Abstract
Compelling data in the literature from the recent years leave no doubt about the pluridimensional nature of G protein-coupled receptor function and the fact that some ligands can couple with different efficacies to the multiple pathways that a receptor can signal through, a phenomenon most commonly known as functional selectivity or biased agonism. Nowadays, transduction coefficients (log(τ/KA)), based on the Black and Leff operational model of agonism, are widely used to calculate bias. Nevertheless, combining both affinity and efficacy in a single parameter can result in compounds showing a defined calculated bias of one pathway over other though displaying varying experimental bias preferences. In this paper, we present a novel scale (log(τ)), that attempts to give extra substance to different compound profiles in order to better classify compounds and quantify their bias. The efficacy-driven log(τ) scale is not proposed as an alternative to the affinity&efficacy-driven log(τ/KA) scale but as a complement in those situations where partial agonism is present. Both theoretical and practical approaches using μ-opioid receptor agonists are presented.
Introduction
G protein-coupled receptors (GPCRs) are membrane receptors responsible for numerous physiological responses in living systems by transducing the signals embodied in the chemical structure of hormones, neurotransmitters and synthetic ligands from outside to inside the cells1. Many diseases are associated with an abnormal functioning of these receptors, which makes them one of the most important target families in drug discovery programs2.
Plasticity is a property inherent to the flexible nature of proteins which GPCRs make proficient use of for signaling purposes3. It is now well established that GPCRs signal not only through G protein-dependent pathways but also through β-arrestin and other accessory proteins4. This multiplicity of signaling pathways has led to a collection of pharmacological concepts (pluridimensional efficacy5,6, stimulus trafficking7, functional selectivity8,9, and biased agonism10) which all share a common link: the differential modulation that ligands may exert on receptor signaling.
Given that ligands may differentially drive receptor signaling, either potentiating or inhibiting one pathway over others, the issue arises of how to measure functional responses in order to establish reproducible scales for drug comparison11. The approach that is currently most commonly used (the log(τ/KA) scale) is based on a combination of estimates of efficacy (τ) and affinity (KA) to cancel the confounding interacting effects between both parameters12. In an attempt to provide new insights on the measurement of receptor signaling bias, this article presents a new scale (the log(τ) scale) that follows from the one described by Kenakin and coworkers to complement it and give extra substance to the classification of compounds’ bias. We use both scales to study a group of μ-opioid receptor ligands as this receptor’s bias behavior has been thoroughly described13,14. We show that two ligands’ properties, efficacy and concentration range, play a role in the results yielded by these two scales, allowing for a better classification of compounds and therefore aiding in the initial stages of drug discovery and hopefully in the development of new therapeutic drugs.
The herein described efficacy-driven log(τ) scale is not proposed as an alternative to the widely used affinity&efficacy-driven log(τ/KA) scale but as a complement in those situations where partial agonism is present. We argue that using only the log(τ/KA) scale is not sufficient for the analysis of biased agonism for compounds with differing maximal responses. In these cases where, according to the operational model15, the expression of the maximum response of a given agonist is Emax = Em * τ/(1 + τ), which does not include affinity, a note of caution seems opportune. As biased agonism is becoming a reality in routine screening studies, increasingly accurate protocols for biased signaling determination are now necessary.
Materials and Methods
Substances
Buprenorphine and fentanyl were supplied by Johnson Matthey (310030 and 310100). Morphine was adquired at Alcaliber. TRV130 was synthesized by Esteve. Finally, endomorphine-2 and Damgo were adquired from Sigma-Aldrich (SCP0133 and E7384).
G protein-independent pathway: β-arrestin-2 recruitment assay
Chinese hamster ovary (CHO)-K1 cells engineered to co-express the ProLink™ (PK) tagged human μ-opioid receptor and the Enzyme Acceptor (EA) tagged β-Arrestin-2 from DiscoverX were used (93-0213C2). 5000 cells/well were seeded in 20 μL of PathHunter Cell Plating Reagent in 384 well plates. Twenty-four hours later, 5 μl ligands (dissolved in Hanks’ balanced salt solution (HBSS) containing 20 mM Hepes) were added to the plate. Cells were incubated for 90 min at 37 °C. 6 μL of detection reagent (PathHunter Detection Reagent) were then added and the incubation continued at room temperature for 60 min. Luminescence was recorded (integration time of 1 s) in a Tecan Infinite M1000 Pro reader.
G protein-dependent pathway: Measurement of cAMP responses by Homogeneous Time Resolved Fluorescence
cAMP measurements on CHO-K1 cells that stably express the human μ-opioid receptor (Perkin Elmer ES-542-C) were performed by using a system based on Homogeneous Time Resolved Fluorescence (HTRF). The HTRF cAMP kit from CisBio (62AM4PEJ) was used according to the manufacturer’s recommendation. 2500 cells/well were seeded the day before the experiment in 10 μl of Opti-Mem (Gibco, 11058-021). On the following day, β-funaltrexamine (β-FNX, Sigma Aldrich, O003) was prepared in OptiMem and cells were treated with 5 μl of either concentration of β-FNX (0, 1, 3, 10, 30, 100, 300 nM) for 2 hours. After that time, cells were washed twice with 40 μl of Optimem. 10 μl of Optimem were finally added and cells were left for one hour at 37 °C. Opioid agonists were prepared in Optimem with 3-isobutyl-1-methyl-xanthine (Sigma-Aldrich, I5879-5G) and forskolin (Tocris, 1099) at 0.5 mM and 7.5 μM respectively and 10 μl added to the cells. After 45 min at 37 °C the reaction was stopped by lysing the cells with a mixture of 10 μl of each HTRF detection reagents. Plates were incubated for an additional hour at room temperature and read at 665 nm/620 nm using a RubyStar Plate reader (BMG LabTech). The conditions were followed as described in ref.16.
Parameter estimation
Curve fitting was performed by using nonlinear least squares regression. The NLIN procedure of SAS statistical package was applied (SAS/STAT 9.2; SAS Institute, Cary, NC, USA). The Gauss iterative method was employed in solving the nonlinear least squares problem. Equation 1 (this article) of the operational model of agonism15 was used for affinity and efficacy parameter estimation. It is known that the operational model cannot be applied to fit a single effect/agonist concentration (E/[A]) curve because there is not a single solution for the estimated parameters12. In this regard, two different fitting procedures, namely, the receptor inactivation and the comparative methods, were followed depending on the experimental assay performed. For the G protein-dependent cAMP assay, the receptor inactivation method17 was used: seven curves for each tested ligand were obtained by varying the concentration (0, 1, 3, 10, 30, 100 and 300 nM, respectively) of the irreversible antagonist β-FNX. Common operational Em, n and KA parameters were shared between curves whereas a τ parameter was defined for each β-FNX concentration-dependent curve15. The τ parameter corresponding to the curve yielded in the absence of β-FNX was used for the biased agonism analysis. For the G protein-independent β-arrestin-2 recruitment assay the same compounds as in the cAMP assay were used. However, because of the absence of an appropriate irreversible antagonist for the β-arrestin-2 assay, an alternative method was necessary. The comparative method18 was considered suitable because the tested compounds behave as partial agonists in the assay. In the comparative method, it is assumed that the maximal response (Emax) and the slope parameter (m) yielded by a full agonist through the Hill equation (E = Emax[A]m/(A50 m + [A]m)) match, respectively, the operational parameters maximum response of the system (Em) and slope parameter (n). Once determined, Emax and m can be used as fixed values in Equation 1 (this article) of the operational model for the estimation of KA and τ parameters of partial agonists. Damgo was used as the full agonist in the present study and its curve data were fitted through the Hill equation. The Emax and m parameters of Damgo curve were then used as fixed Em and n values in the fitting of the selected compounds under the operational model (Equation 1, this article). In all fitting procedures KA and τ were estimated as logarithms to approximate the assumption of normality distribution19.
The two scales compared in the present study are based on log(τ) and log(τ/KA) estimates. Parameter estimates for log(τ) and log(KA) and their corresponding standard errors were obtained from the nonlinear-regression curve fitting described above. Log(τ/KA) was estimated as log(τ) − log(KA). Standard errors for log(τ/KA) were calculated from the standard errors of log(τ) and log(KA) by including the correlation (r) between both parameters because they are not independent properties. Thus, representing log(τ) as x and log(KA) as y, the standard error (se) of log(τ/KA) was calculated as . The 95% confidence intervals of log(τ/KA) were calculated as , where the value of ν for the degrees of freedom of the Student’s t-function depends on whether the variances of x and y are statistically equal or different (F-Fisher test).
In the calculation of bias through both ΔΔlog(τ)G-protein, β-arrestin and ΔΔlog(τ/KA)G-protein, β-arrestin scales we conclude that there is no bias in one or the other when the confidence interval includes zero. However, inasmuch as a collection of compounds is evaluated, the issue of multiple testing appears and a corresponding correction is necessary. The issue of multiple testing was considered by adjusting the significance level through the Holm’s method. To do that we first transformed the IC95% of each of the compounds in a p-value for a t-test with a null hypothesis of μ = 0. Then the p-values for the selected compounds were adjusted according to the Holm’s method. Afterwards, the IC95% were recalculated by adjusting the α value according to the relative position of the previously calculated p-value. This resulted in adjusted IC95% more prone to include the zero value, which parallels the conventional conservative process of multiple testing involving p-values (a lesser propensity to reject the null hypothesis). Due to the statistical consistency of both inference methods, confidence intervals and hypothesis testing produced the same conclusion (biased agonism or not) for each of the compounds.
Results and Discussion
The log(τ/KA) and log(τ) scales
The two scales for biased agonism we discuss herein are based on the operational model of agonism, presented in a seminal work by Black and Leff15. Equation 1 is the general E/[A] equation of the operational model of agonism.
| 1 |
Where E is the pharmacological effect; Em, the maximum effect of the system; [A], the concentration of the agonist A; τ, the operational efficacy of A in the receptor system; KA, the dissociation constant of A for the receptor; and n, a parameter related with the slope of the E/[A] curves. A value of n equal to 1 yields rectangular hyperbolic E/[A] curves whereas n values greater and lower than 1 allow for steeper and flatter curves than rectangular hyperbola, respectively15,20.
Under the operational model of agonism15, τ is defined as τ = [RT]/KE, where [RT] represents the total receptor concentration and KE the value of the concentration of agonist-receptor complex, [AR], for half the maximum possible effect, Em; in other words, the inverse of KE reflects the intrinsic efficacy of the AR complex. Thus, τ contains both tissue and ligand-receptor efficacy parameters. Moreover, KA is not a thermodynamic equilibrium dissociation constant but a conditional or functional constant. This is because KA does not correspond to an individual equilibrium step, the binding of the agonist to the inactive receptor conformation, but it incorporates, in addition, the receptor conformational change associated with receptor activation. From a molecular perspective, the concept of receptor activation is present in both τ and KA parameters. As it has been shown15, τ may reflect the binding of the transducer G protein to the receptor. More precisely, in the presence of GTP and GDP, receptor activation is proportional to the active state of the quaternary complex (AR*G-GDP), with R* indicating the active conformation of the receptor21. In addition, KA is a combined expression of the parameter values for the agonist binding to the bare receptor and the receptor conformational change from the inactive (R) to the active (R*) state22–24.
The asymptotic maximum (Emax in Equation 2) and location (logEC50 in Equation 3) parameters allow for the quantification of E/[A] curve shape20. LogEC50 provides information about the potency of the agonist and Emax reflects agonist efficacy. We see that logEC50, or its commonly used negative value, pEC50, includes operational KA and τ parameters whereas KA is not present in the definition of Emax.
| 2 |
| 3 |
Equation 2 shows that high values of τ are associated with high values of Emax, which at the limit (full agonists) reach Em. On the contrary, low values of τ determine partial agonism: A value of τ as low as one unit makes Emax equal to half Em. With respect to potency, Equation 3 shows that higher potency values (lower logEC50) result from lower KA and higher τ values. Moreover, high values of τ (full agonists) lead to .
Kenakin et al.12, combining the KA and τ parameters of the operational model15, defined a parameter designated the transduction coefficient, , which provides a one-parameter scale able to classify agonists acting through one receptor. They demonstrated that this scale can be transferred between systems with differing receptor densities and, what is more, a ratio of this parameter relative to a reference ligand provides normalization by taking into account the natural bias of the system, and so is useful for comparing experimental and physiological tissues. Precedents of the transduction coefficient can be found in some publications by Ehlert25–27, who used either the ε/KA ratio, with ε being intrinsic efficacy, or the τ/KA ratio.
Is the Δlog(τ/KA) scale sufficient for biased agonism description? Combining the log(τ/KA) and log(τ) scales: A theoretical example
As explained in the Appendix (Supplementary Material), both Δlog(τ) and Δlog(τ/KA) represent useful scales to classify ligands independently of receptor density. An initial look at both scales reveals the fact that while the former only takes into account ligand operational efficacy, the latter also balances this efficacy in respect to ligand affinity, and so naturally these two scales classify ligands differently and the bias calculated from those scales differs as well. Because the Δlog(τ/KA) scale is currently being used in a routine way, the proposal of a complementary scale invites justification. At this point it is worth comparing both scales with a theoretical example:
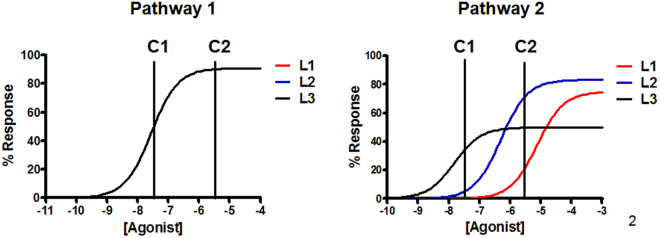
Let us suppose a drug screening study consisting of two pathways that is aimed at identifying ligands with a positive bias effect of Pathway 2 with respect to Pathway 1. Figure 1 shows the concentration-response curves for three ligands with agonistic properties acting through a given receptor in the two pathways, where the values of τ and KA for each ligand at each pathway are displayed in Table 1. We have assumed that the ligands have the same operational affinities and efficacies in Pathway 1 and different operational affinities and efficacies in Pathway 2. A normalized value of 100 has been assumed for Em in both pathways.
Figure 1.
A theoretical example of receptor activation through two different pathways. In the panel on the left it is represented the concentration-response curve of three agonists for a given receptor acting through Pathway 1. In the panel on the right, concentration-response curves are represented for the same agonists acting through Pathway 2. For the sake of clarity, the three agonists show the same effect in Pathway 1 while different behaviors are exerted through Pathway 2. Concentration is the key: Two concentrations are marked (C1 and C2). For both concentrations and in pathway 1, all three ligands show the same response. In pathway 2, differences appear. At concentration C1 the effect observed for agonist 3 is larger than that observed with the two other ligands. However, at concentration C2, the effect of agonist 3 remains greater than that of agonist 1 but smaller than that observed for agonist 2.
Table 1.
Operational parameters for the theoretical example.
| Pathway 1 | Pathway 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Em | τ | KA (nM) | Δlog(τ/KA) | Δlog(τ) | Em | τ | KA (nM) | Δlog(τ/KA) | Δlog(τ) | |
| Ligand 1 | 100 | 10 | 300 | 0 | 0 | 100 | 3 | 30000 | 0 | 0 |
| Ligand 2 | 100 | 10 | 300 | 0 | 0 | 100 | 5 | 3000 | 1.22 | 0.22 |
| Ligand 3 | 100 | 10 | 300 | 0 | 0 | 100 | 1 | 30 | 2.52 | −0.48 |
| Bias: Pathway 2 versus Pathway 1 | ||||||||||
| ΔΔlog(τ/K A ) | ΔΔlog(τ) | |||||||||
| Ligand1 | 0 | 0 | ||||||||
| Ligand2 | 1.22 | 0.22 | ||||||||
| Ligand3 | 2.52 | −0.48 | ||||||||
Theoretical values of the operational parameters for three different ligands at a given receptor capable of signaling through two different pathways. Ligand 1 is used as the reference ligand for bias calculation purposes.
For a proper comparison between a collection of ligands within a single pathway and in various pathways, a reference ligand must be defined. This allows for the cancellation of system effects. Assuming Ligand 1 as the reference ligand, calculated parameters for both scales, Δlog(τ) and Δlog(τ/KA), at each pathway and the bias of Pathway 2 relative to Pathway 1, ΔΔlog(τ) and ΔΔlog(τ/KA), are shown in Table 1. It can be seen that both scales classify ligands in a different order. For the Δlog(τ) scale the order is Ligand 2 > Ligand 1 > Ligand 3 while for the Δlog(τ/KA) scale the order is Ligand 3 > Ligand 2 > Ligand 1. Thus, taking Ligand 1 as the reference ligand whose bias is to be optimized, the second scale, Δlog(τ/KA), would provide that both Ligand 3 and Ligand 2 are optimized to a greater degree and Ligand 3 to a larger extent than Ligand 2, while with the first scale, Δlog(τ), only Ligand 2 is optimized with respect to Ligand 1.
Another practical output from the combination of the two scales comes from the comparison of the results obtained for Ligand 2 and Ligand 3 in both scales. While for Ligand 2 the two parameters ΔΔlog(τ/KA) and ΔΔlog(τ) result in a positive number, suggesting an improvement of the bias with respect to Ligand 1, a different situation is found for Ligand 3. For this last agonist the two parameters show opposing results due to an improvement in affinity but a worsening in efficacy versus Ligand 1.
Although at first sight these results seem contradictory, we understand that the two scales are complementary, each offering information not found in the other. Parameter derivations from the operational model (Equation 1) show that while ΔΔlog(τ/KA) is driven by both potency (EC50) and affinity (KA) (Equation 3) ΔΔlog(τ) is driven by efficacy (Emax) (Equation 2). A closer look at the concentration-response curves of Pathway 2 in Fig. 1 shows that the concentration at which Ligand 2 and Ligand 3 cross separates the relative activity of both ligands, as explained below.
Concentration is the key
Following the example above, the agonist concentration at which the E/[A] curves of two agonists at a given pathway cross can be determined.
Let us consider the E/[A] curves of two agonists: A1 and A2.
| 4 |
| 5 |
where it is assumed that Em and n are system-dependent parameters15.
The curves for Agonists 1 and 2 cross at the ([A], E) point ([A1] = [A2] = [A], E1 = E2 = E). Making Equations 4 and 5 equal and rearranging terms leads to Equation 6.
| 6 |
where [A] is the ligand concentration, τ1 and τ2 the operational agonist efficacies of Ligand 1 and Ligand 2, respectively, and KA1 and KA2 the operational agonist equilibrium dissociation constants.
By substituting the value of τ in Equation 6, we obtain Equation 7.
| 7 |
The ligand concentration ([A]) at which both concentration-response curves cross does not depend upon total receptor concentration ([RT]) and it is constant over the whole range of receptor densities.
Looking at the E/[A] curves for Pathway 2 in Fig. 1 we see that Ligand 3 shows greater effect than Ligand 2 at concentrations below that at which both concentration-response curves cross, in agreement with the Δlog(τ/KA) scale; while at concentrations above that of curve crossing, Ligand 2 shows the greater effect, in agreement with the Δlog(τ) scale.
Represented again by the same concentration-response curves as in the example above, in Fig. 1 we have also marked two concentrations, C1 and C2, below and above the concentration at which both curves cross in Pathway 2. It can be seen that at C1 the effect of Ligand 3 is greater than that of Ligand 2, and because of this the bias favors Ligand 3 versus Ligand 2. The opposite is true at C2. A similar consideration is exemplified in Kenakin and Christopoulos, 2013 (Fig. 5 in cited article)22, where the authors describe how an agonist with a defined calculated bias of one pathway over the other, that is a single value with the Δlog(τ/KA) scale, can show variable effective bias in vivo in tissues with differing receptor density. In their simulations the authors show how the agonist displays a clear bias throughout the full concentration range in the tissue with a high receptor density. However, in the tissue with low receptor density, the agonist exhibits a change of the preference of one pathway over the other at the ligand concentration at which the E/[A] curves for the two pathways cross. Therefore, for this particular ligand in these particular in vivo conditions, the Δlog(τ/KA) scale does not reflect correctly the experimental results. The approximation presented here, a joint consideration of the Δlog(τ) and Δlog(τ/KA) scales aids in identifying experimentally-found differences, provides better fine tuning of the classification of compounds and allows for the calculation of a concentration value that determines the relationship between the scales.
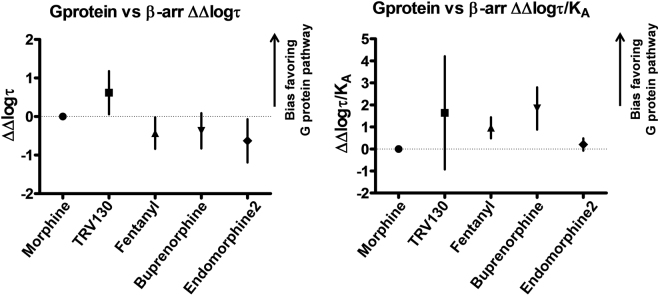
Figure 5.
Graphical representation of ΔΔlog(τ) and ΔΔlog(τ/KA) scales. The left panel represents ligand bias using ΔΔlog(τ) values of all ligands referred to morphine as the common reference ligand for cAMP inhibition versus β-arrestin interaction. Similar results are shown in the right panel using the ΔΔlog(τ/KA) scale.
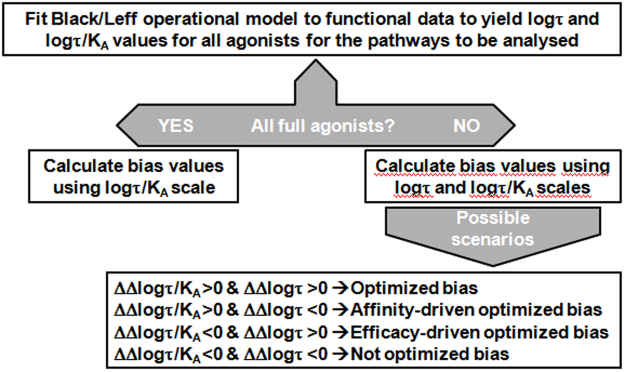
Figure 2 shows a diagram for the analysis of agonist bias using the Δlog(τ) and Δlog(τ/KA) scales calculated by fitting functional data to the Black and Leff operational model15. If all agonists studied behave as full agonists in all the pathways analyzed, then Δlog(τ/KA) scale alone can be used. But, if those agonists are not all full agonists, then both scales should be used, ending up with four different situations. The first situation, where both scales show an improvement in the bias they calculate (ΔΔlog(τ) and ΔΔlog(τ/KA) > 0) meaning that bias has been optimized. A second one where ΔΔlog(τ/KA) > 0 but Δlog(τ) < 0 would represent a situation where only the first scale would point to an improvement in the bias pursued. As the difference between both scales resides in the KA value of the first one, we identify this bias improvement as affinity-driven, due to an increase in affinity (KA) of the ligand studied compared to the reference ligand. A third scenario, where ΔΔlog(τ/KA) < 0 but Δlog(τ) > 0 would represent a situation where only the second scale points to an improvement in bias. In this case we identify this bias improvement as efficacy-driven due to an increase in efficacy (τ) of the ligand studied versus the reference. Finally, in the last situation both scales point out that bias improvement has not been achieved (ΔΔlog(τ) and ΔΔlog(τ/KA) < 0).
Figure 2.
Schematic diagram for analysis of agonist bias using log(τ) and log(τ/KA) scales. Properly designed functional data for the pathways to be studied are fitted to Black & Leff operational model to yield log(τ) and log(τ/KA) values. If all agonists behave as full agonists in all pathways then log(τ/KA) scale alone can be used to classify compounds. If not, then both scales should be used. As a result, four different scenarios may result.
A practical example
Biased signaling has already been analyzed for the μ-opioid receptor13,28 (see also29 as a review). What is more, TRV130, a ligand described as a biased μ-opioid agonist favoring the G protein signaling pathway over that of β-arrestin, is already in Phase II clinical trials30. Bearing this in mind, we used the μ-opioid receptor to apply our proposal for bias calculation.
When µ-opioid receptors couple to Gi/o subtypes they inhibit the production of cAMP and they can also recruit β-arrestins. An HTRF (Cisbio) cAMP determination assay was used to determine the activity of this receptor on the G protein signaling pathway, while an enzyme complementation assay (DiscoverX) was used to determine its ability to recruit and signal through the β-arrestin pathway.
The ligands used in this study were: morphine and fentanyl, two opioids commonly used for pain-relief; buprenorphine, a classically classified partial agonist; and finally endomorphine-2 and TRV130, which are biased agonists for the μ-opioid receptor13,31.
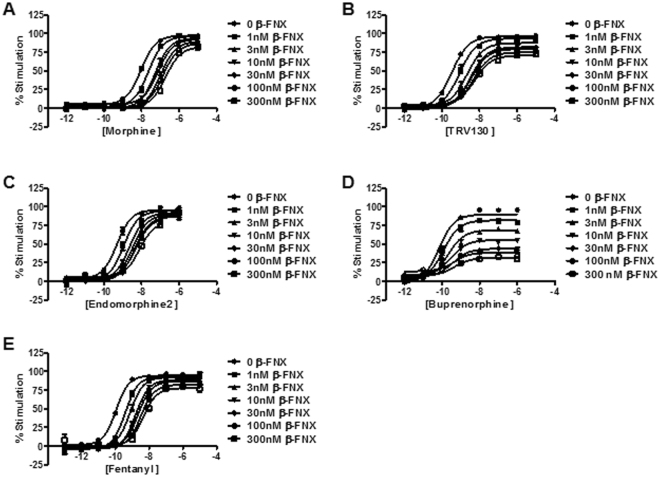
Figure 3 shows the results of the five μ-opioid receptor agonists in the cAMP determination assay. It is known32 that the operational model cannot satisfactorily fit a single experimental E/[A] curve. A solution to the problem can be reached by using the irreversible inactivation method17 (see Parameter estimation in Methods), which produces a collection of experimental curves with lower maximal effect by decreasing receptor density. With this procedure, a single solution for each of the curves, with particular τ and common Em, n and KA parameters, is obtained. We followed this approach for parameter determination in the G protein pathway. Results are shown in Table 2.
Figure 3.
μ-opioid agonist inhibition of forskolin-stimulated cAMP production assay (G protein-dependent pathway). The concentration-response curves for five different opioid agonists were determined in both the absence and presence (1, 3, 10, 30, 100, 300 nM) of the irreversible antagonist β-funaltrexamine. (A) Morphine; (B) TRV130; (C) Endomorphine2; (D) Buprenorphine; (E) Fentanyl. Results were obtained in at least three independent experiments. In each experiment, data points were obtained in duplicates.
Table 2.
Operational parameters (estimates ± standard errors) for the G protein-dependent pathway.
| Em | n | Log (KA) | Log (τ) | Log(τ/KA) | |
|---|---|---|---|---|---|
| Morphine | 98.33 ± 1.41 | 1.04 ± 0.05 | −5.96 ± 0.11 | 2.12 ± 0.12 | 8.08 ± 0.04 |
| Fentanyl | 93.99 ± 1.01 | 1.22 ± 0.08 | −7.74 ± 0.10 | 2.23 ± 0.11 | 9.97 ± 0.05 |
| TRV130 | 98.15 ± 1.44 | 0.90 ± 0.04 | −7.63 ± 0.07 | 1.82 ± 0.09 | 9.45 ± 0.05 |
| Endomorphine-2 | 96.64 ± 2.12 | 1.02 ± 0.07 | −7.38 ± 0.17 | 1.97 ± 0.20 | 9.35 ± 0.07 |
| Buprenorphine | 96.75 | 0.98 ± 0.21 | −9.35 ± 0.23 | 0.85 ± 0.20 | 10.20 ± 0.10 |
Data obtained from concentration-response curves of µ-opioid agonists in presence of various concentrations of the irreversible antagonist β-FNX analyzed with the operational model of agonism (Fig. 3). Parameter estimates and standard errors of operational parameters Em, n, log(KA) and log(τ) were produced by global fitting. Common Em, n and KA parameters were shared between curves whereas a τ parameter was defined for each β-FNX concentration-dependent curve. In the Table, log(τ) for β-FNX concentration equal to 0 is shown. For buprenorphine, the fitting did not converge when Em was included as a free parameter; thus, we set Em equal to the mean of the values obtained for the other ligands (96.75) and kept it fixed as such in the fitting process. Log (τ/KA) values and their standard errors were calculated from estimated τ and KA parameters (see Parameter estimation in Methods).
For the β-arrestin pathway (Fig. 4) we used a different experimental approach: the comparative method18 (see Parameter estimation in Methods). In this method, it is assumed that the maximal response (Emax) and the slope parameter (m) yielded from a full agonist by fitting curve data with the Hill equation match, respectively, the operational Em and n parameters, and, once determined, can be used as fixed values for the estimation of the efficacy and affinity of partial agonists within the operational model. In this assay, we used Damgo as the full agonist. As all the other opioids in the assay behaved as partial agonists, their KA and τ values could be directly calculated using the operational model by substituting Em and n parameters with Damgo Emax and m and keeping them fixed as such in the fitting process. Results are shown in Table 3. It is worth noting the discrepancies in KA between the two pathways for each of the agonists (Tables 2 and 3). This is an acceptable result under the operational model of agonism because KA is a functional affinity of the agonist which includes the interaction of the activated receptor with the signaling protein either the G protein or β-arrestin22.
Figure 4.
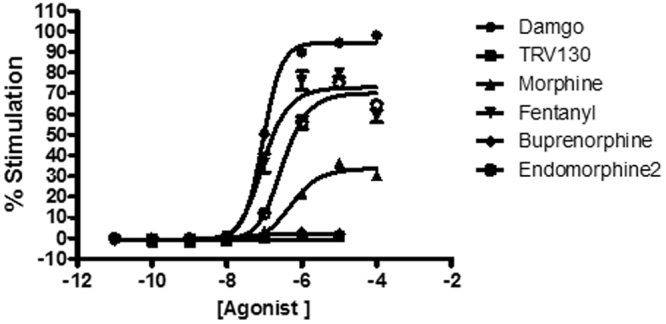
μ-opioid agonist β-arrestin recruitment assay. The concentration-response curves for five different opioid agonists were determined and compared to the concentration-response curve of damgo as the standard full agonist for this assay. Results were obtained in at least three independent experiments. In each experiment, data points were obtained in quadruplicates.
Table 3.
Operational parameters (estimates ± standard errors) for β-arrestin pathway.
| Log (KA) | Log (τ) | Log (τ/KA) | |
|---|---|---|---|
| Morphine | −6.31 ± 0.10 | −0.19 ± 0.02 | 6.12 ± 0.09 |
| Fentanyl | −6.70 ± 0.19 | 0.35 ± 0.08 | 7.05 ± 0.15 |
| TRV130 | −6.96 ± 0.99 | −1.11 ± 0.15 | 5.85 ± 0.92 |
| Endomorphine-2 | −6.90 ± 0.08 | 0.29 ± 0.03 | 7.19 ± 0.06 |
| Buprenorphine | −7.49 ± 0.30 | −1.09 ± 0.04 | 6.40 ± 0.28 |
Data obtained from concentration-response curves of µ-opioid agonists using the comparative method18 with Damgo as full agonist (Fig. 4). The Hill equation was used for fitting to Damgo data. The values obtained for Damgo for maximal response (95.14) and slope parameter (1.48) were used for all ligands in the Table as Em and n parameters in the operational model and kept fixed as such in the fitting process. Parameter estimates and standard errors of operational parameters log(KA) and log(τ) were produced by global fitting. Log (τ/KA) values and their standard errors were calculated from estimated τ and KA parameters (see Parameter estimation in Methods).
At this point it is worth mentioning, for the sake of correct data interpretation, that it is convenient to analyze all the studied pathways in the same cell line to minimize any functional influence of receptor tagging or modification that needs to be performed. Unfortunately, this is not always possible because, depending on the signaling pathway, different receptor or signaling protein constructs must be used33. This is particularly evident when more than two pathways are analyzed, as in the study by Thompson and colleagues28, where bias was calculated for cAMP, GTPγS and pERK1/2 determinations using the wild-type μ-opioid receptor, but for other signaling pathways such as β-arrestin-1, β-arrestin-2 or receptor internalization an Rluc-tagged receptor was used28. It is worth noting that concerns about receptor tagging have been extensively addressed in the literature. Barak and colleagues, back in 199734, described a β2-adrenoceptor variant tagged with eGFP at its C-terminal part which showed ligand binding, second messenger stimulation, receptor phosphorylation and internalization properties closely resembling those of the wild-type receptor. In another example, Scherrer and colleagues compared an eGFP tagged δ-opioid receptor with its wild-type counterpart with both transfected in HEK293 cells35. They showed that the binding of different opioid ligands remained the same between the two receptors and more importantly there was no difference in the capacity of the deltorphin II agonist to stimulate the receptor as measured by using a [35S]-GTPγS binding assay. A final example can be found in the orphan GPR17 receptor36. In this case, a label-free dynamic mass redistribution assay was used to compare the functional response of the wild-type receptor with that of an N-terminal hemagglutinin-tagged receptor, as well as with either a C-terminal Rluc-tagged or a C-terminal GFP2-tagged receptor. Also, the ability of the wild-type receptor to stimulate cAMP response was compared with that of the C-terminal GFP2-tagged receptor. In all cases, the introduction of the corresponding tag had no effect on receptor functionality. Finally, the main concern when using different cells or differently tagged receptors is that system bias and observational bias may differ between the different cell systems used. To address these issues and cancel out both system and observational bias, which can be expected to affect all agonists to the same extent, all bias factors are related to a common reference agonist12,22. In the present work, to calculate bias factors between the two studied pathways, morphine was selected as the reference agonist. The calculation of bias involves parameters (τ and KA) which were estimated as logarithms for reasons of normality. Thus, bias evaluation implies the calculation of differences in logarithmic values and the estimation of their corresponding confidence intervals (see Parameter estimation in Methods). Bias estimates of the (G protein-β-arrestin) − ΔΔlog(τ) and −ΔΔlog(τ/KA) scales for the five opioids used in this study are shown in Table 4 and represented in Fig. 5. As can be seen in the right panel of this figure, all the ligands show a positive bias compared to morphine in the ΔΔlog(τ/KA) scale, though not statistically significant (zero is included in the confidence interval) in the case of TRV130 and endomorphine-2. On the contrary, when analyzing the data in the ΔΔlog(τ) scale (Fig. 5 left panel), a different picture is obtained. In this case, only TRV130 shows a clear positive bias favoring the G protein effect versus that of β-arrestin. Fentanyl, endomorphine-2 and buprenorphine show bias favoring the β-arrestin pathway, though not statistically significant in the last case.
Table 4.
Calculation of (G protein - β-arrestin) ΔΔlog(τ) and ΔΔlog(τ /KA) bias.
| ΔLog (τ) G protein pathway | ΔLog (τ) β-arrestin pathway | ΔΔLog (τ) G protein – β arrestin | ΔLog(τ/KA) G protein pathway | ΔLog(τ/KA) β-arrestin pathway | ΔΔLog(τ/KA) G protein – β arrestin | |
|---|---|---|---|---|---|---|
| Morphine | 0 | 0 | 0 | 0 | 0 | 0 |
| Fentanyl | 0.11 ± 0.16 | 0.54 ± 0.08 | −0.43 ± 0.18 (−0.84, −0.02) * | 1.89 ± 0.06 | 0.93 ± 0.19 | 0.96 ± 0.20 (0.48, 1.44) * |
| TRV130 | −0.3 ± 0.15 | −0.92 ± 0.15 | 0.62 ± 0.21 (0.06, 1.18) * | 1.37 ± 0.06 | −0.27 ± 0.92 | 1.64 ± 0.92 (−0.93, 4.21) |
| Endomorphine-2 | −0.15 ± 0.23 | 0.48 ± 0.04 | −0.63 ± 0.23 (−1.19, −0.07) * | 1.27 ± 0.08 | 1.07 ± 0.11 | 0.20 ± 0.14 (−0.08, 0.48) |
| Buprenorphine | −1.27 ± 0.23 | −0.9 ± 0.04 | −0.37 ± 0.23 (−0.83, 0.09) | 2.12 ± 0.11 | 0.28 ± 0.29 | 1.84 ± 0.38 (0.88, 2.80) * |
Raw data for Log (τ) and log(τ /KA) were taken from Tables 2 and 3. Morphine was taken as the reference compound. Parameter estimates ± standard errors are shown. The confidence intervals of 95% (CI95%) for ΔΔ estimates are shown in parentheses. Multiple testing was considered in the calculation of confidence intervals through Holm’s method (see Parameter estimation in Methods). A * has been added to those CI95% for ΔΔ estimates which do not include zero and show thus statistical significance for bias signaling.
DeWire and colleagues (2013)14 reported a bias favoring the G protein pathway for TRV130 using relative intrinsic activities (RAi) and because RAi values can reduce to τ/KA (when n = 1)12, their data resembles the transduction coefficient scale (ΔΔlog(τ/KA)) that we present here. Regarding endomorphine-2, some studies13,37 have reported a bias favoring the β-arrestin pathway for this ligand. In these studies13,37, τ values were estimated from the operational model using KA values obtained from independent binding experiments. These results for endomorphine-2 favoring the β-arrestin pathway are in agreement with our results in the ΔΔlog(τ) scale, though in our fitting procedure τ and KA are both estimated from the operational model.
Quantitative pharmacology of signaling bias may offer a structure-function framework which can be useful for drug discovery purposes. An elegant study on the M2 muscarinic acetylcholine receptor combining various approaches including mutation and molecular modeling identified orthosteric and allosteric site mutations that contribute to ligand-selective signaling bias38. The authors suggested that the functional selectivity of some of the compound might arise from a bitopic mechanism38. Other examples with similar methodological approaches can be cited as, for example, studies focusing on the glucagon-like peptide-1 receptor39 or the M1 muscarinic acetylcholine receptor40. Moreover, a detailed review on the functional analysis of receptor states can be found in ref.21.
In the theoretical example used, we determined the concentration at which the concentration-response curves of two agonists for a given receptor cross each other at a given signaling pathway. We showed that this concentration does not depend on the total amount of receptor present. In Fig. 6 we have represented the concentration-response curves for morphine and buprenorphine in the cAMP inhibition assay in the presence or absence of β-FNX to illustrate this point. Visual inspection of Fig. 6 shows that the concentration-response curves of morphine and buprenorphine at various β-FNX concentrations cross at similar concentration values (shown by blue dots), in agreement with theoretical predictions (Equation 7). Applying Equation 6 to the data generated from these two agonists gives the following concentration values (critical concentrations) at which the curves cross: 80 nM, 102 nM, 124 nM, 90 nM, 82 nM, 71 nM and 88 nM, respectively, for each of the β-FNX pretreatment conditions. We see that the effect elicited by buprenorphine is always greater than that produced by morphine at concentrations lower than ~100 nM whereas the opposite is true at concentrations above ~100 nM.
Figure 6.
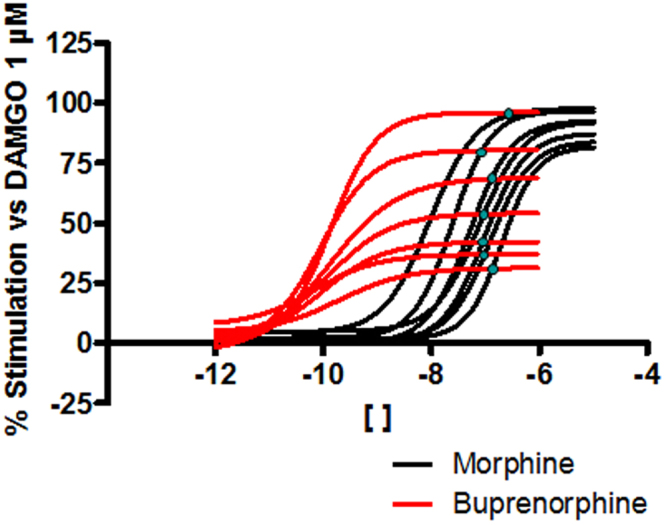
μ-opioid agonist inhibition of forskolin-stimulated cAMP production assay. The concentration-response curves for morphine and buprenorphine determined in both the absence and presence (1, 3, 10, 30, 100, 300 nM) of the irreversible antagonist β-funaltrexamine are represented jointly in the same graph. The concentrations at which the curves for each agonist cross with each other are marked. Results were obtained in at least three independent experiments. In each experiment, data points were obtained in quadruplicates.
Conclusions
Biased agonism is a hot topic in current pharmacologic research with known therapeutic implications. Accurate and standardized measurement of this property is fundamental to drug discovery and development. Currently, the most widely used scale is one based on log(τ/KA). It has the advantage of combining efficacy and affinity properties in a single parameter thus providing simplicity. However, in those situations in which different maximal responses are found, the log(τ/KA) scale appears to be insufficient. In this regard, because the efficacy parameter τ is directly related with the maximal response achieved by an agonist, the log(τ) scale can complement the log(τ/KA) scale in those cases which include ligands with different maximal responses. Of note, we have shown that the log(τ) scale accomplishes the same requirement as that of the log(τ/KA) scale, namely the ratio of τ values for two ligands across receptor systems with varying receptor density remains constant. We have also shown that concentration plays a role in these cases and how the decision of whether to use a biased agonism approach based on either pure efficacy (the ΔΔlog(τ) scale) or a combination of efficacy and affinity parameters (the ΔΔlog(τ/KA) scale) depends on the experimental concentration window used. In this regard, the signs of the (ΔΔlog(τ/KA), ΔΔlog(τ)) pairs provide an indication on whether there is (+, +) or not (−, −) an optimization of the bias in one pathway relative to the other also whether it is mainly affinity- or efficacy-driven, (+, −) and (−, +), respectively. Finally, we have illustrated the application of the proposed methodology to the μ-opioid receptor scenario by considering the G protein and the β-arrestin pathways and selected full and partial agonists.
Electronic supplementary material
Acknowledgements
This study was supported in part by Ministerio de Economía y Competitividad (ERA-NET NEURON PCIN-2013-018-C03-02 and SAF2014-58396-R) and Xunta de Galicia (IN853A2014-08). We thank José Brea and Mabel Loza for carefully reading of the manuscript and helpful comments.
Author Contributions
Contributed to the conception and design of the study: Burgueño and Giraldo. Performed the mathematical modeling: Burgueño and Giraldo. Wrote the manuscript: Burgueño and Giraldo. Carried out the statistical analyses: Roche and Giraldo. Conducted and analyzed the pharmacological assays: Pujol, Varela, and Monroy. Contributed to the design, analysis and interpretation of experiments: Merlos.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15258-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos R, et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Costa-Neto CM, Parreiras-E-Silva LT, Bouvier M. A pluridimensional view of biased agonism. Mol. Pharmacol. 2016;90:587–595. doi: 10.1124/mol.116.105940. [DOI] [PubMed] [Google Scholar]
- 7.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends. Pharmacol. Sci. 1995;16:232–238. doi: 10.1016/S0165-6147(00)89032-X. [DOI] [PubMed] [Google Scholar]
- 8.Lawler CP, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 9.Kilts JD, et al. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol. Exp Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 10.Jarpe MB, et al. [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J Biol Chem. 1998;273:3097–3104. doi: 10.1074/jbc.273.5.3097. [DOI] [PubMed] [Google Scholar]
- 11.Onaran HO, et al. Systematic errors in detecting biased agonism: Analysis of current methods and development of a new model-free approach. Sci. Rep. 2017;7:44247. doi: 10.1038/srep44247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. ACS Chem. Neurosci. 2012. A simple method for quantifying functional selectivity and agonist bias; pp. 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson J, et al. mu-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol. Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeWire SM, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol. Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 15.Black JW, Leff P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B. Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 16.Carliss RD, et al. Receptor reserve reflects differential intrinsic efficacy associated with opioid diastereomers. Pharmacol. Biochem. Behav. 2009;92:495–502. doi: 10.1016/j.pbb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 17. Furchgott, R. F. Advances in Drug Research. Harper, N. J. & Simmonds, A. B. (eds), pp. 21-55 (Academic Press, New York,1966).
- 18.Leff P, Prentice DJ, Giles H, Martin GR, Wood J. Estimation of agonist affinity and efficacy by direct, operational model-fitting. J Pharmacol. Methods. 1990;23:225–237. doi: 10.1016/0160-5402(90)90066-T. [DOI] [PubMed] [Google Scholar]
- 19.Christopoulos A. Assessing the distribution of parameters in models of ligand-receptor interaction: to log or not to log. Trends Pharmacol. Sci. 1998;19:351–357. doi: 10.1016/S0165-6147(98)01240-1. [DOI] [PubMed] [Google Scholar]
- 20.Black JW, Leff P, Shankley NP, Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br. J. Pharmacol. 1985;84:561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlert FJ. Functional studies cast light on receptor states. Trends Pharmacol. Sci. 2015;36:596–604. doi: 10.1016/j.tips.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 23.Roche D, Gil D, Giraldo J. Multiple active receptor conformation, agonist efficacy and maximum effect of the system: the conformation-based operational model of agonism. Drug Discov. Today. 2013;18:365–371. doi: 10.1016/j.drudis.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin MT, Figueroa KW, Liller S, Ehlert FJ. Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o,Gs, and G15. J. Pharmacol. Exp. Ther. 2007;321:1193–1207. doi: 10.1124/jpet.107.120857. [DOI] [PubMed] [Google Scholar]
- 26.Ehlert FJ. On the analysis of ligand-directed signaling at G protein-coupled receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:549–577. doi: 10.1007/s00210-008-0260-4. [DOI] [PubMed] [Google Scholar]
- 27.Figueroa KW, Griffin MT, Ehlert FJ. Selectivity of agonists for the active state of M1 to M4 muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 2009;328:331–342. doi: 10.1124/jpet.108.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson GL, et al. Biased Agonism of Endogenous Opioid Peptides at the mu-Opioid Receptor. Mol. Pharmacol. 2015;88:335–346. doi: 10.1124/mol.115.098848. [DOI] [PubMed] [Google Scholar]
- 29. Thompson, G., Kelly, E., Christopoulos, A. & Canals, M. Novel GPCR paradigms at the mu-opioid receptor. Br. J. Pharmacol. (2014). [DOI] [PMC free article] [PubMed]
- 30.Viscusi ER, et al. A Randomized, Phase 2 Study Investigating TRV130, a Biased Ligand of the μ-opioid Receptor, for the Intravenous Treatment of Acute Pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 31.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol. Sci. 2014;35:308–316. doi: 10.1016/j.tips.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 32. Leff, P. Perspectives on Receptor Classification. Black, J. W., Jenkinson, D. R. & Gerskowitch, V. P. (eds), pp. 157-167 (Alan R. Liss, Inc., New York,1987).
- 33.Rajagopal S, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol. Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barak LS, et al. Internal trafficking and surface mobility of a functionally intact beta2-adrenergic receptor-green fluorescent protein conjugate. Mol. Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- 35.Scherrer G, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc. Natl. Acad. Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hennen S, et al. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci Signal. 2013;6:ra93. doi: 10.1126/scisignal.2004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivero G, et al. Endomorphin-2: a biased agonist at the mu-opioid receptor. Mol. Pharmacol. 2012;82:178–188. doi: 10.1124/mol.112.078659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory KJ, Hall NE, Tobin AB, Sexton PM, Christopoulos A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 2010;285:7459–7474. doi: 10.1074/jbc.M109.094011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koole C, et al. Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) has a critical role in GLP-1 peptide binding and receptor activation. J. Biol. Chem. 2012;287:3642–3658. doi: 10.1074/jbc.M111.309328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keov P, et al. Molecular mechanisms of bitopic ligand engagement with the M1 muscarinic acetylcholine receptor. J. Biol. Chem. 2014;289:23817–23837. doi: 10.1074/jbc.M114.582874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.