Abstract
Antibodies against recombinant proteins can significantly reduce their effectiveness in unanticipated ways. We evaluated the humoral response of mice with the lysosomal storage disease mucopolysaccharidosis type I treated with weekly intravenous recombinant human alpha-l-iduronidase (rhIDU). Unlike patients, the majority of whom develop antibodies to recombinant human alpha-l-iduronidase, only approximately half of the treated mice developed antibodies against recombinant human alpha-l-iduronidase and levels were low. Serum from antibody-positive mice inhibited uptake of recombinant human alpha-l-iduronidase into human fibroblasts by partial inhibition compared to control serum. Tissue and cellular distributions of rhIDU were altered in antibody-positive mice compared to either antibody-negative or naive mice, with significantly less recombinant human alpha-l-iduronidase activity in the heart and kidney in antibody-positive mice. In the liver, recombinant human alpha-l-iduronidase was preferentially found in sinusoidal cells rather than in hepatocytes in antibody-positive mice. Antibodies against recombinant human alpha-l-iduronidase enhanced uptake of recombinant human alpha-l-iduronidase into macrophages obtained from MPS I mice. Collectively, these results imply that a humoral immune response against a therapeutic protein can shift its distribution preferentially into macrophage-lineage cells, causing decreased availability of the protein to the cells that are its therapeutic targets.
Keywords: lysosomal disease, alpha-l-iduronidase, Hurler, Scheie, glycosaminoglycan
Introduction
Recombinant proteins are used clinically to treat dozens of conditions typically caused by a genetic lack of the protein, for example, clotting factor deficiencies and lysosomal storage diseases. If these recombinant proteins are sensed as foreign, humoral immune responses that significantly limit effectiveness can develop. For example, patients with infantile Pompe disease who develop neutralizing antibodies against recombinant human acid alpha-glucosidase can deteriorate and die because the recombinant enzyme is less effective and is unable to prevent disease progression.1, 2 In MPS I, antibodies against recombinant human alpha-l-iduronidase (rhIDU) are associated with reduced clearance of glycosaminoglycans from urine, indicating both a reduced effectiveness of treatment and a significant humoral immune response.3
The humoral immune response to recombinant protein therapies for lysosomal storage diseases can reduce the efficacy of treatment either by neutralizing enzymatic activity directly, presumably by interfering with the enzyme’s active site, or by interfering with binding the mannose 6-phosphate receptor, thus blocking cellular uptake and lysosomal targeting.4 Using the naturally occurring canine model of MPS I, we reported that serum from dogs with a humoral immune response against rhIDU diminished its intracellular uptake into MPS I patient fibroblasts in vitro, suggesting that antibodies may reduce the effectiveness of rhIDU by limiting its uptake into cells.5 Antibodies against rhIDU also altered the distribution of rhIDU in vivo as well. rhIDU preferentially localized to tissues, such as lymph nodes, liver, and spleen, that had greater reticuloendothelial content, and correspondingly less rhIDU distributed to other organs, such as myocardium, lung, and renal medulla.5 In MPS I, heart valves and synovium are particularly difficult to treat, and we found that abnormalities in these tissues did not improve following rhIDU treatment in MPS I dogs with high antibody titers. However, heart valves and synovium from low-titer dogs treated with high-dose intravenous rhIDU appeared morphologically normal.5 These results suggest that enzyme replacement therapy is incompletely effective in patients who develop antibodies against rhIDU because these antibodies divert rhIDU away from tissues that need it most.
One mechanism that could explain the altered distribution of rhIDU that we observed in MPS I dogs is that antibody-bound rhIDU is taken up by reticuloendothelial cells, perhaps via Fc receptors. Uptake of rhIDU normally occurs via the mannose 6-phosphate receptor, and our previous results suggest that antibodies against rhIDU interfere with this receptor-mediated uptake pathway.5 Hence, excess rhIDU might be more likely to be removed from circulation by reticuloendothelial cells. Supporting this notion are data demonstrating that when mannose 6-phosphates are absent from recombinant beta-glucuronidase, which is then administered intravenously to MPS type VII mice, tissue distribution in the liver showed predominant uptake by Kupffer cells rather than hepatocytes, but the intact enzyme was evenly distributed to both cell types.6 The findings from these previous studies suggest that uptake via the mannose 6-phosphate receptor is necessary for even distribution of recombinant lysosomal enzymes to parenchymal tissues.
Results
Modest Humoral Immune Response to rhIDU in MPS I Mice
There were three groups of experimental mice. One “naive” group of MPS I mice (n = 6) received a single 1.57 mg/kg dose of fluorescent-labeled rhIDU at 16 weeks of age. These mice were considered naive because they lacked prior exposure to rhIDU and therefore would not mount a humoral immune response when a single dose of fluorescent-labeled rhIDU was administered. Thirteen MPS I mice received weekly tail vein injections of 1.57 mg/kg rhIDU from 4 to 15 weeks of age, followed by treatment with a single dose of fluorescent-labeled rhIDU at 16 weeks of age. In prior canine studies, intravenous rhIDU administration to MPS I dogs resulted in a humoral immune response in all immune-competent animals. Anti-rhIDU gamma-immunoglobulin (IgG) antibody titers ranged from 16 to 678 optical density (OD) U/μL in dogs that did not receive immune tolerization.7 However, in the present experiment, only eight mice receiving rhIDU developed an anti-rhIDU IgG antibody titer, and these titers ranged from 3 to 30 OD U/μL. We designated these mice as “antibody positive.” Two of these mice died 1 hr after an infusion from presumed infusion reactions and one was found dead 3 days post-infusion. Five MPS I mice receiving rhIDU had titers <1 OD U/μL that were considered negligible. These mice were designated “antibody negative” (Figure S1). Serum from 12 untreated MPS I mice and 10 normal mice were evaluated for anti-rhIDU antibodies as controls. Titers in the 12 naive MPS I mice ranged from 0 to 0.108 OD U/μL undiluted serum (average 0.032 ± 0.040 OD U/μL), and for 10 naive normal mice, titers ranged from 0.001 to 0.577 (average 0.086 ± 0.178) OD U/μL undiluted serum. It is not clear why some mice mounted an immune response and some did not. However, the mice that failed to mount an immune response to rhIDU provided the opportunity to compare rhIDU distribution in naive mice with mice that mounted a humoral immune response and mice that mounted a negligible humoral immune response. We confirmed that the humoral immune response to rhIDU in antibody-positive MPS I mice partially inhibited uptake of rhIDU into MPS I human fibroblasts using an in vitro uptake assay (Figure S2).
Uptake of rhIDU in Liver and Heart Was Significantly Reduced in High-Titer MPS I Mice
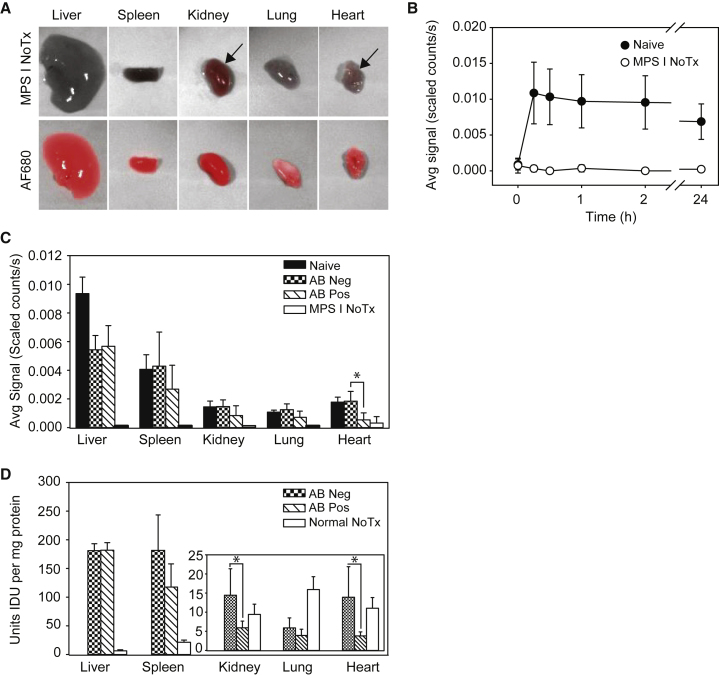
To study the effect of anti-rhIDU antibodies on rhIDU distribution, we administered fluorescent-labeled rhIDU intravenously to adult MPS I mice that were either naive to rhIDU or that had been treated weekly with rhIDU and developed a humoral immune response. We then imaged the mice in vivo and ex vivo using fluorescent imaging (Figure 1). We compared six naive with five antibody-positive MPS I mice and found that antibody-positive MPS I mice had lower fluorescence intensity than naive MPS I mice in all organs by approximately 33%–70% (Figure 1C). Intergroup differences between antibody-positive and naive mice reached statistical significance for the heart (t(7.31) = −3.44; p = 0.010) by a two-sample t test, separate variances. Two of the antibody-positive mice showed zero fluorescence intensity in the lung, kidney, and/or heart, which are organs with relatively low reticuloendothelial content (Figure S3).
Figure 1.
Distribution of Fluorescent-Labeled rhIDU
Distribution of rhIDU labeled with Alexa Fluor 680 (rhIDU-AF680) in naive, antibody-negative (anti-rhIDU IgG antibody < 1 OD U/μL), and antibody-positive (anti-rhIDU IgG antibody > 3 OD U/μL) MPS I mice. (A) False color ex vivo imaging of internal organs from a representative mouse from two untreated MPS I mice (MPS I No Tx; top row) and a representative mouse from six rhIDU-AF680-treated MPS I mice (AF680; bottom row) 24 hr after injection of labeled enzyme. Autofluorescence was apparent in some tissues (arrows). (B) Fluorescence intensity in vivo in the whole mouse. Naive MPS I mice (i.e., mice that had no previous rhIDU exposure; filled circles; n = 6) are compared to untreated MPS I control mice (open circles; n = 2). (C) Distribution of rhIDU to internal organs ex vivo in six naive MPS I mice (Naive), six antibody-negative MPS I mice (AB Neg), five antibody-positive MPS I mice (AB Pos), and two untreated MPS I mice (MPS I NoTx). Average fluorescence intensity was measured 24 hr after injection of rhIDU-AF680. Signals in untreated MPS I mice (MPS I No Tx) represent autofluorescence. (D) IDU enzymatic activity in internal organs of antibody-positive- and antibody-negative-treated MPS I mice (n = 5 per group) after 16 weeks of rhIDU treatment compared to untreated MPS I control mice (n = 10). Untreated normal control mice (we used wild-type littermates) received no treatment (n = 10; Normal No Tx). Error bars represent SD. *p < 0.05.
rhIDU Enzymatic Activity Was Significantly Reduced in High-Titer MPS I Mice
To determine whether there were differences in enzymatic activity of rhIDU as a function of altered biodistribution, we assayed rhIDU enzymatic activity in antibody-negative and antibody-positive mice. Because naive MPS I mice received a single rhIDU dose rather than 16 weekly rhIDU doses, the enzymatic activity of their tissues was not expected to be comparable, and thus the naive mice were not included in studies of rhIDU activity. rhIDU enzymatic activities were generally consistent with ex vivo fluorescence imaging results (Figure 1D). Antibody-positive MPS I mice had 2.4-fold lower rhIDU activity in the kidney (t(4.50) = −2.67; p = 0.049) and 3.7-fold lower rhIDU activity in the heart (t(4.14) = −2.80; p = 0.047) compared to antibody-negative mice (two-sample t test, separate variances). There were no noticeable differences in liver, lung, or spleen rhIDU activity between the antibody-positive and antibody-negative MPS I mice treated with rhIDU.
Fluorescence Labeling Did Not Affect Enzymatic Activity of rhIDU
To exclude the possibility that enzymatic activities of rhIDU and fluorescent-labeled rhIDU systematically differed, we compared rhIDU enzymatic activity (measured by biochemical assay) with fluorescence intensity ex vivo in organs of antibody-negative and antibody-positive mice. Linear regression showed a relationship between ex vivo fluorescence intensity measurements of whole organs and alpha-l-iduronidase (IDU) activity in homogenized tissue in the heart and lung (heart: r2 = 0.86, F(1,8) = 47.94, p < 0.001; lung: r2 = 0.49, F(1,8) = 7.67, p = 0.02; Figure S3). There were no significant mean intergroup differences between fluorescence intensity measurements of fluorescent-labeled rhIDU versus those that we found using rhIDU activity by enzymatic assay (p = non-significant [NS]). There was less agreement in the kidney, where the difference that we measured between antibody-negative and antibody-positive groups using ex vivo fluorescence (0.75 ± 0.56) was smaller than the difference that we measured using the rhIDU activity assay (1.44 ± 1.17; p = NS). The reason for the slight but statistically insignificant disparity may be that the presence of dissociated fluorescent label in the urine was causing a high fluorescence signal in both the antibody-negative and antibody-positive humoral immune response groups, thus diluting the intergroup difference that we observed. To test this hypothesis, we evaluated the bladders and urine of treated mice. Mice that were treated with fluorescent-labeled rhIDU showed a bright fluorescence signal in the bladder, but their urine contained no measurable rhIDU activity, suggesting that the fluorescence intensity that we observed in the bladder was due to free fluorescent label that had been excreted into the urine (Figure S4).
Altered Cellular Distribution of rhIDU in the Liver of Antibody-Negative and Antibody-Positive Mice
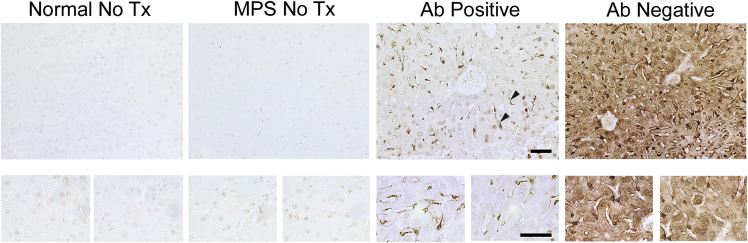
Ex vivo imaging of the whole liver revealed that antibody-negative MPS I mice had similar overall fluorescence intensity as antibody-positive MPS I mice, whereas in other organs, the antibody-negative MPS I mice appeared similar to naive mice, with respect to accumulation of labeled enzyme (Figure 1C). The liver is relatively rich in macrophage-lineage cells, such as sinusoidal Kupffer cells, that may be expected to take up rhIDU even when it is bound to antibodies. We therefore hypothesized that rhIDU might be preferentially localized to sinusoidal cells rather than hepatocytes within the livers of high-titer mice, whereas low-titer mice may show a more even distribution of rhIDU between sinusoidal cells and hepatocytes. To test this hypothesis, antibody-negative mice and antibody-positive mice were compared using immunohistochemistry to determine whether the humoral immune response alters the cellular distribution of rhIDU (Figures 2 and S5). In the liver, rhIDU was found in sinusoidal cells that morphologically appeared to be Kupffer cells in both antibody-negative and antibody-positive MPS I mice. However, antibody-positive MPS I mice showed qualitatively less rhIDU in hepatocytes.
Figure 2.
Altered Cellular Distribution of rhIDU in High-Titer Mice Compared to Low-Titer Mice
Immunohistochemistry of liver tissue sections using anti-rhIDU antibody (1:2,000, brown). Representative images are shown of untreated wild-type mice (Normal NoTx; n = 4), untreated MPS I mice (MPS I NoTx; n = 3), MPS I mice treated with rhIDU with anti-IDU antibody titers 3–30 OD U/μL (AB Positive; n = 5), and MPS I mice treated with rhIDU with anti-IDU antibody titers < 1 OD U/μL (AB Negative; n = 5). Liver sinusoidal cells are indicated by arrowheads. Top row: 20× magnification. Bottom row: 63× magnification. Scale bar, 50 μm.
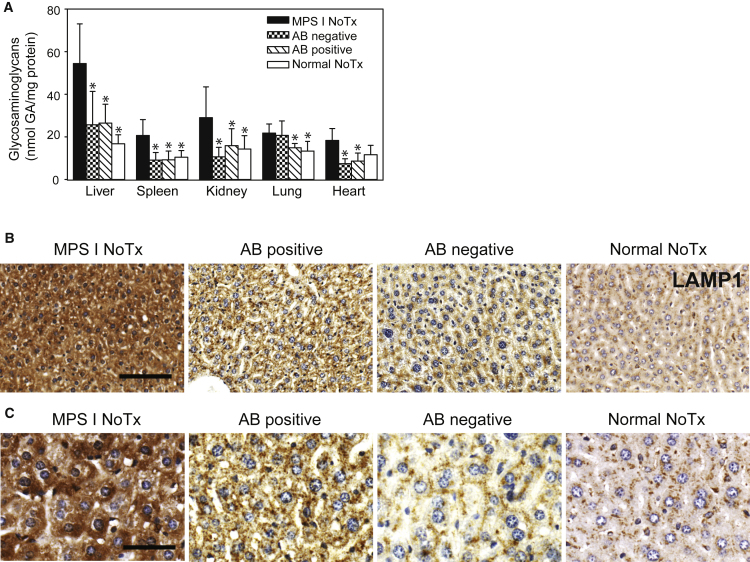
With the observed differences in rhIDU distribution between antibody-negative and antibody-positive mice, we reasoned that the lysosomal accumulation of dermatan and heparan sulfate glycosaminoglycans (the substrates for IDU) would be reduced in low-titer mice compared to high-titer mice in organs. Glycosaminoglycan levels were lower in antibody-positive-treated MPS I mice, antibody-negative-treated MPS I mice, and untreated control mice compared to untreated MPS I mice in the liver (F(3,26) = 14.61; p ≤ 0.001 by ANOVA with post hoc Dunnett’s test), spleen (F(3,16) = 36.79; p < 0.001 by ANOVA with post hoc Dunnett’s test), and kidney (F(3,26) = 9.70; p ≤ 0.001 by ANOVA with post hoc Dunnett’s test). For the lung, a statistically significant difference in glycosaminoglycan levels compared to untreated MPS I mice was found only for control and antibody-positive-treated MPS I mice (F(3,16) = 4.49; p = 0.01 by ANOVA with post hoc Dunnett’s test), and for the heart, significance was found only for antibody-positive- and antibody-negative-treated MPS I mice (F(3,26) = 5.90; p ≤ 0.007 by ANOVA with post hoc Dunnett’s test). We found no difference in glycosaminoglycan values by biochemical assay of whole organ homogenates between antibody-positive and antibody-negative MPS I mice treated with rhIDU (Figure 3A). However, at the tissue level, we found a qualitatively greater reduction in liver staining for lysosomal associated membrane protein-1 (Lamp1) (a marker of lysosomal storage) in antibody-negative MPS I mice compared with antibody-positive MPS I mice treated with rhIDU (Figures 3B and 3C), indicating that anti-rhIDU antibodies may be adversely affecting the ability of rhIDU to reduce lysosomal storage in the liver.
Figure 3.
Lysosomal Storage in MPS I Mice
(A) Glycosaminoglycan levels in internal organs of antibody-positive and antibody-negative MPS I mice (n = 5 per group) after 16 weeks of rhIDU treatment compared to controls. Ten untreated MPS I mice (MPS I NoTx) and ten untreated wild-type normal mice (Normal NoTx) were used as controls (n = 10 per group). Glycosaminoglycans were measured by the carbazole reaction and reported as mol glucuronic acid (GA) per mg protein. Error bars represent SD. *p < 0.05 versus MPS I NoTx. (B and C) Lysosomal-associated membrane protein staining in liver of MPS I and normal mice. Representative images are shown of untreated wild-type mice (Normal NoTx; n = 3), untreated MPS I mice (MPS I NoTx; n = 3), MPS I mice treated with rhIDU with anti-IDU antibody titers 3–30 OD U/μL (AB Positive; n = 4), and MPS I mice treated with rhIDU with anti-IDU antibody titers < 1 OD U/μL (AB Negative; n = 4). 20× magnification (B); scale bar, 100 μm (B). 40× magnification (C); scale bar, 50 µm.
Enhanced rhIDU Uptake into Macrophages in the Presence of Anti-rhIDU Antibodies
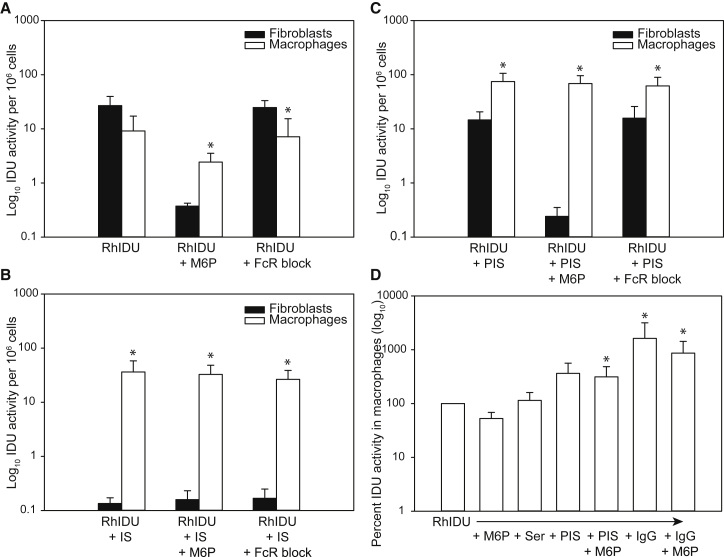
Because rhIDU preferentially localized to sinusoidal cells in antibody-positive mice, we performed an in vitro experiment to determine whether macrophages, which express Fc receptors as well as mannose 6-phosphate receptors, could take up rhIDU in the presence of anti-rhIDU antibodies. We isolated macrophages from bone marrow obtained from MPS I mice and incubated these bone-marrow-derived macrophages with rhIDU in the presence or absence of immunized rabbit serum, a murine Fc receptor blocking agent, and/or mannose 6-phosphate. We evaluated two types of immunized rabbit serum: one that completely abolished rhIDU uptake into human MPS I fibroblasts (inhibiting serum [IS]) and one that only partially inhibited rhIDU uptake into fibroblasts (partially inhibiting serum [PIS]). In the absence of immune serum, uptake of rhIDU into fibroblasts was not significantly different from uptake into murine macrophages (Figure 4A; t(4.59) = −2.54; Bonferroni-adjusted p = 0.54). In the presence of immune serum, uptake of rhIDU into macrophages per cell was greater than uptake into fibroblasts (Figure 4B; t(5.00) = 4.04; Bonferroni-adjusted p = 0.089; and Figure 4C, t(4.57) = 5.53; Bonferroni-adjusted p = 0.04). The uptake of rhIDU into murine macrophages in the presence of immune serum (17.2–118 activity units per 106 cells) was also greater than uptake into murine macrophages in the absence of serum (1.07–18.2 U per 106 cells). The uptake of rhIDU into murine macrophages was only partially inhibited by mannose 6-phosphate, whereas uptake of rhIDU into human fibroblasts was nearly abolished with the addition of mannose 6-phosphate, consistent with prior results.8 Murine Fc receptor blocking agent did not substantially affect the uptake of rhIDU into either murine macrophages or human fibroblasts. However, the blocking agents do not bind to all Fc receptors, limiting our ability to draw conclusions about the mechanism of antibody-bound rhIDU uptake into macrophages.
Figure 4.
Uptake of rhIDU into MPS I Human Fibroblasts and Murine Macrophages
(A–C) Intracellular IDU activity per million cells in the absence of immune serum (A), in the presence of inhibiting immune serum (IS) (anti-rhIDU antibody titer 752 OD U/μL) (B), or in the presence of partially inhibiting immune serum (PIS) (titer 450 OD U/μL) (C), with and without mannose 6-phosphate (M6P) or an Fc receptor blocking agent (FcR block). For all panels, uptake into human fibroblasts is shown as filled bars and uptake into murine macrophages is shown as open bars. (A–C) were run together as a single experiment (replicated five times). Error bars represent SD. *p < 0.05 versus human fibroblasts. (D) Intracellular rhIDU uptake into murine macrophages in the presence of no serum (RhIDU), nonimmune serum (Ser), PIS, or purified rabbit anti-rhIDU IgG antibodies (IgG) with or without M6P. IDU activity is expressed as a percent of uptake into murine macrophages in the absence of any factors. Error bars represent SD. *p < 0.05 versus rhIDU alone.
To confirm that the enhanced macrophage uptake that we observed in the presence of immune serum was due to anti-rhIDU antibodies within the serum, we purified rabbit anti-rhIDU IgG antibodies from serum and applied them to murine MPS I macrophages in the presence of rhIDU. We found a mean 16-fold increase in rhIDU uptake into murine MPS I macrophages in the presence of the purified anti-rhIDU IgG antibodies compared with no antibodies (F(6, 35) = 5.00; p = 0.001; Figure 4D). This result suggests that anti-rhIDU IgG antibodies were the cause of the increased rhIDU uptake into macrophages that we observed in the presence of immune serum.
Discussion
Here, we report the maldistribution of rhIDU with low levels of anti-iduronidase antibodies in MPS I mice receiving intravenous rhIDU. The study demonstrated that even a low-level humoral immune response against rhIDU, which only partially inhibits rhIDU uptake into fibroblasts in vitro, nevertheless altered its tissue distribution in vivo. At the cellular level, antibody-positive mice showed reduced rhIDU distribution in hepatocytes, whereas distribution to tissue macrophages was maintained. In vitro, immunized serum reduced rhIDU uptake into human fibroblasts, but increased uptake into murine macrophages. The altered tissue distribution of rhIDU caused by anti-rhIDU antibodies appeared to be partly due to reduced uptake into fibroblasts and partly due to enhanced uptake into tissue macrophages. These results imply that functional immune assays of rhIDU uptake in vitro into fibroblasts may not completely predict the impact of the humoral immune response against enzyme replacement therapy.
Delivery of recombinant proteins to treat diseases caused by genetic haploinsufficiency or a total lack of a functional protein has proven immensely valuable for treating patients, alleviating suffering and prolonging and improving lifespan. However, the degree of amelioration of symptoms may be limited by a humoral immune response against recombinant proteins that can alter tissue distribution of protein. Why and how this occurs is poorly understood. Designing strategies to alleviate or circumvent the immune response may improve the effectiveness of protein therapeutics for the benefit of patients. Such novel inventions will first require deeper insight into underlying mechanisms.
Our results here show that even a low-level humoral immune response against rhIDU that only partially inhibits rhIDU uptake into fibroblasts in vitro nevertheless may significantly alter its tissue distribution in vivo. In the liver, we found reduced rhIDU distribution to hepatocytes in antibody-positive mice, but distribution to tissue macrophages was maintained. Antibodies also reduced uptake of rhIDU into human fibroblasts, but increased uptake of rhIDU into murine macrophages. Our results also corroborate and extend our previous study in dogs with MPS I that reported that a high-titer humoral immune response against rhIDU altered the tissue distribution of rhIDU preferentially toward organs and tissues with high reticuloendothelial cell content and greatly diminished uptake into human fibroblasts.5 Others have found similar altered biodistribution of rhIDU in immunized rats, with high antibody titers causing enhanced uptake into the liver as well as subcellular distribution into the endosomal compartment.9
In the present study using MPS I mice, we found similar alterations in the tissue distribution of rhIDU, but our in vitro studies comparing the uptake of rhIDU into fibroblasts and macrophages showed surprising results. In the absence of immune serum, the uptake of rhIDU into macrophages was less than the uptake of rhIDU into human fibroblasts when normalized to cell number. However, when anti-rhIDU antibodies were present, the uptake of rhIDU into macrophages was greater even than the uptake of rhIDU into human fibroblasts when no antibodies were present. Collectively then, our results and our previous studies5 indicate that anti-rhIDU antibodies may alter tissue distribution both by suppressing fibroblast uptake and by enhancing tissue macrophage uptake. These results suggest that new strategies to enhance effectiveness of rhIDU might focus on methods that will both improve fibroblast uptake and reduce macrophage uptake of rhIDU. Furthermore, designing in vitro assays that test fibroblast and macrophage uptake of rhIDU (and perhaps other recombinant proteins) could constitute a relatively rapid screening method, as opposed to relying on empiric testing in vivo. Our results also suggest that in vitro assays to assess the likely clinical impact of anti-rhIDU antibodies should not solely focus on the inhibition of uptake into fibroblasts, and underscore the need to develop new strategies to limit humoral responses to enzyme replacement therapy that significantly limits the effectiveness of such therapies in MPS I and probably other lysosomal storage diseases as well.
The extent to which our results can be generalized to other diseases that are treated by replacement of missing proteins with recombinant proteins remains to be determined, but clearly our results are relevant to at least some such therapeutic situations besides MPS I. For example, Fabry disease, which is caused by deficiency of the lysosomal enzyme alpha-galactosidase A, is treated by intravenous delivery of recombinant enzyme. But the therapeutic response varies considerably, and one contributor to that variability is the presence and levels of anti-galactosidase A antibodies that neutralize enzymatic activity and can be associated with higher tissue and urinary levels of globotriaosylceramide substrate.10 Similarly, individuals with forms of Pompe disease (caused by a genetic deficiency of acid alpha-glucosidase) that produce no alpha-glucosidase at all are at particularly high risk of developing adverse immune responses associated with poorer outcomes.11
In contrast, delivery of recombinant human acid alpha-glucosidase to patients with forms of Pompe disease in which minimal or modest amounts of enzyme are produced also induces early production of high titers of antibodies against that protein, but titers decrease over time and do not typically affect uptake or activity of the recombinant enzyme. This seems to be due to a regulatory T cell-mediated immunosuppression.12 That interpretation was also supported by a report that anti-CD3 antibodies reduced anti-human alpha-glucosidase antibody titers in mice and prevented hypersensitivity reactions, and these effects were accompanied by reduced numbers of both CD4+ and CD8+ T cells and an increased ratio of regulatory T cells (i.e., CD4+ CD25+ FoxP3+) to CD4+ T cells.13 In addition, antibodies against glucocerebrosidase are not usually a problem in enzyme replacement therapy for type 1 Gaucher disease, in part because these patients are not null for glucocerebrosidase and in part because macrophages are the target of therapy.14
Furthermore, mechanisms by which antibodies might interfere with efficacy of recombinant proteins differ. For some therapeutic enzymes, the immune response hinders efficacy by abolishing enzymatic activity, whereas in other cases, the mechanism is inhibition of uptake and lysosomal targeting. Antibodies to rhIDU do not affect enzymatic activity, unlike the immune response to recombinant human alpha-galactosidase A for Fabry disease.15 Rather, anti-rhIDU antibodies interfere with uptake via mannose 6-phosphate receptors.5 Clinically, patients with anti-rhIDU antibodies show less reduction in urinary glycosaminoglycan excretion following rhIDU treatment, implying a possible impairment of biochemical efficacy.3, 16 Uptake inhibition by anti-drug antibodies has been shown for other recombinant enzymes, such as recombinant alpha-galactose 6-sulfatase for Morquio A, although there is no evidence that the antibodies impact clinical outcomes in this disorder.17
Notwithstanding the complexities and variability in immunogenic responses to administration of recombinant therapeutic proteins,18, 19, 20 our results provide important new insights that will prove useful in designing new therapeutic strategies that maximize therapeutic effects of rhIDU in MPS I. Furthermore, our results might guide the design of therapies against other genetic diseases, in which delivery of recombinant proteins is effective, for example, the six lysosomal storage disorders, in which US Food and Drug Administration (FDA)-approved recombinant enzyme replacement therapy is available and used clinically.18
Materials and Methods
Study Animals and Enzyme Replacement Therapy
Animal experiments were approved by the Institutional Animal Care and Use Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
The MPS I knockout mouse (B6.129-Iduatm1Clk/J) was acquired from Jackson Laboratory (Bar Harbor, ME) and maintained on an inbred background (C57BL/6).21 Heterozygous males and females were used to generate mutant (Idua−/−) and wild-type (Idua+/+) mice with mixed gender for this study. Genotype was determined with primers: oIMR1451: 5′-GGAACTTTGAGACTTGGAATGAACCAG-3′; oIMR1452: 5′-CATTGTAAATAGGGGTATCCTTGAACTC-3′; and oIMR1453: 5′-GGATTGGGAAGACAATAGCAGGCATGCT-3′. In order to avoid the interference of infrared signals caused by chlorophyll-degraded products, animals received a special chlorophyll-free diet AIN-76A (Research Diet, New Brunswick, NJ) and water ad libitum and were housed in constant temperature/humidity, with a 12 hr light:12 hr dark cycle.
MPS I mice (n = 13) were sensitized to the enzyme by a weekly intravenous dose of 1.57 mg/kg body weight rhIDU (formulated as laronidase, lot #V011004 and V011005, 0.58 mg/mL in 150 mM NaCl, 100 mM sodium phosphate, 0.001% polysorbate 80, pH ∼5.8, BioMarin Pharmaceutical, Novato, CA) from 4 weeks to 15 weeks of age via tail vein injection. This dose was selected based on previous studies in dogs.22 Three mice with an anti-rhIDU IgG antibody titer greater than 15 OD U/μL died prior to the completion of the experiment, apparently from infusion reactions. Specifically, one mouse was found dead 3 days after the 12th week of infusion. Two mice died from anaphylaxis that occurred immediately following infusions at week 12 (n = 1) and week 14 (n = 1). Anaphylaxis manifested as labored breathing and lack of response to stimulus and tail pinch. We began subsequent infusions with injections of diphenhydramine (5 mg/kg body weight) 30 min prior to the procedure via intraperitoneal injection to prevent the anaphylaxis reactions. At 16 weeks of age, MPS I mice previously sensitized to rhIDU (n = 10) and naive to rhIDU (n = 6) were administrated one dose of 1.57 mg/kg body weight of Alexa Fluor 680 labeled rhIDU (AF680-rhIDU; degree of labeling 1.3 mol label/mol protein), which was previously showed to retain its biochemical properties (including enzymatic activity, cellular uptake, and lysosomal delivery).8
Sample Collections and Fluorescent Imaging
Blood samples were collected through the facial submandibular vein every 4 weeks beginning at week 4. In vivo fluorescence intensity was measured in un-injected control mice (n = 2) and naive MPS I (n = 6) mice at 0, 15, and 30 min and 1, 2, and 24 hr after the AF680-rhIDU injection (at week 16) using the Maestro EX in vivo fluorescence imaging system (Cambridge Research & Instrumentation [Cri], now PerkinElmer [Caliper Life Sciences], Waltham, MA), with red excitation and emission filters for wavelengths at a range of 720–850 nm for 2,000 ms exposure. Average fluorescence intensity was calculated using Maestro software (version 3.012).
24 hr after the injection of AF680-rhIDU, mice were sacrificed by CO2 asphyxiation and terminal blood samples were collected by cardiac exsanguination. For biochemical evaluation, a portion of the organs (hearts, lungs, spleens, kidneys, and livers) were rapidly frozen in dry ice and stored at −80°C before homogenization. The rest of the organs were imaged by Maestro EX imaging system to quantify the distribution of AF680-rhIDU labeled enzyme and their fluorescence intensity before fixation in 10% buffered formalin and further processed for paraffin embedding and histology.
Biochemical Evaluations
For biochemical assays, organs were weighed (wet weight) and homogenized in 3 volumes of PAD buffer (10 mM sodium phosphate, pH 5.8, 0.02% sodium azide, 0.1 mM dithiothreitol, and 0.1% Triton X-100). Iduronidase activity was assessed using 250 μmol/L 4-methylumbelliferyl α-l-iduronide substrate (4-MUI) (Glycosynth, Warrington, Cheshire, UK), as previously described, but with a modified incubation temperature (37°C) and incubation time (30 min).23 Net fluorescence was determined by RF-1501 spectrofluorophotometer (Shimadzu, Columbia, MD) at the wavelength of 365 nm and 440 nm for excitation and emission, respectively. One activity unit is defined as the activity catalyzing the hydrolysis of 1 nmol substrate in 1 hr at 37°C. Protein concentrations in the extracts were determined by the Bradford method (Bio-Rad, Irvine, CA). Results were expressed as U/mg of protein. GAG amount in tissue was assessed using the carbazole reaction with glucuronic acid as the standard.24 Tissue results were expressed as nmol GAG (glucuronic acid) per mg protein.
Immunohistochemistry
Immunohistochemistry was performed with the EXPOSE IHC detection kit (Abcam, Cambridge, MA) on 10-μm-thick organ sections with antibody against rhIDU (BP13, kindly donated by BioMarin Pharmaceutical) and LAMP1 (ab24170, Abcam, Cambridge, MA) following the manufacturer’s instructions. Slides were counterstained with hematoxylin for light microscopic evaluation.
Cellular Uptake of rhIDU in MPS I Fibroblasts and Macrophages
MPS I mouse macrophages were derived from bone marrow monocytes following the protocol of Zhang et al.,25 with minor modifications. In brief, bone marrow monocytes were collected from the femurs and cultured in the medium containing 10 ng/mL macrophage colony-stimulating factor (M-CSF) (PeproTech, Rocky Hill, NJ) for 7 days. After eliminating non-adherent cells, adherent macrophages were harvested for subsequent assays.
Freshly cultured bone-marrow-derived macrophages from MPS I mouse and human skin fibroblasts from an individual with MPS I Hurler (GM 1391; Coriell Institute for Medical Research, Camden, NJ) were seeded in 12-well cell culture plates at the concentration of 300,000 cells/wells. RhIDU was diluted to 18 U/mL in MEM plus 1% l-glutamine. Rabbit anti-rhIDU IgG was obtained from two different immunized rabbits by purification of serum on a protein A column (Thermo Fisher Scientific, Waltham, MA). Pre-immune rabbit serum, immune serum, or purified anti-rhIDU IgG were added at 1:1,000 dilution and preincubated for 1 hr at 37°C with diluted rhIDU at the presence or absence of 5 mM mannose 6-phosphate (Sigma-Aldrich, St. Louis, MO) or 0.5 μg/mL murine Fc receptor blocking agent (eBioscience, San Diego, CA). The diluted serum-rhIDU mixture (0.5 mL) with or without inhibitor(s) was added to human MPS I fibroblasts or murine MPS I bone-marrow-derived macrophages and incubated for 1 hr at 37°C and 5% CO2. After 1 hr, cells were harvested and counted before they were lysed in 60 μL of PAD. Intracellular iduronidase activity was measured as described above and results were expressed as U/million cells.
Measurement of rhIDU-Specific IgG
Specific IgG antibodies against rhIDU were measured by ELISA. Briefly, 96-well plates (Corning, Corning, NY) were coated overnight with 0.2 μg rhIDU in acidic sodium phosphate buffer (pH 5.8). Following blocking with 3% BSA in acidic phosphate-buffered saline (pH 5.8), serial dilutions of serum were added in triplicate to rhIDU-coated plates and incubated at 37°C for 1 hr. The plates were washed, and alkaline-phosphatase-conjugated goat anti-mouse IgG secondary antibody (Southern Biotechnology Associates, Birmingham, AL) was added and allowed to incubate for 1 hr at 37°C. Following a final wash, 1 mg/mL p-nitrophenol phosphate substrate (Sigma-Aldrich, St. Louis, MO) was added and allowed to develop at room temperature. The reaction was stopped after 1 hr by the addition equal volume of 0.1 M EDTA, and absorbance values were read at 405 nm as OD units using a Synergy 2 spectrophotometer (BioTek, Winooski, VT). The calculated OD value for any sample was taken from dilutions within the linear signal range.
Determination of Uptake Inhibition of rhIDU in Mouse Serum Samples
MPS I human skin fibroblasts GM 1391 were seeded in 12-well cell culture cluster plates for a concentration of 300,000 cells/wells. Serum samples collected at week 12 of the mice in the study was added at 1:1,000 dilution and preincubated for 1 hr at 37°C with 18 U/mL rhIDU. The serum-rhIDU mixture (0.5 mL) was then added to MPS I fibroblasts and incubated for 1 hr at 37°C and 5% CO2. Cells were lysed in 60 μL of PAD buffer and centrifuged. Intracellular iduronidase activity was measured with 15 μL cell lysate in triplicate as described above, and results were normalized to pooled serum-rhIDU samples from non-immunized controls and expressed as a percentage toward the control sample.
Statistics
We performed statistical analyses using SYSTAT13 (Systat, Chicago, IL). We used two-sample, two-tailed t tests, without adjustment for multiple comparisons, or ANOVA with post hoc pairwise comparisons, as specified in the results. Where noted, we adjusted p values using the Bonferroni method to correct for multiple comparisons. Linear regression analyses were performed using SYSTAT13 by the least-squares method.
Author Contributions
S.Q.L., S.-h.K., D.C., V.S., K.N.V., M.E., and M.U.V. performed experiments. S.-h.K., D.C., V.S., T.M.D., M.U.V., M.I., M.S.S., M.E., J.D.C., and P.I.D. designed experiments and analyzed data. S.Q.L, S.-h.K., T.M.D., J.D.C., M.S.S., and P.I.D. wrote the manuscript.
Conflicts of Interest
P.I.D., M.S.S., and J.D.C. receive research support from BioMarin Pharmaceutical Inc., which manufactures laronidase.
Acknowledgments
Thanks to Catalina Guerra and Jenny Dancourt (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center) for assistance with the animals. Funding was provided by the Eunice Kennedy Shriver Institute for Child Health and Human Development and the National Institute for Neurological Disorders and Stroke at the NIH (R03 HD074907 and R01 NS085381 to P.I.D. and R01 NS043205 to M.S.S.), the American Heart Association (12SDG9260007 to M.I.), and by support from the Los Angeles Biomedical Research Institute and the Department of Pediatrics at Harbor-UCLA Medical Center. S.-h.K. was supported by a T32 fellowship in the UCLA Medical Genetics Training Program (GM008243). RhIDU in the form of laronidase was donated by BioMarin Pharmaceutical Inc.
Footnotes
Supplemental Information includes five figures and can be found with this article online at https://doi.org/10.1016/j.omtm.2017.09.008.
Supplemental Information
References
- 1.Banugaria S.G., Prater S.N., Patel T.T., Dearmey S.M., Milleson C., Sheets K.B., Bali D.S., Rehder C.W., Raiman J.A., Wang R.A. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: a step towards improving the efficacy of ERT. PLoS One. 2013;8:e67052. doi: 10.1371/journal.pone.0067052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elder M.E., Nayak S., Collins S.W., Lawson L.A., Kelley J.S., Herzog R.W., Modica R.F., Lew J., Lawrence R.M., Byrne B.J. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J. Pediatr. 2013;163:847–854.e1. doi: 10.1016/j.jpeds.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giugliani R., Rojas V.M., Martins A.M., Valadares E.R., Clarke J.T.R., Góes J.E.C., Kakkis E.D., Worden M.A., Sidman M., Cox G.F. A dose-optimization trial of laronidase (Aldurazyme) in patients with mucopolysaccharidosis I. Mol. Genet. Metab. 2009;96:13–19. doi: 10.1016/j.ymgme.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Lozier J., Johnson G., Kirshner S., Verthelyi D., Pariser A., Shores E., Rosenberg A. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat. Biotechnol. 2008;26:901–908. doi: 10.1038/nbt.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson P., Peinovich M., McEntee M., Lester T., Le S., Krieger A., Manuel H., Jabagat C., Passage M., Kakkis E.D. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands M.S., Vogler C.A., Ohlemiller K.K., Roberts M.S., Grubb J.H., Levy B., Sly W.S. Biodistribution, kinetics, and efficacy of highly phosphorylated and non-phosphorylated β-glucuronidase in the murine model of mucopolysaccharidosis VII. J. Biol. Chem. 2001;276:43160–43165. doi: 10.1074/jbc.M107778200. [DOI] [PubMed] [Google Scholar]
- 7.Dickson P.I., Ellinwood N.M., Brown J.R., Witt R.G., Le S.Q., Passage M.B., Vera M.U., Crawford B.E. Specific antibody titer alters the effectiveness of intrathecal enzyme replacement therapy in canine mucopolysaccharidosis I. Mol. Genet. Metab. 2012;106:68–72. doi: 10.1016/j.ymgme.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tippin B.L., Troitskaya L., Kan S.H., Todd A.K., Le S.Q., Dickson P.I. Biochemical characterization of fluorescent-labeled recombinant human alpha-L-iduronidase in vitro. Biotechnol. Appl. Biochem. 2011;58:391–396. doi: 10.1002/bab.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner C.T., Hopwood J.J., Brooks D.A. Enzyme replacement therapy in mucopolysaccharidosis I: altered distribution and targeting of alpha-L-iduronidase in immunized rats. Mol. Genet. Metab. 2000;69:277–285. doi: 10.1006/mgme.2000.2979. [DOI] [PubMed] [Google Scholar]
- 10.Oder D., Nordbeck P., Wanner C. Long term treatment with enzyme replacement therapy in patients with Fabry disease. Nephron. 2016;134:30–36. doi: 10.1159/000448968. [DOI] [PubMed] [Google Scholar]
- 11.Berrier K.L., Kazi Z.B., Prater S.N., Bali D.S., Goldstein J., Stefanescu M.C., Rehder C.W., Botha E.G., Ellaway C., Bhattacharya K. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet. Med. 2015;17:912–918. doi: 10.1038/gim.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masat E., Laforêt P., De Antonio M., Corre G., Perniconi B., Taouagh N., Mariampillai K., Amelin D., Mauhin W., Hogrel J.Y., French Pompe Registry Study Group Long-term exposure to Myozyme results in a decrease of anti-drug antibodies in late-onset Pompe disease patients. Sci. Rep. 2016;6:36182. doi: 10.1038/srep36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohashi T., Iizuka S., Shimada Y., Higuchi T., Eto Y., Ida H., Kobayashi H. Administration of anti-CD3 antibodies modulates the immune response to an infusion of α-glucosidase in mice. Mol. Ther. 2012;20:1924–1931. doi: 10.1038/mt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishnani P.S., Dickson P.I., Muldowney L., Lee J.J., Rosenberg A., Abichandani R., Bluestone J.A., Burton B.K., Dewey M., Freitas A. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol. Genet. Metab. 2016;117:66–83. doi: 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Linthorst G.E., Hollak C.E.M., Donker-Koopman W.E., Strijland A., Aerts J.M. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004;66:1589–1595. doi: 10.1111/j.1523-1755.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- 16.Clarke L.A., Wraith J.E., Beck M., Kolodny E.H., Pastores G.M., Muenzer J., Rapoport D.M., Berger K.I., Sidman M., Kakkis E.D. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 17.Melton A.C., Soon R.K., Jr., Tompkins T., Long B., Schweighardt B., Qi Y., Vitelli C., Bagri A., Decker C., O’Neill C.A. Antibodies that neutralize cellular uptake of elosulfase alfa are not associated with reduced efficacy or pharmacodynamic effect in individuals with Morquio A syndrome. J. Immunol. Methods. 2017;440:41–51. doi: 10.1016/j.jim.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Broomfield A., Jones S.A., Hughes S.M., Bigger B.W. The impact of the immune system on the safety and efficiency of enzyme replacement therapy in lysosomal storage disorders. J. Inherit. Metab. Dis. 2016;39:499–512. doi: 10.1007/s10545-016-9917-1. [DOI] [PubMed] [Google Scholar]
- 19.De Groot A.S., Scott D.W. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Vinuesa C.G., Chang P.-P. Innate B cell helpers reveal novel types of antibody responses. Nat. Immunol. 2013;14:119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- 21.Clarke L.A., Russell C.S., Pownall S., Warrington C.L., Borowski A., Dimmick J.E., Toone J., Jirik F.R. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum. Mol. Genet. 1997;6:503–511. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- 22.Dierenfeld A.D., McEntee M.F., Vogler C.A., Vite C.H., Chen A.H., Passage M., Le S., Shah S., Jens J.K., Snella E.M. Replacing the enzyme α-l-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakkis E.D., McEntee M.F., Schmidtchen A., Neufeld E.F., Ward D.A., Gompf R.E., Kania S., Bedolla C., Chien S.L., Shull R.M. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem. Mol. Med. 1996;58:156–167. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- 24.Manzi A., Esko J. Direct chemical analysis of glycoconjugates for carbohydrates. Curr. Protoc. Mol. Biol. 2001;32:17.9.1–17.9.11. doi: 10.1002/0471142727.mb1709s32. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., Goncalves R., Mosser D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008;83:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.