Abstract
Background:
Pain alleviation and improvement of functional status are the main objectives in the treatment of osteoarthritis. Artemisia absinthium (AA) was used traditionally in reducing pain and inflammation. The aim of the present study was to compare the effects of topical formulations of AA and piroxicam gel (PG) among patients with knee osteoarthritis.
Methods:
In total, 90 outpatients aged 30-70 years with the diagnosis of primary osteoarthritis in at least one knee were enrolled in a randomized double-blind clinical trial. The patients referred to the Rheumatology Clinic at Shahid Beheshti Hospital in Hamadan province during 2012-2013. The patients were randomly assigned into three groups, 30 patients per group, and respectively received AA ointment (AAO) 3%, AA liniment (AAL) 3%, and PG; three times daily (TID) for 4 weeks. The patients were visited at baseline, week 4, and week 6. The effectiveness criteria were pain severity which was assessed with a 10-point visual analog scale (VAS), the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) for total pain score (WTPS), total physical function score (WTPFS), and total stiffness score (WTSS). Repeated measure ANOVA, paired t test and post hoc were used to compare variables. Statistical analysis was performed using the SPSS software, version 13.0 (SPSS Inc., Chicago, Illinois).
Results:
All groups had similar patient demographics. The administration of PG significantly improved all tested criteria with no recurrence after discontinuing the treatment protocol. AAO alleviated all tested factors except for WTSS. Alleviation was comparable to PG. AAL only reduced pain factors (VAS, WTPS) in week 4 with recurrence in week 6.
Conclusion:
Administration of Artemisia ointment may have beneficial effects in the treatment of osteoarthritis. Trial Registration Number: IRCT201202123109N3
Keywords: Artemisia absinthium, Osteoarthritis, Piroxicam
What’s Known
The anti-inflammatory effects of wormwood in some diseases such as Crohn’s disease have been known.
What’s New
Considering the inflammatory mechanism involved in the disease, wormwood exerts beneficial effects in osteoarthritis when compared with piroxicam as NSAID.
Introduction
Knee osteoarthritis (KOA) is one of the most prevalent types of OA. KOA occurs in about 6% of people over 30 years and increases to 10% in adults above 60 years of age, especially in women at menopause period with overweight.1 Because the lifespan of the general population is increasing, the prevalence of OA is rising.2 The main clinical symptoms of KOA are joint pain, swelling, stiffness, loss of movement, and reduction of participation in daily activities.3 These physical damages and incapabilities may have psychological effects and increase the risk of comorbidities.1
There are inter-individual differences in the course of OA progression, manifestations, and functional limitations.4 Regardless of personal differences, the main objectives in KOA management are pain reduction and improvement of life quality.3 Nowadays, topical NSAIDs are available with reduced potential of systemic adverse effects.5 According to recent research studies, inflammatory cytokines including IL-1b, TNFa, IL-6, IL-15, IL-17, and IL-18 as well as anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 are involved in the initiation and progression of KOA.6-9
Natural products consist the major components of 50% current drugs for the management of various ailments.10 Topical herbal therapies for the management of osteoarthritis are also available. Arnica gel may alleviate pain and functional status comparable to that of NSAIDs. Comfrey may have analgesic effect more than placebo. Capsicum gel is the same as placebo and has no beneficial effects in this regard.11
Artemisia absinthium (Asteraceae) is locally called afsantin and wormwood in Persian and English, respectively. Its use in medicine dates back to Roman times. It is a medicinal herb with many ethnopharmacological uses, such as antihelmintic, antiseptic, reducing muscle pain, easing the symptoms of depression, and to improve memory.12 Moreover, using A. absinthium ointment externally can reduce the stiffness of muscles and joints as well as help in healing bruises.12 On human breast cancer cells, it showed antiproliferative effects as well as potent antioxidant properties which may be used in the prevention of oxidative stress-induced disorders.13 Some studies reported that A. absinthium extract may suppress TNFα and other interleukins.13,14
In a double-blind study, treatment with capsules containing A. absinthium whole plant reduced TNFα level and also improved the mood of patients suffering from Crohn’s disease.14 Alcoholic and aqueous extracts and essential oil of A.absinthium exhibited analgesic and anti-inflammatory activities.12 Also, the extract obtained by maceration of A. absinthium in acetic acid produced topically antinociceptive activity, which was significantly lessened by pretreatment with naloxone, ondansetrone, metoclopramide, and atropine. The result of the latter study recommended that these effects are probably produced by the involvement of opioidergic, dopaminergic, and serotonergic systems.13 Considering the ongoing use of plants and the rising prevalence of KOA, the aim of the present study was to evaluate the effect of A. absinthium topical formulations (ointment and liniment) versus piroxicam gel in patients with KOA.
Patients and Methods
Preparation of Wormwood Ointment and Liniment
Wormwood extract was prepared according to guidelines by Zeraati et al.13 Briefly, the plant was grown in Avicenna Medicinal Plants Research Station of Hamadan Agricultural and Natural Resources Research Center, Hamadan, Iran. The plant was harvested in August 2012 and authentication was done by Dr. Kalvandi at the herbarium of the above-mentioned center. After drying, the powdered plant material was soaked in 8% acetic acid. The extract was filtered and dried by means of rotary evaporation. Wormwood ointment and liniment had 3% w/w of wormwood extract in ointment base and olive oil, respectively, that were prepared by a pharmacist and packaged alike.
Participants
Patients aged 30-70 years with the final diagnosis of primary osteoarthritis in at least one knee were consecutively recruited into the study. The patients referred to the Rheumatology Clinic at Shahid Beheshti Hospital in Hamadan province (Iran) during 2012-2013.
The diagnosis of primary osteoarthritis was established by clinical and radiological assessments according to the guidelines of the American College of Rheumatology.15,16 To rule out the inflammatory disease such as rheumatoid arthritis, routine laboratory examinations including cell blood count, rheumatic factor analysis, and 2ME test were performed. The values of C- reactive protein, erythrocyte sedimentation rate, alkaline phosphatase, and serum levels of calcium and phosphorus were determined. The inclusion criterion was primary osteoarthritis of at least one knee that has been established by clinical and radiological assessments. The exclusion criteria were confirmed or suspected secondary osteoarthritis, trauma, congenital abnormalities, endocrinological impairments, metabolic disturbances, other musculoskeletal disorders, administration of oral corticosteroids and analgesics within 10 days before admission, long-acting corticosteroids, and pregnancy and nursing. For women of childbearing age, representing adequate evidence of contraception prior to inclusion was necessary. Patients that fulfilled the inclusion criterion, after signing the informed consent form, were randomized into three groups. Patients that had severe pain were excluded from the study.
Study Design and Treatment
This randomized, double-blind, parallel groups, non-inferiority trial was conducted to compare the efficacy and safety of Artemisia absinthium ointment and liniment versus piroxicam gel. Patients that fulfilled the inclusion criteria, after signing the informed consent form, were randomized into three groups. Randomization was done by permuted-block design that involves randomizing patients to treatment groups in sequential blocks. Patients were then treated under double-blind conditions for up to 4 weeks. Different formulations (ointment, liniment, and gel) were packaged alike and only marked with A, B, and C letters. Patients received the order of drugs by a collaborator that was not involved with patients or data analysis.
The patients were advised to rub topical formulations on the affected knee (front, back, and sides) three times a day for 28 days and then re-visit the clinic for further assessments. In addition, all participants visited again two weeks after treatment discontinuation for the evaluation of disease recurrence. In cases with bilateral involvement, both knees were treated; however, only the knee with more severe pain was evaluated.
In the present study, we did not categorize patients into control or placebo group, instead, each patient was his/her control. Each group was examined before and after treatment and was compared at different intervals. All patients were informed about the possible side effects or lack of response to treatment. They were reminded that their participation in the study is voluntary. All patients were also recommended that in case of appearing redness, itching, or burning at the site of topical application of the drug, they should terminate its use and referred to the clinic. The patients were asked to avoid the use of other analgesic drugs within the study period without prior consultation.
The number of patients who abandoned the treatment protocol was recorded to evaluate the level of patient satisfaction. Ethical approval was obtained from the local Research Ethics Committee of Hamadan University of Medical Sciences (number: P/16/35/9/57231). Moreover, the study was performed in accordance with the principles of the Helsinki Declaration. This study was registered at the Iranian Registry of Clinical Trials with IRCT registration number: IRCT201202123109N3.
Statistical Analyses
Due to the nature of the present clinical trial (non-inferiority trial), the sample size was calculated according to G*Power software (version 3.0.10 for Windows). Accordingly, 3*25 patients were required to ensure that 80% of the lower limit of a two-sided 95% confidence interval would be above the non-inferiority limit of -8. The efficacy analyses were performed based on the intention-to-treat (ITT) analysis. ITT population was defined as randomized patients who took the medication and provided baseline and post-baseline efficacy assessment.
The primary outcome measure of the study was the reduction in the mean pain score from baseline, as assessed by the VAS (0-10) points. This visual analog scale ranged from 0 up to 10 that corresponds to no pain and the worst imaginable pain, respectively. To measure secondary outcome, the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) was used. The WOMAC consists of three dimensions: pain (5 items), stiffness (2 items), and physical functioning (17 items). The five-point Likert version 9 of the WOMAC was used. An item response can range from ‘‘none’’ to ‘‘extreme’’. The Persian version of WOMAC has been tested previously and its reliability, validity, and responsiveness were satisfactory.15 Evaluation of patients, including assessment of pain severity, stiffness, and physical activity were performed at baseline, 4 weeks after starting treatment (week 4), and 2 weeks after treatment completion (week 6).
The results were presented as mean±SD (standard deviation) for quantitative variables and summarized by absolute frequencies and percentages for categorical variables. Categorical variables were compared using the chi-square or Fisher’s exact test when more than 20% of cells with an expected count of less than 5 were observed. To compare variables between the three groups, repeated measure ANOVA was conducted for comparing the effect of the AAO, AAL, and PG on VAS pain, total WOMAC, WOMAC pain, WOMAC stiffness, and WOMAC physical function scores at different intervals. Moreover, paired t test was used to perform post hoc comparison within each group (end of treatment vs. onset and 2 weeks after treatment vs. end of treatment). P≤0.05 were considered statistically significant. Statistical analysis was performed using the SPSS software, version 13.0 (SPSS Inc., Chicago, Illinois).
Results
The present study was conducted during June 2012-2013. Ninety patients with diagnosed primary osteoarthritis were included in the study. Patients’ demographic, baseline VAS and WOMAC, and demographic properties (sex, weight, and age) were not significantly different between the three treatment groups (table 1). The flow chart of patient enrollment and disposition is shown in figure 1. Discontinuation rate was higher in the AAO group. In total, 90 eligible patients were enrolled in the study in which 80 patients (88%) completed the process.
Table 1.
Demographic and baseline characteristics of the patients in the three study groups. Values are presented as mean±SD
| Variables | Artemisia absinthium ointment | Artemisia absinthium liniment | Piroxicam gel | P value |
|---|---|---|---|---|
| Weight (kg) | 74±11 | 74±8 | 71±9 | 0.597 |
| Age (year) | 57±9 | 53±13 | 60±10 | 0.22 |
| Baseline VAS score | 6.0±2.5 | 6.1±1.8 | 6.0±2.6 | 0.983 |
| Baseline WOMAC score | 49.0±18.3 | 42.0±15.2 | 50.3±18.6 | 0.231 |
Figure 1.
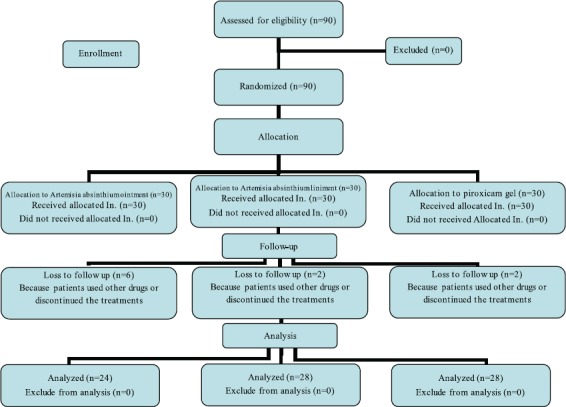
The CONSORT diagram for patient enrollment and disposition in the Artemisia absinthium ointment and liniment, and piroxicam gel groups.
Comparing WOMAC total, pain, and physical function scores and VAS pain score at different intervals showed significant differences before treatment, end of treatment, and two weeks after treatment in the AAO group (P<0.001). There was no significant difference in WOMAC stiffness score in terms of treatment time in the AAO group.
In the AAL group, comparison of the outcome measure showed that there were significant differences in terms of treatment time in the VAS and WOMAC pain scores (P<0.001). In the PG group, all scores were significantly different in terms of treatment time (P<0.001). The trend of changes in pain severity and physical function state across the three groups was compared using three-way repeated measure ANOVA; the result showed no differences between the groups. In case of significant difference in each group, two-paired sample t test was used to perform post hoc comparison between different intervals (week 4 vs. before treatment and week 6 vs. week 4) (table 2). For the evaluation of recurrence, the scores in weeks 4 and 6 were compared with each other (table 2).
Table 2.
VAS and WOMAC scores in different treatment groups at different time intervals, values are presented as mean±SD
| Time point | Wormwood ointment (n=24) | Wormwood liniment (n=28) | Piroxicam gel (n=28) | P value between effective groups (base comparison) |
|---|---|---|---|---|
| Total WOMAC score | ||||
| Before treatment | 49.1±18.3 | 42.2±15.2 | 50.3±18.6 | 0.265 |
| End of treatment | 42.0±15.6* | 36.5±18.1 | 40.4±17.9* | |
| 2 weeks after treatment | 41.0±13.0 | 36.8±17.8 | 40.1±17.4 | |
| P value | 0.017/0.783 | <0.001/0.493 | ||
| WOMAC pain score | ||||
| Before treatment | 11.1±3.51 | 9.35±3.10 | 10.3±4.05 | 0.194 |
| End of treatment | 8.00±3.96* | 7.14±3.81* | 8.07±4.29* | |
| 2 weeks after treatment | 8.10±3.72 | 8.04±352** | 8.08±3.75 | |
| P value | 012/0.804 | 0.001/0.041 | <0.001/0.674 | |
| WOMAC stiffness score | ||||
| Before treatment | 3.14±2.08 | 3.00±1.96 | 3.66±2.26 | 0.494 |
| End of treatment | 2.96±1.91 | 2.82±1.46 | 2.60±1.81* | |
| 2 weeks after treatment | 3.22±2.35 | 2.84±1.81 | 2.75±1.58 | |
| P value | 0.049/0.490 | |||
| WOMAC physical function score | ||||
| Before treatment | 34.1±13.1 | 29.9±11.7 | 36.3±13.9 | 0.216 |
| End of treatment | 31.1±11.6* | 26.6±13.6 | 28.9±12.3* | |
| 2 weeks after treatment | 29.7±9.43 | 26.6±13.5 | 29.4±11.8 | |
| P value | 0.039/0.509 | <0.001/0.367 | ||
| VAS pain score | ||||
| Before treatment | 5.99±2.47 | 6.11±1.89 | 6.05±2.6 | 0.992 |
| End of treatment | 5.07±2.22* | 4.79±2.18* | 4.12±2.22* | |
| 2 weeks after treatment | 4.93±2.52 | 5.51±2.20** | 4.6±2.53 | |
| P value | 0.039/0.472 | <0.001/0.01 | <0.001/0.056 |
Significant for comparing week 4 and week 0;
Significant for comparing week 6 and week 4; Week 0: Before treatment; Week 4: End of treatment; Week 6: Two weeks after ending treatment; First P value/second P value; Comparing week 4 and week 0/comparing week 6 and week 4
Discussion
The present study aimed to compare beneficial effects of topical formulations containing wormwood with piroxicam gel, in patients with primary knee osteoarthritis at different intervals. The other objective was to evaluate the carryover effect of these formulations after completion of the treatment.
Although only PG had appropriate effects on joint stiffness, analgesic effects and improvements in physical function were the same for groups receiving AAO and PG. There was no recurrence 2 weeks after treatment completion. AAL was only effective on pain severity in a certain period of the treatment.
According to recent research studies, inflammatory cytokines, including IL-1b, TNFa, IL-6, IL-15, IL-17, IL-18, and anti-inflammatory cytokines such as IL-4, IL-10, and IL-13 are involved in the initiation and progression of KOA.6-9 Although few experimental trials have shown the efficacy of blocking pro-inflammatory cytokine in the treatment of OA;17 however, Chevalier et al. revealed that TNFa blockers (adalimumab, two SC injections) had no beneficial effects in treating hand OA.18 Hence, OA treatment with blocking inflammatory cytokines is not proven and requires further investigations.
The genus Artemisia has many uses in traditional medicine, among which antinociceptive and anti-inflammatory activities have been reported for several species of the genus. Recent research studies have reported that Artemisia herba alba,10 Artemisia dracunculus,10 and Artemisia deserti,19 have clear potentials. Artemisia absinthium and Artemisia copa also showed topical analgesic and anti-inflammatory activities, respectively.10 Artemisia absinthium showed anti-inflammatory effect in some in vitro and in vivo studies.12,20 In a controlled clinical trial, Krebs et al. confirmed that AA can accelerate healing in Crohn’s disease by suppressing tumor necrosis factor alpha14. Omer and colleagues suggested that wormwood has a steroid sparing effect in patients with Crohn’s disease.21 The patients suffering from Crohn’s disease received prednisone for 3 weeks. After tapering the steroid, in week 10, they were free of steroids. Then, they received herbal blend containing wormwood herb (3×500 mg/day) or placebo for 10 weeks. Unlike the group receiving herbal blend, restarting steroid for the prevention of disease deterioration was necessary for the placebo group.21 Zeraati et al. (2014) revealed that the extract of A. absinthium caused topical antinociceptive effect on mice. This effect was alleviated by using naloxone, ondansetrone, metoclopramide, and atropine as pretreatment. In addition, the results suggested that the involvement of opioidergic, dopaminergic, and serotonergic produced the effects.13 Choi et al. reported a flavone from Artemisia absinthium which induces the production of IL-10 and anti-inflammatory cytokine, and thus suppressing collagen-induced arthritis in mice.20
Ahmad et al. reported that the methanolic extract of A. absinthium displayed significant analgesic action at different dose levels (from 300 to 1000 mg/kg). The duration and intensity of analgesia were dose-dependent. The plant extract showed a rapid onset and less potent analgesia when compared with acetylsalicylic acid (ASA) at 300 mg/kg dose. Also, the anti-inflammatory effects in the dose of 1000 mg/kg was comparable to that of ASA.22
Taken together, based on these observations, the therapeutic effects of this agent in osteoarthritis can be generalized. Although we focused on a specific species of Artemisia, anti-inflammatory effects of other species of this plant with different mechanisms have also been discussed. Koo HN and his colleagues showed that Artemisia capillaris inhibits TNF-α and IL-α cytokines in human hepatica cell line and thus could prevent the ethanol-induced cytotoxicity.23 Also, Choi and his colleagues demonstrated that DA-9601, a standardized aqueous extract of Artemisia Asiatica, blocks TNF-α-induced IL-8 and CCL20 production by inhibiting p38 kinase and NFқB pathways in human gastric epithelial cells.24
Giangaspero et al. isolated eupatilin from Artemisia umbelliformis which has topical anti-inflammatory activity comparable to that of indomethacin and hydrocortisone.25 Eupatilin inhibit the expression of cyclooxygenase-2 and different pro-inflammatory cytokines, such as interleukins (IL-4, IL-6, and IL-8) and tumor necrosis factor-R (TNF-R).25 Thus, anti-inflammatory impacts of Artemisia may be mediated by various suppressive effects of inflammatory cells and mediators, even if the drug is administered topically. The formulations tested in the present study contained 3% w/w of wormwood extract. Although the results showed appropriate effects on pain and physical function, none of the tested formulations could alleviate stiffness. Hence, further investigation is required to obtain the optimal dosage.
In the present study, liniment and Absinthium ointment showed similar effects in reducing pain. However, liniment was ineffective on physical activity. Two weeks after treating with Absinthium ointment, the patients had no pain until the end of the treatment. However, in the liniment group, the effect of the drug was alleviated two weeks after the treatment and showed a significant difference by the time of treatment completion. Liniment was produced by injecting its extract into olive base in a liquid form, while a semi-solid base of the ointment was used for the ointment formulation. Based on previous findings, it is expected that liniment to have a faster onset and ends the action, while the drug absorption from the ointment base to be gradual and the duration of action to be longer. This is in-line with the results of the present study. However, liniment and Absinthium ointment have an equal intensity of action. Further investigation is required for the justification of liniment being ineffective on physical activity.
The most important limitation of the present study was the pain assessing method that introduced information bias. Necessary actions were taken to minimize the bias through appropriate methodological design. Adjustment of extract content in the formulation and isolation of bioactive components is under investigation.
Conclusion
Topical administration of ointment containing Artemisia absinthium extract may improve clinical manifestation of patients suffering from KOA. The result of the present study is useful in producing topical formulations to alleviate OA symptoms.
Acknowledgement
The present study was sponsored by a grant (number: P/16/35/9/57231) from the Vice Chancellor of Research and Technology, Hamadan University of Medical Sciences, Hamadan, Iran.
Conflict of Interest: None declared.
References
- 1.Ferreira AH, Godoy PB, Oliveira NR, Diniz RA, Diniz RE, Padovani Rda C, et al. Investigation of depression, anxiety and quality of life in patients with knee osteoarthritis: a comparative study. Rev Bras Reumatol. 2015;55:434–8. doi: 10.1016/j.rbr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Giordano N, Fioravanti A, Papakostas P, Montella A, Giorgi G, Nuti R. The efficacy and tolerability of glucosamine sulfate in the treatment of knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Curr Ther Res Clin Exp. 2009;70:185–96. doi: 10.1016/j.curtheres.2009.05.004. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett MS, Rice SJ, Madurasinghe V, Slack R, Fayter DA, Harden M, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage. 2013;21:1290–8. doi: 10.1016/j.joca.2013.05.007. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls E, Thomas E, van der Windt DA, Croft PR, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22:2041–50. doi: 10.1016/j.joca.2014.09.026. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanos S. Osteoarthritis guidelines: a progressive role for topical NSAIDs. J Am Osteopath Assoc. 2013;113:123–7. [PubMed] [Google Scholar]
- 6.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westacott CI, Barakat AF, Wood L, Perry MJ, Neison P, Bisbinas I, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthritis Cartilage. 2000;8:213–21. doi: 10.1053/joca.1999.0292. [DOI] [PubMed] [Google Scholar]
- 8.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–7. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6:95–105. doi: 10.5312/wjo.v6.i1.95. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoaib M, Shah I, Ali N, Shah WA. A mechanistic approach to anti-nociceptive potential of Artemisia macrocephala Jacquem. BMC Complement Altern Med. 2016;16:141. doi: 10.1186/s12906-016-1114-0. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013:CD010538. doi: 10.1002/14651858.CD010538. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi A, Hossein N, Shirin P, Najmeh N, Abolfazl M. Anti-inflammatory and analgesic activities of Artemisia absinthium and chemical composition of its essential oil. Int J Pharm Sci Rev Res. 2014;38:237–44. [Google Scholar]
- 13.Zeraati F, Esna-Ashari F, Araghchian M, Emam AH, Rad MV, Seif S, et al. Evaluation of topical antinociceptive effect of Artemisia absinthium extract in mice and possible mechanisms. African Journal of Pharmacy and Pharmacology. 2014;8:492–6. [Google Scholar]
- 14.Krebs S, Omer TN, Omer B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn's disease - A controlled clinical trial. Phytomedicine. 2010;17:305–9. doi: 10.1016/j.phymed.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006;65:1363–7. doi: 10.1136/ard.2006.051482. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki T, Inoue K, Ushiyama T, Fukuda S. [Assessment of the American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee] Ryumachi. 1998;38:2–5. [PubMed] [Google Scholar]
- 17.Calich AL, Domiciano DS, Fuller R. Osteoarthritis: can anti-cytokine therapy play a role in treatment? Clin Rheumatol. 2010;29:451–5. doi: 10.1007/s10067-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 18.Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergniaud P, et al. a randomized, multicentre, double blind, placebo-controlled trial of anti TNF alpha (adalimumab) in refractory hand osteoarthritis. Osteoarthritis Cartilage. 2013;21:S146. doi: 10.1136/annrheumdis-2014-205348. [DOI] [PubMed] [Google Scholar]
- 19.Karimi H, Monajemi R, Amjad L. Analgesic and Anti-Inflammatory Effects of Artemisia Deserti Krasch (Extract in Rats) Int J Sci Basic Appl Res. 2014;3:1–6. [Google Scholar]
- 20.Choi KM, Hwang DH, Shin KM, Yoon D-Y, Kim DS. Effect of a Flavon Extracted from Artemisia absinthium on Collagen Induced Arthritis in Mice. Korean J Pediatr. 2004;47:677–84. [Google Scholar]
- 21.Omer B, Krebs S, Omer H, Noor TO. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn's disease: a double-blind placebo-controlled study. Phytomedicine. 2007;14:87–95. doi: 10.1016/j.phymed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad F, Khan RA, Rasheed S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. Journal of Islamic Academy of Sciences. 1992;5:111–14. [Google Scholar]
- 23.Koo HN, Hong SH, Jeong HJ, Lee EH, Kim NG, Choi SD, et al. Inhibitory effect of Artemisia capillaris on ethanol-induced cytokines (TNF-alpha, IL-1alpha) secretion in Hep G2 cells. Immunopharmacol Immunotoxicol. 2002;24:441–53. doi: 10.1081/IPH-120014728. [DOI] [PubMed] [Google Scholar]
- 24.Choi S-C, Choi E-J, Oh H-M, Lee S, Lee J-K, Lee M-S, et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-α-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-κB pathways in human gastric epithelial cells. World J Gastroenterol. 2006;12:4850–8. doi: 10.3748/wjg.v12.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giangaspero A, Ponti C, Pollastro F, Del Favero G, Della Loggia R, Tubaro A, et al. Topical anti-inflammatory activity of Eupatilin, a lipophilic flavonoid from mountain wormwood (Artemisia umbelliformis Lam.) J Agric Food Chem. 2009;57:7726–30. doi: 10.1021/jf901725p. [DOI] [PubMed] [Google Scholar]


