Abstract
More new diagnosed syphilis cases were reported in china, the incidence and relevant factors of asymptomatic neurosyphilis (ANS) in serofast syphilis patients were unclear. Clinical and laboratory data of 402 Human Immunodeficiency Virus (HIV) negative, serofast syphilis patients, who underwent lumbar puncture at the Peking University Ditan Teaching Hospital between September 2008 and August 2016, were collected. Incidence of ANS was verified and the relevant factors were further analyzed. According to the ANS criteria, 139 (34.6%) patients had ANS. Of these, 40 (28.8%) had reactive cerebrospinal fluid (CSF), rapid plasma reagin (RPR) positive, 115 (82.7%) had CSF white blood cell (WBC) count > 5 × 106/L, 28 (20.1%) had CSF protein concentration > 45 mg/dL (without other neurological diseases). Patients aged 51–60 years, of non-Han ethnicity, with serum RPR titer 1:32 and ≥ 1:64 were 2.28-fold, 9.11-fold, 5.12-fold and 5.69-fold, respectively, more likely to have ANS. The incidence of ANS was 34.6% among Chinese serofast syphilis patients. Age, ethnicity and serum RPR titer were associated with high risk of ANS.
Introduction
The incidence of syphilis is rapidly increasing in China1. Syphilis is a systemic and infectious disease caused by Treponema pallidum, which can disseminate to any organ shortly after infection. Neurosyphilis can occur at any stage of syphilis, after invasion of the central nervous system by Treponema pallidum, and its most common form is asymptomatic neurosyphilis (ANS)2. ANS shows one or more CSF abnormalities (pleocytosis, or elevated protein concentration, or reactive cerebrospinal fluid (CSF) Venereal Disease Research Laboratory (VDRL) in patients with serological evidence of syphilis but no neurological signs or symptoms3. According to the World Health Organization (WHO), an estimated 12 million new cases of syphilis occur each year4, The incidence of neurosyphilis is also increasing, with an annual incidence of 0.16–2.1 per 100,000 population5,6. About 13.5% of latent syphilis patients without neurological symptoms had ANS, and these patients were more likely to have late neurological complications7. About 35% ANS patients developed symptomatic neurosyphilis8. In China, the incidence of serofast state was 34.4%, but the rate of ANS in the asymptomatic serofast syphilis patients remains unknown9,10. This study explored the incidence of ANS and relevant factors through analysis of clinical and laboratory data of 402 asymptomatic serofast syphilis patients.
Results
Characteristics of study patients
A total of 402 eligible patients were enrolled in the study. There were 142 and 260 male and female patients, respectively. Most patients were of Han ethnicity (293). The median age was 33 years (range: 17–79 years). A total of 139 (34.6%) patients were diagnosed with ANS according to the above criteria. Of these, 40 had reactive CSF RPR titer, of which 26 had RPR 1:1; nine had RPR 1:2, three had RPR 1:4; and two had RPR 1:8. A total of 115 patients had CSF WBC > 5 × 106/L, the median was 10 × 106/L (range: 0–216 × 106/L); 28 patients had CSF protein concentration > 45 mg/dL, the median was 29.9 mg/dL (range: 7.2–216 mg/dL). Pretreatment serum RPR titer, serum RPR titer (when lumbar puncture was performed), fold decline of serum RPR titer after initial treatment, therapeutic regimen time between treatment and lumbar puncture and other detailed information are shown in Table 1.
Table 1.
Patient Demographics and Baseline Characteristics (N = 402).
| Variables (Units) | ANS (n = 139) | Serofast (n = 263) |
|---|---|---|
| Sex | ||
| Male (n, %) | 56(40.3) | 86(32.7) |
| Female (n, %) | 83(59.7) | 177(67.3) |
| Age, (yrs, %) | ||
| ≤30 | 47(33.8) | 115(43.7) |
| 31–40 | 42(30.2) | 78(29.7) |
| 41–50 | 7(12.2) | 31(11.7) |
| 51–60 | 22(15.8) | 24(9.1) |
| ≥61 | 11(7.9) | 15(5.7) |
| Ethnic (n, %) | ||
| Han | 132(95.0) | 261(99.2) |
| Others | 7(0.5) | 2(0.8) |
| Marital status (n, %) | ||
| Married | 104(95.0) | 189(71.9) |
| Single | 28(20.1) | 61(23.2) |
| Divorced | 5(3.6) | 9(3.4) |
| Widowed | 1(0.7) | 1(0.4) |
| Unknown | 1(0.7) | 3(1.1) |
| Pretreatment serum RPR titer (n, %) | ||
| ≤1:2 | 3(2.2) | 16(6.1) |
| 1:4 | 7(5.0) | 24(9.1) |
| 1:8 | 17(12.2) | 40(15.2) |
| 1:16 | 22(15.8) | 45(67.2) |
| 1:32 | 26(18.7) | 41(17.1) |
| 1:64 | 24(17.3) | 28(10.6) |
| 1:128 | 14(10.1) | 20(7.6) |
| ≥1:256 | 4(2.9) | 8(3.0) |
| Unknown | 22(15.8) | 41(15.6) |
| Current serum RPR titer (n, %) | ||
| 1:1 | 4(2.9) | 19(7.2) |
| 1:2 | 13(9.35) | 45(17.1) |
| 1:4 | 20(14.4) | 52(19.8) |
| 1:8 | 33(23.7) | 65(24.7) |
| 1:16 | 24(17.3) | 50(19.0) |
| 1:32 | 28(20.1) | 21(8.0) |
| ≥1:64 | 17(12.2) | 11(4.2) |
| Decline fold of serum RPR titer after initial treatment (n, %) | ||
| <4-fold | 74(53.2) | 123(46.8) |
| ≥4-fold | 43(30.9) | 99(37.6) |
| Unknown | 22(15.8) | 41(15.6) |
| Therapeutic regimen (n, %) | ||
| Benzathine | 129(92.8) | 241(91.6) |
| Ceftriaxone | 8(5.8) | 12(4.6) |
| Tetracycline | 1(0.7) | 9(3.4) |
| Erythrocin | 1(0.7) | 1(0.4) |
| Duration between syphilis diagnose and lumber puncture (n, %) | ||
| <12 m | 21(15.1) | 33(12.5) |
| ≥12 m and <18 m | 43(30.9) | 77(29.3) |
| ≥18 m and <24 m | 14(10.1) | 23(8.7) |
| ≥24 m | 61(43.9) | 130(49.4) |
Abbreviation: ANS = asymptomatic neurosyphilis; RPR = rapid plasma regain.
Some ANS patients met two or three diagnostic criteria. A total of 25 patients had both reactive CSF RPR titer and CSF WBC > 5 × 106/L, nine patients had both reactive CSF RPR titer and CSF protein concentration > 45 mg/dL; 17 patients had both CSF WBC > 5 × 106/L and CSF protein concentration >45 mg/dL and seven patients had CSF WBC > 5 × 106/L, CSF protein > 45 mg/dL and the CSF RPR titer was reactive. 82.7% of ANS patients had CSF WBC > 5 × 106/L, higher than those of reactive CSF-RPR (28.8%) and high CSF protein concentration (20.1%); the rate of ANS with at least two CSF abnormalities was 26.6%, and the rate of ANS with three abnormalities was 5.0% (Table 2).
Table 2.
CSF characteristics of ANS patients.
| CSF characteristics | CSF RPR (+) N(%) | CSF RPR (−) N(%) | |
|---|---|---|---|
| Reactive RPR titer | |||
| WBC > 5 × 106/L and protein > 45 mg/dL | 7(5.0) | 10(7.2) | |
| WBC > 5 × 106/L and protein ≤ 45 mg/dL | 18(12.9) | 80(57.6) | |
| protein > 45 mg/dL and WBC ≤ 5 × 106/L | 2(1.4) | 9(6.5) | |
| protein ≤ 45 mg/dLand WBC ≤ 5 × 106/L | 13(9.4) | — | |
| Total | 40(28.7) | 99(71.3) | 139(100) |
Abbreviation: CSF = cerebrospinal fluid; ANS = asymptomatic neurosyphilis; RPR = rapid plasma reagin; WBC = white blood cell.
Relationship between serum RPR titer and ANS in asymptomatic serofast syphilis patients
The proportions of ANS in asymptomatic serofast syphilis patients increased with the serum RPR titers (Table 1), the proportion of ANS ranged from 17.4% in the patients with RPR titers 1:1 to 60.7% in the patients with RPR titers ≥ 1:64. From the ROC curve, serum RPR titer 1:16 with the highest Youden index was the best cutoff (sensitivity 32.4%, specificity 87.8%). The area under the curve (95% CI) was 0.639 (0.610–0.668) (Fig. 1).
Figure 1.
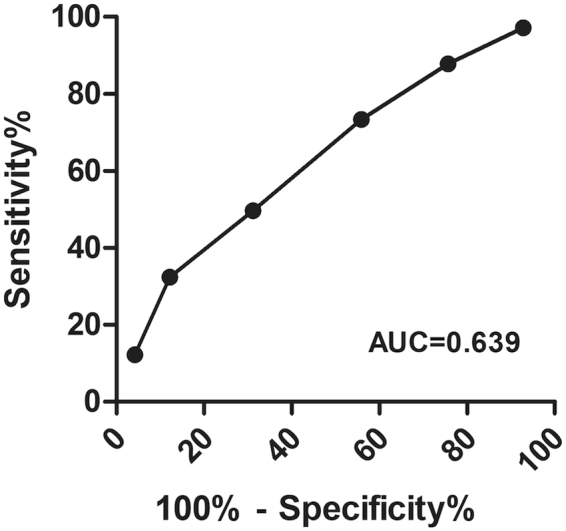
Receiver operating characteristic curve using serum RPR titer to distinguish ANS from asymptomatic serofast syphilis.
After the initial recommended treatment, 142 patients had a ≥4-fold decline of serum titer, of which 30.3% had ANS; 197 patients had a <4-fold decline of serum titer, and the proportion of ANS was 37.6%, which was higher than 30.3% but not statistically significant (p > 0.05). Hence, a 4-fold decline of serum titer after recommended treatment had no apparent influence on ANS, and could not be used to predict its occurrence.
Relevant factors of asymptomatic neurosyphilis
The incidence of neurosyphilis and related factors has been extensively studied, but the literature on ANS is very limited. Our study explored the association of the above-mentioned factors with the risk of ANS. A total of 402 patients were first identified by the bivariate analysis, factors with p < 0.1 were further identified in the multivariate analysis. In the bivariate analysis, ANS was significantly more common in patients aged 51–60 years (OR,2.24; CI,1.15–4.39; p = 0.02) (as compared to age ≤ 30 years), non-Han ethnicity (OR,6.92; CI,1.42–33.78; p = 0.02) (as compared to Han ethnicity); pretreatment serum RPR titer 1:32 (OR,6.33; CI,1.87–21.40; p = 0.00), serum RPR titer 1:64 (OR,4.57; CI,1.19–17.6; p = 0.03) (as compared to ≤ 1:2), and ≥ 1:64 (OR,7.34; CI,1.96–27.43; p = 0.00) (as compared to 1:1). In the multivariate analysis, ANS was not significant in patients with pretreatment serum RPR titer 1:64 (p > 0.05). ANS remained significantly more common in patients aged 51–60 years (AOR,2.28; CI,1.11–4.66; p = 0.03) (as compared to age ≤ 30 years), non-Han ethnicity (AOR,9.11; CI,1.78–46.78; p = 0.01) (as compared to Han ethnicity), serum RPR titer 1:32 (AOR,5.12; CI,1.34–19.47; p = 0.02) and ≥ 1:64 (AOR,5.69; CI,1.34–24.20; p = 0.02) (as compared to 1:1). Patients aged 51–60 years, of other ethnicity, and with serum RPR titer 1:32 and ≥ 1:64 were 2.28-fold, 9.11-fold, 5.12-fold and 5.69-fold, respectively, more likely to have ANS. However, other factors, such as gender, marital status, fold decline of serum RPR titer after treatment, therapeutic regimen, time between treatment and lumbar puncture were not relevant factors for ANS.
Discussion
Studies have documented the incidence of neurosyphilis in different stages of syphilis (primary, secondary, latent) or among various populations (HIV-negative, HIV-positive), and relevant factors of neurosyphilis among HIV-negative and/or HIV-positive syphilis patients6,10,11. However since ANS is difficult to diagnose12. its incidence and relevant factors remain largely unknown. Some studies had demonstrated that serofast state was common in syphilis with an incidence rate of 35.2–44.4%13. There was a certain association between ANS and serofast state, but the incidence of ANS among asymptomatic serofast syphilis patients in China requires further studies9,10,14. Therefore, we examined the incidence of ANS in asymptomatic syphilis patients with serofast state. Our results indicated that 139 of 402 patients had ANS, the incidence was 34.6%, higher than that of ANS among latent syphilis (13.5%) and syphilis with all stages (13.5%)7, which proved that ANS had a high incidence among serofast syphilis patients. About 35% of ANS patients developed symptomatic neurosyphilis8. Hence, ANS among serofast syphilis patients should be given more attention by clinicians. Of the 139 ANS patients, CSF WBC abnormality had the highest proportion of 82.7%, which was consistent with the highest proportion of CSF WBC abnormality in neurosyphilis patients with serofast state10. CSF WBC abnormality had high diagnostic value both in ANS and neurosyphilis. Our results were inconsistent with some studies on neurosyphilis in untreated early syphilis patients, which showed that CSF WBC abnormality accounted for the highest proportion among HIV-positive and total population, but protein abnormality had the highest proportion among HIV-negative patients11.
From the ROC curve, serum RPR titer 1:16 was the best cutoff value (sensitivity 32.4%, specificity 87.8%) for the diagnosis of ANS. Serofast syphilis patients with a serum RPR titer ≥1:16 had high risk of ANS. Some researches used a ≥4-fold decline as serological cure criteria15,16, but in our study, patients with a 4-fold decline of serum RPR titer after treatment who became serofast for more than one year showed similar chances of developing ANS, which was inappropriate as serological cure criteria.
Many studies showed that HIV-positive syphilis patients were more likely to be complicated by neurosyphilis, especially ANS17. Neurosyphilis in HIV-positive patients was related to CD4 ≤ 350 cells/ml, serum RPR titer ≥ 1:32, male gender, and an HIV viral load (VL) above 10000 copies/mL12,17–19. Recent researches showed CSF CXCL13 >10 pg/ml as a predictor of neurosyphilis20. Our results indicated that the patients with serum RPR titer 1:32 and ≥1:64 had 5.12-fold and 5.69-fold increased risk of ANS, respectively; and patients aged 51–60 years had a 2.28-fold increased risk of ANS. Furthermore, 60.9% of these patients had ≥2 years syphilis history between initial treatment and lumbar puncture. Unlike neurosyphilis, male patients did not have a high incidence of ANS in our study. Although the incidence of ANS in males (39.4%) was higher than in females (31.9%), it was not statistically significant.
This study had several limitations. Prognosis, which was obviously influenced by the presence and extent of CSF abnormalities7, could not be observed in our study. Also, other ethnicity patients were 9.11-fold more likely to develop ANS, but there were only nine other ethnicity patients, and more patients are needed to confirm this finding. Further investigations of ANS in different stages and HIV-positive serofast syphilis patients are essential. Patients >51–60 years with serum RPR titer ≥1:16 are more likely to have ANS. These patients should be recommended to undergo lumbar puncture and neurosyphilis treatment to prevent them from developing symptomatic neurosyphilis. Our findings can be used to guide clinicians in the identification of ANS among syphilis patients with serofast state.
Methods
Study Design and Ethics Statement
All patients in this study were enrolled from the clinical database of Peking University Ditan Teaching Hospital between September 2008 and August 2016. According to the 2008 European Guidelines on the Management of Syphilis and CDC guidelines in USA21,22, All of the enrolled patients were HIV-negative, and both of serological TPPA and RPR positive at any stage of syphilis; Serofast status was defined as (1) in the early syphilis patients who have been at least six months after initial recommended treatment and regular follow-up, a 4-fold decline in serum RPR titer was not observed even after additional treatment; (2) RPR titer was less than 4-fold decline more than one year after treatment in late latent syphilis; and (3) those who had 4-fold decline of serum RPR titer after initial treatment, but became no seroreversion and any decline in nontreponemal antibody titers for more than one year13,15,21. All patients underwent lumbar puncture and CSF tests for ANS at the Peking University Ditan Teaching Hospital. Patients with HIV-infection, reinfection of syphilis, symptomatic neurosyphilis, or other neurological diseases were excluded. Asymptomatic neurosyphilis is defined by the presence of one or more CSF abnormalities (pleocytosis, elevated protein concentration, or reactive CSF Rapid plasma regain card test [CSF RPR] test) in persons with serologic evidence for syphilis but no neurologic signs or symptoms3,22,23. ANS is characterized by reactive CSF RPR, or negative CSF RPR but CSF WBC count >5 × 10 6/L, or negative CSF RPR, CSF WBC count ≤5 × 10 6/L but CSF protein concentration >45 mg/dL, and without any neurological signs and symptoms. The other neurological diseases were excluded based on clinical history and physical examination. According to the diagnostic criteria, the patients were divided into two groups: the ANS group, which met the ANS criteria listed above, and the serofast syphilis group (excluding neurosyphilis). All methods were carried out in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants, This study was approved by the Institutional Ethics Committee of Peking University Ditan Teaching Hospital.
Laboratory methods
Laboratory analyses, including RPR (Shanghai Rongsheng Biological Pharmaceutical Co., Ltd, China) and Treponema pallidum particle agglutination (TPPA) (Fujirebio, Tokyo, Japan), were conducted as per the manufacturers’ instructions.
Analysis and Statistics
Data was analyzed by IBM SPSS version 19.0. Figures were drawn using GraphPad Prism 5. Continuous variables were described using median and interquartile range (IQR), while categorical variables were described by numbers and percentages. Associations between categorical variables were assessed by the chi-square test. The ROC curve of the serum RPR titer was drawn and the best cutoff was found. Bivariate analysis was utilized to determine the factors associated with ANS. The odds ratios (OR) was estimated with 95% confidence intervals (CIs) from the bivariate analysis, and factors with p < 0.1 were further identified in the multivariate analysis. Adjusted odds ratios (AOR) with 95% CIs were also estimated from the logistic regression analysis. All hypotheses testing was two-sided, and p < 0.05 was considered to be statistical difference.
Acknowledgements
We thank the colleagues in the Department of dermatology, Peking University Ditan Teaching Hospital for partially supporting this study and also the general patients included in the study. This study is currently funded through the National Clinical Key Department of Infectious Diseases.
Author Contributions
C.S.N. and L.W.H. wrote the main manuscript text, L.J. and C.C. collected the clinical data, W.G. was in charge of statistics anlaysis. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang S, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2017;17:S1473–3099. doi: 10.1016/S1473-3099(17)30227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, J. R., & Dean, D. Neurosyphilis. Handbook of clinical neurology121, 1461–1472 (2013). [DOI] [PubMed]
- 3.Bennett J. E. et al. Principles and practice of infectious diseases. Elsevier Health Sciences, Canada, 2685–2702 (2014)
- 4.Qin. J, et al. Potential predictors for serofast state after treatment among HIV-negative persons with syphilis in China: a systematic review and meta-analysis. Iranian journal of public health. 2015;44:155–169. [PMC free article] [PubMed] [Google Scholar]
- 5.Sabre L, Braschinsky M, Taba P. Neurosyphilis as a great imitator: a case report. BMC Research Notes. 2016;9:372. doi: 10.1186/s13104-016-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong ML, et al. Spectrum and characterization of movement disorders secondary to neurosyphilis. Parkinsonism & related disorders. 2013;19:441–445. doi: 10.1016/j.parkreldis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem KG. REVIEW: Neurosyphilis: A historical perspective and review. CNS neuroscience & therapeutics. 2010;16:e157–e168. doi: 10.1111/j.1755-5949.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastuszczak M, et al. Cerebrospinal fluid abnormalities in HIV-negative patients with secondary and early latent syphilis and serum VDRL ≥ 1: 32. Indian journal of dermatology. 2013;58(4):325. doi: 10.4103/0019-5154.113941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong ML, et al. Factors associated with serological cure and the serofast state of HIV-negative patients with primary, secondary, latent, and tertiary syphilis. PLoS One. 2013;8(7):e70102. doi: 10.1371/journal.pone.0070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SL, Zhang HS. Relationship between syphilis serofast reaction and neurosyphilis[J] Chin J Nosocomiol. 2012;20:2235–2238. [Google Scholar]
- 11.Rolfs RT, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. New England Journal of Medicine. 1997;337:307–314. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem KG, Workowski KA. Management of adult syphilis. Clinical infectious diseases. 2011;53(suppl 3):S110–S128. doi: 10.1093/cid/cir701. [DOI] [PubMed] [Google Scholar]
- 13.Seña AC, et al. A systematic review of syphilis serological treatment outcomes in HIV-infected and HIV-uninfected persons: rethinking the significance of serological non-responsiveness and the serofast state after therapy. BMC infectious diseases. 2015;15:479. doi: 10.1186/s12879-015-1209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, et al. Risk profiles of neurosyphilis in HIV‐negative patients with primary, secondary and latent syphilis: implications for clinical intervention. Journal of the European Academy of Dermatology and Venereology. 2016;4:659–666. doi: 10.1111/jdv.13514. [DOI] [PubMed] [Google Scholar]
- 15.Seña AC, et al. Predictors of serological cure and Serofast State after treatment in HIV-negative persons with early syphilis. Clinical infectious diseases. 2011;53:1092–1099. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren RX, et al. No improvement in serological response among serofast latent patients retreated with benzathine penicillin. International journal of STD & AIDS. 2016;27:58–62. doi: 10.1177/0956462415573677. [DOI] [PubMed] [Google Scholar]
- 17.Chahine LM, et al. The changing face of neurosyphilis. International Journal of Stroke. 2011;6:136–143. doi: 10.1111/j.1747-4949.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang YJ, et al. Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan. Journal of Microbiology, Immunology and Infection. 2012;45:337–342. doi: 10.1016/j.jmii.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Merins V, Hahn K. Syphilis and neurosyphilis: HIV-coinfection and value of diagnostic parameters in cerebrospinal fluid. European journal of medical research. 2015;20:81. doi: 10.1186/s40001-015-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu RX, et al. Value of CXCL13 in diagnosing asymptomatic neurosyphilis in HIV-infected patients. International journal of STD & AIDS. 2016;27:141–146. doi: 10.1177/0956462415577229. [DOI] [PubMed] [Google Scholar]
- 21.Workowski, K. A., Berman, S. M. Sexually transmitted diseases treatment guidelines. Morbidity and Mortality Weekly Report55(RR11), 1–94 (2006). [PubMed]
- 22.French P, et al. IUSTI: 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS. 2009;20:300–309. doi: 10.1258/ijsa.2008.008510. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, et al. Comparison of the cerebrospinal fluid (CSF) toluidine red unheated serum test and the CSF rapid plasma reagin test with the CSF venereal disease research laboratory test for diagnosis of neurosyphilis among HIV-negative syphilis patients in China. J Clin Microbiol. 2014;52(3):736–40. doi: 10.1128/JCM.02522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


