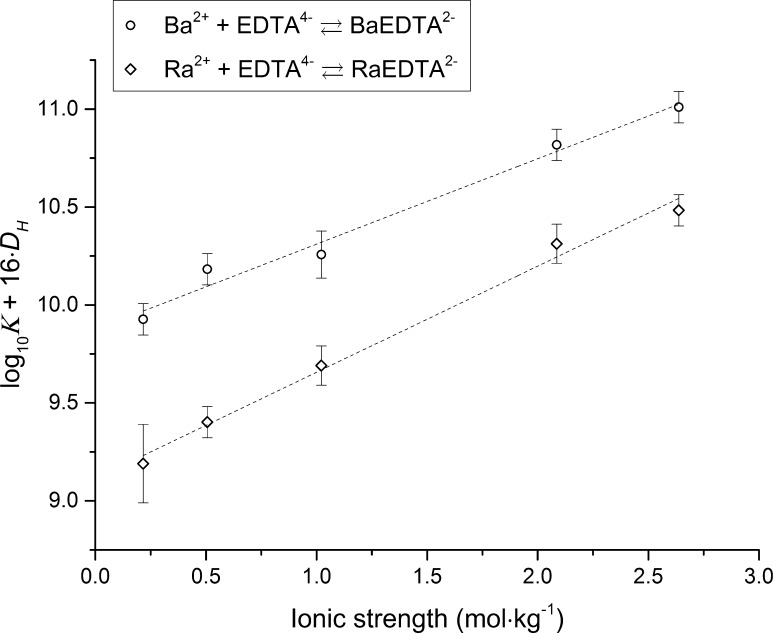
The stability constants obtained are listed in Table 3 and extrapolation of the BaEDTA2− and RaEDTA2− stability constants to zero ionic strength (non-weighted linear regression) using the SIT are shown in Fig. 2.
Table 3.
Apparent stability constants of BaEDTA2− and RaEDTA2− aqueous complexes in NaCl media at 25 °C formed via reaction 1
| I m (mol·kg−1) | log10 K BaEDTA | log10 K RaEDTA |
|---|---|---|
| 0 | 9.88 ± 0.11 | 9.11 ± 0.09 |
| 0.22 | 7.70 ± 0.08 | 6.96 ± 0.20a |
| 0.51 | 7.38 ± 0.08 | 6.60 ± 0.08 |
| 1.02 | 6.99 ± 0.12 | 6.42 ± 0.10 |
| 2.09 | 7.10 ± 0.08 | 6.60 ± 0.10 |
| 2.64 | 7.16 ± 0.08 | 6.63 ± 0.08 |
Ionic strengths were adjusted from the mol·L−1 to mol·kg−1 scale using the appropriate conversion factors [25]. Uncertainties correspond to 95% confidence intervals
aEstimated uncertainty
Fig. 2.
Extrapolation of BaEDTA2− and RaEDTA2− apparent stability constants (NaCl media, reaction 1) to zero ionic strength using SIT
As can be observed from Fig. 2, the fits are satisfactory and the experimental data are accurately modelled by the SIT. According to the calculations, the effect of Na+ complex formation with EDTA4− (Eq. 9) is significant and the difference between the corrected and uncorrected stability constants of both BaEDTA2− and RaEDTA2− at zero ionic strength is more than 1 log10 unit. The difference between the slopes (with and without correction for Na complex formation with EDTA), which corresponds to the ion interaction parameter term, was also significant and the deviation of the experimental data points from the regression line was higher at increased ionic strength. This strongly indicates that the complex formation between sodium and EDTA is significant, which is in agreement with previous studies [28].
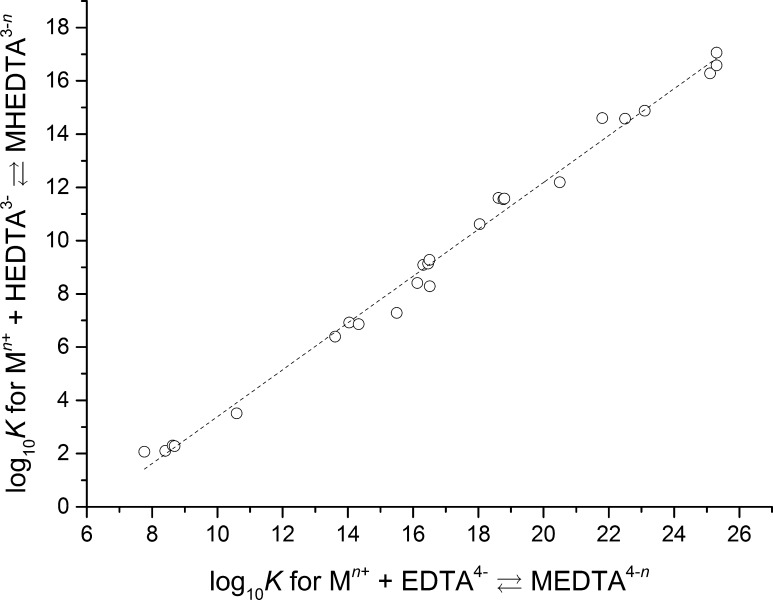
The apparent stability constants, assuming only the formation of the BaHEDTA− and RaHEDTA− complexes [according to reaction 1 (r = 1)], were derived from the experiments conducted at −log10 [H+] of 7.9–8.3 with the mole fraction of HEDTA3− being more than 98% using the same method as used for derivation of the BaEDTA2− and RaEDTA2− complex stability constants. The apparent stability constants obtained were extrapolated to zero ionic strength using the SIT that resulted in stability constants of log10 K° = 7.34 ± 0.30 (for BaHEDTA−) and log10 K° = 6.57 ± 0.30 (for RaHEDTA−). Schwarzenbach and Ackermann [36] have previously given a log10 K value for the same reaction (BaHEDTA− complex) of 2.07 at 20 °C and an ionic strength of 0.1 mol·L−1. This value, when extrapolated to zero ionic strength, results in log10 K° = 3.15, which is much lower than the value obtained in the present work. It can be seen that the value from this study is more than four orders of magnitude larger than the value given by Schwarzenbach and Ackermann. There are two probable reasons for the disagreement between these two values: either the assumption that the BaHEDTA− complex is formed according to reaction 1 (r = 1) at −log10 [H+] of 7.9–8.3 is not valid or the data from Schwarzenbach and Ackermann are inconsistent. The latest hypothesis can be verified by combining the data from Schwarzenbach and Ackermann [36] with other literature data [37, 38], where the stability constants for the reaction of various metals with EDTA4− and HEDTA3− are reported for the same experimental conditions (20 °C and an ionic strength of 0.1 mol·L−1) and performing a linear free energy analysis of the data. This analysis (i.e., a plot of the log10 K values of Mn+–EDTA4− complexes against the log10 K of Mn+–HEDTA3− complexes, where Mn+ is a metal ion with n ≥ 2 (reaction 1 with r = 0 and r = 1, respectively)) is shown in Fig. 3.
Fig. 3.
Linear free energy analysis of available literature data [36–38] for the decadic logarithm of Mn+EDTA(4−n) and Mn+HEDTA(3−n) apparent stability constants (n ≥ 2) at the same experimental conditions (20 °C, I = 0.1 mol·L−1)
As shown in Fig. 3, there is a strong relationship between the magnitude (log10 K values) of the Mn+EDTA(4−n) and Mn+HEDTA(3−n) stability constants (n ≥ 2), and consequently, the available literature data [36–38] are consistent. Therefore, the assumption that only the BaHEDTA− or RaHEDTA− complexes are formed at a −log10 [H+] of 7.9–8.3 is not valid. The stability constant for the BaHEDTA− complex derived in the present study is more than four orders of magnitude larger when compared to those values available in the literature, which indicates that another stronger complex dominates at a −log10 [H+] of 7.9–8.3. The only other strong complex that could be formed in the studied system is BaEDTA2− (or RaEDTA2−). The likely mechanism of the formation of these two complexes at a −log10 [H+] of 7.9–8.3, where the mole fraction of HEDTA3− is more than 98% is as follows:
| 13 |
| 14 |
If the proposed reactions 13 and 14 occur in the studied system, then Eq. 7 can be adapted to reactions 13 and 14 to describe the experimental data obtained at a −log10 [H+] of 7.9–8.3:
| 15 |
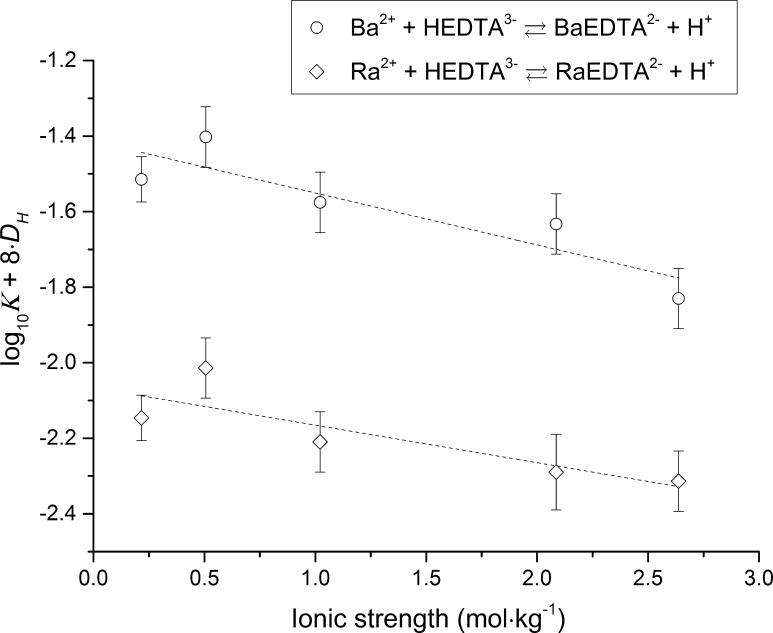
According to Eq. 15, the concentration of the free HEDTA3− must be divided by the H+ concentration to obtain the apparent stability constant for the BaEDTA2− or RaEDTA2− complex via reactions 13 and 14 under these lower −log10 [H+] conditions. Moreover, it can be shown that the sum of the decadic logarithm of obtained stability constants for reactions 13 and 14 and the decadic logarithm of the protonation constant of EDTA4− results in the decadic logarithm of the stability constant for the BaEDTA2− or RaEDTA2− complexes formed via reaction 1 with r = 0. The stability constants for reactions 13 and 14 at a −log10 [H+] of 7.9–8.3 and the associated standard deviations were derived using the same method as was used to derive stability constants and standard deviations for reaction 1 with r = 0 at a −log10 [H+] of 12.4. These stability constants and the calculated stability constants for reaction 1 with r = 0, using the derived constants and the protonation constants of EDTA4− from Table 1, are listed in Table 4. Extrapolation of the BaEDTA2− and RaEDTA2− stability constants to zero ionic strength using the SIT is shown in Fig. 4.
Table 4.
Apparent stability constants of BaEDTA2− and RaEDTA2− aqueous complexes in NaCl media at 25 °C formed via reactions 13 and 14 and 1
| I m (mol·kg−1) | log10 K BaEDTA (formed via reaction 13) | log10 K BaEDTA (formed via reaction 1) | log10 K RaEDTA (formed via reaction 14) | log10 K RaEDTA (formed via reaction 1) |
|---|---|---|---|---|
| 0 | −1.41 ± 0.12 | 9.83 ± 0.14 | −2.07 ± 0.11 | 9.17 ± 0.13 |
| 0.22 | −2.63 ± 0.06 | 7.61 ± 0.08 | −3.26 ± 0.06 | 6.98 ± 0.08 |
| 0.51 | −2.80 ± 0.08 | 7.32 ± 0.10 | −3.42 ± 0.08 | 6.71 ± 0.10 |
| 1.02 | −3.21 ± 0.08 | 7.00 ± 0.11 | −3.85 ± 0.08 | 6.37 ± 0.11 |
| 2.09 | −3.49 ± 0.08 | 7.02 ± 0.14 | −4.15 ± 0.10 | 6.36 ± 0.15 |
| 2.64 | −3.75 ± 0.08 | 6.99 ± 0.18 | −4.24 ± 0.08 | 6.50 ± 0.18 |
Fig. 4.
Extrapolation of BaEDTA2− and RaEDTA2− apparent stability constants (NaCl media, reactions 13 and 14) to zero ionic strength using the SIT
As can be observed in Fig. 4, the experimental data are accurately described by Eq. 15. A comparison of the stability constants of the BaEDTA2− and RaEDTA2− complexes formed via reaction 1 listed in Table 4 with the same stability constants listed in Table 3 shows that all the values are within the 95% confidence intervals. This strongly indicates that the proposed reactions 13 and 14 occur at the pH region where the HEDTA3− species dominates. The effect of Na+ complex formation with HEDTA3− (Eq. 10) was not as significant as in the case of EDTA4− due to the fact that the NaHEDTA2− complex is much weaker than NaEDTA3− (Tables 1, 2).
A comparison of the average value of the obtained metal–EDTA stability constants at zero ionic strength with data available in the literature is shown in Table 5. The data from the literature were, where necessary, extrapolated to zero ionic strength using the Davies equation [39] (in the last term 0.2·I was used instead of 0.3·I, the latter as proposed by Davies [40]) for activity coefficient corrections. The weighted mean and associated 95% confidence intervals of the BaEDTA2− and RaEDTA2− stability constants at zero ionic strength were calculated from the values listed in Tables 3 and 4.
Experimental data for the stability constant of BaEDTA2− [36, 41–46] and reviews of relevant stability constants [38, 51] are available in the literature. The data given in Table 5 for extrapolation of the literature data for the stability constant of BaEDTA2− to zero ionic strength are in very good agreement with the value determined in the present work.
The complex formation of radium with EDTA has been studied by several researchers using the ion exchange or solvent extraction methods and the experimental data have been reviewed [51, 52]. Nikolsky and co-workers were the first to study RaEDTA2− complex formation and obtained a log10 K value of 7.12 for RaEDTA2− [47]. The value was extrapolated to zero ionic strength assuming a temperature of 20 °C and an ionic strength of 0.1 mol·L−1. Baetsle and Bengsch studied RaEDTA2− complex formation using an ion exchange resin (Amberlite IR120) at 20 °C and an ionic strength of 0.1 mol·L−1 (sodium salt) and reported a log10 K value of 7.07 ± 0.06 [48]. The concentration of EDTA4− was 0.01 mol·L−1 and an acetate buffer was used. Such a high concentration of EDTA4− has a significant influence on the ionic strength, and therefore, the actual ionic strength used was 0.19 mol·L−1 and this value has been used to extrapolate the reported value to zero ionic strength. Sekine and co-workers used solvent extraction (a mixture of 0.1 mol·L−1 thenoyltrifluoroacetone and 0.1 mol·L−1 tributylphosphate in CCl4) to study Ra2+ complex formation with various amino carboxylic acids at 25 °C and 0.1 mol·L−1 NaClO4 and obtained a log10 K value of 7.7 for the RaEDTA2− complex [49]. A log10 K value for RaEDTA2− was also estimated to be 7.4 for 25 °C and an ionic strength of 0.1 mol·L−1 by Nelson and co-workers [50]. The RaEDTA2− stability constant obtained in this work is in very good agreement with those of the other studies when taking into account differences in temperature, ionic strength and difficulties in analyzing the literature data (experimental details missing, high EDTA concentrations affecting the ionic media etc.). Probably the best comparison of the RaEDTA2− stability constants obtained in this work is with work of Sekine and co-workers and values obtained for zero ionic strength from the two studies are in very good agreement.
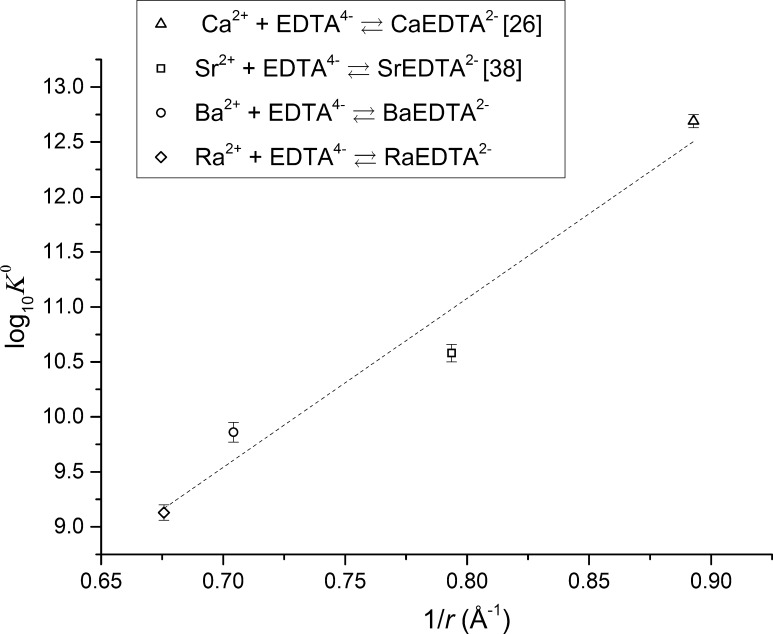
The difference between log10 K°2−BaEDTA and log10 K°2−RaEDTA is 0.73 log10 units. The difference is relatively small which may indicate that the speciation of Ba2+, Ra2+, and potentially other alkaline earth metals with EDTA4−, depends on the ionic radius of the metal ion. Extrapolation of the thermodynamic properties of radium, including stability constants, from the property values of other alkaline-earth metals using an electrostatic model is a widely used method [8]. A plot of the decadic logarithm of stability constants of calcium (taken from [26]), strontium (taken from [38] and extrapolated to zero ionic strength using the Davies equation), barium and radium with EDTA4− at zero ionic strength and 25 °C against the effective ionic radii of these elements in 8-fold coordination (taken from Shannon [15]) is shown in Fig. 5.
Fig. 5.
Comparison of alkaline-earth metal–EDTA4− stability constants at zero ionic strength using their effective ionic radii in 8-fold coordination (ionic radii taken from Shannon [15])
As shown in Fig. 5, the fit is good for all alkaline-earth metals which likely indicates that the bonding between these alkaline-earth metals and EDTA4− is similar and relativistic or other effects do not occur. It also confirms that the electrostatic model is a useful tool for extrapolation of radium thermodynamic properties and obtaining a first estimate of stability constants for radium complexation.
SIT Ion Interaction Parameters of Ba2+ and Ra2+
According to the SIT model (Eq. 5), the slopes are equal to the ion interaction parameters between oppositely charged ions. The slopes for the extrapolation to zero ionic strength in Figs. 1 and 3 yield the SIT ion interaction parameter terms shown in Eqs. 16, 17, 18, and 19, respectively:
| 16 |
| 17 |
| 18 |
| 19 |
The SIT ion interaction parameters determined for Eqs. 16–19 and some other ion interactions relevant to the studied systems are listed in Table 6.
Table 6.
SIT ion interaction parameters kg·mol−1 of some metal ions and ligands relevant to the studied systems at 25 °C
| Interaction | SIT parameters (kg·mol−1) | References |
|---|---|---|
| Δε 1(MgEDTA2−) | −(0.52 ± 0.04) | [26] |
| Δε 1(CaEDTA2−) | −(0.5 ± 0.5) | [26] |
| Δε 1(BaEDTA2−) | −(0.44 ± 0.07) | Equation 16 (this work) |
| Δε 1(RaEDTA2−) | −(0.54 ± 0.06) | Equation 17 (this work) |
| Δε 2(BaEDTA2−) | 0.14 ± 0.08 | Equation 18 (this work) |
| Δε 2(RaEDTA2−) | 0.10 ± 0.07 | Equation 19 (this work) |
| ε(Na+, EDTA4−) | 0.32 ± 0.14 | [25] |
| ε(Na+, HEDTA3−) | −(0.10 ± 0.14) | [25] |
| ε(H+, Cl−) | 0.12 ± 0.01 | [25] |
| ε(Ba2+, Cl−) | 0.07 ± 0.01 | [25] |
| ε(Na+, MgEDTA2−) | −(0.01 ± 0.15) | [25] |
| ε(Na+, BaEDTA2−) | −(0.03 ± 0.11) | This work |
| ε(Na+, RaEDTA2−) | −(0.10 ± 0.11)a | This work |
Uncertainties correspond to 95% confidence interval
aThis value has been calculated using ε(Ba2+, Cl−) as a substitute for ε(Ra2+, Cl−)
As shown in Table 6, the SIT parameters for all of the listed alkaline-earth metal ions are very similar. According to the SIT, interactions occur only between ions of opposite charge, which means that the alkaline-earth metal ions undergo similar short- and long-range electrostatic interactions with EDTA4− and Cl−. The SIT ion interaction parameters between Na+ and BaEDTA2− can be calculated as a weighted mean (Eqs. 16 and 18) and using the derived Δε 1(BaEDTA2−) or Δε 2(BaEDTA2−) and previously established ion interaction parameters: ε(Ba2+, Cl−), ε(H+, Cl−), ε(Na+, EDTA4−) and ε(Na+, HEDTA3−) [25]. The SIT ion interaction parameters between Na+ and RaEDTA2− can be calculated using the same method, with ε(Ba2+, Cl−) continuing to substitute for ε(Ra2+, Cl−). All parameters are listed in Table 6 and a comparison of the computed ε(Na+, BaEDTA2−) and ε(Na+, RaEDTA2−) parameters with ε(Na+, MgEDTA2−), taken from the literature [25], shows that all parameters are within the 95% confidence intervals.
The barium ion interaction parameters are often used as a substitute for the radium parameters due to a lack of experimental data in the case of radium [5, 16, 17]. It is possible to verify this methodology by calculation of Δε 1(RaEDTA2−) or Δε 2(RaEDTA2−) (Eqs. 17 and 19) using ε(Na+, EDTA4−), ε(Na+, HEDTA3−) and the barium SIT parameters listed in Table 6 as substitutes for unknown radium parameters (i.e., ε(Na+, BaEDTA2−) instead of ε(Na+, RaEDTA2−) and ε(Ba2+, Cl−) instead of ε(Ra2+, Cl−)). This results in Δε 1(RaEDTA2−) = −(0.42 ± 0.18) and Δε 2(RaEDTA2−) = −(0.08 ± 0.18) which are within the 95% confidence intervals of the experimentally determined Δε 1(RaEDTA2−) and Δε 2(RaEDTA2−) SIT parameters. This indicates that the method of using the barium SIT parameters as a substitute for those of radium is valid for the Ra2+–NaCl–EDTA4− system at ionic strengths below 3.5 mol·kg−1.
Conclusion
The apparent stability constants of the BaEDTA2− and RaEDTA2− complexes were determined over a wide range of NaCl concentrations (0.2–2.5 mol·L−1) at 25 °C and in two pH regions where the EDTA4− and HEDTA3− species dominate. The obtained constants were extrapolated to zero ionic strength using the SIT and compared with available literature data. It was found that in the pH region where the HEDTA3− species dominates, the reaction of Ba2+ or Ra2+ with the HEDTA3− ligand results in the formation of the BaEDTA2− and RaEDTA2− complexes and a proton release and that formation of BaHEDTA− or RaHEDTA− does not occur in alkaline media. The similarity of the barium and radium ion interaction parameters indicates that both metal ions undergo almost identical short- and long-range electrostatic interactions with EDTA4− and Cl−. The results also show that using the SIT interaction parameters of Ba2+ as a substitute for missing Ra2+ SIT interaction parameters is a useful tool for the Ra2+–NaCl–EDTA4− system.
Acknowledgements
This work has received funding from the Swedish Radiation Protection Authority (SSM). The authors are grateful to Dr. Stellan Holgersson and Dr. Kastriot Spahiu for help with experimental work and valuable discussions.
References
- 1.Winter, M.: WebElements periodic table. https://www.webelements.com (1993) Accessed 27 Dec 2016
- 2.Eisenbud M, Gesell TF. Environmental Radioactivity from Natural, Industrial & Military Sources: From Natural, Industrial and Military Sources. San Diego: Academic Press; 1997. [Google Scholar]
- 3.Nirdosh I, Muthuswami S. Distribution of 230Th and other radionuclides in Canadian uranium mill streams. Hydrometallurgy. 1988;20:31–47. doi: 10.1016/0304-386X(88)90025-4. [DOI] [Google Scholar]
- 4.Tripathi R, Sahoo S, Jha V, Khan A, Puranik V. Assessment of environmental radioactivity at uranium mining, processing and tailings management facility at Jaduguda, India. Appl. Radiat. Isot. 2008;66(11):1666–1670. doi: 10.1016/j.apradiso.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Paige C, Kornicker W, Hileman O, Snodgrass W. Solution equilibria for uranium ore processing: the BaSO4–H2SO4–H2O system and the RaSO4–H2SO4–H2O system. Geochim. Cosmochim. Acta. 1998;62(1):15–23. doi: 10.1016/S0016-7037(97)00320-7. [DOI] [Google Scholar]
- 6.Carvalho F, Madruga M, Reis M, Alves J, Oliveira J, Gouveia J, Silva L. Radioactivity in the environment around past radium and uranium mining sites of Portugal. J. Environ. Radioact. 2007;96(1):39–46. doi: 10.1016/j.jenvrad.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Gregory K, Hammack RW, Vidic RD. Co-precipitation of radium with barium and strontium sulfate and its impact on the fate of radium during treatment of produced water from unconventional gas extraction. Environ. Sci. Technol. 2014;48(8):4596–4603. doi: 10.1021/es405168b. [DOI] [PubMed] [Google Scholar]
- 8.Langmuir D, Riese AC. The thermodynamic properties of radium. Geochim. Cosmochim. Acta. 1985;49(7):1593–1601. doi: 10.1016/0016-7037(85)90264-9. [DOI] [Google Scholar]
- 9.Matyskin AV, Ebin B, Tyumentsev M, Allard S, Skarnemark G, Ramebäck H, Ekberg C. Disassembly of old radium sources and conversion of radium sulfate into radium carbonate for subsequent dissolution in acid. J. Radioanal. Nucl. Chem. 2016;310(2):589–595. doi: 10.1007/s10967-016-4927-x. [DOI] [Google Scholar]
- 10.Brown PL, Ekberg C, Ramebäck H, Hedström H, Matyskin A. Solubility of radium and strontium sulfate across the temperature range of 0 to 300° C. In: Merkel BJ, Arab A, editors. Uranium-Past and Future Challenges. Berlin: Springer; 2015. pp. 553–564. [Google Scholar]
- 11.Monnin C. A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200 C and to 1 kbar. Chem. Geol. 1999;153(1):187–209. doi: 10.1016/S0009-2541(98)00171-5. [DOI] [Google Scholar]
- 12.Kozempel J, Vlk M, Floriánová M, Drtinová B, Němec M. Dissolution of [226Ra] BaSO4 as part of a method for recovery of 226Ra from aged radium sources. J. Radioanal. Nucl. Chem. 2015;304(1):337–342. doi: 10.1007/s10967-014-3433-2. [DOI] [Google Scholar]
- 13.Nixon A, Keller D, Fritze K, Pidruczny A, Corsini A. Radium removal from Elliot Lake uranium-mill solids by EDTA leaching. Hydrometallurgy. 1983;10(2):173–186. doi: 10.1016/0304-386X(83)90004-X. [DOI] [Google Scholar]
- 14.Kuznetsov R, Butkalyuk P, Butkalyuk I. A rapid method for radium regeneration from its sulfate. Radiochemistry. 2013;55(1):112–115. doi: 10.1134/S1066362213010220. [DOI] [Google Scholar]
- 15.Shannon RT. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976;32(5):751–767. [Google Scholar]
- 16.Rosenberg Y, Metz V, Oren Y, Volkman Y, Ganor J. Co-precipitation of radium in high ionic strength systems: 2. Kinetic and ionic strength effects. Geochim. Cosmochim. Acta. 2011;75(19):5403–5422. doi: 10.1016/j.gca.2011.07.013. [DOI] [Google Scholar]
- 17.Rosenberg YO, Metz V, Ganor J. Co-precipitation of radium in high ionic strength systems: 1. Thermodynamic properties of the Na–Ra–Cl–SO4–H2O system–estimating Pitzer parameters for RaCl2. Geochim. Cosmochim. Acta. 2011;75(19):5389–5402. doi: 10.1016/j.gca.2011.06.042. [DOI] [Google Scholar]
- 18.Matyskin AV, Ylmen R, Lagerkvist P, Ramebäck H, Ekberg C. Crystal structure of radium sulfate: an X-ray powder diffraction and density functional theory study. J. Solid State Chem. 2017;253:15–20. doi: 10.1016/j.jssc.2017.05.024. [DOI] [Google Scholar]
- 19.Gran G. Determination of the equivalence point in potentiometric titrations. Part II. Analyst. 1952;77(920):661–671. doi: 10.1039/an9527700661. [DOI] [Google Scholar]
- 20.Decay Data Evaluation Project, L.N.H.B., France. http://www.nucleide.org/DDEP_WG/DDEPdata.htm. Accessed 27 Dec 2016
- 21.Brønsted JN. Studies on solubility. IV. The principle of the specific interaction of ions. J. Am. Chem. Soc. 1922;44(5):877–898. doi: 10.1021/ja01426a001. [DOI] [Google Scholar]
- 22.Brønsted JN. Calculation of the osmotic and activity functions in solutions of uni-univalent salts. J. Am. Chem. Soc. 1922;44(5):938–948. doi: 10.1021/ja01426a003. [DOI] [Google Scholar]
- 23.Scatchard G. Concentrated solutions of strong electrolytes. Chem. Rev. 1936;19(3):309–327. doi: 10.1021/cr60064a008. [DOI] [Google Scholar]
- 24.Guggenheim E, Turgeon J. Specific interaction of ions. Trans. Faraday Soc. 1955;51:747–761. doi: 10.1039/tf9555100747. [DOI] [Google Scholar]
- 25.Guillaumont R, Fanghänel T, Neck V, Fuger J, Palmer DA, Grenthe I, Rand MH. Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. Boston: Elsevier; 2003. [Google Scholar]
- 26.Hummel W, Anderegg G, Rao L, Puigdomenech I, Tochiyama O. Chemical Thermodynamics of Compounds and Complexes of U, Np, Pu, Am, Tc, Se, Ni and Zr with Selected Organic Ligands. Boston: Elsevier; 2005. [Google Scholar]
- 27.Brown PL, Ekberg C. Hydrolysis of Metal Ions. Weinheim: Wiley VCH; 2016. [Google Scholar]
- 28.Felmy AR, Mason MJ. An aqueous thermodynamic model for the complexation of sodium and strontium with organic chelates valid to high ionic strength. I. Ethylenedinitrilotetraacetic acid (EDTA) J. Solution Chem. 2003;32(4):283–300. doi: 10.1023/A:1023716703517. [DOI] [Google Scholar]
- 29.Botts J, Chashin A, Young HL. Alkali metal binding by ethylenediaminetetraacetate, adenosine 5’-triphosphate, and pyrophosphate*. Biochemistry. 1965;4(9):1788–1796. doi: 10.1021/bi00885a015. [DOI] [Google Scholar]
- 30.Daniele PG, Rigano C, Sammartano S. Ionic strength dependence of formation constants. Alkali metal complexes of ethylenediaminetetraacetate nitrilotriacetate, diphosphate, and tripolyphosphate in aqueous solution. Anal. Chem. 1985;57(14):2956–2960. doi: 10.1021/ac00291a046. [DOI] [Google Scholar]
- 31.Palaty V. Sodium chelates of ethylenediaminetetraacetic acid. Can. J. Chem. 1963;41(1):18–20. doi: 10.1139/v63-004. [DOI] [Google Scholar]
- 32.Sal’nikov Y, Boos G, Gibadullina K, Basyrova R, Shakirova N. Ethylenediaminetetraacetate complexes of patassium and sodium and dissociation of EDTA in aqueous–acetonitrile and aqueous–dioxane media. Russ. J. Inorg. Chem. 1991;36(5):745–749. [Google Scholar]
- 33.Daniele PG, Foti C, Gianguzza A, Prenesti E, Sammartano S. Weak alkali and alkaline earth metal complexes of low molecular weight ligands in aqueous solution. Coord. Chem. Rev. 2008;252(10):1093–1107. doi: 10.1016/j.ccr.2007.08.005. [DOI] [Google Scholar]
- 34.Marcus Y, Hefter G. Ion pairing. Chem. Rev. 2006;106(11):4585–4621. doi: 10.1021/cr040087x. [DOI] [PubMed] [Google Scholar]
- 35.Allard S, Ekberg C. Complexing properties of α-isosaccharinate: stability constants, enthalpies and entropies of Th-complexation with uncertainty analysis. J. Solution Chem. 2006;35(8):1173–1186. doi: 10.1007/s10953-006-9048-7. [DOI] [Google Scholar]
- 36.Schwarzenbach GV, Ackermann H. Komplexone V. Die Äthylendiamin-tetraessigsäure. Helv. Chim. Acta. 1947;30(6):1798–1804. doi: 10.1002/hlca.19470300649. [DOI] [Google Scholar]
- 37.Schwarzenbach G, Gut R, Anderegg G. Komplexone XXV. Die polarographische Untersuchung von Austauschgleichgewichten. Neue Daten der Bildungskonstanten von Metallkomplexen der Äthylendiamin-tetraessigsäure und der 1,2-Diaminocyclohexan-tetraessigsäure. Helv. Chim. Acta. 1954;37(4):937–957. doi: 10.1002/hlca.19540370402. [DOI] [Google Scholar]
- 38.Martell AE, Smith RM. Critical Stability Constants. Berlin: Springer; 1974. [Google Scholar]
- 39.Davies CW. Ion Association. London: Butterworths; 1962. [Google Scholar]
- 40.Stumm W, Morgan JJ. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York: John Wiley and Sons; 2012. [Google Scholar]
- 41.Carini FF, Martell AE. Thermodynamic quantities associated with the interaction between ethylenediaminetetraacetate and alkaline earth ions. J. Am. Chem. Soc. 1954;76(8):2153–2157. doi: 10.1021/ja01637a032. [DOI] [Google Scholar]
- 42.Schmid R, Reilley CN. A rapid electrochemical method for the determination of metal chelate stability constants. J. Am. Chem. Soc. 1956;78(21):5513–5518. doi: 10.1021/ja01602a016. [DOI] [Google Scholar]
- 43.Astakhov K, Fomenko M. The use of the Ca-45 isotope in the determination of the instability constants of intracomplex compounds formed by alkali earth metals with ethylenediaminotetraacetic acid. Zh. Fiz. Khim. 1957;31(9):2110–2120. [Google Scholar]
- 44.Bohigian, T., Martell, A.: Progress Report US Atomic Energy Commission Contract No. AT,(30-1)-1823 (1960)
- 45.Jokl V, Majer J. Investigation of complex compounds in solution using paper electrophoresis. IV. Complexes of 1,3-diamino-2-propanol-N,N,N′,N′-tetraacetic acid. Chem. Vesti. 1965;19:249–258. [Google Scholar]
- 46.Delgado R, Da Silva JF. Metal complexes of cyclic tetra-azatetra-acetic acids. Talanta. 1982;29(10):815–822. doi: 10.1016/0039-9140(82)80251-8. [DOI] [PubMed] [Google Scholar]
- 47.Nikolsky BP, Trofimov AM, Vysokoostrovskaya NB. Complex formation of barium and radium in Trilon B solutions. Radiochemistry. 1959;1(2):147–154. [Google Scholar]
- 48.Baetsle L, Bengsch E. Ion-exchange characteristics of the radium–ethylene-diaminetetraacetate complex. J. Chromatogr. A. 1962;8:265–273. doi: 10.1016/S0021-9673(01)99257-X. [DOI] [PubMed] [Google Scholar]
- 49.Sekine T, Kawashima Y, Unnai T, Sakairi M. Studies of the alkaline earth complexes in various solutions. IV. Solvent extraction study of radium(II) complexes with some aminocarboxylic acids in perchlorate media. Bull. Chem. Soc. Jpn. 1968;41(12):3013–3015. doi: 10.1246/bcsj.41.3013. [DOI] [Google Scholar]
- 50.Nelson F, Day R, Kraus K. Anion exchange studies—XXX A number of elements in ethylenediaminetetraacetic acid solutions. J. Inorg. Nucl. Chem. 1960;15(1–2):140–150. doi: 10.1016/0022-1902(60)80022-X. [DOI] [Google Scholar]
- 51.Anderegg G. Critical Survey of Stability Constants of EDTA Complexes: Critical Evaluation of Equilibrium Constants in Solution: Stability Constants of Metal Complexes. New York: Elsevier; 2013. [Google Scholar]
- 52.Vdovenko VM, Dubasov YV. Analytical Chemistry of Radium. New York: Wiley; 1976. [Google Scholar]