Abstract
Background:
Saponins identified from fenugreek (Trigonella foenum-graecum) seeds are reported effective on dyslipidemia. However, the definite mechanism is still not elucidated systematically. In this study, we evaluate the effects of saponin extract on cholesterol absorption, metabolism, synthesis, and reverse cholesterol transport in vivo.
Methods:
Saponin extract was prepared according to a craft established in our previous study. After the establishment of dyslipidemia model, 40 male Sprague-Dawley rats were divided into five groups, namely the control group (normal diet plus normal saline), HFD group (high fat diet plus normal saline), Lipitor group (high fat diet plus Lipitor (2 mg/kg)), and L, M, and H-saponin groups (high fat diet plus saponin in dosages of 6, 12, and 24 mg/kg, respectively). Rats were sacrificed at the end of the 9th week after treatment. Biochemical characteristics of rats were tested, histopathological sections of liver tissue were observed, and the protein and mRNA expression of related factors of cholesterol in the intestine and liver were determined. One-way ANOVA test (SPSS software version 11.5, Chicago, IL, USA) was used to determine statistically significant differences between the HFD and other groups.
Results:
In saponin groups, the serum lipid, bile acid efflux, anti-peroxide activities, and lipid area of liver tissue improved. Cholesterol 7alpha-hydroxylase and scavenger receptor class B type I elevated in the liver. 3-hydroxy-3-methylglutaryl coenzyme A reductase levels were suppressed in both the serum and liver. However, significant cholesterol efflux was not found and Niemann-Pick C1-Like 1 levels elevated in the intestine.
Conclusion:
The mechanisms of saponin in Fenugreek effect on ameliorating dyslipidemia are probably related to accelerated cholesterol metabolism, inhibited cholesterol synthesis, and facilitated reverse cholesterol transport, but not cholesterol absorption.
Keywords: Fenugreek, Saponins, Dyslipidemias, Cholesterol
What’s Known
Trigonella foenum-graecum seeds can ameliorate dyslipidemia, and saponins may constitute one of the most effective ingredients.
Mechanism may be related to accelerating cholesterol and bile acid efflux and suppressing the expression of SREBP-1 in the liver.
What’s New
Mechanisms of dyslipidemia-ameliorating effects of saponins from Trigonella foenum-graecum seeds were firstly explicated through accelerating cholesterol metabolism and reverse cholesterol transport and inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase in both serum and liver.
Introduction
Trigonella foenum-graecum, also called fenugreek, has been widely cultivated in the Mediterranean region and Asia for manufacturing spices since ancient times.1 Its crude extract has been reported to prevent hepatotoxicity2 and diabetic3,4 successfully. To evaluate the effects of Trigonella foenum-graecum seeds (TFGs) on cardiovascular disease, studies on the effect of TFGs on dyslipidemia are still to proceed. Etsuko et al. reported TFGs aqueous extract was effective in lowering serum lipid in rats fed with high-fat-diet and accelerated the cholesterol and bile acid efflux.5 Kumar et al. found that after treating TFGs aqueous extract on high-fat-diet rats, several antioxidant enzymes were elevated and fatty acid synthetase was stored at the normal level. They believe that TFGs modulated dyslipidemia through inhibition of impaired lipid digestion and absorption.6 Vijayakumar et al. analyzed lowered sterol regulatory element-binding protein-1 (SREBP-1) and elevated low-density lipoprotein (LDL) receptor after treated with aqueous extract in rats fed with high-fat-diet, inferred TFGs had a potential effect on dyslipidemia due to inhibition for lipid accumulation.7 Taku et al. prepared saponin fraction from TFGs and treated on rats fed with high-fat-diet. They observed lowered serum triglyceride (TG) level. Hence, they proved that diosgenin was the main effective saponin on inhibiting liver X receptor-alpha and SREBP-1c levels in HepG2 cells.8
Saponin in TFGs is a promising candidate for ameliorating dyslipidemia. However, most relevant studies on dyslipidemia-modulating activities of TFGs were using aqueous extract. Specific studies about saponin fraction are still required to illustrate its lipid-lowering activities. On the other hand, the definite mechanisms linking saponin to modulating dyslipidemia are not fully understood yet.
In the present study, we prepared high purity saponin extract from TFGs and designed a systematic mechanism study in vivo. We considered four aspects of the mechanisms of dyslipidemia-modulating effects. Firstly, cholesterol absorption from daily diet is mainly depended on Niemann-Pick C1-Like 1 (NPC1L1) and ATP-binding cassette transporters G5/G8 (ABCG5/G8) in the intestine. As a pair of opposing gatekeeper, NPC1L1 and ABCG5/ABCG8 control cholesterol intake and outflow in the small intestine, respectively.9 Secondly, extrahepatic cholesterol returns to the liver for excretion to the bile and ultimately to the feces, which is known as the process of reverse cholesterol transport (RCT). The RCT process is mainly performed by two crucial cellular factors, transporter (ATP-binding cassette transporter member A1 (ABCA1) and receptor (scavenger receptor class B type I (SR-BI)). ABCA1 is abundantly expressed in both the liver and peripheral macrophages among other tissues.10,11 Cellular cholesterol and phospholipids can be efflux and transformed into high-density lipoprotein (HDL), mediated by ABCA1. Thus, HDL finally combines mainly with SR-BI, a high specific receptor of HDL.11 Thirdly, serum lipid is mainly from synthesis of cholesterol and triglyceride in vivo are corresponding to 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMG-CoAR) and sterol regulatory element-binding proteins-1c (SREBP-1c) expressions, respectively.8,12 Finally, cholesterol is transformed into bile acid and efflux outside of the body, which is also an important cholesterol-eliminating way and the transformation is mainly controlled by cholesterol 7alpha-hydroxylase (CYP7A1).13
With great appreciation of fundamental contributions by other researchers, we further extracted and purified saponin from TFGs, investigated its dyslipidemia-modulating activities in obese rats’ models, and systematically illustrated their lipid-lowering mechanisms.
Materials and Methods
Extraction and Purification of Saponin from TFGs
TFGs were provided by Bozhou-ShuZhong Chinese herbal medicine industry Ltd., China. Diosgenin was extracted and purified as reported previously.14 All steps were completed at the Key Lab of Hui Ethnic Medicine Modernization, Ministry of Education (Ningxia, China). Briefly, TFGs were extracted by 75% (v/v) ethanol with material-liquid ratio of 1:12 (w/v) three times (1.5, 1, and 0.5 hours, respectively) at 75 ºC. The liquid extract was then concentrated by vacuum distillation and freeze-dried ground. The powdered extract was then dealt with different solvents systematically, including n-hexane, chloroform, ethyl acetate, and n-buthanol. Chloroform fraction was dried and further purified through HPD-400 macroreticular resins. The purity of saponin in the final dry product was determined by ultraviolet spectroscopy and reached 80.50%. Hence, the final product was used in the present study.
Animals
The present study was conducted in conformity with the PHS policy on Use of Laboratory Animals and WMA Declaration of Helsinki, as well as approval and authorization by the Animal Ethical Committee of Ningxia Medical University (Ningxia, China). Four-week-old male Sprague-Dawley rats of specific pathogen-free level were recruited from the Laboratory Animal Center of Ningxia Medical University (License number: SCXY (Ning) 2005-0001). All rats were housed in a temperature-controlled room (22±1 ºC) with a 12-h light cycle (6 am to 6 pm) and 12-h dark cycle (6 pm to 6 am) and kept three per cage.
After 1-week adapting period, 40 male rats were given high-fat-diet (HFD) as the model group, and 8 other rats were given a normal diet (ND) every day as the control group. Both groups were fed with 18.5 g HFD or ND (D12451 and GB13078, respectively) (Beijing Keaoxieli Ltd., China) on a daily basis. After 4 weeks, TC and TG levels of blood from the eye fundus were tested. Then, the model group was divided into five subgroups and treated in different conditions daily; the HFD group (HFD plus normal saline (NS)), Lipitor group (HFD with 10 mg/kg Lipitor, oral administration), and L-saponin, M-saponin, and H-saponin groups (oral administration, HFD with 6, 12, and 24 mg/kg saponin, respectively). Initially, the groups were almost of identical average weight and activity. The control group started to be irrigated with normal saline. All rats were further treated for 9 weeks and the dosages of Lipitor or saponin was weekly adjusted according to individual weight. In the 9th week, feces of rats in each group were collected and kept in -20 ºC for further study. At the end of the experiment, after fasting for 24 h, all rats were anesthetized with chloral hydrate injection in the abdomen cavity and sacrificed. Livers and intestines were separated, immediately froze by liquid nitrogen and kept in -70ºC for further measurement.
Morphological Observation
Food consumption was monitored every day. The weight of all rats was determined every 7 days. Liver weights were measured as soon as they were separated out and the liver index was used as a morphological parameter.
Liver index=(liver weight/body weight)×100%
Blood Sample Assays
Blood from femoral artery was collected and serum was separated by centrifugation for 15 minutes at 4,000 rpm and stored at -20 ºC. Serum malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and HMG-COAR levels were determined by each kit. All procedures were performed according to the manufacturers’ instructions. TC, TG, LDL, HDL, glutamic-pyruvic transaminase (ALT), and glutamic oxalacetic transaminase (AST) levels were determined at the Biochemistry Study Institution, Affiliated Hospital of Ningxia Medical University (Ningxia, China). Total cholesterol (TC), total glyceride (TG), SOD, and MDA kits were from Nanjing Jiancheng Bioengineering Research Institute, China. HMG-CoA reductase kit was procured from Beijing Chenglin Biotechnology Ltd., China. GSH-PX kit was obtained from Beijing Yonghui Biotechnology Ltd., China.
Feces Assays
Before the end of treatment, feces in each cage were collected for seven days. Cholesterol and bile acid were extracted according to Carr’s method.15 Briefly, after lyophilization, 5 g feces was added into 100 mL mixture of 66.7 mL chloroform and 33.3 mL methanol, shook thoroughly for 3 minutes and filtrated through degreased filter paper. The filtrated mixture was then mixed with 20 mL 0.05% sulfuric acid in a separating funnel for 20 minutes and the lower layer was collected. One-milliliter liquid in lower layer was mixed with 1 mL 1% TritonX-100 and then dried at 45ºC. Total cholesterol (TC) and total bile acid (TBA) levels were tested by each kit (Nanjing Jiancheng Bioengineering Research Institute, China). All procedures were performed according to the manufacturer’s instructions. The output of cholesterol and bile acid was expressed as mmol cholesterol (or bile acid) per gram feces.
Histopathological Assay
Hepatic lobule from fresh liver was taken and fabricated into histopathological sections through hematoxylin-eosin staining. It was then observed by the Pathodology Department, Affiliated Hospital of Ningxia Medical University (Ningxia, China).
Western Blotting
About 50 μg frozen intestine was ground with liquid nitrogen and the total protein was extracted by a protein extracting kit (KeyGEN Whole Cell Lysis Assay, KeyGEN BioTECH Ltd. China.) according to the manufacturer’s description. The supernatant was collected by centrifugation for 5 minutes at 10,000 rpm. After quantification using a protein quantification kit (KeyGEN BCA Protein Quantitation Assay, KeyGEN BioTECH Ltd. China) according to the manufacturer’s description, the protein of each group was diluted into almost the same concentration (5 μg/μL). Total protein (~ 80 μg) from each specimen was loaded onto 8% SDS-PAGE. After electrophoresis at 80V~ 120V for 80 minutes, fractionated proteins were transferred to pure nitrocellulose blotting membrane (Pall Gelman Laboratory, USA) at 200 mA for 2 hours. Membranes were cut into strips and blocked by 5% skim milk for 1 hour before incubating with Anti-ABCG8 antibody (sc-30111, Santa Cruz Ltd.), Anti-NPC1L1 antibody (TA309769, Origene Ltd.), and mouse anti-beta actin antibody monoclonal antibody (ZSGB-BIO Ltd.) at 4 °C overnight. The strips were subjected to 10 minutes washing 3 times with PBS containing 0.05% Tween 20. This procedure was followed by incubation with peroxidase-conjugated affinipure goat anti-rabbit IgG (H+L) or peroxidase-conjugated affinipure goat anti-mouse IgG (H+L) (ZSGB-BIO Ltd.) for 2 hours, and by 5 minutes washing 6 times. The strips were immersed in Lu-Mino substrate buffer and gently shaken. After exposure and fixing, protein bands were semi-quantitatively analyzed by the QuantityOne software.
Quantitative Real-Time RT-PCR
RNA was prepared from rats’ tissues using a total RNA isolation kit (AxyPrep Multisource Total RNA Miniprep Kit, Corning Incorporated (Wujiang), China.) according to the manufacturer’s instructions. Total RNA was reverse transcribed using a total RNA reverse transcription kit (One-Step gDNA Removal and cDNA Synthesis SuperMix, Beijing TransGen Biotech Co., Ltd., China). The cDNA was used as a template for quantitative real-time RT-PCR using Tip Green qPCR SuperMix. To select the optimal primer and annealing temperature for each gene, three pairs of primers were designed. Before qRT-PCR, cDNA templates were dealt with amplification in gradient temperature with each primer and the optimal conditions were selected after electrophoresis and were further validated by analyzing amplification curve and melt curve. The selected optimal primers are shown in table 1. The relative mRNA expression of each group versus HFD group is expressed with 2-ΔΔCt after qRT-PCR.16
Table 1.
Designed primers of each gene for qRT-PCR. Optimized primers and annealing temperatures for each gene with different product size
| Gene | Primer (5’-3’) | Annealing temperature (°C) | Product size (bp) | |
|---|---|---|---|---|
| HMG-CoAR | Forward | AGGTCATGGCTGAGGTGAAC | 60 | 103 |
| Reverse | TCCATCTCTTGGCTGCTCTC | |||
| CYP7A1 | Forward | CTGGCTCAACTTCCAAGGAG | 60 | 122 |
| Reverse | GTGCGATCTTCCCATTCAGT | |||
| SREBP-1c | Forward | TTTATCTGGGTAGGCGGATG | 59 | 105 |
| Reverse | CAGAAGCCAGCAAACACTTG | |||
| ABCA1 | Forward | TCAGCCTACAGAGCCAGTGA | 59 | 106 |
| Reverse | CCAAAGGAGCCCATAAATGA | |||
| ABCG8 | Forward | CCTTCGTTTCCAGCCAGAC | 60 | 100 |
| Reverse | CTTGTCCTCCATCATCACTGC | |||
| SR-BI | Forward | GGGAGTGCCATTTACTTGGA | 60 | 118 |
| Reverse | CCTCCTTAGCTGTGCGGATA | |||
| β-actin | Forward | CCCATCTATGAGGGTTACGC | 60 | 150 |
| Reverse | TTTAATGTCACGCACGATTTC | |||
Statistics Analyses
All analyses were performed using the SPSS software version 11.5 (SPSS, Chicago, IL, USA). One-way ANOVA test was used to determine statistically significant differences between the HFD and other groups. P values less than 0.05 were considered significant and P values less than 0.01 were considered extremely significant.
Results
Establishment of Dyslipidemia Rat Model
After one-month HFD feeding, TC and TG in serum were observed slightly and significantly higher in the HFD group than the control group, respectively (1.61, 0.69 mmol/L vs. 1.45, 0.46 mmol/L, P=0.490 and 0.023, data not shown).
Biochemical Characteristics of Rats
Dyslipidemia rat model was divided into different groups as described above. After being sacrificed, the total body weight, gained weight after treating, liver index, serum lipid levels, ALT and AST levels, malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) levels, HMG-CoAR levels in serum, cholesterol (TC) and total bile acid (TBA) levels in feces were tested (table 2).
Table 2.
Biochemical characteristics of rats according to different groups. Data are reported as mean±SD of eight rats carried out in duplicate
| Groups | Control | HFD | Lipitor | L-saponin | M-saponin | H-saponin |
|---|---|---|---|---|---|---|
| Liver index | 2.03%±0.06%* | 2.18%±0.13% | 2.06%±0.18%* | 2.07%±0.09% | 2.07%±0.09% | 2.06%±0.07%* |
| Final weight (g) | 450±43** | 576±37 | 532±77 | 568±65 | 579±56 | 581±68 |
| Gained weight (g) | 77±19* | 113±28 | 93±45 | 123±32 | 128±25 | 127±58 |
| TC (mmol/L) | 1.449±0.071** | 1.831±0.176 | 1.364±0.180** | 1.506±0.150** | 1.499±0.299** | 1.528±0.171** |
| TG (mmol/L) | 0.455±0.040** | 0.755±0.240 | 0.576±0.192** | 0.616±0.063** | 0.573±0.155** | 0.581±0.090** |
| LDL (mmol/L) | 0.150±0.022** | 0.288±0.055 | 0.186±0.039** | 0.249±0.047 | 0.254±0.058 | 0.213±0.047** |
| HDL (mmol/L) | 1.430±0.126** | 0.934±0.300 | 1.159±0.103* | 1.009±0.178 | 1.195±0.274** | 1.186±0.108** |
| ALT (U/L) | 35.525±4.362** | 73.850±28.292 | 46.263±6.901** | 54.088±14.198** | 38.788±16.593** | 36.063±8.772** |
| AST (U/L) | 88.925±20.541 | 109.150±16.449 | 107.438±24.674 | 90.150±10.144 | 97.113±21.672 | 100.925±16.595 |
| MDA (nmol/L) | 1.600±0.289 | 2.500±0.367 | 2.266±1.004 | 2.414±0.512 | 2.460±0.301 | 2.400±1.065 |
| SOD (U/L) | 243.939±14.979* | 222.479±11.844 | 232.619±17.457 | 230.798±23.572 | 247.445±27.572** | 241.753±22.158* |
| GSH-PX (mmol/L) | 20.111±0.008 | 20.080±0.073 | 20.110±0.005 | 20.020±0.135 | 20.114±0.007 | 20.093±0.046 |
| HMG-COAR (U/L) | 19.219±0.335 | 19.007±0.501 | 18.854±0.445 | 18.306±0.261** | 17.628±0.540** | 17.698±0.826** |
| TC in feces (μmol/g) | 1.412±0.090* | 1.206±0.042 | 0.993±0.117* | 1.113±0.103 | 1.193±0.099 | 1.173±0.181 |
| TBA in feces (μmol/g) | 2.380±0.338** | 3.586±0.259 | 3.422±0.230 | 3.679±0.118 | 4.333±0.205** | 5.253±0.096** |
P values are obtained from the independent sample t-test between two groups (HFD group and another group).
P<0.05: Indicate significant difference from the HFD group;
P<0.01: Indicate extremely significant difference from the HFD group; ALT: Glutamic-pyruvic transaminase; AST: Aspartate transaminase; GSH-PX: Glutathione peroxidase; HFD: High-fat-diet; HMG-CoAR: 3-hydroxy-3-methyl glutaryl coenzyme A reductase; MDA: Malondialdehyde; TBA: Total bile acid; TC: Total cholesterol; TG: Triglyceride
Histopathological Sections of the Liver Tissue
Figure 1 shows the histopathological morphology of the liver tissue of each group after stained with H&E. Restored lipid areas in the liver tissue were observed in saponin groups and showed a dose-dependent tendency.
Figure 1.
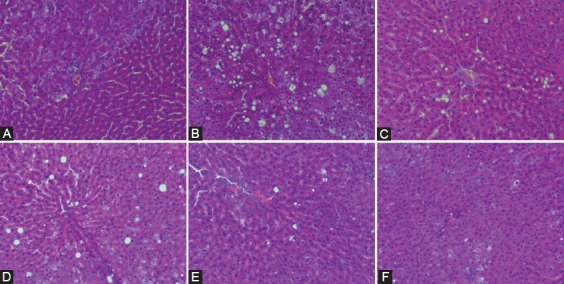
Histopathological analysis image of liver tissue sections observed through optical microscope (H&E, 400×). A: Control group; B: HFD group; C: Lipitor group; D: L-saponin group; E: M-saponin group; F: H-saponin group
Protein and mRNA Expression of Related Factors of Cholesterol
Figure 2 shows NPC1L1 and ABCG8 protein expression in the intestine detected by Western blot method as described. Figure 3 shows CYP7A1, ABCG8, NPC1L1, HMG-CoAR, SREBP-1c, ABCA1, and SR-BI mRNA levels in the liver determined by qRT-PCR.
Figure 2.
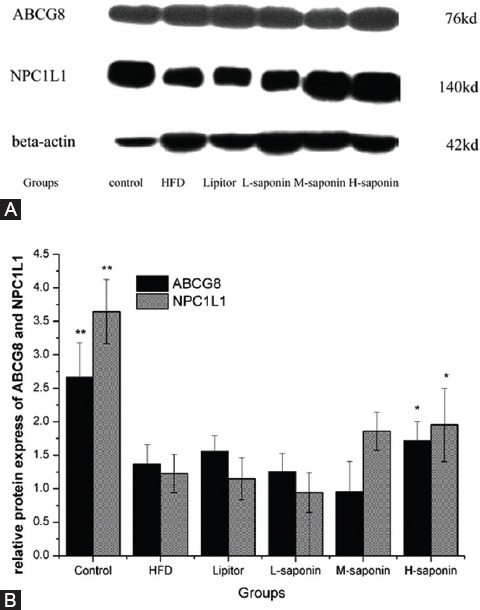
ABCG8 and NPC1L1 protein expression in intestine. (2A) The protein bands of ABCG8, NPC1L1 and beta-actin. (2B) The data of the protein bands semi-quantitatively analyzed by QuantityOne. Data are reported as mean±SD of six rats carried out in duplicate. P values are obtained from the independent sample t-test between two groups (HFD group and another group). *P<0.05: Indicate significant difference from the HFD group; **P<0.01: Indicate extremely significant difference from the HFD group; ABCG8: ATP-binding cassette transporter G8, NPC1L1: Niemann-Pick C1-Like 1
Figure 3.
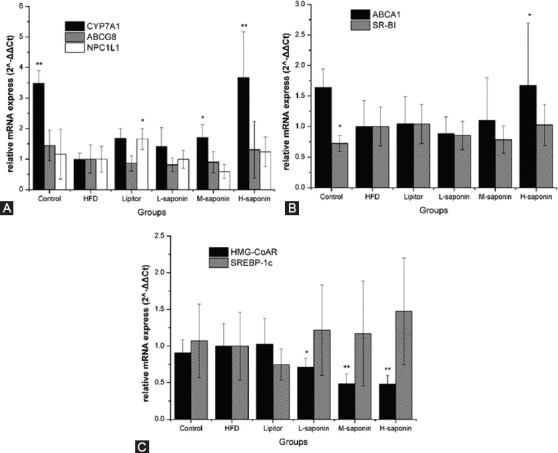
The CYP7A1, ABCG8, NPC1L1, HMG-CoAR, SREBP-1c, ABCA1, and SR-BI mRNA expression in liver. Data are reported as mean±SD of eight rats carried out in duplicate. P values are obtained from the independent sample t-test between two groups (HFD group and another group). *P<0.05: Indicate significant difference from the HFD group; **P<0.01: Indicate extremely significant difference from the HFD group; ABCA1: ATP-binding cassette transporter A1; ABCG8: ATP-binding cassette transporter G8; CYP7A1: Cholesterol 7alpha-hydroxylase; HMG-CoAR: 3-hydroxy-3-methyl glutaryl coenzyme A reductase; NPC1L1: Niemann-Pick C1-Like 1; SR-BI: Scavenger receptor class B type I; SREBP-1: Sterol regulatory element-binding protein-1
Discussion
Saponin is the main effective ingredient from fenugreek on modulating dyslipidemia.8,17,18 In this article, we further confirmed the effectiveness of fenugreek saponin on dyslipidemia and explored possible mechanisms. Saponin was effective in modulating serum lipid, although not apparently influenced the weight in rats fed with HFD. There was no significant difference in the food intake and water intake during the experiment. Saponin could not successfully decrease the abnormal weight of rats fed with HFD, but it modulated serum lipid levels by decreasing TC, TG, LDL levels and elevated HDL levels (table 2). Consistent with the present study, the extract of TFGs is reported to decrease serum lipid in diabetic rabbits,19 patients,18 and rats.20 Different from other studies on water extract of trigonella foenum-graecum seeds (e.g. Mohammadi et al. and Kumar et al.21-23), the saponin extract made by our craft influenced the body weight of rats fed with high-fat-diet. Punna Ramulu et al.24 found that the percent gain in body weight was significantly lowered after treated with soluble dietary fiber isolated from fenugreek seeds. Perhaps, purified saponin ingredient that contains very little soluble dietary fiber has little impact on the weight gain of the rats in our study. Considering lowered liver index in the saponin groups, we presumed that saponin could also restore liver tissue of rats fed with HFD.
Saponin also restored liver tissue and elevated anti-peroxidation activities in vivo. Rats of dyslipidemia were also benefited from fanugreek saponin in two auxiliary aspects; restored liver tissue and elevated anti-peroxidation activities. Saponin lowered abnormal serum ALT levels (table 2) and eliminated lipid areas in the liver tissue (figure 1) of rats fed with HFD. This finding was consistent with those by Parveen Kumar et al.6 On the other hand, accompanying dyslipidemia, peroxidation probably induces atherosclerosis which endangers the cardiovascular system.25 Elevated MDA level and lowered SOD level indicated that HFD might aggravate dyslipidemia by inducing peroxidation. Saponin treatment could obviously elevate serum SOD levels in rats fed with HFD. Restored SOD levels in serum can be found after healing with saponin (table 2). This result can also be supported by the study of Belguith-Hadriche et al. that used ethyl acetate extract of fenugreek25 and studies on diosgenin, the main saponin in fenugreek.26,27
Saponin might not eliminate serum lipid through reducing cholesterol absorption in the intestine. Based on the above results, mechanisms and pathways of lipid-lowering effect were further studied. With respect to cholesterol absorption and efflux, previous reports5,28 have shown that water extracts of TFGs could accelerate the outputting of TC and TBA into feces. In our study, saponin dose-dependently increased TBA level but showed no obvious influences on the TC level in feces (table 2). This is possibly due to using saponin ingredient, but not the whole water extract. Being a pair of gate-keeper, which control cholesterol intake and efflux of intestinal cells, NPC1L1 and ABCG89 were employed to explain the performance of TC and TBA efflux. The Western blot results showed saponin treatment induced higher NPC1L1 protein express and no obvious change on ABCG8 (figure 2). This means that cholesterol absorption in the intestine was facilitated without influencing its excretion. Tang et al.29 reported that the final excretion of cholesterol was strongly correlated with the expression of NPC1L1 in the intestine. In the present study, no change of excretion of cholesterol was observed, although the expression of NPC1L1 in the intestine was elevated. Where did the absorbed cholesterol go and why the excretion of total bile acid increased? In this regard, we further studied the effect of cholesterol metabolism.
Saponin probably accelerates TBA efflux through facilitating cholesterol metabolism involve with RCT. Considering the above observation, we presumed that excess TBA in feces correlated with cholesterol metabolism. To confirm the up-regulatory activity of saponin for cholesterol metabolism, we tested the CYP7A1, ABCG8, and NPC1L1 levels in the liver by qRT-PCR. CYP7A1 is the rate-limiting enzyme in the bile acid biosynthetic pathway in the liver and thus controls cholesterol and bile acid homeostasis. ABCG8 and NPC1L1 influence the rate of cholesterol metabolism by accelerating and inhibiting, respectively.9,13 Under the dual function of elevated NPC1L1 in the intestine and CYP7A1 in the liver (figures 2 and 3A), both the cholesterol absorption and metabolism were simultaneously facilitated. That explained why TBA level in feces was obviously elevated, whereas TC level had no significant change.
Besides, RCT can also facilitate cholesterol metabolism and it is the most important way to eliminate peripheral cholesterol.10 This process involves two main factors, namely ABCA1 and SR-BI. ABCA1 mRNA expression in the liver was dose-dependently increased in saponin-treated groups (figure 3B), which is probably the main reason for elevated HDL level in the serum of saponin groups (table 2). However, there were no significant changes in SR-BI mRNA expression. These results revealed that high-dose saponin might partly facilitate RCT process by escalating ABCA1 mRNA expression. As a result, excess TBA levels in feces of rats treated with saponin might mainly be a response to cholesterol metabolism.
Diongenin could also inhibit cholesterol synthesis in vivo. Saponin also contributes to modulate serum lipid through inhibiting cholesterol synthesis in vivo, but it might induce excess triglyceride synthesis in high dosage. The synthesis processes of cholesterol and triglyceride, as the main sources of serum TC and TG, are characterized by HMG-CoAR and SREBP-1c, respectively. HMG-CoAR activity in serum (table 2) and mRNA expression in the liver (figure 3C) were decreased for all saponin-treated groups. This result is also supported by a study focused on HMG-CoAR inhibiting effect of diosgenin by Hao et al.30 However, SREBP-1c mRNA expression in the liver elevated when high-dose saponin was treated (figure 3C). This was in contrast to a similar study that fed KK-Ay mice with the crude extract of TFGs and treated HepG2 cells with diogenin.8 Perhaps such discrepancy is due to different ingredients used. Hence, fenugreek saponin can suppress cholesterol synthesis, but it might accelerate the synthesis of triglyceride under high-dose treatment.
Conclusion
For the first time, the present study demonstrates that saponin from TFGs can effectively accelerate cholesterol metabolism and RCT process, meanwhile inhibit cholesterol synthesis. This multi-pathway action of fanugreek saponin on ameliorating dyslipidemia is preferable in preventing cardiovascular disease effectively.
Acknowledgement
The authors would like to thank Pathodology Department and Biochemistry Study Institution (Affiliated Hospital of Ningxia Medical University) for supporting the present study. This research was also supported by grants from the Animal Ethical Committee of Ningxia Medical University and National Science Foundations of China. Zhi Chen and Yan-li Lei contributed equally to this work.
Conflict of Interest: None declared.
References
- 1.Mehrafarin A, Rezazadeh Sh, Naghdi Badi H, Noormohammadi Gh, Zand E, Qaderi A. A Review on Biology, Cultivation and Biotechnology of Fenugreek (Trigonella foenum-graecum L.) as a Valuable Medicinal Plant and Multipurpose. Journal of Medicinal Plants. 2011;10:6–24. doi: 10.4172/2157-7110.1000181. [DOI] [Google Scholar]
- 2.Sushma N, Devasena T. Aqueous extract of Trigonella foenum graecum (fenugreek) prevents cypermethrin-induced hepatotoxicity and nephrotoxicity. Hum Exp Toxicol. 2010;29:311–9. doi: 10.1177/0960327110361502. [DOI] [PubMed] [Google Scholar]
- 3.Haeri MR, Limaki HK, White CJ, White KN. Non-insulin dependent anti-diabetic activity of (2S, 3R, 4S) 4-hydroxyisoleucine of fenugreek (Trigonella foenum graecum) in streptozotocin-induced type I diabetic rats. Phytomedicine. 2012;19:571–4. doi: 10.1016/j.phymed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Hamza N, Berke B, Cheze C, Le Garrec R, Umar A, Agli AN, et al. Preventive and curative effect of Trigonella foenum-graecum L. seeds in C57BL/6J models of type 2 diabetes induced by high-fat diet. J Ethnopharmacol. 2012;142:516–22. doi: 10.1016/j.jep.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Muraki E, Matsuoka C, Oikawa R, Sato S, Chiba H, Tsunoda N, et al. Fenugreek attenuates lipid accumulation in normal rats. J Jpn Nutr Food Sci. 2011;64:99–106. doi: 10.4327/jsnfs.64.99. [DOI] [Google Scholar]
- 6.Kumar P, Bhandari U, Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed Res Int. 2014;2014:606021. doi: 10.1155/2014/606021. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayakumar MV, Pandey V, Mishra GC, Bhat MK. Hypolipidemic effect of fenugreek seeds is mediated through inhibition of fat accumulation and upregulation of LDL receptor. Obesity (Silver Spring) 2010;18:667–74. doi: 10.1038/oby.2009.337. [DOI] [PubMed] [Google Scholar]
- 8.Uemura T, Goto T, Kang MS, Mizoguchi N, Hirai S, Lee JY, et al. Diosgenin, the main aglycon of fenugreek, inhibits LXRalpha activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr. 2011;141:17–23. doi: 10.3945/jn.110.125591. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM, Yu L. Opposing Gatekeepers of Apical Sterol Transport: Niemann-Pick C1-Like 1 (NPC1L1) and ATP-Binding Cassette Transporters G5 and G8 (ABCG5/ABCG8) Immunol Endocr Metab Agents Med Chem. 2009;9:18–29. doi: 10.2174/187152209788009797. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, et al. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–47. doi: 10.1194/jlr.M044198. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sporstol M, Mousavi SA, Eskild W, Roos N, Berg T. ABCA1, ABCG1 and SR-BI: hormonal regulation in primary rat hepatocytes and human cell lines. BMC Mol Biol. 2007;8:5. doi: 10.1186/1471-2199-8-5. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canovas A, Quintanilla R, Gallardo D, Diaz I, Noguera JL, Ramirez O, et al. Functional and association studies on the pig HMGCR gene, a cholesterol-synthesis limiting enzyme. Animal. 2010;4:224–33. doi: 10.1017/S1751731109991145. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, et al. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. doi: 10.1002/hep.24107. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hei J, Chen Z, Lei Y, Wang W, Sui H. Investigation of Extraction and Purification Process for Total Saponins in Trigonellae Semen. Chinese Journal of Experimental Traditional Medical Formulae. 2014;20:11–3. [Google Scholar]
- 15.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 16.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller S, Stephens JM. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: mechanisms of actions and potential effects on metabolic syndrome. Adv Nutr. 2015;6:189–97. doi: 10.3945/an.114.007807. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma N, Usman K, Patel N, Jain A, Dhakre S, Swaroop A, et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro) in patients with type 2 diabetes. Food Nutr Res. 2016;60:32382. doi: 10.3402/fnr.v60.32382. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puri D, Prabhu KM, Dev G, Agarwal S, Murthy PS. Mechanism of Antidiabetic Action of Compound GII Purified from Fenugreek (Trigonella foenum graecum) Seeds. Indian J Clin Biochem. 2011;26:335–46. doi: 10.1007/s12291-011-0150-2. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haritha C, Reddy AG, Reddy YR, Anilkumar B. Pharmacodynamic interaction of fenugreek, insulin and glimepiride on sero-biochemical parameters in diabetic Sprague-Dawley rats. Vet World. 2015;8:656–63. doi: 10.14202/vetworld.2015.656-663. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi A, Gholamhosseinian A, Fallah H. Trigonella foenum-graecum water extract improves insulin sensitivity and stimulates PPAR and gamma gene expression in high fructose-fed insulin-resistant rats. Adv Biomed Res. 2016;5:54. doi: 10.4103/2277-9175.178799. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P, Kale RK, McLean P, Baquer NZ. Antidiabetic and neuroprotective effects of Trigonella foenum-graecum seed powder in diabetic rat brain. Prague Med Rep. 2012;113:33–43. doi: 10.14712/23362936.2015.35. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Kale RK, Baquer NZ. Antihyperglycemic and protective effects of Trigonella foenum graecum seed powder on biochemical alterations in alloxan diabetic rats. Eur Rev Med Pharmacol Sci. 2012;16(Suppl3):18–27. [PubMed] [Google Scholar]
- 24.Ramulu P, Giridharan NV, Udayasekhararao P. Hypolipidemic effect of soluble dietary fiber (galactomannan) isolated from fenugreek seeds in WNIN (GR-Ob) obese rats. J Med Plant Res. 2011;5:4804–13. [Google Scholar]
- 25.Belguith-Hadriche O, Bouaziz M, Jamoussi K, El Feki A, Sayadi S, Makni-Ayedi F. Lipid-lowering and antioxidant effects of an ethyl acetate extract of fenugreek seeds in high-cholesterol-fed rats. J Agric Food Chem. 2010;58:2116–22. doi: 10.1021/jf903186w. [DOI] [PubMed] [Google Scholar]
- 26.Kalailingam P, Kannaian B, Tamilmani E, Kaliaperumal R. Efficacy of natural diosgenin on cardiovascular risk, insulin secretion, and beta cells in streptozotocin (STZ)-induced diabetic rats. Phytomedicine. 2014;21:1154–61. doi: 10.1016/j.phymed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Son IS, Kim JH, Sohn HY, Son KH, Kim JS, Kwon CS. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci Biotechnol Biochem. 2007;71:3063–71. doi: 10.1271/bbb.70472. [DOI] [PubMed] [Google Scholar]
- 28.Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Nagar BJ, Naikwadi NN, et al. Galactomannan: a versatile biodegradable seed polysaccharide. Int J Biol Macromol. 2013;60:83–92. doi: 10.1016/j.ijbiomac.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Jia L, Ma Y, Xie P, Haywood J, Dawson PA, et al. Ezetimibe restores biliary cholesterol excretion in mice expressing Niemann-Pick C1-Like 1 only in liver. Biochim Biophys Acta. 2011;1811:549–55. doi: 10.1016/j.bbalip.2011.05.013. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao S, Xu R, Li D, Zhu Z, Wang T, Liu K. Attenuation of Streptozotocin-Induced Lipid Profile Anomalies in the Heart, Brain, and mRNA Expression of HMG-CoA Reductase by Diosgenin in Rats. Cell Biochem Biophys. 2015;72:741–9. doi: 10.1007/s12013-015-0525-8. [DOI] [PubMed] [Google Scholar]


