Abstract
Background:
Deltamethrin (DM) is a synthetic pyrethroid insecticide which can lead to pathological effects in mammals through oxidative stress. On the other hand, virgin olive oil (VOO) is a rich source of phenolic compounds with antioxidants. The aim of the present study was to determine the protective effects of VOO against DM-induced hepatotoxicity.
Methods:
Thirty-six mice were randomly separated into 4 groups: vehicle group, VOO group, DM group, and DM plus VOO group. Immunohistochemistry of PARP, COX-2, and caspase-3 with the biochemical analysis of malondialdehyde and total antioxidant capacity levels were performed in the liver samples 5 weeks after gavaging. Statistical analysis was performed using SPSS, version 15. The data were compared between the groups using the Tukey multiple comparison tests and the analysis of the variance. A P value <0.05 was considered significant.
Results:
The malondialdehyde level in the liver was increased in the DM group (71.18±0.01), whereas it was significantly (P=0.001) decreased after VOO administration in the DM plus VOO group (39.59±2.43). While the total antioxidant capacity level in the liver was decreased in the DM group (3.05±0.05), it was significantly increased (P=0.03) after VOO administration in the DM plus VOO group (3.95±0.04). A greater expression of caspase-3 (P=0.008), COX-2 (P =0.004), and PARP (P 0.006) could be detected in the DM group, while it was significantly (P=0.009) attenuated in the DM plus VOO group. Also, the degeneration of hepatocytes, which was detected in the DM group, was attenuated after VOO consumption.
Conclusions:
VOO exerted protective effects against DM-induced hepatotoxicity, which might be associated with its anti-apoptotic, anti-inflammatory, and antioxidative properties.
Keywords: Decamethrin, Olive oil, Antioxidants, Inflammation, Apoptosis, Hepatotoxicity
What’s Known
Deltamethrin is an insecticide used worldwide as a major class of insecticides in agriculture. Deltamethrin can elicit pathophysiological effects through oxidative stress in non-targeted organisms such as mammals.
It is essential that antioxidant supplements be used to reduce the side effects. There is accumulating evidence that virgin olive oil, a rich source of polyphenolic components, has antioxidant properties.
What’s New
Dietary virgin olive oil can significantly attenuate the indicators of the deltamethrin-induced toxicity. Thus, it can be recommended as a dietary supplement to reduce the side effects of synthetic pyrethroid insecticides.
Introduction
Relatively safe pyrethroid insecticides have been classified as type I and type II according to their chemical structure.1 Deltamethrin (DM), a type II synthetic pyrethroid insecticide with relatively low mammalian toxicity, is used worldwide in agriculture.2 It is well documented that DM is readily absorbed through contaminated food and water2 with high bioavailability in urine and feces.3 In this regard, research has documented that long-term exposure to DM among mammals has many side effects such as neurotoxicity,4 genotoxicity,5 hemolysis,6 reproductive damage,7 pulmonary disorders,8 and nephrotoxicity.9 Recently, it was also reported that exposure to DM could elicit histopathological changes in the liver.10-12 Several mechanisms account for DM toxicity such as the production of free radicals, induction of lipid peroxidation, disturbance of the total body’s antioxidant capacity, inflammation, and apoptosis.13,14 Therefore, it seems that the use of antioxidant supplements is essential to subside the side effects. Within the previous decades, a rapidly growing number of natural polyphenols, secondary metabolites of plants, with antioxidant and anti-apoptotic effects have been described. One of the main sources of these molecules is olive oil. Olive oil is a rich source of polyphenolic components, which have many beneficial health effects in humans.15 There is accumulating evidence attributing the beneficial effects of olive oil to a variety of biological activities such as free radical scavenging action, which is mediated by the chelation of metal ions and provision of the hydroxyl group for the quenching and neutralization of free radicals,16,17 anti-inflammatory potency, which is mediated by the attenuation of anti-inflammatory mediators, and anti-apoptotic property, which is mediated by the inhibition of the proapoptotic and induction of anti-apoptotic proteins.18,19 Meanwhile, total plasma antioxidant capacity increases after olive oil consumption.20
Accordingly, in the present study, we investigated the protective effects of virgin olive oil (VOO) consumption against DM-induced hepatotoxicity.
Materials and Methods
Animals
The present experimental study used 36 adult male mice (25±5.0 g) (Laboratory Animal Research Center, Sari, Iran). The mice were kept under constant conditions of temperature (23±1 ºC) and light/dark cycle (12 h/12 h) in the process of investigation. All the procedures were done according to the guidelines of the university’s animal care codes to minimize the animals’ suffering. The animals were fed a standard mouse chow and drinking water ad libitum throughout the study period. DM was purchased from Sigma-Aldrich (Germany) and VOO from Giah Essence Phytopharm Co. (Tehran, Iran).
The mice were randomly allocated to 4 equal groups, each containing 9 mice: I) the DM-treated group, which received 5 mg/kg/d of DM, diluted in dimethyl sulfoxide, for a period of 5 weeks by gavage;21 ΙΙ) the DM plus VOO-treated group, which received VOO (0.4 mL) for 5 weeks after 2 hours of DM gavage;22 ΙΙΙ) the VOO-treated group, which received VOO (0.4 mL) for 5 weeks by gavage; and ΙV) the vehicle group, which received dimethyl sulfoxide for 5 weeks by gavage. At the end of the 5th week, the animals’ livers were removed for immunohistochemical, biochemical, and histopathological assessments. The doses and treatment schedules were based on previous studies21,22 and pilot experiments in our laboratory.
Biochemistry
The obtained samples (sections of the right lobe of the liver) were thoroughly cleaned of blood and were then immediately frozen and stored in a -80 °C freezer for assays of tissue malondialdehyde (MDA) levels as a product of lipid peroxidation23 and total antioxidant capacity (TAC).24 The absorbance of the supernatant was measured by spectrophotometry. The data regarding MDA and TAC levels are expressed as micromoles per milligram of protein (µmol/mg-protein) and nanomoles Trolox equivalent per milligram of protein (nmol Trolox equivalent/mg-protein), respectively.
Histopathology
The obtained samples (3-mm thick sections of the left lobe of the liver)25 were collected, thoroughly cleaned of blood, and then were immediately fixed in 10% (w/v) PBS-buffered formaldehyde and embedded in paraffin. Five-micrometer serial sections were prepared from the paraffin-embedded blocks using a microtome. Some tissue sections were deparaffinized with xylene, stained with hematoxylin and eosin (H&E), and studied by light microscopy (DME; Leica Microsystems Inc., Buffalo, NY, USA) to assess the histopathological changes. All the histological studies were performed in a blinded fashion.
Immunohistochemistry
For immunohistochemistry, some sections were incubated in goat normal serum (in order to block the nonspecific site), and subsequently with anti-caspase 3 rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam, Cambridge, USA), anti-COX 2 rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam, Cambridge, USA), and anti-PARP rabbit polyclonal antibody (1:100 in PBS, v/v, Abcam, Cambridge, USA) overnight at 4 °C. The sections were washed with PBS and then incubated with secondary antibody conjugated with horseradish peroxidase (goat anti-rabbit IgG, Abcam, Cambridge, USA) for 2 hours and detected by diaminobenzidine tetrahydrochloride for 5 minutes. Afterward, they were dehydrated and mounted. For negative controls, primary antibodies were omitted. For quantitative analysis, immunohistochemical photographs (n=5 photos from each sample were collected from all the mice in each experimental group) were assessed by densitometry using MacBiophotonics ImageJ 1.41a software on an ASUS personal computer. The data are expressed as a percentage of the total tissue area.
Statistical Analysis
The statistical analyses were carried out in SPSS, version 15 (Chicago, IL, USA). The results are presented as means±SDs. The Kolmogorov–Smirnov test was used in order to evaluate the normality of the data. Also, the Tukey multiple comparison tests and the analysis of the variance were used to compare each 2 groups and the data between the groups, respectively. A P<0.05 was considered significant.
Results
Biochemical Analysis
The MDA levels for all groups at the end of the experiment are shown in table 1. The administration of DM in the DM group produced a significant elevation (P=0.003) in the lipid peroxidation level compared to the other groups. The MDA level in the DM plus VOO group was significantly (P=0.001) lower than that in the DM group, while the differences between the DM plus VOO group, vehicle group, and VOO group were not significant (P=0.31) (figure 1).
Table 1.
Effect of virgin olive oil on the biochemical markers of the liver affected by eltamethrin-induced hepatotoxicity
| Experimental groups | MDA | P value µmol/mg-protein | TAC nmol Trolox equivalent/mg-protein | P value |
|---|---|---|---|---|
| Vehicle | 38.94±0.01 | 3.74±0.05 | ||
| VOO | 39.24±0.26 | 4.08±0.28 | ||
| DM | 71.18±0.01 | 0.003 | 3.05±0.05 | 0.02 |
| DM+VOO | 39.59±2.43 | 0.001 | 3.95±0.04 | 0.01 |
DM: Deltamethrin; VOO: Virgin olive oil
Figure 1.
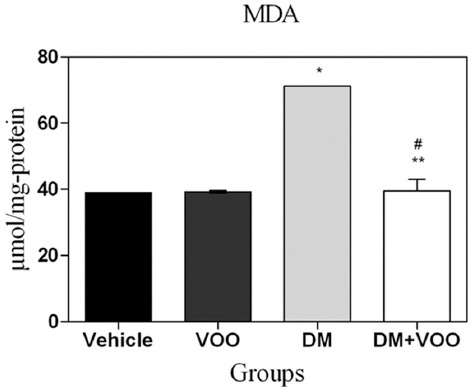
Histogram shows the levels of malondialdehyde (MDA) at the end of the experiment. Values are expressed as micromole per milligram protein. *P=0.001 versus the vehicle and VOO groups; **P=0.002 versus the DM group; #P=0.36 versus the vehicle and VOO groups.
The TAC levels for all the groups at the end of the experiment are shown in table 1. The administration of DM in the DM group produced a significant (P=0.02) decrease in the TAC level compared to the other groups. We found a significantly (P=0.01) increased level of TAC in the DM plus VOO group compared to the DM group, while the differences between the DM plus VOO group, vehicle group, and VOO group were not significant (P=0.45) (figure 2).
Figure 2.
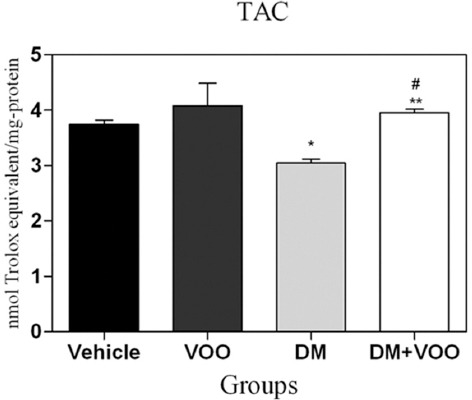
Histogram shows the levels of total antioxidant capacity (TAC) at the end of the experiment. Values are expressed as nanomoles Trolox equivalent per milligram protein. *P=0.003 versus the vehicle and VOO groups; **P=0.001 versus the DM group;#P=0.09 versus the vehicle and VOO groups.
Histopathologic Changes
The results of the histopathological examination are depicted in figure 3. Cytoplasmic hypereosinophilia, extensive nuclear pyknosis, and loss of intercellular borders in some of the hepatocytes were detected in the sections of the liver obtained from the DM group (figure 3A). Treatment with VOO reduced the changes, so that only focal nuclear pyknosis and cytoplasmic vacuolation were detected in the DM plus VOO group (figure 3B). No detectable injury was shown in the vehicle and VOO groups.
Figure 3.
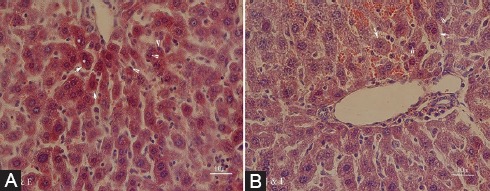
Photomicrographs of the hepatic section of the DM group (A) with cytoplasmic hypereosinophilia (arrows), extensive nuclear pyknosis (arrowheads), and loss of intercellular borders in some of the hepatocytes (asterisks), and the DM plus VOO group (B) with only focal nuclear pyknosis (arrowheads) and cytoplasmic vacuolation (arrows) (stained with hematoxylin and eosin, ×400).
Immunohistochemical Assessment
Figures 4, 5, and 6 show the immunohistochemical staining of caspase-3, COX-2, and PARP, respectively. The administration of DM in the DM group increased the expression of caspase-3 (figure 4A), COX-2 (figure 5A), and PARP (figure 6A), while VOO treatment in the DM plus VOO group reduced the degree of positive staining for caspase-3 (figure 4B), COX-2 (figure 5B), and PARP (figure 6B) compared to the DM group. The histograms of the quantitative analysis of caspase-3, COX-2, and PARP positive staining in the experimental groups are shown in figures 4C, 5C, and 6C, respectively.
Figure 4.
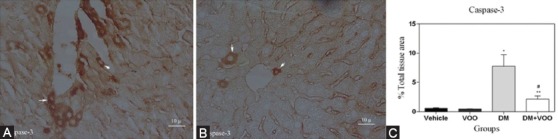
Light photomicrographs show the immunohistochemical expression of caspase-3 in the DM (A) and DM plus VOO (B) groups (magnification, ×400). The positive staining of caspase-3 is presented by a brown color of cytoplasm (arrows). Densitometry analysis of immunohistochemical photomicrographs for caspase-3 was conducted. Data are expressed as a percentage of the total tissue area (C). *P=0.01 versus the vehicle and VOO groups; **P=0.02 versus the DM group;#P=0.15 versus the vehicle and VOO groups.
Figure 5.
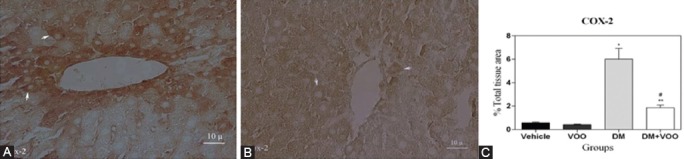
Light photomicrographs show the immunohistochemical expression of COX-2 in the DM (A) and DM plus VOO (B) groups (magnification, ×400). The positive staining of COX-2 is presented by a brown color of cytoplasm (arrows). Densitometry analysis of immunohistochemical photomicrographs for COX-2 was conducted. Data are expressed as a percentage of the total tissue area (C). *P=0.001 versus the vehicle and VOO groups; **P=0.004 versus the DM group;#P=0.09 versus the vehicle and VOO groups.
Figure 6.
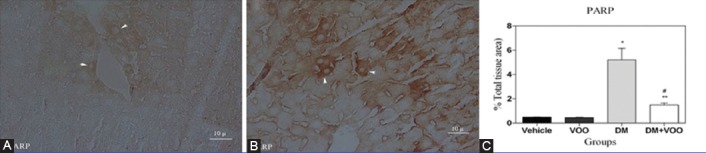
Light photomicrographs show the immunohistochemical expression of PARP in the DM (A) and DM plus VOO (B) groups (magnification, ×400). The positive staining of PARP is presented by a brown color of cytoplasm (arrows). Densitometry analysis of immunohistochemical photomicrographs for PARP was carried out. Data are expressed as a percentage of the total tissue area (C). *P=0.001 versus the vehicle and VOO groups; **P=0.008 versus the DM group; #P=0.08 versus the vehicle and VOO groups.
Discussion
The main findings of the current study showed that the administration of VOO attenuated (I) histopathological changes, (II) apoptosis, (III) inflammation, and (IV) lipid peroxidation, while it (IV) improved TAC in the liver against DM-induced hepatotoxicity.
Despite its low mammalian toxicity and worldwide use in agriculture, chronic exposure to DM exerts some undesirable effects on different organs, including the liver. Studies have shown that DM administration significantly increases the liver MDA content, as an indicator of lipid peroxidation, in rats compared with the control group.10,26,27 Lipid peroxidation is an important pathologic event, the breakdown of polyunsaturated fatty acids, which is induced by free radicals.28 Our results showed that elevated MDA levels were attenuated significantly after the administration of VOO in the treatment group. Olive oil contains a large number of molecules such as several different combinations of phenolic antioxidants, which are free radical scavengers that neutralize toxic species and sometimes even prevent the early stages of their formation.29,30 In this regard, studies have documented the protection of the liver cell membrane lipid peroxidation with olive oil after partial hepatectomy31 and in aging-related changes32 compared to control groups. Endogenous antioxidants prevent cellular oxidative damage caused by free radicals. Studies have shown that TAC in the liver tissues is significantly decreased after DM administration compared to control groups,26,27 meanwhile these studies have documented a significant decrease in the liver glutathione, catalase, and superoxide dismutase. Our results showed that the administration of DM decreased TAC levels in the liver, meanwhile the decrease was somewhat attenuated after the administration of VOO in the treatment group. It has been documented that olive oil and phenolics such as hydroxytyrosol and tyrosol can restore the antioxidant status in the liver after TCDD-induced hepatotoxicity30 and partial hepatectomy.31
Apoptosis is a key mechanism of degenerative diseases and is triggered by some factors such as toxins. In vivo and in vitro studies have revealed that exposure to DM significantly affects cell survival and induces apoptosis in thymic cells,14 neuronal cells,32 renal tubular cells,33 splenocytes,34 PC12 cells,35 and hepatocytes.36 Our immunohistochemical assessments revealed that the gavaging of DM significantly increased the expression of caspase-3, PARP, and COX-2, which are involved in the production of inflammatory mediators. Also, VOO consumption significantly decreased these upregulations. It is well documented that VOO and its phenolics have inhibitory effects against apoptosis. In this regard, Pan et al.37 documented that hydroxytyrosol, a main olive oil phenolic compound, exerted protective effects against hepatic ischemia/reperfusion injury through the attenuation of parenchymal apoptosis in mice. Another study demonstrated that the consumption of olive oil and its phenolics ameliorated apoptosis in TCDD-induced hepatotoxicity in rats.30
Conclusion
In sum, our results support the notion that dietary VOO, a good source of phytochemicals, can significantly attenuate the indicators of DM-induced hepatotoxicity. It can, therefore, be recommended as a dietary supplement to reduce the side effects of synthetic pyrethroid insecticides.
Acknowledgment
This work was supported financially by Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences (grant #1516).
Conflict of Interest: None declared.
References
- 1.Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, et al. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 2.Barlow SM, Sullivan FM, Lines J. Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem Toxicol. 2001;39:407–22. doi: 10.1016/s0278-6915(00)00152-6. [DOI] [PubMed] [Google Scholar]
- 3.El-Maghraby S. Metabolism of deltamethrin in rats. Biomed Environ Sci. 2007;20:212–6. [PubMed] [Google Scholar]
- 4.Patro N, Shrivastava M, Tripathi S, Patro IK. S100beta upregulation: a possible mechanism of deltamethrin toxicity and motor coordination deficits. Neurotoxicol Teratol. 2009;31:169–76. doi: 10.1016/j.ntt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Ismail MF, Mohamed HM. Deltamethrin-induced genotoxicity and testicular injury in rats: comparison with biopesticide. Food Chem Toxicol. 2012;50:3421–5. doi: 10.1016/j.fct.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Vani T, Saharan N, Mukherjee S, Ranjan R, Kumar R, Brahmchari R. Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pesticide biochemistry and physiology. 2011;101:16–20. doi: 10.1016/j.pestbp.2011.05.007. [DOI] [Google Scholar]
- 7.Abdallah FB, Slima AB, Dammak I, Keskes-Ammar L, Mallek Z. Comparative effects of dimethoate and deltamethrin on reproductive system in male mice. Andrologia. 2010;42:182–6. doi: 10.1111/j.1439-0272.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 8.Erdogan S, Zeren EH, Emre M, Aydin O, Gumurdulu D. Pulmonary effects of deltamethrin inhalation: an experimental study in rats. Ecotoxicol Environ Saf. 2006;63:318–23. doi: 10.1016/j.ecoenv.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Daim MM, El-Ghoneimy A. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren Fail. 2015;37:297–304. doi: 10.3109/0886022X.2014.983017. [DOI] [PubMed] [Google Scholar]
- 10.Xu MY, Wang P, Sun YJ, Wang HP, Liang YJ, Zhu L, et al. Redox status in liver of rats following subchronic exposure to the combination of low dose dichlorvos and deltamethrin. Pestic Biochem Physiol. 2015;124:60–5. doi: 10.1016/j.pestbp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Tos-Luty S, Haratym-Maj A, Latuszynska J, Obuchowska-Przebirowska D, Tokarska-Rodak M. Oral toxicity of deltamethrin and fenvalerate in Swiss mice. Ann Agric Environ Med. 2001;8:245–54. [PubMed] [Google Scholar]
- 12.Dubey N, Khan AM, Raina R. Sub-acute deltamethrin and fluoride toxicity induced hepatic oxidative stress and biochemical alterations in rats. Bull Environ Contam Toxicol. 2013;91:334–8. doi: 10.1007/s00128-013-1052-1. [DOI] [PubMed] [Google Scholar]
- 13.Yousef MI, Awad TI, Mohamed EH. Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicology. 2006;227:240–7. doi: 10.1016/j.tox.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Sasmal D, Sharma N. Deltamethrin induced an apoptogenic signalling pathway in murine thymocytes: exploring the molecular mechanism. J Appl Toxicol. 2014;34:1303–10. doi: 10.1002/jat.2948. [DOI] [PubMed] [Google Scholar]
- 15.Visioli F, Galli C. Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr. 2002;42:209–21. doi: 10.1080/10408690290825529. [DOI] [PubMed] [Google Scholar]
- 16.Visioli F, Poli A, Gall C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev. 2002;22:65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 17.Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papageorgiou VP. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J Med Food. 2002;5:1–7. doi: 10.1089/109662002753723160. [DOI] [PubMed] [Google Scholar]
- 18.Erol-Dayi O, Arda N, Erdem G. Protective effects of olive oil phenolics and gallic acid on hydrogen peroxide-induced apoptosis. Eur J Nutr. 2012;51:955–60. doi: 10.1007/s00394-011-0273-5. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Correa JA, Munoz-Marin J, Arrebola MM, Guerrero A, Narbona F, Lopez-Villodres JA, et al. Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia-reoxygenation. Lipids. 2007;42:921–9. doi: 10.1007/s11745-007-3097-6. [DOI] [PubMed] [Google Scholar]
- 20.Salvini S, Sera F, Caruso D, Giovannelli L, Visioli F, Saieva C, et al. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. Br J Nutr. 2006;95:742–51. doi: 10.1079/bjn20051674. [DOI] [PubMed] [Google Scholar]
- 21.Ben Slima A, Ali MB, Barkallah M, Traore AI, Boudawara T, Allouche N, et al. Antioxidant properties of Pelargonium graveolens L'Her essential oil on the reproductive damage induced by deltamethrin in mice as compared to alpha-tocopherol. Lipids Health Dis. 2013;12:30. doi: 10.1186/1476-511X-12-30. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour SW, Sangi S, Harsha S, Khaleel MA, Ibrahim AR. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pac J Trop Biomed. 2013;3:563–8. doi: 10.1016/S2221-1691(13)60114-8. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin Chem. 2001;47:1725–7. [PubMed] [Google Scholar]
- 24.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunduz E, Ulger BV, Ibiloglu I, Ekinci A, Dursun R, Zengin Y, et al. Glutamine provides effective protection against deltamethrin-induced acute hepatotoxicity in rats but not against nephrotoxicity. Med Sci Monit. 2015;21:1107–14. doi: 10.12659/MSM.893180. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuzmen N, Candan N, Kaya E, Demiryas N. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct. 2008;26:119–24. doi: 10.1002/cbf.1411. [DOI] [PubMed] [Google Scholar]
- 27.Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin S. The modulatory effect of deltamethrin on antioxidants in mice. Clin Chim Acta. 2006;369:61–5. doi: 10.1016/j.cca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–72. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 29.Mateos R, Martinez-Lopez S, Baeza Arevalo G, Amigo-Benavent M, Sarria B, Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016;205:248–56. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Kalaiselvan I, Samuthirapandi M, Govindaraju A, Sheeja Malar D, Kasi PD. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharm Biol. 2016;54:338–46. doi: 10.3109/13880209.2015.1042980. [DOI] [PubMed] [Google Scholar]
- 31.Kirimlioglu V, Kirimlioglu H, Yilmaz S, Ozgor D, Coban S, Karadag N, et al. Effect of fish oil, olive oil, and vitamin E on liver pathology, cell proliferation, and antioxidant defense system in rats subjected to partial hepatectomy. Transplant Proc. 2006;38:564–7. doi: 10.1016/j.transproceed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Wu A, Ren T, Hu Q, Liu Y. Deltamethrin induces altered expression of P53, Bax and Bcl-2 in rat brain. Neurosci Lett. 2000;284:29–32. doi: 10.1016/s0304-3940(00)00952-6. [DOI] [PubMed] [Google Scholar]
- 33.Liu FJ, Chou CT, Cheng JS, Chang HT, Liang WZ, Kuo CC, et al. Ca(2+) movement and apoptosis induced by deltamethrin in Madin-Darby canine kidney canine renal tubular cells. Kaohsiung J Med Sci. 2015;31:1–8. doi: 10.1016/j.kjms.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Sharma N. Comparative efficacy of piperine and curcumin in deltamethrin induced splenic apoptosis and altered immune functions. Pestic Biochem Physiol. 2015;119:16–27. doi: 10.1016/j.pestbp.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Park YS, Park JH, Ko J, Shin IC, Koh HC. mTOR inhibition by rapamycin protects against deltamethrin-induced apoptosis in PC12 Cells. Environ Toxicol. 2017;32:109–21. doi: 10.1002/tox.22216. [DOI] [PubMed] [Google Scholar]
- 36.Das PC, Streit TM, Cao Y, Rose RL, Cherrington N, Ross MK, et al. Pyrethroids: cytotoxicity and induction of CYP isoforms in human hepatocytes. Drug Metabol Drug Interact. 2008;23:211–36. doi: 10.1515/dmdi.2008.23.3-4.211. [DOI] [PubMed] [Google Scholar]
- 37.Pan S, Liu L, Pan H, Ma Y, Wang D, Kang K, et al. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol Nutr Food Res. 2013;57:1218–27. doi: 10.1002/mnfr.201300010. [DOI] [PubMed] [Google Scholar]


