Abstract
Schizophrenia Spectrum Disorders (SSD) are known to be characterised by abnormalities in attentional processes, but there are inconsistencies in the literature that remain unresolved. This article considers whether perceptual resource limitations play a role in moderating attentional abnormalities in SSD. According to perceptual load theory, perceptual resource limitations can lead to attenuated or superior performance on dual-task paradigms depending on whether participants are required to process, or attempt to ignore, secondary stimuli. If SSD is associated with perceptual resource limitations, and if it represents the extreme end of an otherwise normally distributed neuropsychological phenotype, schizotypal traits in the general population should lead to disproportionate performance costs on dual-task paradigms as a function of the perceptual task demands. To test this prediction, schizotypal traits were quantified via the Schizotypal Personality Questionnaire (SPQ) in 74 healthy volunteers, who also completed a dual-task signal detection paradigm that required participants to detect central and peripheral stimuli across conditions that varied in the overall number of stimuli presented. The results confirmed decreasing performance as the perceptual load of the task increased. More importantly, significant correlations between SPQ scores and task performance confirmed that increased schizotypal traits, particularly in the cognitive-perceptual domain, are associated with greater performance decrements under increasing perceptual load. These results confirm that attentional difficulties associated with SSD extend sub-clinically into the general population and suggest that cognitive-perceptual schizotypal traits may represent a risk factor for difficulties in the regulation of attention under increasing perceptual load.
Keywords: Schizotypy, Perception, Attention, Perceptual load, Selective attention
1. Introduction
Recent evidence suggests that the clinically defining positive (hallucinations, disorganised thought, delusions) and negative (apathy, impoverished speech, lack of drive, anhedonia, social withdrawal) symptoms of Schizophrenia Spectrum Disorders (SSD) represent the extreme ends of otherwise normally distributed schizotypal traits in three independent functional domains: Cognitive-perceptual, Interpersonal, and Disorganisation (Raine, 1991). If this spectrum view of SSD is correct, schizotypal traits in the general population should be associated with changes in associated cognitive domains (Ettinger et al., 2015).
One of the abnormal cognitive domains that has been frequently reported in SSD is selective attention (Andreasen, 1999, Ettinger et al., 2015). For instance, slower responses have been reported in visual search tasks where participants have to locate a target stimulus among varying numbers of distracters (Carr et al., 1998). Difficulties in the shifting of attention (Bellgrove et al., 2003) and in inhibiting task-irrelevant information have also been documented (Fuentes et al., 1999, Fuller et al., 2000, MacQueen et al., 2003, Salo et al., 1996). Gold et al., 2007 have argued that the source of these abnormalities does not lie in the selection of information for attention, but in the top-down regulation of selective attention by executive control processes. On Posner cueing tasks (Posner, 1980), in which participants need to respond as quickly as possible to the location of a target stimulus that is preceded by a cue, patients with schizophrenia demonstrate response time benefits and costs for valid and invalid cues that are comparable to control participants (Bustillo et al., 1997, Gouzoulis-Mayfrank et al., 2007, Pardo et al., 2000). Although their response times are slower overall, this sensitivity to cues demonstrates a preserved ability to selectively attend to likely target locations, which has also been shown on visual search tasks (Elahipanah et al., 2008, Gold et al., 2009). By contrast, abnormalities tend to arise when tasks require top-down regulation of attention, such as when cues in the Posner paradigm are designated to indicate the opposite location for the target (Maruff et al., 1998) or when attention to salient cues needs to be inhibited in anti-saccade (Radant et al., 2010) or negative priming tasks (Fuller et al., 2000, Salo et al., 1996).
Despite considerable evidence that the executive control of attention is a source of difficulty in SSD (Gold et al., 2007), some evidence suggests that additional factors contribute to atypical patterns of selective attention. Specifically, under some circumstances individuals with SSD are, seemingly, better at allocating attention to task-relevant stimuli. In rapid serial visual presentation (RSVP) paradigms, where participants need to identify a target among a series of rapidly presented distractors, individuals with schizophrenia are less likely than matched controls to miss-report the distractors as targets (Boucart et al., 2000). Giersch et al. (2002) also observed reduced interference from non-pertinent information in SSD during certain orientation matching tasks in which pertinent and non-pertinent information was physically separated. From an executive function perspective, this would indicate enhanced rather than reduced ability to inhibit task-irrelevant information.
Lavie, 2005, Lavie, 1995 perceptual load theory of selective attention may help to reconcile the above pattern of findings. According to Lavie, our ability to effectively allocate attention to task-relevant information (and filter irrelevant information) critically depends on the perceptual demands of the task. A low perceptual load results in an automatic ‘spill over’ of available resources to the processing of task-irrelevant information, whereas a high perceptual load prevents such spill-over and processing is limited to basic task-relevant features. Lavie's load theory has been supported by a number of behavioural (Murphy et al., 2016) and neurophysiological studies (Muggleton et al., 2008, Rees et al., 1999) and has proven valuable for understanding attention abnormalities in other disorders such as Autism Spectrum Disorder (ASD; Fairnie et al., 2016, Remington et al., 2009, Remington et al., 2012, Remington and Fairnie, 2017).
Interestingly, there have been reports that ASD is associated with increased perceptual capacity (Remington et al., 2009, Remington et al., 2012, Remington and Fairnie, 2017), whereas the evidence in SSD would lead to the prediction of decreased perceptual capacity. Specifically, limitations in perceptual capacity could explain the paradoxical finding of seemingly enhanced inhibition of task-irrelevant information when, in fact, there may simply be insufficient perceptual resources available to ‘spill over’ to the processing of such information in the first place. Minassian et al. (2004) have provided preliminary support for this by measuring pupil dilation to stimuli under conditions of varying perceptual load. Pupil dilation is thought to reflect attentional resource allocation (Beatty and Jackson, 1982) and compared to controls, participants with schizophrenia exhibit larger pupil dilation to low-load stimuli.
To our knowledge, only one study has examined perceptual load in SSD. Ducato et al. (2008) asked participants either to identify a black square (low-load task), to locate the larger number between two one-digit numbers (medium-load condition), or to locate the larger number between two several digit numbers (high-load condition) whilst two disks (distractors) simultaneously moved across the screen. All groups showed greater response times in the low load condition, indicating that perceptual resources spilled over to distractor processing, which thus caused interference with the principal task. However, whereas controls only resisted such interference in the high-load condition, patients with schizophrenia and those high on schizotypal traits demonstrated release from interference in both the medium and high load conditions. This suggests that the full spectrum of schizotypy is characterised by reduced perceptual capacities that are exceeded at lower levels of perceptual load.
The aim of the present study is to further test if limited perceptual capacities contribute to abnormal selective attention in schizotypy, but using a dual task paradigm that tests the effects of perceptual load on the ability to detect a peripheral stimulus (rather than to inhibit distracters). Typically, higher perceptual load in such paradigms leads to longer reaction latencies on the central task and also reduced peripheral stimulus detection (Macdonald and Lavie, 2008). Schizotypal traits should therefore be associated with even longer reaction latencies and poorer peripheral target detection at lower levels of perceptual load. Given the observation of the opposite pattern in ASD (Remington et al., 2009, Remington et al., 2012), and the suggestion that ASD and SSD share common etiological mechanisms (Fatemi et al., 2005, King and Lord, 2011, Rapoport et al., 2009), we tested this prediction whilst controlling for autistic traits.
2. Method
2.1. Participants
Seventy-four adults with normal or corrected to normal vision participated in this study (46 female: 28 male, age M = 27.5 years, SD = 8.03 years). The majority were recruited from the student population at the host institution where they were reimbursed with course credits. All procedures were approved by the host Department's ethics committee and all participants provided informed consent.
2.2. Materials & design
Schizotypal traits were measured using the 74-item Schizotypal Personality Questionnaire (SPQ: Raine, 1991) which has a robust three-factor structure to measure cognitive-perceptual, interpersonal and disorganisation schizotypal traits (Raine et al., 1994, Wuthrich and Bates, 2006). An SPQ total of 41 is typically used as the cut-off point for significant high Schizotypy (Raine, 1991). To control for the possible influence of sub-clinical autistic traits on task-performance (Remington et al., 2009, Remington et al., 2012, Remington and Fairnie, 2017), participants completed the 50-item Autism-Spectrum Quotient (AQ; Baron-Cohen et al., 2001), which examines traits commonly associated with ASD.
The experimental task was modelled on the study of Remington et al. (2012) and was presented using E-prime 2.0 on a Dell 17-inch LCD monitor with a 2 ms refresh rate at a viewing distance of 60 cm. Each trial involved the presentation of either 1, 3 or 6 letters around the circumference of an imaginary circle in the centre of the monitor with a radius of 1.7° visual angle (VA). One of these items was a target letter (X or N) with the other positions occupied either by distracter letters (Z, H, K, Y or V) or a dot (.) place holder. Letters measured 0.6° × 0.6° VA and the place holder 0.2° × 0.2° VA. The locations of the target, the distracters and the place holders were counterbalanced across trials.
On 50% of trials, a peripheral stimulus (PS: #) measuring 0.3° × 0.3° VA, was randomly presented in one of six positions of a larger imaginary circle with a radius of 5.4° VA. Items in the inner circle were presented in black on a light grey background (RGB values: 204, 204, 204) and the PS was a darker grey (RGB: 153,153,153). A total of 72 unique trials could be generated, comprising 12 PS-absent and 12 PS-present trials for each set size, and for each of the target letters (X or N) in the 6 possible central circle positions. These 72 trials were repeated across three runs separated by a short break. Within runs, trials of different set sizes were grouped into mini blocks, the order of which was counterbalanced across runs using a Latin square. 12 additional trials at each set size served for practice at the beginning of the experiment and another block of 12 trials of each set size was presented at the end as a control condition to ensure participants could detect the PS when ignoring the central task.
2.3. Procedure
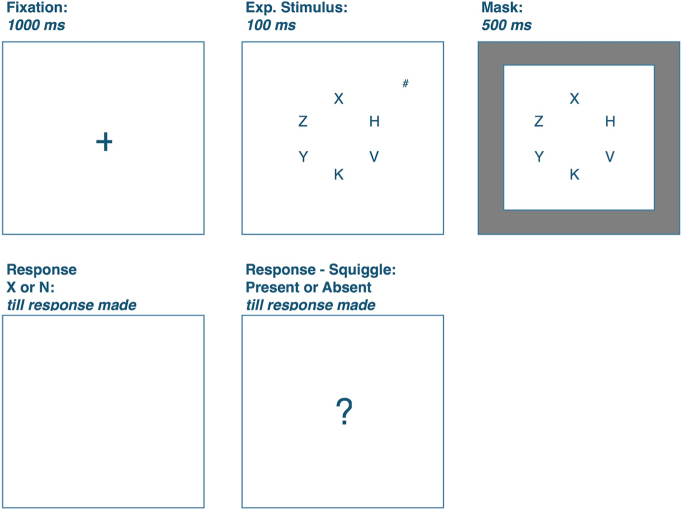
Each trial started with a 1000 ms fixation cross. The central circle of letters then followed for 600 ms and within the first 100 ms of this display the PS square could also appear before being masked by a peripheral black mesh pattern. A blank screen then cued participants to indicate whether the X or N had appeared in the central locations by pressing the ‘A’ or ‘S’ keys on the keyboard as quickly and accurately as possible. Following this response, a screen with a question mark served as the cue for participants to indicate whether the PS was absent or present by pressing the letters ‘K’ or ‘L’ on the keyboard (see Fig. 1). Response accuracy and time (RT) were recorded for both response screens but because the letter task was responded to first, only RTs from this task were analysed. The experiment began with 12 practice trials, followed by the 3 experimental runs and ended with a final control block of trials during which participants were instructed to focus only on the peripheral stimulus.
Fig. 1.
Summary of the trial procedure as illustrated with a PS-present experimental trial of set size 6.
3. Results
On the last control block of trials, 14 participants were unable to detect the peripheral stimulus (PS) above the 50% chance level (M = 44.3%; SD = 7.4%). Since their PS detection was also at chance during the experimental trial blocks (M = 50.3%; SD = 1.3%), they were excluded from subsequent analyses.
The remaining 60 participants (37 female: 23 male; age M = 27.50 years, SD = 8.40 years) reliably detected the PS during the control block (M = 89.8%; SD = 12.4%). Performance on the experimental trials is summarised in Table 1. For the reaction time (RT) analysis of the letter identification task, only trials were considered where both letter and PS detection were accurate and where the RT < 4000 ms (2% of trials failed this cut-off). A repeated-measures ANOVA demonstrated significant increases in RT as a function of set size (F(1.66, 98.0) = 121.40, p < 0.001, n2 = 0.67), with longer RTs for set size 3 compared to set size 1 (t = 8.07, df = 59, p < 0.001, d = 0.47) and longer RTs for set size 6 compared to set size 3 (t = 9.65, df = 59, p < 0.001, d = 0.39). This result confirms an effective perceptual load manipulation, which was further supported by a significant set size effect on letter identification accuracy (F(1.29, 75.94) = 87.74, p < 0.001, n2 = 0.60). Participants demonstrated significantly lower accuracy at set size 6 compared to set size 3 (t = 10.54, df = 59, p < 0.001, d = 1.34) and also lower accuracy at set size 3 compared to set size 1 (t = 2.03, df = 59, p = 0.047, d = 0.30). Finally, a perceptual load effect was also evident in PS detection accuracy (F(1.84, 108.76) = 9.59, p < 0.001, n2 = 0.14), where detection rates were significantly lower at set size 3 compared to set size 1 (t = 3.72, df = 59, p < 0.001, d = 0.17), with no additional performance decrement at set size 6 compared to set size 3 (t = 0.98, df = 59, p = 0.33, d = 0.04).
Table 1.
Summary statistics for the letter identification accuracy and response times and the peripheral stimulus detection accuracy as a function of set size.
| Set size |
||||||
|---|---|---|---|---|---|---|
| 1 |
3 |
6 |
||||
| Measure | Mean | SD | Mean | SD | Mean | SD |
| Letter identification | ||||||
| RT (MS) | 684.62 | 328.42 | 862.21 | 423.33 | 1030.86 | 446.14 |
| Mean accuracy (%) | 97.92 | 2.34 | 96.88 | 4.28 | 87.16 | 9.32 |
| PS detection | ||||||
| Mean accuracy (%) | 82.40 | 1.55 | 79.56 | 1.68 | 78.69 | 1.60 |
Table 2 summarises participant's SPQ and AQ scores. There were no gender differences for either the SPQ (t = 1.04; df = 58; p = 0.30; d = 0.27) or AQ total scores (t = 0.18; df = 58, p = 0.86; d = 0.05) or any of their sub-factors (all ps > 0.12; ds < 0.4) and responses for all scales were normally distributed (Skewness < 1.02; Kurtosis < 0.96). As expected, there was a strong positive correlation between the AQ and SPQ total scores (r(60) = 0.57, p < 0.001), which was primarily a reflection of associations between the SPQs interpersonal sub-factor and the AQs social (r(60) = 0.59, p < 0.001), communication (r(60) = 0.45, p < 0.001) and imagination (r(60) = 0.55, p < 0.001) sub-factors.
Table 2.
Summary statistics for the SPQ and AQ total and sub-factor scores.
| Mean | SD | Range | |
|---|---|---|---|
| SPQ total | 18.9 | 12.2 | 0–45 |
| Disorganised | 2.60 | 2.1 | 0–8 |
| Interpersonal | 7.1 | 5.3 | 0–21 |
| Cognitive-perceptual | 9.2 | 7.9 | 0–28 |
| AQ total | 15.7 | 6.5 | 0–31 |
| Social | 2.3 | 2.0 | 0–7 |
| Attention switching | 4.3 | 2.3 | 0–10 |
| Attention to detail | 4.4 | 2.2 | 0–9 |
| Communication | 2.3 | 1.7 | 0–8 |
| Imagination | 2.5 | 2.1 | 0–8 |
To test the prediction that schizotypal traits are associated with relatively greater decrements in task performance under increasing perceptual load, the proportional increase in RT from set size 1 to set size 6 (i.e., [(RT set 6 − RT set 1) / RT set 1]) was used as an index of the influence of perceptual load on letter identification. The proportional difference between PS detection at set size 6 and set size 1 was used as an index of the influence of perceptual load on PS detection accuracy. For the letter identification task, the RT difference score was positively correlated with total SPQ scores (r(60) = 0.35, p < 0.01), confirming the prediction that higher schizotypal traits are associated with greater response time costs as perceptual load increases (see Fig. 2). At the sub-factor level, this association was significant for the cognitive-perceptual (r(60) = 0.36, p < 0.01), but not the interpersonal (r(60) = 0.17, p = 0.16) or disorganisation domain (r(60) = 0.24, p = 0.07) and these correlations held when controlling for total AQ scores, which were not correlated with the increase in response time as a function of perceptual load (r(60) = 0.14, p = 0.27). Thus, despite strong associations between schizotypal and autistic traits, only the former were uniquely associated with performance decrements under increasing perceptual load. A parallel analysis on the PS detection difference score, however, yielded no associations with SPQ total or sub-factor scores (r(60) < 0.18, p > 0.18).
Fig. 2.
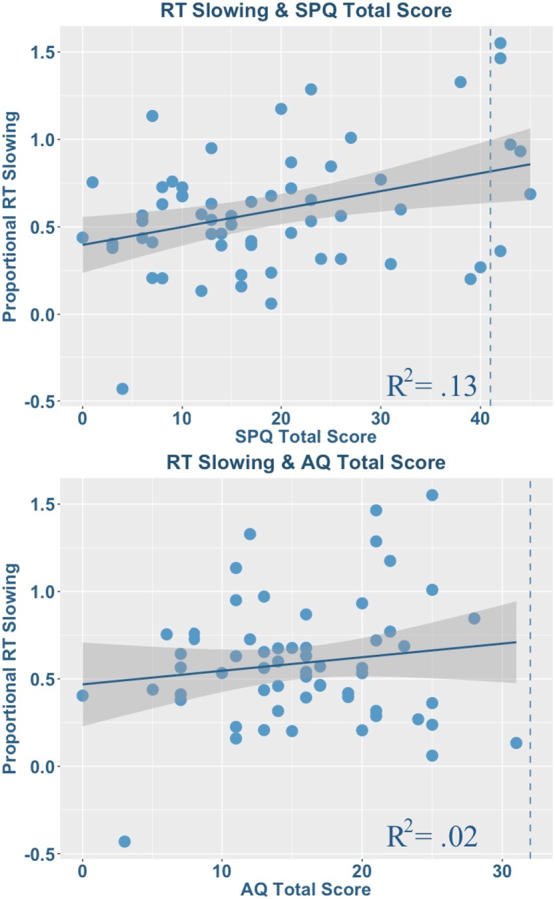
Simple correlations between the difference in reaction time between set size 6 and set size 1 and total SPQ score (Top) and total AQ score (Bottom). The dashed lines represent the respective threshold for clinically significant traits.
4. Discussion
The current study aimed to investigate whether schizotypal traits are associated with disproportionate performance decrements under increasing perceptual demands in a dual-task paradigm that required participants to detect, rather than to ignore, a peripheral stimulus. Although 90% of participants had SPQ scores that fell below the threshold for clinical significance, higher schizotypal traits were associated with more pronounced response time slowing as a function of perceptual load on the central letter identification task. Contrary to predictions however there was no association between schizotypal traits and the influence of perceptual load on the ability to detect a peripheral stimulus. A number of explanations can be offered for this pattern of results.
One possibility is that SSD is not really associated with a limitation in perceptual capacity, but rather with a problem in processing efficiency. In other words, individuals with higher schizotypal traits may extract the same amount of information as those with lower schizotypal traits, but they may be less efficient (i.e., slower) at processing this information. This “slowed processing” may be a manifestation of generally reduced processing speed, which would be in line with observations of slower response times in patients with schizophrenia on visual search (e.g., Elahipanah et al., 2008) and Posner type cueing tasks (e.g., Pardo et al., 2000). However, Badcock et al. (2015) showed that the reduced processing speed that is evident in clinical psychosis does not extend to schizotypal traits in the general public, which makes this explanation unlikely.
An alternative explanation, that would be compatible with the notion of reduced perceptual capacity in SSD, is that schizotypal traits are associated with abnormalities in the distribution or division of attention under increasing perceptual demands. Gray et al. (2014) recently reached this conclusion in relation to patients with schizophrenia or schizoaffective disorder who were significantly slower than controls on the ‘Useful Field of View’ task that requires participants to discriminate a central target stimulus whilst simultaneously locating a peripheral target. These demands are very similar to those in the current paradigm and may suggest that abnormalities in the distribution of attention that are evident in SSD extend sub-clinically into the general population. A valid concern that could be raised about this explanation is why abnormalities in the distribution of attention should affect only response times on the letter identification task but not also the detection accuracy of the peripheral stimulus. One possibility is that participants in the current study prioritised the detection of the peripheral stimulus because this was more difficult than correctly identifying the central target letter (see Table 1). Such a strategy could render the PS detection rate less sensitive to the perceptual load manipulation than performance on the central task, which is, precisely what was observed. The relevant effect size for the perceptual load manipulation on PS detection accuracy was only n2 = 0.14, compared to effects of n2 = 0.60 for letter identification accuracy and n2 = 0.67 for letter identification RT.
For future studies it would be useful to include intelligence as a general measure and investigate the links with perceptual load in relation to schizotypy and autism traits.
It is important to acknowledge that the current study cannot rule out the possible influences of other cognitive domains on task performance, such as task switching or other executive functions. Further work is required to investigate the possible role of such domains and also to clarify the downstream consequences of attentional difficulties on other cognitive abilities. Despite these open issues, however, the current observations make an important contribution to the literature in suggesting that attentional difficulties associated with SSD extend sub-clinically into the general population and that, in particular, cognitive perceptual schizotypal traits, may represent a risk factor for difficulties in the regulation of attention under increasing perceptual load.
Author disclosure
Funding body agreements and policies
We have received no external funding.
Contributors
Authors HS, SG, & CH designed the study and wrote the protocol. Authors HS & SG programmed the perceptual load tasks. Authors HS & SK collected the data. Authors HS & SG undertook the statistical analysis, and authors HS, SG, CH wrote the paper. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare they have no conflicts of interest.
Acknowledgements
We thank Prof. Philip Corr and Ms. Jenny Murphy who provided invaluable comments on drafts of the manuscript.
References
- Andreasen N.C. A unitary model of schizophrenia: Bleuler's “fragmented phrene”; as schizencephaly. Arch. Gen. Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Badcock J.C., Clark M.L., Pedruzzi R.A., Morgan V.A., Jablensky A. Intact speed of processing in a community-based sample of adults with high schizotypy: a marker of reduced psychosis risk? Psychiatry Res. 2015;228:531–537. doi: 10.1016/j.psychres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beatty J., Jackson Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol. Bull. 1982;91:276–292. [PubMed] [Google Scholar]
- Bellgrove M.A., Vance A., Bradshaw J.L. Local–global processing in early-onset schizophrenia: evidence for an impairment in shifting the spatial scale of attention. Brain Cogn. 2003;51:48–65. doi: 10.1016/s0278-2626(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Boucart M., Rascle C., Lang J.P., Thomas P. Adv. Psychol. Res Novascience. Vol. 2. Elsevier; 2000. Dissociation between spatial and temporal disengagement of attention in schizophrenia; pp. 161–184. [Google Scholar]
- Bustillo J.R., Thaker G., Buchanan R.W., Moran M., Kirkpatrick B., Carpenter W.T. Visual information-processing impairments in deficit and nondeficit schizophrenia. Am. J. Psychiatry. 1997;154:647–654. doi: 10.1176/ajp.154.5.647. [DOI] [PubMed] [Google Scholar]
- Carr V.J., Dewis S.A.M., Lewin T.J. Preattentive visual search and perceptual grouping in schizophrenia. Psychiatry Res. 1998;79:151–162. doi: 10.1016/s0165-1781(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Ducato M.-G., Thomas P., Monestes J.-L., Despretz P., Boucart M. Attentional capture in schizophrenia and schizotypy: effect of attentional load. Cogn. Neuropsychiatry. 2008;13:89–111. doi: 10.1080/13546800701707371. [DOI] [PubMed] [Google Scholar]
- Elahipanah A., Christensen B.K., Reingold E.M. Visual selective attention among persons with schizophrenia: the distractor ratio effect. Schizophr. Res. 2008;105:61–67. doi: 10.1016/j.schres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Ettinger U., Mohr C., Gooding D.C., Cohen A.S., Rapp A., Haenschel C., Park S. Cognition and brain function in schizotypy: a selective review. Schizophr. Bull. 2015;41:S417–S426. doi: 10.1093/schbul/sbu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairnie J., Moore B.C.J., Remington A. Missing a trick: auditory load modulates conscious awareness in audition. J. Exp. Psychol. Hum. Percept. Perform. 2016;42:930–938. doi: 10.1037/xhp0000204. [DOI] [PubMed] [Google Scholar]
- Fatemi S.H., Pearce D.A., Brooks A.I., Sidwell R.W. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fuentes L.J., Boucart M., Alvarez R., Vivas A.B., Zimmerman M.A. Inhibitory processing in visuospatial attention in healthy adults and schizophrenic patients. Schizophr. Res. 1999;40:75–80. doi: 10.1016/s0920-9964(99)00044-4. [DOI] [PubMed] [Google Scholar]
- Fuller R., Frith C.D., Jahanshahi M. Reduced negative priming does indicate reduced cognitive inhibition in schizophrenia. Cogn. Neuropsychiatry. 2000;5:21–35. [Google Scholar]
- Giersch A., Danion J.M., Boucart M., Roeser C., Abenhaim K. Reduced or increased influence of non-pertinent information in patients with schizophrenia? Acta Psychol. 2002;111:171–190. doi: 10.1016/s0001-6918(02)00048-3. [DOI] [PubMed] [Google Scholar]
- Gold J.M., Fuller R.L., Robinson B.M., Braun E.L., Luck S.J. Impaired top-down control of visual search in schizophrenia. Schizophr. Res. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Hahn B., Strauss G.P., Waltz J.A. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol. Rev. 2009;19:294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E., Balke M., Hajsamou S., Ruhrmann S., Schultze-Lutter F., Daumann J., Heekeren K. Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophr. Res. 2007;97:35–42. doi: 10.1016/j.schres.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Gray B.E., Hahn B., Robinson B., Harvey A., Leonard C.J., Luck S.J., Gold J.M. Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr. Bull. 2014;40:1462–1471. doi: 10.1093/schbul/sbu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.H., Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011 doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. J. Exp. Psychol. Hum. Percept. Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn. Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Macdonald J.S.P., Lavie N. Load induced blindness. J. Exp. Psychol. Hum. Percept. Perform. 2008;34:1078–1091. doi: 10.1037/0096-1523.34.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G.M., Galway T., Goldberg J.O., Tipper S.P. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychol. Med. 2003;33:121–129. doi: 10.1017/s0033291702006918. [DOI] [PubMed] [Google Scholar]
- Maruff P., Danckert J., Pantelis C., Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol. Med. 1998;28:1091–1100. doi: 10.1017/s0033291798007132. [DOI] [PubMed] [Google Scholar]
- Minassian A., Granholm E., Verney S., Perry W. Pupillary dilation to simple vs. complex tasks and its relationship to thought disturbance in schizophrenia patients. Int. J. Psychophysiol. 2004:53–62. doi: 10.1016/j.ijpsycho.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Muggleton N., Lamb R., Walsh V., Lavie N. Perceptual load modulates visual cortex excitability to magnetic stimulation. J. Neurophysiol. 2008;100:516–519. doi: 10.1152/jn.01287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Groeger J.A., Greene C.M. Twenty years of load theory—where are we now, and where should we go next? Psychon. Bull. Rev. 2016 doi: 10.3758/s13423-015-0982-5. [DOI] [PubMed] [Google Scholar]
- Pardo P.J., Knesevich M.A., Vogler Q.P., Pardo J.V., Towne B., Cloninger C.R., Posner M.I. Genetic and state variables of neurocognitive dysfunction in schizophrenia: a twin study. Schizophr. Bull. 2000;26:459–477. doi: 10.1093/oxfordjournals.schbul.a033466. [DOI] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Radant A.D., Dobie D.J., Calkins M.E., Olincy A., Braff D.L., Cadenhead K.S., Freedman R., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Light G.A., Meichle S.P., Millard S.P., Mintz J., Nuechterlein K.H., Schork N.J., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Swerdlow N.R., Tsuang M.T., Turetsky B.I., Tsuang D.W. Antisaccade performance in schizophrenia patients, their first-degree biological relatives, and community comparison subjects: data from the COGS study. Psychophysiology. 2010;47:846–856. doi: 10.1111/j.1469-8986.2010.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Raine A., Reynolds C., Lencz T., Scerbo A., Triphon N., Kim D. Cognitive-perceptual, interpersonal, and disorganized features of schizotypal personality. Schizophr. Bull. 1994;20:191–201. doi: 10.1093/schbul/20.1.191. [DOI] [PubMed] [Google Scholar]
- Rapoport J., Chavez A., Greenstein D., Addington A., Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:10–18. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G., Russell C., Frith C.D., Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–2507. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- Remington A., Fairnie J. A sound advantage: increased auditory capacity in autism. Cognition. 2017;166:459–465. doi: 10.1016/j.cognition.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Remington A., Swettenham J., Campbell R., Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychol. Sci. 2009;20:1388–1393. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- Remington A.M., Swettenham J.G., Lavie N. Lightening the load: perceptual load impairs visual detection in typical adults but not in autism. J. Abnorm. Psychol. 2012;121:544–551. doi: 10.1037/a0027670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R., Robertson L.C., Nordahl T.E. Normal sustained effects of selective attention are absent in schizophrenic patients withdrawn from medication. Psychiatry Res. 1996;62:121–130. doi: 10.1016/0165-1781(96)02804-1. [DOI] [PubMed] [Google Scholar]
- Wuthrich V.M., Bates T.C. Confirmatory factor analysis of the three-factor structure of the schizotypal personality questionnaire and chapman schizotypy scales. J. Pers. Assess. 2006;87:292–304. doi: 10.1207/s15327752jpa8703_10. [DOI] [PubMed] [Google Scholar]