Abstract
Erythropoietin-producing hepatocellular carcinoma A2 (EphA2) is overexpressed in more than 90% of non-small cell lung cancer (NSCLC) but not significantly in normal lung tissue. It is therefore an important tumor antigen target for chimeric antigen receptors (CAR)-T-based therapy in NSCLC. Here, we developed a specific CAR targeted to EphA2, and the anti-tumor effects of this CAR were investigated. A second generation CAR with co-stimulatory receptor 4-1BB targeted to EphA2 was developed. The functionality of EphA2-specific T cells in vitro was tested with flow cytometry and real-time cell electronic sensing system assays. The effect in vivo was evaluated in xenograft SCID Beige mouse model of EphA2 positive NSCLC. These EphA2-specifc T cells can cause tumor cell lysis by producing the cytokines IFN-γ when cocultured with EphA2-positive targets, and the cytotoxicity effects was specific in vitro. In vivo, the tumor signals of mice treated with EphA2-specifc T cells presented the tendency of decrease, and was much lower than the mice treated with non-transduced T cells. The anti-tumor effects of this CAR-T technology in vivo and vitro had been confirmed. Thus, EphA2-specific T-cell immunotherapy may be a promising approach for the treatment of EphA2-positive NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related mortality among men and the second leading cause of cancer death among women worldwide [1]. The 5-year relative survival rate of patients diagnosed with lung cancer was less than 19%, while the average rate of cancer patients at all site was 70% [2]. Non-small cell lung cancer (NSCLC) accounts for nearly 85% of all cases of lung cancer [3], in which adenocarcinoma will be the predominant histological subtype [4], [5]. The current treatments including surgery, radiotherapy, chemotherapy and targeted therapy has helped improve the survival in patients with NSCLC. However, the average 5-year survival rate of lung adenocarcinoma was only 15% [6], mainly because of the poor prognosis and lack of effective treatment in late-stage, highlighting the unmet need for new therapeutic paradigms for this disease.
Immunotherapy with chimeric antigen receptor (CAR)-engineered T cells is a breakthrough treatment in hematology, such as anti-CD19 CAR-T cells in treating acute lymphoblastic leukemia (ALL) [7], [8], chronic lymphocytic leukemia (CLL) [9] and B cell lymphomas [10]. In recent years, much more progress has been made in solid tumors, including colorectal cancer [11], metastatic ovarian cancer [12], glioblastoma [13]. Immunotherapy with CARs targeting epidermal growth factor receptor (EGFR) in a clinical trial showed good response with EGFR-expressing advanced relapsed/refractory NSCLC [14]. CAR glypican 3 (CARgpc3) T cells was also proved to be a novel potential therapeutic agent for the treatment of patients with lung squamous cell carcinoma (LSCC) [15]. However, the studies with regards to NSCLC were still limited.
The ephrin receptors (Ephs) are the largest group within the family of receptor tyrosine kinases (RTKs) [16]. Erythropoietin-producing hepatocellular carcinoma A2 (EphA2) play critical roles in many developmental processes and are implicated in a number of cancers [17], [18]. EphA2 is overexpressed in more than 90% of NSCLC but not significantly in normal lung tissue [19], and correlates with tumor malignancy and poor patient survival [20]. In addition, we have found EphA2-positive cells in malignant pleural effusion of lung adenocarcinoma patients. One study using EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer have shown improved clinical outcome [21]. Hence, EphA2 is supposed to be an important marker with potential clinical utility in the immunotherapy of NSCLC [22].
Here, we report the development of an EphA2-specifc CAR to redirect T cells to EphA2-positive NSCLC. These T cells are able to recognize and kill EphA2-positive lung cancer cells. Furthermore, we have found that INF-γ paly role in EphA2-CAR-T therapies. The effect of EphA2-specifc CAR in vivo was also evaluated in xenograft SCID Beige mouse model of lung cancer.
Materials and Methods
Cell Lines, Pleural Effusions, and Media
Three NSCLC cell lines (A549, PC9, H1650), the leukemia cell line K562 and 293 T cell line were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Thirteen samples of pleural effusions were obtained from patients diagnosed with lung adenocarcinoma in the First Affiliated Hospital of Zhejiang University. Peripheral blood mononuclear cells (PBMCs) derived from human donors were collected by Ficoll–Hypaque density-gradient centrifugation provided by the Zhejiang Blood Center. All cell lines were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum(FBS) and100 μg/ml penicillin. Medium with recombinant human interleukin-2 (IL-2) of 300 U/ml was used for the expansion of T cells.
Flow Cytometric Analysis
Flow cytometric analysis (BD, Mountain View, CA) was used to detect the expression of EphA2 on tumor cells and to detect the expression of CAR on EphA2-positive T cells. EphA2 expression in cell lines (A549, PC9, H1650, K562) was tested using a EphA2-PE antibody (BioLegend, San Diego, CA). Cells collected from pleural effusions were stained with both EphA2-PE antibody and CD45-APC antibody (BD, San Jose, CA). Cells were collected and washed once with phosphate buffered saline(PBS) containing 2% FBS prior to the addition of antibodies, and were incubated for 20 minutes on ice in the dark, washed twice prior to analysis.
Generation of CAR-Expressing T Cells
The EphA2-specific single chain variable fragment was derived from the EphA2 MAb 4H5, a humanized version of the EphA2 MAb EA2 [23], [24]. A codon-optimized synthetic gene encoding 4H5 in single chain variable fragment format was cloned into a SFG retroviral vector upstream of an IgG1-CH2CH3 domain, a CD8α transmembrane domain, and costimulatory domains derived from 4-1BB and CD3-ζ. Lentiviral particles were generated by transient transfection of 293 T cells with the EphA2-specific CAR encoding SFG retroviral vector. PBMC were stimulated on 6-well plates in RPMI 1640 medium containing 1x107/beads/ml dynabeads human T-activator CD3/CD28 (Invitrogen, Carlsbad, CA). On day 2, vector encoding the EphA2-specific CAR were used to transduce CD3/CD28-activated T cells. On day 3 or 4, T cells were transferred into new wells and subsequently expanded with 300 IU/ml IL-2. Nontransduced T cells, used as controls, were activated with CD3/CD28 and expanded in parallel. EphA2-specific CAR expression was determined 5 to 6 days posttransduction. In all experiments, the functionality of matched (from the same donor) transduced and nontransduced T cells was compared.
Cytokine Release Assays
The transduced and nontransduced T cells were co-cultured with EphA2-positive cell A549 or negative cell k562 at the effector:target ratio of 5:1, 1:1 and 1:5 for 5 h, separately. Each ratio repeated three times. The expression of cytokines IFN-γ were measured by flow cytometry(FCM). BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit and APC mouse anti-human IFN-γ (BD Bioscience,USA) were used for staining.
Cytotoxicity Assay
The xCELLigence system, also known as the Real-Time Cell Electronic Sensing System (RT–CES, ACEA Biosciences), was used to test the time- and dose-dependent lung cancer cell line response profiles to EphA2-specific T cells in vitro. Briefly, 50 μL of the medium was added to E-plates to obtain background readings, followed by the addition of 50 μL of the target cell (A549, 2 x105/ml) suspension. The E-plates containing the indicated initial number of cells were incubated at room temperature for 30 min before being placed onto the reader in the incubator. The cells were allowed to attach and grow for 24 h to reach a stable baseline before the addition of the different number of CAR-T and T cells. The CAR-T and T cells were co-cultured with A549 at the effector:target ratio of 1:1, 5:1 and 20:1 for 48 h, separately.Each ratio repeated three times.The target cells were monitored every 15 min for 48 h.
Establishment of Xenograft SCID Beige Mouse Model of Lung Cancer
A total 2.5 × 106 A549.GFP. Luc cells were injected into the caudal vein of six to eight week female SCID Beige mice on day 0. On day 7, the tail vein injection was performed with 1 × 107 non-transduced T cells or EphA2-specific T cells in 200 μL into the previous stereotactic tumor coordinates. The mice were anesthetized with rapid sequence inhalation isoflurane after 150 mg/kg D-luciferin was injected per mouse intraperitoneally. The mice were then photoed with serial noninvasive in vivo bioluminescence imaging system (Berthold LB983, Germany) in 10–15 minutes. The photons emitted from the luciferase-expressing tumor cells were quantified using NightOWLindiGo™ software (Berthold technologies, Germany). Animals were imaged on day 0 and day 7 after injections, then once a week thereafter. The mice were treated and housed according to protocols approved by the Medical Experimental Animal Care Commission of Zhejiang University.
Statistical Analysis
For the mouse experiments, differences in tumor radiance from baseline at each time point were calculated and compared between groups using t test. Overall survival determined from the time of tumor cell injection was analyzed by the Kaplan–Meier method and by the log-rank test. GraphPad Prism 5.0 was used for statistical calculations. P < .05 were considered significant.
Results
EphA2 is Expressed in Lung Cancer Cell Lines and Malignant Pleural Effusions
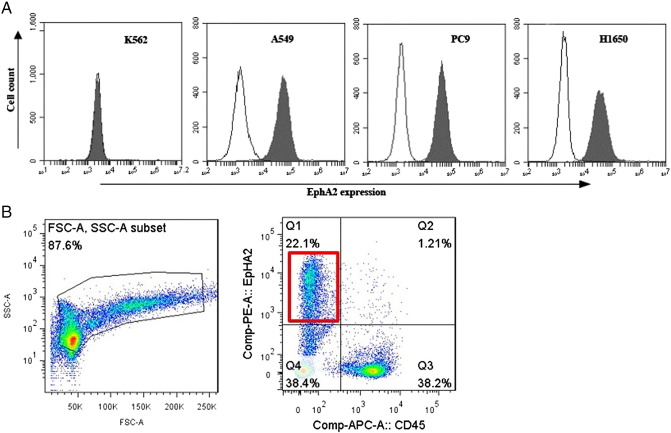
To evaluate whether EphA2 function as surface phenotypic hallmarks of lung cancer cells, we detected the distribution of this molecule on the lung cancer cell lines (A549, PC9, H1650) and leukemia cell line K562 by FCM. The results showed that EphA2 was expressed in the lung cancer cell lines but not in the leukemia cell line K562 (Figure 1A). 13 samples of pleural effusions were collected from patients diagnosed with lung adenocarcinoma. 5.4–22.1% EphA2 expression was found in 5 pleural effusions, while the other cells expressing CD45 indicated the white blood cells. Because of a shortage of pleural effusion, only one sample tested for EphA2 expression was identified with microscopic cancer cells (Figure 1B).
Figure 1.
Erythropoietin-producing hepatocellular carcinoma A2 (EphA2) is expressed in lung cancer cell lines and malignant pleural effusions. (A) FCM showed that EphA2 was expressed in the lung cancer cell lines but not in the leukemia cell line K562. (B) EphA2 were found in cancerous pleural effusion of lung adenocarcinoma patients.
Generation of EphA2-Specific CAR-Modified T Cells
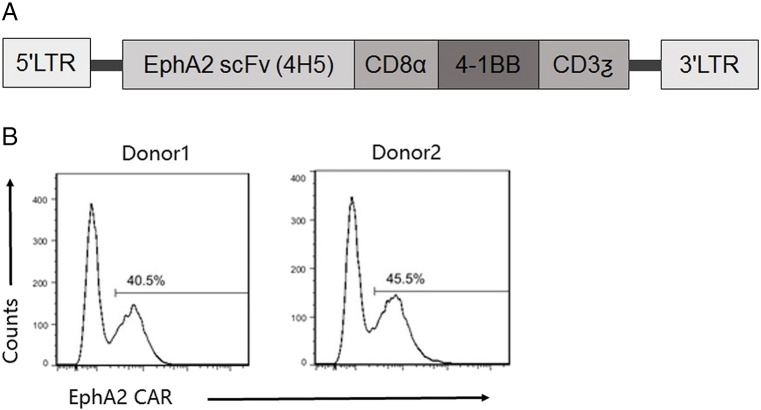
To redirect T cells to the EphA2 receptor, a second-generation EphA2-specific CAR was designed based on the humanized EphA2 monoclonal antibody (MAb) 4H5. A codon-optimized synthetic gene encoding 4H5 in single chain variable fragment format was cloned into a SFG retroviral vector upstream of an IgG1-CH2CH3 domain, a CD8α transmembrane domain, and costimulatory domains derived from 4-1BB and CD3-ζ (Figure 2A). Lentiviral vector encoding the EphA2-specific CAR were used to transduce CD3/CD28-activated T cells from normal healthy donors. Following T-cell transduction, FCM analysis was used to determine the cell surface expression of the EphA2-specific CAR. The percentage of CAR-expressing T cells were 40.5% and 45.5% in two normal healthy domors (Figure 2B).
Figure 2.
Generation of erythropoietin-producing hepatocellular carcinoma A2 (EphA2)-specific T cells. (A) The EphA2-specific chimeric antigen receptors (CAR) was generated by cloning a single chain variable fragment derived from the EphA2 monoclonal antibody 4H5 upstream of an IgG1-CH2CH3 domain, a CD8α TM domain, and costimulatory domains derived from 4-1BB and CD3-ζ into an SFG retroviral vector. (B) EphA2-CAR expression was detected by staining T cells with a corresponding antibody. Fluorescence activated cell sorting analysis revealed expression of EphA2-specific CARs on the cell surface of transduced T cells as compared with controls. The percentage of CAR-expressing T cells were 40.5% and 45.5% in two normal healthy donors LTR, long terminal repeats; TM, transmembrane.
EphA2-Specific T Cells Recognize and Kill EphA2-Positive Lung Cancer Cells
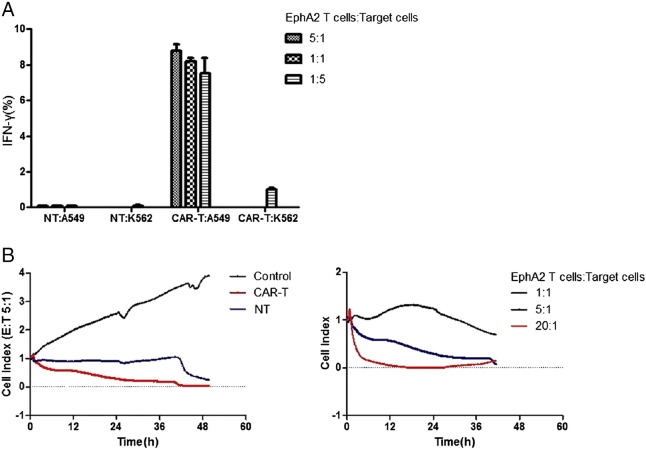
We used FCM and RT–CES assays to test the functionality of EphA2-specific T cells in vitro. EphA2-specific T cells were cocultured with EphA2-positive and negative target cells, and the percentage of interferon-γ-expressing(IFN-γ) T cells was determined after 5 hours by FCM. EphA2-specific T cells recognized EphA2-positive lung cancer cell lines A549 as evidenced by a higher percentage of IFN-γ-expressing T cells in comparison to the EphA2-negative T cells and K562 (Figure 3A). None IFN-γ-expressing T cells was observed in non-transduced-T cells in response to any targets (Figure 3A). The cell index(CI) of A549 cocultured with different concentrations of effector cells were monitored continuously in real-time by RT-CES. In standard 48-hour assays, EphA2-specific T cells showed a significant lower CI against A549 whereas non-transduced T cells (Figure 3B), and a rapid and precipitous increase in CI occurred in the control group (only A549). And the higher effector to target (E:T) ratio showed the stronger cytotoxic activity against A549.
Figure 3.
Erythropoietin-producing hepatocellular carcinoma A2 (EphA2)-specific T cells recognize and kill EphA2-positive lung cancer cells. (A) Non-transduced (NT) or EphA2-specific T cells were cocultured with target cells at 5:1, 1:1 and 1:5 ratio, and after 24 hours, percentage of IFN-γ-expressing T cells was determined by FCM. A higher percentage of IFN-γ-expressing T cells was occurred in comparison to the EphA2-negative T cells and K562. (B) EphA2-specific T cells showed a significant lower CI against A549 whereas non-transduced T cells, and a rapid and precipitous increase in CI occurred in the control group (only A549). And the stronger cytotoxic activity against A549 was observed at the higher effector to target (E: T) ratio of 20:1.
EphA2-Specific T Cells Inhibit the Growth of Lung Cancer In Vivo
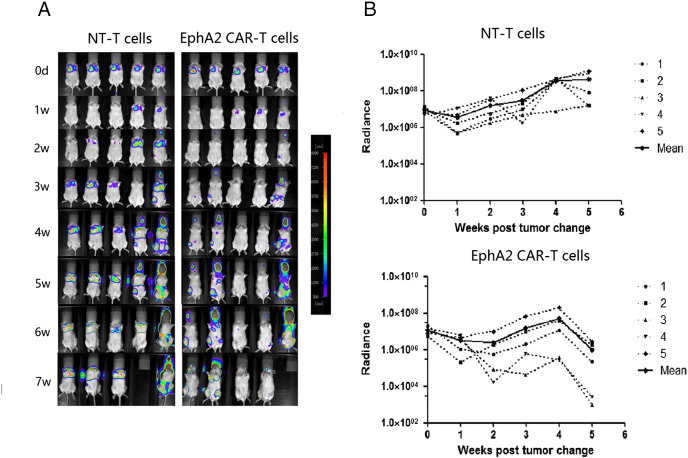
We used an orthotopic xenograft mouse model to evaluate the therapeutic effect of lung cancer of EphA2-specific T cells in vivo. A549 lung cancer cells were modified to express an GFP-Luciferase fusion protein (A549.GFP. Luc), allowing us to track tumor growth using serial noninvasive in vivo bioluminescence imaging (Berthold LB983, Germany). 2.5 × 106 A549.GFP Luc cells were injected into the caudal vein of SCID Beige mice on day 0. Tumors engrafted and grew exponentially in the week post-tumor cell injection. On day 7, the tail vein injection was performed with 1 × 107 non-transduced T cells or EphA2-specific T cells into the previous stereotactic tumor coordinates. Although the mice treated with EphA2-specifc T cells and non-transduced T cells both showed continuous tumor growth, the tumor signals of mice treated with EphA2-specifc T cells presented the tendency of decrease, and was significantly lower than the mice treated with non-transduced T cells at the fourth week (n = 5, P = .02; Figure 4, A and B). In addition, one mouse treated with EphA2-specifc T cells had a complete response (CR). Two groups of mice die within 7–8 weeks successively, and no significant difference of mice survival was observed.
Figure 4.
Erythropoietin-producing hepatocellular carcinoma A2 (EphA2)-specific cells induce regression of established lung cancer in vivo. (A)2.5 × 106 A549.GFP. Luc cells were injected into the caudal vein of SCID Beige mice on day 0 and treated with 1 × 107 EphA2-specific T cells into the previous stereotactic tumor coordinates on day 7. Mice treated with non-transduced (NT) T cells served as controls. Bioluminescence imaging was used to follow tumor progression. All mice had detectable tumors just prior to treatment (day 7). (B) The bioluminescence signal from the tumors over time. Dotted lines represent each individual mouse while the solid black line represents the mean radiance for the group at the given time.
Discussion
In this study, the development and characterization of a novel CAR specific for the EphA2 receptor has been described. These EphA2-specifc T cells can cause tumor cell lysis by producing the cytokines IFN-γ when cocultured with EphA2-positive targets, and the cytotoxicity effects was specific in vitro. The antitumor activity in vivo of these EphA2-specifc T cells has also been demonstrated.
On 171 (61%)adenocarcinomas and 108 (39%)squamous cell carcinomas tissue microarrays (TMA) samples, EphA2 expression was detected in >90% of NSCLC samples [20]. Likewise, EphA2 expression was detected in all nine NSCLC cell lines assayed by Western blotting [25]. Epidermal growth factor receptor (EGFR) is expressed in majority of NSCLC. EGFR mutations are present in approximately 10 to 15% of Caucasian patients [26] and 51.4% of Asian patients [27]. The prolonged progression-free survival (PFS) and increased disease control rates were observed on treatment with EGFR tyrosine kinase inhibitor (EGFR TKI), however, inevitable drug resistance was also observed after 6–12 months' treatment [28]. Recent studies indicated that EphA2 was more than 10-fold overexpressed in EGFR-TKI-resistant cells suggesting a potential role in developing resistance [29]. On the other hand, NSCLC patients for smokers with Kirsten rat sarcoma (K-Ras) mutations didn't enjoy the same benefit from EGFR-TKI, and the treatment options are very limited. While the overexpression of EphA2 has been found to correlate with a poor prognosis on smokers with K-Ras mutations [20]. All the results indicate that EphA2 may be a potential molecular target for treatment of most NSCLC, including the special patients resistant to EGFR-TKI and smokers with K-Ras mutations. Based on the above researches, we didn't test the expression of EphA2 in tumor tissue in this study, but 5.4–22.1% EphA2 expression was found in 5 of 13 pleural effusions diagnosed with lung adenocarcinoma. As is well known, approximately 15% of lung cancer patients show a pleural effusion at the time of initial diagnosis, and 50% develop a pleural effusion later in their progress [30], [31]. Hence, EphA2-specifc T cells developed in this study can be injected into the thoracic cavity to treat patients with EphA2-positive malignant pleural effusions, and the subsequent researches have been started.
Actually, EphA2-specific T-cell immunotherapy has been reported in glioblastoma [13], and secretion of IFN-γ was observed when EphA2-specifc T cells cocultured with EphA2-positive target cells. In another study, patients with renal cell carcinoma(RCC), who expressing high levels of EphA2, exhibit both CD8 and CD4 T-cell responses to novel EphA2-derived epitopes [32]. The results of this study demonstrated that the EphA2-specific T-cells can be activated and expanded in the presence of EphA2-positive lung cancer cells. The in vitro cytotoxicity assay further supported that the CAR T cells could specifically eliminate EphA2-positive lung cancer cells by secretion of IFN-γ. The cytotoxic effect increased at the higher effector to target (E: T) ratio.
In vivo, the mice treated with EphA2-specifc T cells and non-transduced T cells both die within 7–8 weeks. Especially, one of the five mice treated with EphA2-specifc T cells, in which nearly no tumor signal was detected since the fifth week also died in the eighth week. The cause of death could not be related to tumor progress. Although no significant difference of mice survival was observed, the tumor signals of mice treated with EphA2-specifc T cells was much lower than the mice treated with non-transduced T cells at the fourth week (n = 5, P = .02).
Conclusions
In this study, we provide evidence that T cells redirected to EphA2 by an EphA2-specifc CAR have potent antitumor activity against NSCLC in vitro and in vivo, and might be novel therapeutic agents for patients with NSCLC.
Funding
This work was supported by the National Key R&D Program of China (No.2016YFC1303403), the National Natural Science Foundation of China (No.31501082) and the Natural Science Foundation of Zhejiang Province (No.LY15H160011).
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of The First Affiliated Hospital of Zhejiang University for the use of clinical materials for research purpose. And animal use and experiment protocol were approved by the Institutional Animal Care and Use Committee of Tongji University School of Medicine.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgments
Not applicable.
Contributor Information
Ning Li, Email: lining198684@163.com.
Shaohui Liu, Email: shaohui999999@163.com.
Mingjiao Sun, Email: smjaa@163.com.
Wei Chen, Email: zhizhuo_huanxiang@126.com.
Xiaogang Xu, Email: xuxg@zju.edu.cn.
Zhu Zeng, Email: drzengzhu@sina.cn.
Yemin Tang, Email: 13136158098@163.com.
Yongquan Dong, Email: ahui_008@163.com.
Alex H. Chang, Email: alexhchang@yahoo.com.
Qiong Zhao, Email: zhaoqiong@zju.edu.cn.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . 2016. SEER Cancer Statistics Review, 1975-2013. [Available online: https://seer.cancer.gov/csr/1975_2013/browse_csr.php?sectionSEL=1&pageSEL=sect_01_table.04.html] [Google Scholar]
- 3.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61(2):91–112. doi: 10.3322/caac.20102. [DOI] [PubMed] [Google Scholar]
- 4.Kaisermann MC, Trajman A, Madi K. Evolving features of lung adenocarcinoma in Rio de Janeiro, Brazil. Oncol Rep. 2001;8(1):189–192. doi: 10.3892/or.8.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer. 1999;86(9):1867–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 7.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S. Infusion of donor-derived CD19-redirected-virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase I study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magee MS, Kraft CL, Abraham TS, Baybutt TR, Marszalowicz GP, Li P, Waldman SA, Snook AE. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. Oncoimmunology. 2016;5(10):e1227897. doi: 10.1080/2162402X.2016.1227897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, Han W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci. 2016;59(5):468–479. doi: 10.1007/s11427-016-5023-8. [DOI] [PubMed] [Google Scholar]
- 15.Li K, Pan X, Bi Y, Xu W, Chen C, Gao H, Shi B, Jiang H, Yang S, Jiang L. Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget. 2016;7(3):2496–2507. doi: 10.18632/oncotarget.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 17.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19(3):275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Brannan JM, Sen B, Saigal B, Prudkin L, Behrens C, Solis L, Dong W, Bekele BN, Wistuba I, Johnson FM. EphA2 in the early pathogenesis and progression of non-small cell lung cancer. Cancer Prev Res (Phila) 2009;2(12):1039–1049. doi: 10.1158/1940-6207.CAPR-09-0212. [DOI] [PubMed] [Google Scholar]
- 20.Brannan JM, Dong W, Prudkin L, Behrens C, Lotan R, Bekele BN, Wistuba I, Johnson FM. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res. 2009;15(13):4423–4430. doi: 10.1158/1078-0432.CCR-09-0473. [DOI] [PubMed] [Google Scholar]
- 21.Patel AR, Chougule M, Singh M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm Res. 2014;31(10):2796–2809. doi: 10.1007/s11095-014-1377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T, Yamada H, Kuroki M, Kodama S, Tamura K, Takamatsu Y. A modified adenovirus vector-mediated antibody screening method identifies EphA2 as a cancer target. Transl Oncol. 2017;10(4):476–484. doi: 10.1016/j.tranon.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63(22):7907–7912. [PubMed] [Google Scholar]
- 24.Damschroder MM, Widjaja L, Gill PS, Krasnoperov V, Jiang W, Dall'Acqua WF, Wu H. Framework shuffling of antibodies to reduce immunogenicity and manipulate functional and biophysical properties. Mol Immunol. 2007;44(11):3049–3060. doi: 10.1016/j.molimm.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, Lynch T, Johnson BE, Miller VA. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koch H, Busto ME, Kramer K, Medard G, Kuster B. Chemical proteomics uncovers EPHA2 as a mechanism of acquired resistance to small molecule EGFR kinase inhibition. J Proteome Res. 2015;14(6):2617–2625. doi: 10.1021/acs.jproteome.5b00161. [DOI] [PubMed] [Google Scholar]
- 30.Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer. 1974;33(4):916–922. doi: 10.1002/1097-0142(197404)33:4<916::aid-cncr2820330405>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Memon A, Zawadzki ZA. Malignant effusions: diagnostic evaluation and therapeutic strategy. Curr Probl Cancer. 1981;5(8):1–30. doi: 10.1016/s0147-0272(81)80012-8. [DOI] [PubMed] [Google Scholar]
- 32.Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E, Storkus WJ. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63(15):4481–4489. [PubMed] [Google Scholar]