Abstract
Background and Objective
Extended-release (ER) opioids are associated with high rates of abuse. Recreational opioid users often manipulate ER formulations to achieve a high plasma concentration in a short amount of time, resulting in a more rapid and intense high. Patients may also manipulate ER tablets to facilitate swallowing, without recognizing that manipulation could increase release rate. The goal of this study was to assess the ability of oxycodone DETERx (Xtampza® ER, Collegium Pharmaceutical, Inc., Canton, MA, USA) and other commercially available ER opioid formulations with and without physicochemical abuse-deterrent characteristics to be manipulated by crushing in an in vitro setting.
Methods
In vitro dissolution techniques were used to compare the opioid release from a variety of ER opioid formulations. Dissolution was assessed for intact and crushed dosage forms. Opioid release was quantified using high-performance liquid chromatography.
Results
Intact formulations exhibited drug release rates characteristic of 12- or 24-h dosage forms. After crushing using commonly available household tools, only Xtampza ER maintained ER of opioid.
Conclusions
Xtampza ER maintained its ER characteristics after crushing, unlike many other commercially available opioid formulations, including some formulated with abuse-deterrent properties. As such, Xtampza ER may be less appealing to abusers and offer a margin of safety for patients who manipulate dosage forms to facilitate swallowing.
Key Points
| When assessed using in vitro dissolution techniques, Xtampza® ER (oxycodone DETERx®), an abuse-deterrent formulation of oxycodone, maintained its extended-release characteristics after crushing with commonly available household tools. |
| Other commercially available extended-release opioids, including some formulated with abuse-deterrent properties, did not maintain their extended-release properties after crushing. The distinguishing features of the Xtampza ER formulation make it less susceptible to crushing. |
| Xtampza ER may be less appealing to abusers and offers a margin of safety for patients who manipulate dosage forms to facilitate swallowing. |
Introduction
Extended-release (ER) opioids are used for the management of pain severe enough to require daily, around-the-clock, long-term treatment. However, because they contain a relatively high opioid dose per tablet or capsule, ER opioids are attractive to recreational opioid abusers [1]. To achieve a more rapid high, a recreational opioid user may manipulate (e.g. crush) ER opioids to increase the surface area and the amount of drug available for immediate release [2]. In addition, ER formulations may pose a safety threat to patients even if they do not intend abusing the drug. For example, patients may misuse ER opioids by cutting or crushing them to facilitate swallowing, thus putting themselves at risk of exposure to rapid release of opioid. Although different approaches have been used to combat abuse of ER opioid formulations, including the development of “crush-resistant” tablets and agonist/antagonist formulations, these approaches do not entirely deter abuse, nor do they all offer protection for patients who misuse the drug via manipulation [3]. For example, reformulated OxyContin® [oxycodone hydrochloride (HCl), Purdue Pharma, Stamford, CT, USA] was introduced in 2010, and although there was a decrease in abuse of OxyContin via snorting and injecting, abuse via these routes is still reported [3–5]. Additionally, many recreational opioid users still abuse OxyContin by manipulating the formulation prior to oral administration (e.g. chewing, dissolving in the mouth) [5, 6]. Further, OxyContin and other abuse and non-abuse-deterrent formulations all carry language in the boxed warning against crushing/chewing, as it can lead to rapid release of opioid and potentially fatal opioid exposure [4, 7–14].
Xtampza® ER (Collegium Pharmaceutical, Inc., Canton, MA, USA) is an ER oxycodone formulation that uses the DETERx® (Collegium Pharmaceutical, Inc., Canton, MA, USA) technology [15–17]. Xtampza ER is a microsphere-in-capsule formulation in which each microsphere contains oxycodone homogeneously dispersed within a hydrophobic matrix of fatty acids and waxes. Xtampza ER is designed to be abuse deterrent, with each microsphere functioning as an individual abuse-deterrent, ER, drug-delivery system [15–17]. Xtampza ER is the only opioid formulation available without a boxed warning against crushing or chewing [16]. In vitro studies have previously shown that Xtampza ER is less susceptible to the effects of grinding, crushing, and extracting using many commonly available beverages and solvents when compared with immediate-release (IR) oxycodone and OxyContin [18–20]. Pharmacokinetic studies have demonstrated that manipulation of Xtampza ER by crushing or chewing does not increase the peak plasma concentration (C max) or time to C max (T max) of oxycodone, indicating that Xtampza ER maintains its ER properties after manipulation [19, 21]. Intranasal administration of crushed Xtampza ER does not result in higher C max than that of orally administered Xtampza ER [22]. Further, intranasal administration of crushed Xtampza ER is associated with significantly lower mean “drug liking” and “take drug again” scores when compared with intranasal administration of crushed IR oxycodone [22]. In addition, oral administration of chewed and intact Xtampza ER has been shown to have a lower abuse potential than oral administration of crushed IR oxycodone [23].
Results from prior studies have demonstrated that Xtampza ER maintains its ER profile after manipulation to a greater degree than does OxyContin [19–21]. The goal of this study was to assess the susceptibility of Xtampza ER and other commercially available ER opioid formulations, including some formulated with abuse-deterrent properties, to crushing in an in vitro setting. These data have implications for both opioid abuse and misuse.
Methods
Particle Size Reduction
The following ER opioid products were compared: oxycodone DETERx 36-mg (equivalent to 40 mg oxycodone HCl) capsules (Xtampza ER), oxycodone HCl ER 40-mg tablets (OxyContin), morphine sulfate ER 100-mg capsules (Kadian®, Actavis Pharma, Inc., Parsippany, NJ, USA) [7], morphine sulfate ER 100-mg tablets (MS Contin®, Purdue Pharma, Stamford, CT, USA) [10], oxymorphone HCl ER 40-mg tablets (Opana® ER, Endo Pharmaceuticals, Inc., Malvern, PA, USA) [8], generic oxymorphone HCl ER 40-mg tablets (Impax Laboratories, Inc., Hayward, CA, USA) [9], hydromorphone HCl ER 32-mg tablets (Exalgo®, Mallinckrodt Brand Pharmaceuticals, Inc., Hazelwood, MO, USA) [11], generic hydromorphone HCl ER 16-mg tablets (Watson Laboratories, Inc., Corona, CA, USA) [13], and hydrocodone bitartrate ER 50-mg tablets (Zohydro® ER, Pernix Therapeutics, LLC, Morristown, NJ, USA) [12]. The highest marketed dose strength for each ER opioid was generally studied with the exception of OxyContin, which was tested at the 40-mg dose strength to directly compare its performance with Xtampza ER, and both Kadian and MS Contin, which were tested at a 100-mg dose, although a 200-mg dose is available for each.
The ability to reduce particle size was qualitatively assessed using five different tools (all commonly available household items). The selected tools cover a range of possible manipulation techniques, including pulverizing, chopping, grinding, grating, etc. (all methods are referred to as “crushed”). The identity of the specific utensils used is concealed due to public health concerns. All capsule dosage forms were opened first and the contents of the capsules were crushed. Each manipulation method was applied for 2 min, where possible; a shorter duration of manipulation was used when particle size reduction was complete and it was no longer possible to apply the utensil to the product. Qualitative observations of particle size reduction (i.e. crushing) were made, noting whether or not a given tool produced particle size reduction and which tools produced the finest particles. The percentage yield was determined for each dosage form by measuring weight before and after particle size reduction.
Dissolution was performed on intact products and on crushed products. For Xtampza ER and OxyContin, results from a previous in vitro particle-size reduction and dissolution study are presented [19]; the best tools for each respective product were determined based on the percent increase in the in vitro dissolution release rate and the percentage recovery. For the remaining products, dissolution was performed using the two best tools identified based on qualitative observations of particle size reduction and the percentage recovery (note that the two tools could be different for each product). Six replicates were tested for each product and each condition (intact and crushed). Dissolution data are presented for the most effective tool for each respective product as determined in dissolution studies.
Dissolution Methods and Analysis
The dissolution medium, medium volume, and sampling time points used to test the various ER products were based on the FDA-recommended dissolution method database [24]. A summary of the dissolution conditions is presented in Table 1. A Distek dissolution Apparatus 2 (paddles) with an autosampler (Distek, Inc., North Brunswick, NJ, USA) was used for both intact and crushed dosages. USP Apparatus 2 (paddles at 50 RPM) was used for all products due to the difficulty in adding crushed product to a USP Apparatus 1 (basket). Samples were separately tested in the designated dissolution media at 37 °C, and samples were collected at varying time points with an autosampler (Distek 4300) to give a dissolution profile. For encapsulated products, dissolution was performed on the capsule contents directly without the capsule.
Table 1.
Dissolution parameters
| Drug product | Dissolution medium (volume) | Sampling times, h |
|---|---|---|
| Xtampza® ER (oxycodone DETERx) | pH 4.5 acetate with 0.03% Tween 20 (900 mL) | 0.25, 1, 2, 4, 8, 12, 16, 20, 24 |
| OxyContin® (oxycodone ER) | Simulated gastric fluid (900 mL) | 0.25, 1, 2, 4, 6, 8, 12 |
| MS Contin® (morphine sulfate) | Deaerated deionized water (900 mL) | 0.25, 1, 2, 3, 6, 9, 12 |
| Kadian® (morphine sulfate) | 0.1 N HCl (500 mL) for 1 h/pH 7.5 phosphate buffer (500 mL) for 8 h | 0.25, 1, 2, 4, 6, 9 |
| Opana® ER (oxymorphone ER) | pH 4.5, 45 mM phosphate buffer (900 mL) | 0.25, 1, 2, 4, 6, 8, 10 |
| Generic Oxymorphone HCl ER | pH 4.5, 45 mM phosphate buffer (900 mL) | 0.25, 1, 2, 4, 6, 8, 10 |
| Exalgo® (hydromorphone ER) | Deionized water (900 mL) | 0.25, 1, 2, 4, 6, 8, 10, 12, 16, 20, 24 |
| Generic Hydromorphone HCl ER | Deionized water (900 mL) | 0.25, 1, 2, 4, 6, 8, 10, 12, 16, 20, 24 |
| Zohydro® ER (oxycodone ER) | pH 6.8 phosphate buffer (900 mL) | 0.25, 1, 2, 4, 6, 8, 12 |
ER Extended release
Samples were analyzed by high-performance liquid chromatography (HPLC; 1200 series, Agilent Technologies, Santa Clara, CA, USA) for oxycodone using a 4.6-mm × 50-mm, 2.6-µm, 100-Å Kinetex® C18 column (Phenomenex, Inc., Torrance, CA, USA), a mobile phase of 30 mM hexanesulfonate buffer to acetonitrile (78:22), and a flow rate of 1.5 mL/min. The monitoring wavelength was 225 nm. Samples were analyzed for morphine sulfate using a 3.9-mm × 300–mm, µ-Bondapak® C18 column (Waters, Milford, MA, USA), a mobile phase of 30 mM hexanesulfonate buffer:methanol:acetic acid buffer (72:28:1), a flow rate of 1.3 mL/min, and a monitoring wavelength of 284 nm. Samples were analyzed for oxymorphone HCl using a 4.6-mm × 50-mm, 3.0-µm Gemini® NX C18 column (Phenomenex, Inc.), a mobile phase of 30 mM sodium hexanesulfonate buffer to acetonitrile (84:16), a flow rate of 1.2 mL/min, and a monitoring wavelength of 225 nm. Samples were analyzed for hydromorphone HCl using a 4.6-mm × 150-mm, 5-µm Zorbax® C18 column (Agilent Technologies, Santa Clara, CA, USA), a mobile phase of sodium dodecyl sulfate with glacial acetic acid buffer to acetonitrile (33:17), a flow rate of 2.0 mL/min, and a monitoring wavelength of 280 nm. Samples were analyzed for hydrocodone using a 4.6-mm × 50-mm, 2.6-µm Kinetex C18 column (Phenomenex, Inc.), with a mobile phase of 30 mM sodium hexanesulfonate buffer to acetonitrile (78:22), a flow rate of 0.5 mL/min, and a monitoring wavelength of 280 nm.
The dissolution profiles were compared graphically and using descriptive statistics. The percent of opioid released over time and the percent difference in average dissolution between crushed and intact formulations were calculated and compared across formulations.
Results
Particle Size Reduction
For tablets (OxyContin, MS Contin, Opana ER, generic oxymorphone HCl ER, Exalgo, and generic hydromorphone HCl ER) and bead-in-capsule products (Kadian and Zohydro ER), the ability of the various tools to reduce the particle size of the dosage forms was visually apparent. Xtampza ER, a microsphere-in-capsule formulation, had a significantly smaller starting particle size (median particle size of ~300 microns), and the effectiveness of crushing was not readily visually apparent because of the small starting particle size. Figure 1 shows the appearance of the various products when crushed with the most effective of the five applied tools.
Fig. 1.
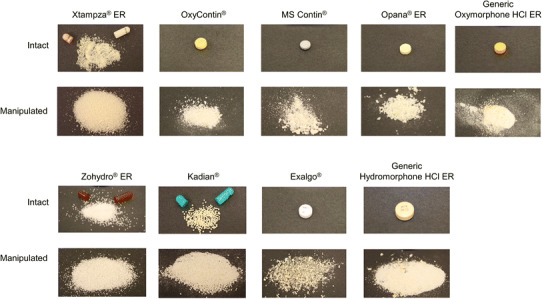
Photos of intact and manipulated opioids. The tool that was most effective at crushing each product was used for the images of manipulated products
As the crushing of Xtampza ER microspheres could not be visually assessed, the particle size before and after crushing with the five tools was measured using a laser diffraction technique, as reported previously [19]. None of the tools produced a meaningful decrease in median particle size for Xtampza ER. For all other products, at least two tools among the five tested were able to reduce the dosage forms to small particles. Formulations designed to be crush resistant (OxyContin and Opana ER) were effectively reduced to small particles (similar to Fig. 1) by three (OxyContin) or two (Opana ER) tools; other tools were ineffective or were able to reduce the tablets only to large fragments. MS Contin and generic oxymorphone tablets were readily crushed into a powder with all five tools. The other products (Zohydro ER, Exalgo, generic hydromorphone tablets, and Kadian) could be manipulated into finer particles with four of the five tools tested, and did not provide any meaningful degree of crush-resistance on manipulation.
Dissolution of Intact and Manipulated Extended-Release Opioids
When assessed as intact formulations, all formulations demonstrated ER dissolution profiles (Fig. 2). The 12-h formulations (Xtampza ER, OxyContin, generic Oxymorphone ER, Opana ER, MS Contin, and Zohydro ER) achieved 80–100% of opioid released after approximately 12 h of dissolution. The 24-h formulations (generic Hydromorphone ER and Exalgo) reached ≥90% of opioid release after approximately 24 h of dissolution. Kadian, which is indicated for dosing either every 12 or 24 h, exhibited an opioid-release profile consistent with products indicated for dosing every 12 h.
Fig. 2.
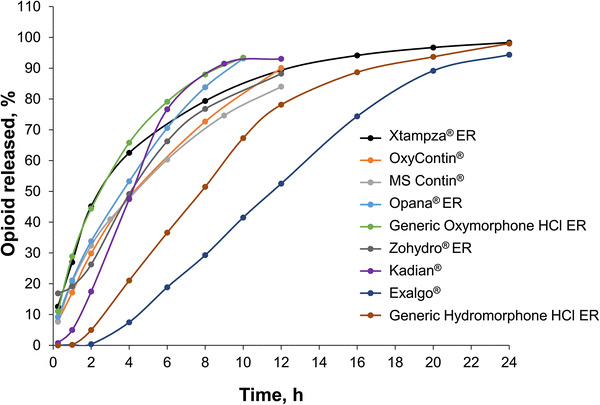
Dissolution profiles of intact extended release (ER) opioid products. The percent of the total amount of opioid released over 24 h from each intact product is shown
The calculated differences in average percent drug release for manipulated versus intact conditions during early timepoints of the dissolution study are shown in Fig. 3. Manipulated opioid formulations generally demonstrated very different dissolution profiles from those observed when they were intact. Most of the crushed opioid formulations released ≥60% (range 60–88%) more opioid after 15 min of dissolution compared with intact formulations. In contrast, manipulated Xtampza ER released just 10% more opioid after 15 min of dissolution compared with intact Xtampza ER. Similar results were observed after 1 and 2 h of dissolution, where crushed Xtampza ER released an average of 17% more opioid, and all other crushed opioid formulations released 50–97% more opioid when crushed than when intact.
Fig. 3.
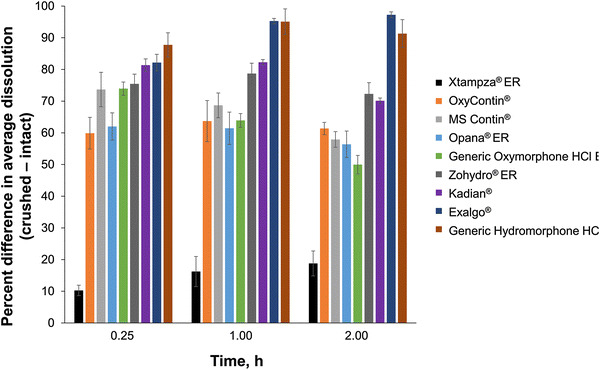
Mean percent difference (± standard deviation) in average dissolution between manipulated and intact extended release (ER) opioid products
Figure 4 displays the percent opioid released in dissolution over the first 15 min in vitro, comparing manipulated to intact dosage forms. Only Xtampza ER maintained a slow release of drug early in the dissolution time-course with 23% released at 15 min when manipulated. All other opioid formulations released 67–100% of opioid after only 15 min of dissolution.
Fig. 4.
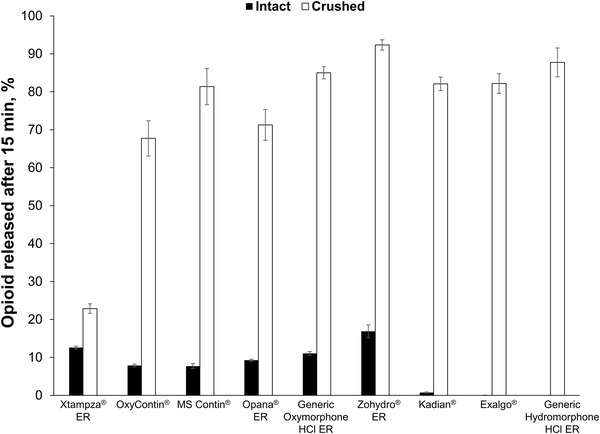
Dissolution profiles of intact versus crushed extended release (ER) opioid products. The mean (± standard deviation) percent of the total amount of opioid release over 15 min is shown
Discussion
Recreational opioid users frequently target ER formulations because of the high quantity of opioid in each dose [1]. Crushing a susceptible ER formulation before oral or other routes of administration can increase the bioavailability of the opioid, making it similar to that of an IR formulation [21]. The accelerated absorption of opioid leads to a more rapid high [2].
It is thought that nonmedical use of prescription opioids often begins with oral administration of the intact pill and that drug abusers evolve their habits to use more efficient routes of administration (e.g. manipulation prior to oral ingestion, then intranasal or intravenous use, both of which require manipulation prior to abuse) [25]. Thus, there is a need for ER formulations that interrupt the “abuse trajectory” before progressing past the oral-abuse phase.
In addition to being manipulated for abuse, ER opioids may also be manipulated by patients or their caregivers, not to get high, but for any number of reasons, including to allow ease of swallowing [26]. Crushing of ER formulations to facilitate administration may inadvertently put the patient at risk of exposure to rapid release of drug, potential withdrawal, or limited pain control [4, 7–13], but the dangers of this practice are not always appreciated by patients and caregivers [26]. Taken together, abuse and misuse of ER opioids via product manipulation is an important public health issue that is not adequately addressed by many current treatment options.
ER opioid tablets that incorporate crush-resistant properties are still vulnerable to manipulation by common household tools that can be used to break down the tablets into smaller particles [19, 21, 27]. As such, the boxed warnings of opioids, including all crush-resistant tablets, commonly include language instructing patients to swallow tablets/capsules intact and whole (i.e. not to crush or chew the product before ingesting) [4, 8, 11, 12, 14]. Notably, the prescribing information for Xtampza ER does not include a statement regarding swallowing the capsule whole (i.e. not crushing, breaking, or chewing) in its boxed warning [16].
Although some crush-resistant products (e.g. OxyContin) have curbed abuse to a certain degree, there are still reports of oral abuse, including manipulation of the drug prior to oral abuse [5, 6, 26, 28]. As the current study has demonstrated that a variety of household tools were capable of crushing several formulations, one might expect that OxyContin, as well as other opioid formulations with or without crush-resistant technology, may still be manipulated prior to oral abuse.
In vitro dissolution results demonstrated that Xtampza ER maintained its ER characteristics after manipulation. The amount of opioid released in relatively short timeframes (within 15 min of dissolution) from all other formulations was dramatically increased after manipulation. For 24-h products, which contain greater amounts of drug than 12-h products, rapid release of the full opioid load is even more dramatic and potentially dangerous to both recreational users and patients.
The relatively small effect of crushing on drug release from Xtampza ER is due to essentially three unique physicochemical characteristics. The first feature is the waxy nature of the formulation, which can cause the microspheres to smear rather than break into small particulates, potentially resulting in a reduction in drug release. Other formulations tend to break into smaller particles with considerably faster drug release. Second, Xtampza ER microspheres are of uniform composition where the active pharmaceutical ingredient (API) is distributed as a solid solution in waxy, hydrophobic components that do not provide direct fluid contact with the API. Both the hydrophobic nature of the formulation and the uniform distribution of the API as a solid solution combine to limit the rate of extraction of the API. Finally, Xtampza ER contains microspheres with a median particle size of approximately 300 µm, which limits the ability to impart a consequential change in surface area by crushing. This effect is illustrated in the minimal difference in the dissolution profiles when comparing crushed with intact product (Fig. 3).
One limitation of the current study is that correlations between the in vitro dissolution rate and in vivo drug exposure have not been established across all the products tested. However, results from previous in vivo pharmacokinetic studies have demonstrated that manipulation of Xtampza ER by crushing or chewing does not significantly increase the C max of oxycodone or reduce the T max of oxycodone, indicating that Xtampza ER maintains its ER properties after manipulation [19, 21]. For the other products, the in vitro rapid drug release and loss of ER characteristics indicate that dose dumping may occur in vivo. Rapid in vivo drug release after crushing has been demonstrated for OxyContin [21], thus supporting the predictability of in vitro IR profiles translating to in vivo IR profiles. An additional potential limitation of the study was the lack of statistical comparison across formulations; however, the drastic change (or lack thereof) in opioid dissolution after crushing some formulations clearly identified the formulations that are more susceptible to manipulation.
Although abuse-deterrent opioid formulations are available, they account for a small fraction of opioids—just 22% of ER opioids and 2% of all opioids (ER and IR) have abuse-deterrent labeling [29]. Further, generic (non-abuse–deterrent) products are more widely used (as much as 67% of prescriptions) than branded opioid products, including abuse-deterrent formulations [29]. A general lack of understanding of the abuse trajectory and extent of abuse (or misuse) by product manipulation is a barrier that hinders appropriate prescribing of abuse-deterrent opioids. Complicating matters is the fact that access to abuse-deterrent formulations is often limited. For example, some insurers (particularly Medicare and Medicaid plans) require patients to first fail generic (non-abuse-deterrent formulation) products before using an abuse-deterrent formulation [25]. In addition, there is a general rule that fee-for-service Medicaid patients must first try and fail (“fail first”) preferred drugs before they can obtain coverage for non-preferred products [30]. Most fee-for-service Medicaid plans require patients to try and fail fentanyl ER, morphine sulfate, or methadone before patients can use an abuse-deterrent formulation or another branded opioid. As a result, fentanyl ER, morphine sulfate ER, and methadone account for 93% of all generic treatments in Medicare Part D access plans [29, 30]. Because manipulation prior to opioid abuse is wide spread across all routes of administration [6], improving access to manipulation-resistant abuse-deterrent opioid formulations may help prevent recreational users from moving to more dangerous routes of abuse.
Conclusion
Results from this study illustrate that Xtampza ER maintains its ER properties after physical manipulation, unlike other commercially available ER opioids, including formulations that are marketed as being crush resistant. As such, Xtampza ER is an abuse-deterrent opioid that may be less appealing to recreational users and may provide added protection for patients with chronic pain who misuse opioids via product manipulation.
Acknowledgements
This study was supported by Collegium Pharmaceutical, Inc., Canton, MA, USA. Medical writing assistance was provided by Kelly M Cameron, PhD, of JB Ashtin, who, on behalf of Collegium Pharmaceutical, Inc., developed the first draft based on an author-approved outline and implemented author revisions. Analytical testing support was provided by Michael Grima, John Ojih, and Svetlana Shlyakhover. The authors thank Christy Thompson and Michael DeGeorge for their critical review of the manuscript.
Compliance with Ethical Standards
Conflicts of interest
S Mayock, S. Saim, and A. Fleming are full-time employees of Collegium Pharmaceutical, Inc., and hold stock and/or stock options.
Source of funding
This study was funded by Collegium Pharmaceutical, Inc.
References
- 1.Webster L. Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009;10(Suppl 2):S124–S133. doi: 10.1111/j.1526-4637.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 2.Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. Pharm Ther. 2012;37(7):412–418. [PMC free article] [PubMed] [Google Scholar]
- 3.Hale ME, Moe D, Bond M, Gasior M, Malamut R. Abuse-deterrent formulations of prescription opioid analgesics in the management of chronic noncancer pain. Pain Manag. 2016;6(5):497–508. doi: 10.2217/pmt-2015-0005. [DOI] [PubMed] [Google Scholar]
- 4.OxyContin® [package insert]. Purdue Pharma L.P., Stamford, CT; August 2015. http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o. Accessed 10 Nov 2016.
- 5.Butler SF, Cassidy TA, Chilcoat H, Black RA, Landau C, Budman SH, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14(4):351–358. doi: 10.1016/j.jpain.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Butler SF, Black RA, Fleming AB. Relative abuse of abuse deterrent formulations via alternative oral routes. In: 35th Annual Scientific Meeting of the American Pain Society May 11–14, 2016; Austin, TX.
- 7.Kadian® ER [package insert]. Actavis Pharma, Inc., Parsippany, NJ; April 2014. http://www.allergan.com/assets/pdf/kadian_pi. Accessed 10 Nov 2016.
- 8.Opana® ER [package insert]. Endo Pharmaceuticals, Inc., Malvern, PA; April 2014. http://www.endo.com/File%20Library/Products/Prescribing %20Information/OpanaER_prescribing_information_newformulation.html. Accessed 10 Nov 2016.
- 9.Oxymorphone HCl ER [package insert]. Impax Laboratories, Inc., Hayward, CA; September 2016. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=557e9610-62d7-42bf-90c1-44215bd8c1f8. Accessed 10 Nov 2016.
- 10.MS Contin® [package insert]. Purdue Pharma L.P., Stamford, CT; June 2014. http://app.purduepharma.com/xmlpublishing/pi.aspx?id=ms. Accessed 10 Nov 2016.
- 11.Exalgo® [package insert]. Mallinckrodt Brand Pharmaceuticals, Inc., Hazelwood, MO; April 2014. http://www2.mallinckrodt.com/workarea/downloadasset.aspx?id=2147483728. Accessed 10 Nov 2016.
- 12.Zohydro® [package insert]. Pernix Therapeutics, LLC, Morristown, NJ; January 2016. http://www.zohydroer.com/downloads/ZOHYDROERFullPrescribingInformation.pdf. Accessed 10 Nov 2016.
- 13.Hydromorphone HCl ER [package insert]. Watson Laboratories, Inc., Corona, CA; May 2014. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a717315-250b-4e2d-b315-3ae1b6c337f9. Accessed 10 Nov 2016.
- 14.Hysingla® [package insert]. Purdue Pharma L.P., Stamford, CT; February 2015. http://app.purduepharma.com/xmlpublishing/pi.aspx?id=h. Accessed 10 Nov 2016.
- 15.Lamb YN, Garnock-Jones KP, Keam SJ. Oxycodone DETERx(R) ER capsules: a review in severe. Chronic Pain. Drugs. 2016;76(18):1759–1769. doi: 10.1007/s40265-016-0660-6. [DOI] [PubMed] [Google Scholar]
- 16.Xtampza® ER [package insert]. Collegium Pharmaceutical, Inc., Canton, MA; April 2016. http://www.xtampzaer.com/pdf/xtampza-pi.pdf. Accessed 10 Nov 2016.
- 17.Gudin J. Oxycodone DETERx®: a novel abuse-deterrent, extended-release analgesic option for the treatment of patients with chronic pain. Pain Ther. 2016;5(2):171–186. doi: 10.1007/s40122-016-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming AB, Scungio TA, Grima MP, Mayock SP. In vitro assessment of the potential for abuse via the intravenous route of oxycodone DETERx® microspheres. J Opioid Manag. 2016;12(1):57–65. doi: 10.5055/jom.2016.0312. [DOI] [PubMed] [Google Scholar]
- 19.Kopecky EA, Fleming AB, Noonan PK, Varanasi RK, Grima M, Saim S, et al. Impact of physical manipulation on in vitro and in vivo release profiles of oxycodone DETERx(R): an extended-release, abuse-deterrent formulation. J Opioid Manag. 2014;10(4):233–246. doi: 10.5055/jom.2014.0211. [DOI] [PubMed] [Google Scholar]
- 20.Fleming AB, Mayock SP, Kopecky EA, Saim S, Varanasi RK. Abuse-deterrent properties of oxycodone DETERx, an extended-release formulation for chronic pain management. In: American Association of Pharmaceutical Scientist Annual Meeting and Exposition; 2013; San Antonio, TX; 2013.
- 21.Gudin J, Levy-Cooperman N, Kopecky EA, Fleming AB. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med. 2015;16(11):2142–2151. doi: 10.1111/pme.12834. [DOI] [PubMed] [Google Scholar]
- 22.Webster LR, Kopecky EA, Smith MD, Fleming AB. A randomized, double-blind, double-dummy study to evaluate the intranasal human abuse potential and pharmacokinetics of a novel extended-release abuse-deterrent formulation of oxycodone. Pain Med. 2016;17(6):1112–1130. doi: 10.1093/pm/pnv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopecky EA, Fleming AB, Levy-Cooperman N, O’Connor M, Sellers EM. Oral human abuse potential of oxycodone DETERx(R) (Xtampza(R) ER) J Clin Pharmacol. 2017;57(4):500–512. doi: 10.1002/jcph.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dissolution Methods Database. US. Food and Drug Administration Web Site. http://www.accessdata.fda.gov/scripts/cder/dissolution/. Accessed 16 Jan 2017.
- 25.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pergolizzi JV, Jr, Taylor R, Jr, Nalamachu S, Raffa RB, Carlson DR, Varanasi RK, et al. Challenges of treating patients with chronic pain with dysphagia (CPD): physician and patient perspectives. Curr Med Res Opin. 2014;30(2):191–202. doi: 10.1185/03007995.2013.854197. [DOI] [PubMed] [Google Scholar]
- 27.Omidian A, Mastropietro DJ, Omidian H. Reported methods of abuse for common prescription analgesic opioids. J Dev Drugs. 2014;3(2):120. [Google Scholar]
- 28.McCarberg BH, Kopecky EA, O’Connor M, Marseilles A, Varanasi RK, Thompson C, et al. An abuse-deterrent, microsphere-in-capsule formulation of extended-release oxycodone: alternative modes of administration to facilitate pain management in patients with dysphagia. Curr Med Res Opin. 2016;32(12):1975–1982. doi: 10.1080/03007995.2016.1222517. [DOI] [PubMed] [Google Scholar]
- 29.IMS Xponent [database online]. Danbury, CT: QuintilesIMS; 2016. Accessed 23 Dec 15–23 Dec 16.
- 30.MMIT Network [database online]. Yardley, PA: Managed Markets Insight & Technology, LLC; 2017. Accessed 10 Jan 2017.


