Abstract
Using a TALEN-mediated gene-editing approach, we have previously described a process for the large-scale manufacturing of “off-the-shelf” CAR T cells from third-party donor T cells by disrupting the gene encoding TCRα constant chain (TRAC). Taking advantage of a previously described strategy to control TALEN targeting based on the exclusion capacities of non-conventional RVDs, we have developed highly efficient and specific nucleases targeting a key T cell immune checkpoint, PD-1, to improve engineered CAR T cells’ functionalities. Here, we demonstrate that this approach allows combined TRAC and PDCD1 TALEN processing at the desired locus while eliminating low-frequency off-site processing. Thus, by replacing few RVDs, we provide here an easy and rapid redesign of optimal TALEN combinations. We anticipate that this method can greatly benefit multiplex editing, which is of key importance especially for therapeutic applications where high editing efficiencies need to be associated with maximal specificity and safety.
Keywords: multiplex gene editing, genotoxicity, TALEN, immunotherapy, chimeric antigen receptors, checkpoint inhibitors
Introduction
Recent advancement in genome-engineering technology is changing the landscape of biological research, especially for the development of new therapeutic applications. This groundbreaking technology provides scientists with novel opportunities to develop new methodologies to ask and answer critical questions. This advancement is highlighted by the increased use of programmable DNA-binding agents such as transcription activator-like effector (TALE) and RNA-guided CRISPR/CRISPR-associated (Cas) systems that became part of the most powerful gene-editing tools.1, 2, 3 These novel and robust DNA-targeting platforms allow a wide use of gene manipulation in both research and the development of novel therapies. These engineered enzymes can introduce DNA double-strand breaks (DSBs) with high efficiency and specificity into desired target sequences, and their ability to precisely cleave a specific locus is being used to disrupt genes via mutagenic non-homologous end joining (NHEJ).
Adoptive immunotherapy is a new paradigm for treating cancer. Recent reports have highlighted the synergistic possibilities of genome editing and the chimeric antigen receptor (CAR) T cell technology to develop the next generation of therapeutic applications. Although CAR-engineered T cells infiltrate the tumor tissue and persist over long periods,4 tumor reduction is frequently transient mainly due to tumor-associated immune repression. The latter is mediated, at least in part, by regulatory T cells (Tregs), which heavily infiltrate solid tumor lesions, and by the upregulation of multiple immune-checkpoint molecules (CTLA-4, PD-1, LAG3, or TIM-3) on tumor-infiltrating lymphocytes. Immune checkpoint pathways strongly downregulate T cell activation, carrying the important function of keeping nascent T cell responses in check and reducing the likelihood of an immune attack against normal tissues. During tumorigenesis, however, cancer cells may exploit these co-inhibitory pathways to resist detection or avoid elimination by the adaptive immune system.5 The PD-1 checkpoint pathway is thought to act primarily in peripheral tissues to dampen ongoing immune responses and/or to prevent damage to self-tissues. The ability of non-immune cells to express ligands for PD-1 such as PD-L1 is exploited by tumors as a way to avoid immune attack.6, 7 Preliminary clinical findings with blockers of immune-checkpoint proteins such as CTLA-4 and PD-1 indicate broad and diverse opportunities to enhance anti-tumor immunity with the potential to produce durable clinical responses.8, 9, 10, 11, 12, 13 However, most tissues rely on PD-L1 expression to limit T cell responses.14 Thus, the systemic administration of PD-L1/PD-1-blocking antibodies carries a risk of breaking peripheral tolerance, which might lead to autoimmune responses. Recent studies have demonstrated the feasibility and potency of approaches incorporating advanced gene-editing technologies into adoptive cell therapy protocols to silence immune checkpoints as a strategy to overcome locally active immune escape pathways.15, 16 We have previously described a platform for the production of “off-the-shelf” CAR T cells (UCAR T cells) from unrelated third-party donor T cells by disrupting TCRα constant gene TRAC.17 By combining the beneficial effects of both PD-1 blockade and CAR therapy, we have not only reduced the effective cost of this adoptive immune therapy but also simplified the protocol of administration as compared to a therapy that would combine the injection of both UCAR T cells and anti-PD-1 antibody.
Here, we used the latest in the transcription activator-like effector nuclease (TALEN) technology to develop highly efficient and specific nucleases targeting a key T cell immune checkpoint, PD-1, to improve engineered CAR T cells’ functionalities. By taking advantage of unique features of the TALE DNA targeting, its modularity, associated with the independence of the targeting modules, we aimed at replacing natural targeting modules, the repeat variable diresidues (RVDs), with appropriately chosen non-conventional RVDs (RVDs not found in the natural repertoires of TALE) to improve the specificity of targeting for multiplex genome editing. Our results demonstrate that our strategy based on exclusion properties of these non-conventional RVDs allowed combined TRAC and PDCD1 TALEN processing at the desired locus while eliminating low-frequency off-site processing. These results further confirmed previous reports showing that strategies replacing a few RVDs allowed the easy and rapid redesign of optimal TALEN nuclease combinations (so-called multiplex editing),18, 19, 20 a feature of prime importance especially for therapeutic applications where high editing efficiencies associated with maximal specificity and safety is of prime importance.
Results
PDCD1 Locus Is Efficiently Processed Using TALEN T3v1
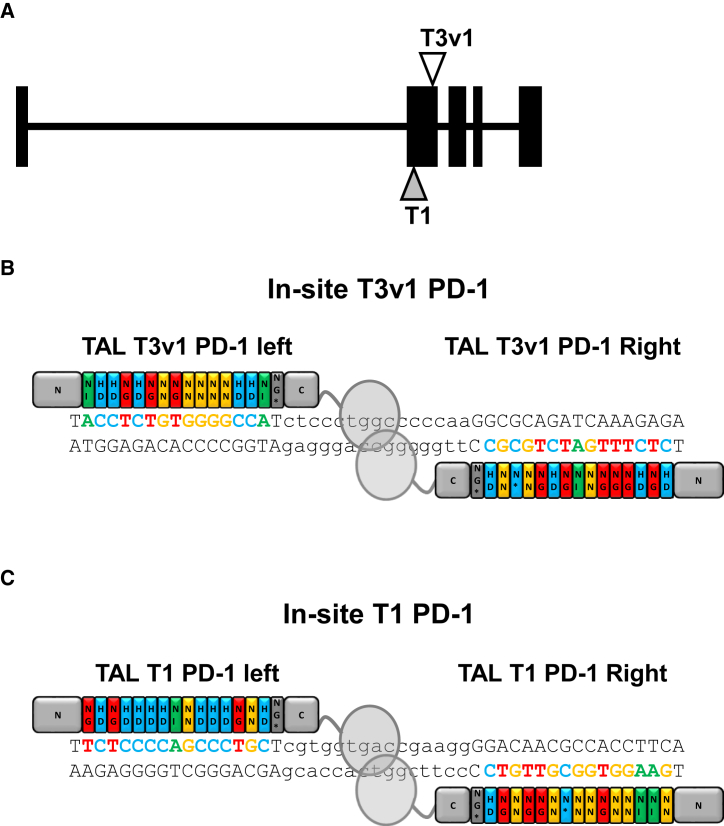
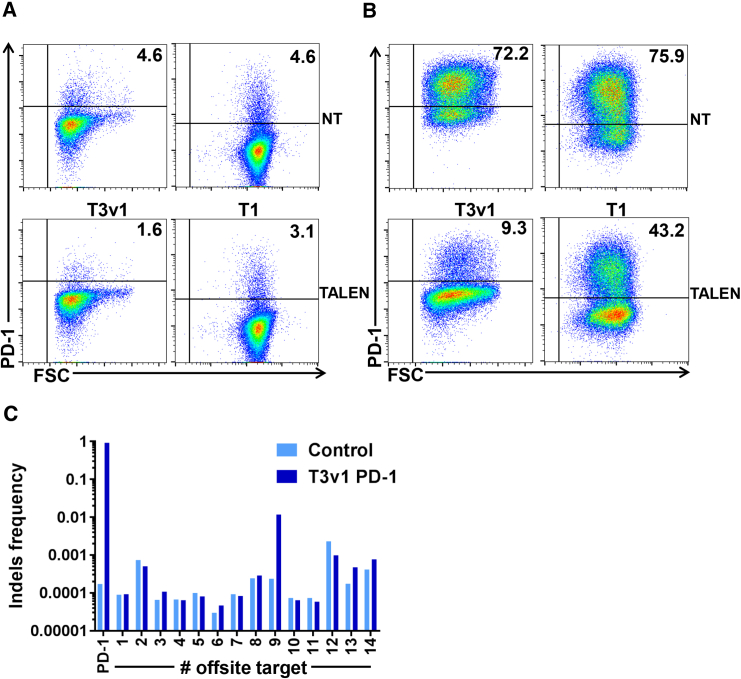
Today, four RVDs are mainly implemented and used, NI, HD, NN, and NG, to target an adenine, a cytosine, a guanine, and a thymine, respectively. Using features from our TALEN scaffold (TAL DNA binding array of 15.5 RVDs and spacer length of 15 base pairs) we designed and synthesized 2 TALEN T3v1 and T1 (first version of TALEN design) targeting the second exon of the PDCD1 locus where the PD-L1 binding site is located (Figures 1A–1C). For more clarity throughout this manuscript, the term TALEN represents the nuclease entity composed of two engineered TALE fused to the FokI catalytic domain. In order to evaluate the efficiency of our TALEN, we performed targeted mutagenesis experiments at the PDCD1 locus. Thirteen days post-mRNA electroporation, PD-1 production was assessed on non-transfected or TALEN-transfected T cells by flow cytometry after exclusion of non-viable cells (Figure 2A). PD-1 production is strongly disrupted on the surface of T3v1-transfected T cells as compared to non-transfected T cells (Figure 2A, left panels). Indeed, the surface detection is reduced by about 65% (from 4.6% to 1.6%) after mRNA TALEN transfection. As demonstrated in the literature, PD-1 is one of the key-inhibitory receptor expressed by activated T cells, and its expression is upregulated following antigen- and ligand-receptor engagement.21 Thus, PD-1 production increases early after activation and decreases about a week after the initial activation. By reactivating non-transfected T cells, PD-1 is markedly re-induced at their surface, while its production remains very low without additional reactivation. Indeed, PD-1 is only detected on 4.6% of non-transfected T cells 17 days after their initial activation, while we observe a frequency of 72.2% of PD-1+ T cells 3 days after reactivation (Figure 2B, left panels). We observe a reduction of about 85% (from 72.2% to 9.3%) when T3v1-transfected T cells were reactivated. Even though our second available TALEN to knock out PDCD1 (T1) is efficient at processing PDCD1 locus, its efficiency remains lower than T3v1 TALEN (Figure 2B, right panels). Indeed, PD-1 surface detection on T1-transfected and -reactivated T cells is reduced by 43% (from 75.9% down to 43.2% after reactivation). T3v1 TALEN being our lead candidate, we characterized in depth by high-throughput DNA sequencing (454 method) the molecular events generated by this TALEN at its target locus. Genomic DNA, recovered from T cells grown for more than 6 days after electroporation of PD-1 TALEN was used to generate specific PCR amplicons. Our sequencing results reveal insertion/deletion (indel) frequencies of ∼70% to 80% at the locus of interest for T3v1 TALEN (Figure 2C), confirming that TALEN-mediated processing of PDCD1 gene is very highly efficient under our experimental conditions. We also characterized in depth the molecular events generated by this TALEN at potential off-site targets. These off-site targets were systematically defined as genomic sequences bearing any combinations of TALEN binding sites containing ≤4 mismatches with respect to the sequence to target and separated from one another by 9 to 30 bp. The lists of potential off-site targets were generated and scored taking into account the nature and position of the substitutions as described previously.22 The 14 (T3v1) targets with the highest scores regardless of their genomic position, as well as the top four targets located in (or within 200 bp from) a coding sequence, were chosen for high-throughput DNA sequencing analysis. One off-site target (v1OS9) is found to be processed at low frequency (>2 orders of magnitude lower than the in-site, 0.5%) for T3v1 TALEN, the other sites tested do not show mutagenesis above background (Figure 2C). All together, these data confirm the possibility to design TALEN that present very high levels of activity on their cognate in-site target while sparing potential off-site targets.
Figure 1.
Design of Two PD-1 TALEN Targeting the First Exon of PDCD1 Locus
(A) Schematic representation of the PDCD1 (PD-1) genomic sequence. Black boxes represent exons, and triangles represent the approximate TALEN-binding sites. (B and C) Schematic representations of the loci and T3v1 (B) and T1 (C) PD-1 TALEN used to knock out PDCD1 in primary T cells.
Figure 2.
T3v1 PD-1 TALEN Is Highly Efficient to Disrupt PDCD1 Gene Expression in Primary T Cells
Four days after activation, 5 million T cells were transfected with 10 μg of each mRNA encoding the left and right arms of T3v1 or T1 PD-1 TALEN. Ten days after transfection, T cells were reactivated (B) or not (A) using Dynabeads human T activator CD3/CD28. Three days later, PD-1 production was assessed by flow cytometry on viable non-transfected (NT) T cells (upper panels) and transfected (TALEN) T cells (bottom left panels corresponding to T3v1 and right panels to T1) using PD-1 monoclonal antibody (mAb) in combination with a live/dead cell marker. The frequency of positive cells is indicated in each panel. Dot plots are representative of at least three independent experiments. (C) The efficiency of TALEN-mediated gene processing was analyzed by high-throughput DNA sequencing analysis of engineered T cell genomic DNA harvested 6 days after transfection. Potential in-site and off-site targets were carefully evaluated for these loci.
The Use of Non-conventional RVDs Improves Targeting Specificity in the Context of a Single Knockout
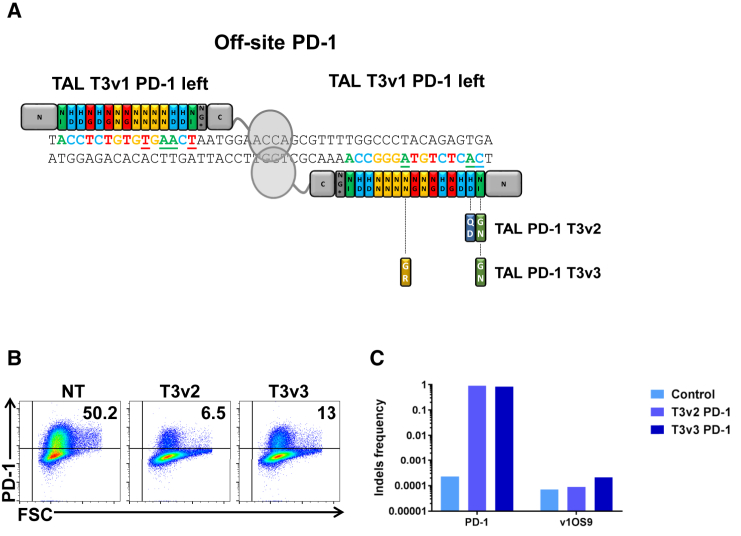
Considering the very high efficiency of the T3v1 TALEN at the desired PDCD1 locus, we decided to investigate whether we could modify this TALEN using non-conventional RVDs to maintain the same high level of activity at the desired locus but completely prevent the TALEN activity at the identified off-site sequence.18 The OS9 detected is mostly probably due to a homodimer association of the left half TALEN T3v1. Using the same strategy as previously presented,18 we first identified the mismatches between the in-site and off-site binding sequences. Then we identified non-conventional RVDs that allow discrimination at the mismatch position, between the in-site and the off-site nucleotides. To narrow the choice of non-conventional RVDs, we focused on non-conventional RVDs that will present minimal (or preferably no) relaxed specificities for the two remaining nucleotides to prevent any new off-site targeting. We further favored modification in the first half of the TALE array since this portion of the TALE array has previously been shown to have a higher impact on the targeting specificity.22, 23 Thus, after having identified the mismatches between the in-site and off-site binding sequences of the left arm of the TALEN, we designed two new TALEN (v2 and v3) by incorporating two non-conventional RVDs at the position 1 and 2 (T3v2) and 1 and 9 (T3v3) (Figure 3A). In these specific examples, NI and HD were replaced by GN and QD, respectively (T3v2) or NI and NN were replaced by GN and GR (T3v3). We then investigated the activity and specificity of both TALEN T3v2 and v3. As shown by flow cytometry, PD-1 surface detection after T cell reactivation is reduced by 87% (from 50.2% down to 6.5%; T3v2) and 74% (from 50.2% down to 13%; T3v3). These efficiencies of PD-1 TALEN activities are very similar to the efficiency of T3v1 PD-1 TALEN. Having demonstrated that a very high activity of PD-1 TALEN was maintained at the in-site locus, we further investigated the capacity of the new TALEN to process the previously defined off-site 9 (v1OS9) target. As expected from the phenotypic results obtained (Figure 3B) our sequencing results reveal indel frequencies of ∼80% to 90% at the locus of interest, confirming that TALEN-mediated processing of PDCD1 gene by T3v2 and T3v3 PD-1 TALEN are highly efficient. More importantly, the v1OS9 tested does not show mutagenesis above background level (Figure 3C), indicating that our strategy to improve TALEN specificity by replacing conventional RVDs with non-conventional RVDs is highly efficient.
Figure 3.
T3v2 and T3v3 PD-1 TALEN Are as Efficient as T3v1 PD-1 TALEN at Disrupting PDCD1 Gene Expression in Primary T Cells and Do Not Induce Mutagenesis at the Off-Site Sequence Tested
Four days after activation, 5 million T cells were transfected with 10 μg of each mRNA encoding the left and right arms of TALEN T3v2 or T3v3 PD-1. (A) Schematic representation of the locus targeted by a homodimer left of the T3v1 PD-1 TALEN and of the new TALEN used in the primary T cell target discrimination experiment.18 (B) Nine days post-transfection, T cells were reactivated using Dynabeads human T activator CD3/CD28. Two days later, surface PD-1 was measured by flow cytometry on viable non-transfected T cells (left panel) and transfected (T3v2 and T3v3) T cells (middle and right panels) using PD-1 mAb in combination with a live/dead cell marker. The frequency of positive cells is indicated in each panel. Dot plots are representative of at least three independent experiments. (C) The efficiency of TALEN-mediated gene processing was analyzed by high-throughput DNA sequencing analysis of engineered T cell genomic DNA harvested 6 days after transfection. Potential in-site and off-site 9 targets were carefully evaluated for these loci.
The Use of Non-conventional RVDs Improves Targeting Specificity in the Context of a Double Knockout
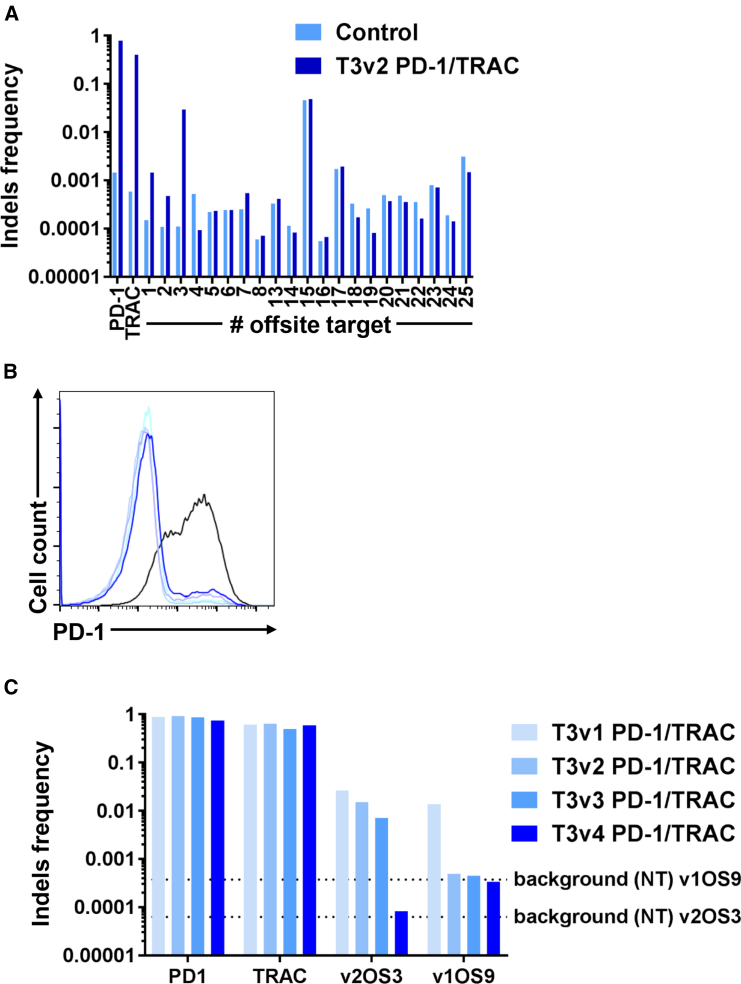
To align with the allogeneic approach we developed,17, 24 we combined both TRAC and T3v2 PD-1 TALEN. We then characterized in depth by high-throughput DNA sequencing the molecular events generated by those two TALEN at their respective target loci and their potential off-site targets. In this particular example, the two TALEN were designed separately to present individual optimal (high in-site and no or low off-site processing) activities, without taking into account the possibility of a simultaneous use (multiplex gene editing) in their respective design. To analyze the activity profile of the combined two TALEN, we further increased the number of analyzed off-sites to the 25 targets with the highest scores. This allowed us to also include the first 10 ranked targets that would be the result of the combination of one of each arm of the two TALEN (PD-1 and TRAC) used (Figure 4A). The sequencing results reveal high indel frequencies at both loci of interest (PDCD1 and TRAC), demonstrating that TALEN-mediated processing of PDCD1 and TRAC gene is highly efficient. Nevertheless, 2.9% of indels are detected at one of the highest ranked off-sites studied, the v2OS3, which corresponds to an off-site induced by a heterodimer TRAC left/PD-1 left. Using the same design strategy, we elaborated one new TALEN T3v4 by replacing the first and seventh RVDs by non-conventional RVDs. By design, we chose a combination of non-conventional RVDs; NI and NN were replaced by KL and YK, respectively. That would allow preventing processing of the previously identified v1OS9 and the newly identified v2OS3. We focus our TALEN reengineering efforts on the PD-1 left arm, as the number and type of mismatches observed with the TRAC left arm were expected to be already more impactful on the TALEN activity. We thus investigated the specificity and activity of each of these new TALEN as compared to T3v1 PD-1 original TALEN. As shown by an overlay analysis of PD-1 production on the surface of reactivated cells, we observed a similar reduction of surface PD-1 for T cells transfected with each of the different PD-1 TALEN (Figure 4B). We then monitored the capacity of the new TALEN to edit the previously defined off-site targets v1OS9 (due to the homodimer association) and v2OS3 (due to the heterodimer association). In this experiment, we obtain indel frequencies of ∼70% to 80% at the PDCD1 locus and ∼60% at the TRAC locus (Figure 4C). As expected, the v1OS9 tested does not show mutagenesis above background level for any of the new TALEN tested, and the v2OS3 tested does not show mutagenesis above background for the last T3v4 PD-1 TALEN produced (Figure 4C), indicating that our strategy to improve TALEN specificity by replacing conventional RVDs with non-conventional RVDs is highly efficient and can be translated to prevent off-site processing while maintaining high levels of activity at the different in-site loci. Importantly, as expected, the other potential off-site targets that were previously studied were unaffected (no processing) by the incorporation of the non-conventional RVDs.
Figure 4.
T3v4 PD-1 TALEN Is as Efficient as T3v1 PD-1 TALEN at Disrupting PDCD1 Gene Expression in Primary T Cells and Does Not Induce Mutagenesis at the Off-Site Sequences Tested
Four days after activation, 5 million T cells were transfected with 10 μg of each mRNA encoding the left and right arms of TALEN T3v2 PD-1 (A) or TALEN T3v1, T3v2, T3v3, or T3v4 (B and C) in combination with 10 μg of each mRNA encoding the left and right arms of TALEN TRAC. (A) The efficiency of TALEN-mediated gene processing was analyzed by high-throughput DNA sequencing analysis of engineered T cell genomic DNA harvested 6 days after transfection. Potential in-site and off-site targets were carefully evaluated for these loci. The off-site OS9, OS10, OS11, and OS12 have identical potential TALEN binding sites as the OS8. The PCR products generated for deep-sequencing analysis for OS8, OS9, OS10, OS11, and OS12 are more than 99.8% identical. Thus, OS8 represents the pooled data of the off-sites mentioned above. (B) Nine days after transfection, T cells were reactivated using Dynabeads human T activator CD3/CD28. Two days later, the mean fluorescence intensity (MFI) of surface PD-1 detection was assessed by flow cytometry on viable non-transfected T cells and transfected T cells using PD-1 mAb in combination with a live/dead cell marker. Histograms are representative of at least two independent experiments. (C) The efficiency of TALEN-mediated gene processing was analyzed by high-throughput DNA sequencing analysis of engineered T cell genomic DNA harvested 6 days after transfection. Potential in-site and off-site 9 and off-site 3 targets were carefully evaluated for these loci.
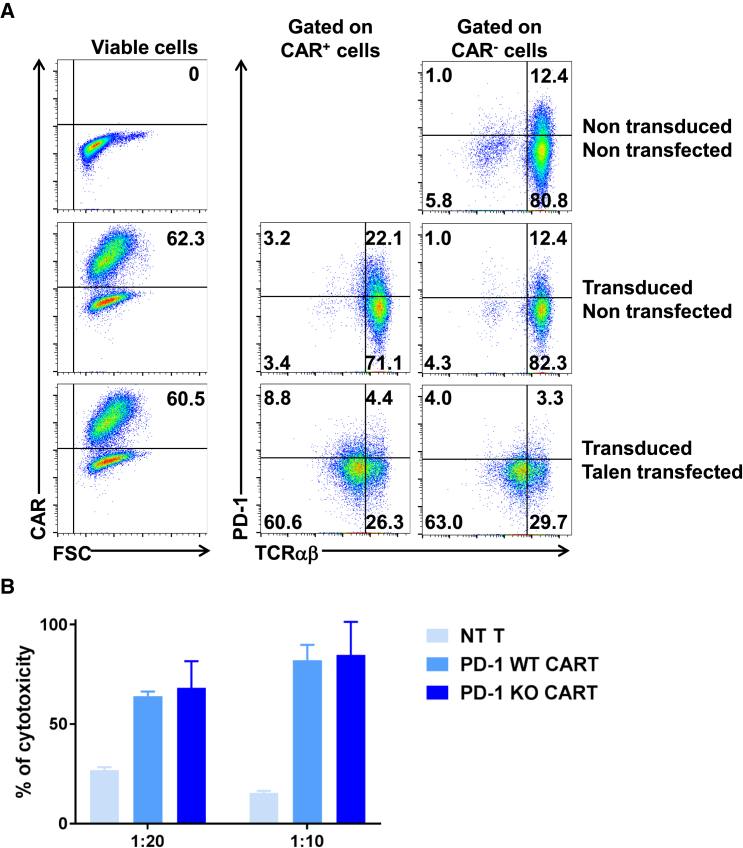
PDCD1 and TRAC Double Knockout CAR T Cells Are Highly Efficient to Eliminate Target Cells In Vitro
Having demonstrated that the PDCD1 locus could be efficiently edited using TALEN, containing or not non-conventional RVDs, we further investigated whether the in vitro cytolytic activity was affected by the double knockout of PDCD1 and TRAC. T cells were transduced or not with lentiviral particles encoding CD20 CAR and transfected or not with mRNA encoding T3v4 PD-1 and TRAC TALEN. The efficiencies of transduction and transfection were assessed by flow cytometry 4 days after transduction. When transduced at a MOI of 5, more than 60% of the cells express CD20 CAR on their surface (Figure 5A, left panels), while surface PD-1 is reduced by almost 50% when CAR T cells are transfected with mRNA encoding T3v4 PD-1 TALEN (Figure 5A, middle panels). As observed in Figure 5A, PD-1 surface detection on CAR T cells is higher compared to that on CAR negative T cells (middle versus right columns), reflecting the activation level of T cells. These observations are in accordance with previous studies that demonstrated that PD-1 is not detected on resting T cells but is inducibly re-induced within 24 h after stimulation, the highest level of expression being reached 2 to 4 days after activation.21, 25 These two sets of T cells (wild-type [WT] and dual PDCD1/TRAC knockout) were then challenged in an in vitro long-term cytotoxicity assay using target cells presenting the CAR antigen. As anticipated, we observed that independently of the ratio of T cells and target cells, the dual knockout CAR T cells are at least as efficient as WT CAR T cells at eradicating the targets cells in vitro (Figure 5B). At a ratio of 1:20 (T cell:target cell), 64% of the target cells are killed in co-culture with WT CAR T cells, while 68.3% are eliminated in co-culture with PDCD1 knockout CAR T cells. At a ratio of 1:10, the cytotoxic activity of WT CAR T cells toward their specific antigen reaches 82%, while it reaches 84.8% for PDCD1 knockout CAR T cells. All together, these data demonstrate that the dual knockout of the PDCD1 and TRAC genes is not impacting the cytolytic properties of CAR T cells.
Figure 5.
The In Vitro Anti-tumor Activity of PDCD1 Knockout CAR T Cells Is at Least as Efficient as the Activity of PDCD1 WT CAR T Cells
CD20 CAR T cells were transfected or not with 10 μg of each mRNA encoding the left and right arms of TALEN T3v4 PD-1 in combination with TRAC TALEN. (A) Transduction and transfection efficiencies were assessed 4 days post-transduction on viable T cells using PD-1 and TCRαβ mAb, and CAR expression was reflected by the level of BFP expression. (B) The effect of PDCD1 knockout on the CAR T cells toward antigen-presenting cells overexpressing PD-L1 was assessed in a flow-based cytotoxicity assay. The CD20+ and CD20− target cell viability was measured after 3 days of co-culture with engineered CAR T cells at ratio set to 1:20 and 1:10 effector:target. Data are shown as mean ± SD of triplicates per point of two independent experiments.
The study we present here reports the fine and precise “multi-layer” genome editing of primary T cells. Such reengineering of the TALEN DNA binding motives allows the reliable serial and sequential generation of precise designer nucleases from already existing TALEN. In particular, we demonstrated that the UCAR T cell (TRAC knockout CAR T cell) platform can be further rapidly, efficiently, and safely engineered using TALEN containing non-conventional RVDs. The multiplexed engineered UCAR T cells (TRAC and PDCD1 knockout) maintained a potent in vitro anti-tumor function. Different recent studies have demonstrated that the disruption of PD-1 on CAR T cells enhanced the anti-tumor activity in vitro and in animal models.15, 16, 26, 27 Beyond our proof-of-concept study, further additional in vitro and in vivo experiments will be desirable to fully assess the benefits of such multiplex engineered UCAR T cells with adequate tumor models.
Discussion
One of the major challenge of gene editing using designer nuclease, especially for clinical applications, is the risk that off-target cleavage can occur at sequences within the genome that contain a few mismatches related to the targeted sequence of interest. Thus, the possibility to control and finely tune the targeting specificity of such platforms represents a key issue. Regarding TALEs, the DNA-binding domain consists of highly conserved 33–35 amino acid sequence repeats with divergent 12th and 13th amino acids in each repeat.28, 29 These so-called RVDs are highly variable and show a strong correlation with specific nucleotide recognition. So far, four DNA targeting modules (RVDs) have been used by most researchers (NI, HD, NN, and NG), but these naturally found RVDs only explore about 5% of the possible diversity repertoire at these two key positions.30, 31 Using this four-RVD repertoire, TALEN has already proven to be specific and compatible to ex vivo engineering of primary T cells for therapeutic applications with no or very low (background) levels of off-site processing.17 Specificity of targeting being a key factor, especially for therapeutic application, the use of the NN canonical RVD to target a guanine was questioned due to its ability to target both an adenine and a guanine.28, 29 Several research groups have therefore studied the implementation of alternative natural RVDs, such as NK and NH to improve the specificity of guanine targeting.30, 31 Recent studies took advantage of the vast repertoire offered by DNA-targeting characteristics of RVDs (positions 12 and 13), to identify and characterize new non-conventional RVDs with novel intrinsic targeting specificity features.18, 19, 20 This strategy to exclude the targeting of specific genomic sequences (so-called off-site targets) by TALEN is based on the unique targeting properties of non-conventional RVDs. In particular, the mapping of their binding properties (affinity for the different nucleotides) revealed that the nature of the amino acid at position 13 of these non-conventional RVDs largely defined the base preference (as already observed for canonical RVDs)28, 29, 32, 33, 34, 35 with some effects on the affinity. The identity of the amino acid at position 12 was described to be the major contributor to the RVD binding strength (with possible minimal effect on specificity). In addition, studies on the sensitivity of TALE array to epigenetic modifications (e.g., 5-methylcytosine) highlighted how steric hindrance between the amino acids at positions 12 and 13 and the targeted nucleotide is affecting the recognition and binding of an RVD array to its target sequence.36, 37 The overall better understanding of the interaction between the RVD array and the targeted sequence allowed the implementation of such non-conventional RVDs, improving the discrimination between different nucleotides and therefore further increasing the specificity of TALE-based molecular tools.18, 19, 20
Better mastering the targeting properties (e.g., specificity) of designer nuclease is of prime interest for clinical application, as demonstrated, for example, by the synergy that could be obtained between gene editing and adoptive immunotherapies (e.g., CAR T cells) developed to fight cancer.17, 38 The current optimized version of our process is initiated by CD3-dependent T cell activation at time 0, incorporates a transduction step at 72 hr to allow for the expression of a CAR, and finally concludes with a TALEN mRNA electroporation step for TRAC/CD52 gene disruption at 120 hr. Subsequent to the TALEN electroporation step, the cells are cultured in a closed system for 10 to 12 days followed by magnetic depletion of remaining TCRαβ-positive cells. Using this process, TCR/CD52-deficient CAR T cells were manufactured with highly reproducible yields. Since there will not be any possibility to deplete PD-1+ cells because of the lack of good manufacturing practice (GMP) magnetic depletion system for PD-1, the TALEN that induces the highest level of knockout was chosen. In such a case where a TALEN is highly efficient but one off-site target is found to be processed even though at low frequency, it is extremely useful to have the possibility to design TALEN that maintain such high levels of activity on their cognate in-site target while sparing the potential off-site target.
CAR T cells have demonstrated significant responses in patients with treatment-refractory hematologic malignancies but have only modest results in solid tumors. This is likely due to a host of hurdles encountered in the tumor microenvironment of solid tumors,39, 40 especially intrinsic inhibitory pathways mediated by upregulated inhibitory receptors such as PD-1 reacting with their cognate ligand within the tumor. Many studies have demonstrated that advanced-generation human CAR T cells are reversibly inactivated within the solid tumor microenvironment.41 Thus, PD-1 pathway antagonism can augment human CAR T cell function.42, 43 Recently, we and others underlined that disruption of endogenous PDCD1 using designer nuclease enhances the efficacy of gene-disrupted CAR T cell therapy or the function of tumor-reactive T cells used for adoptive cell transfer (ACT) in tumor models.15, 26, 27 However, disrupting PD-1 expression on CAR T cells that could eventually express autoreactive T cells might lead to autoimmune adverse events such as those described with systemic PD-1 antibody blockade. Here, we implemented the non-conventional RVD approach to edit the genome of human primary T cells with TALEN presenting improved discrimination between the desired on-site and the off-sites targets. We previously described a platform for the production of “off-the-shelf” CAR T cells from unrelated third-party donor T cells by disrupting TCRα constant gene TRAC.17 We therefore focused on optimizing a TALEN targeting the PDCD1 locus that can be used in combination with the previously reported TRAC TALEN, such a multiplex genome-editing strategy could allow the generation of highly potent and tumor-specific UCAR T cells and prevent the risk of adverse events previously observed.8 By incorporating non-conventional RVDs on the left T3v1 PD-1 half TALEN, we were able to produce an optimized and highly efficient TALEN that is not processing the low-frequency PD-1 off-sites identified with first generations of TALEN (for use of the PD-1 TALEN alone or simultaneously with the TRAC TALEN). In this study, we decided to use simultaneously two TALEN pairs that were designed separately. In such case, the combination of arms of the different TALEN may lead to the targeting of off-site sequences that were not taken into account in the original TALEN design. The a posteriori strategy of educated TALEN reengineering that we presented here can be further broadly adapted to multiplex TALEN gene editing. All together, these results showed that a very fine and predictable tuning of a TALEN targeting can be obtained by incorporating a few non-conventional RVDs. This approach could positively impact the development of new generations of treatments involving gene editing for many diseases. Whether the identification of potential off-sites is performed in silico or experimentally, once the evaluation of genomic toxicity is performed during clinical trials, the detection of off-target mutagenesis could block further development of a candidate product. Here, our approach enables fine tuning of the TALEN used to abrogate off-target activity specifically where it is detected. In addition, one can imagine combining this approach with the use of engineered obligated heterodimeric FokI cleavage domains44, 45, 46 to prevent the pairing of the specific half TALEN molecules responsible for off-site activity. Moreover, we demonstrated that the in vitro anti-tumor activity of PDCD1 knockout CAR T cells is not impaired. This development validated in human primary T cells will enable TALEN tools for gene editing to be applied more broadly and safely in basic research and disease treatment.
Materials and Methods
Antibodies
The following monoclonal antibodies and reagents were used: for surface staining, anti-PD-1 (clones PD1.3.1.3 and EH12.2H7 from Miltenyi Biotech and Biolegend, respectively), anti-TCRαβ (clone BW242/412 from Miltenyi Biotech), and fixable viability dye eFluor 780 from eBioscience. All the cytometry analyses were performed on a FACSCanto II (BD Biosciences).
Cell Culture
Frozen peripheral blood mononuclear cells (PBMC) were obtained from healthy volunteer donors (Allcells). T lymphocytes were activated directly from PBMCs using Dynabeads human T activator CD3/CD28 (Invitrogen), taking into account the frequency of CD3+ cells at a ratio of 1:1 bead:cell. Activated T cells were then immediately diluted in X-Vivo-15 (Lonza) media supplemented by 20 ng/mL IL-2 (final concentration) (Miltenyi Biotech) and 5% human serum AB (Seralab). Jeko-PD-L1 (ATCC-CRL3006) were generated by lentiviral transduction with an expression cassette for PD-L1. PD-L1+ cells were single cell sorted by high-speed flow cytometry on a SH800 (Sony) at a purity >98%. Jeko and Jeko-PD-L1 were cultured in RPMI 1640 containing Glutamax (Gibco) supplemented by 20% fetal bovine serum (FBS) (Lonza), 100 U/mL Penicillin, and 100 μg/mL Streptomycin (Lonza).
TALEN
TRAC, T3v1 PD-1, T3v2 PD-1, T3v3 PD-1, and T3v4 PD-1 TALEN were obtained from Cellectis. The target sequences for each TALEN were as follows:
TRAC: 5′-TTGTCCCACAGATATCCagaaccctgaccctgCCGTGTACCAGCTGAGA-3′
PDCD1: 5′-TACCTCTGTGGGGCCATctccctggcccccaaGGCGCAGATCAAAGAGA-3′
Two 17-bp recognition sites (upper case letters) are separated by a 15-bp spacer.
mRNA
mRNAs were synthesized using the mMessage mMachine T7 Ultra Kit (Life Technologies). RNAs were purified with RNeasy columns (QIAGEN) and eluted in cytoporation medium T (Harvard Apparatus). Following process optimization, TALEN mRNAs were produced by a commercial manufacturer (Trilink Biotechnologies).
Cell Transfection and Investigation of Knockout Efficiency
Four to five days after activation, T cells were transfected with 10 μg of each mRNA encoding the left and right arms of TALEN. Transfection was performed using Agilpulse technology. Cells were then immediately diluted in X-Vivo-15 media supplemented by 20 ng/mL IL-2 (final concentration) and 5% human serum AB. Transfected T cells were eventually diluted at 1 × 106 cells/mL and kept in culture at 37°C in the presence of 5% CO2 and 20 ng/mL IL-2 (final concentration) and 5% human AB serum for further characterization.
Nine days post-transfection, T cells were reactivated or not using Dynabeads human T activator CD3/CD28 at a bead:cell ratio of 0.5:1. Three days later, production of PD-1 at the membrane was assessed by flow cytometry. Knockout efficiencies can be investigated by flow cytometry starting from day 2 post-transfection, but PDCD1 knockout is more easily detected after reactivation.
Cell Transduction
Three days after activation, 6 × 106 primary T cells resuspended in 3.6 mL of X-Vivo-15 media were cultured in a T25 flask pre-coated by 30 μg/mL of Retronectine in the presence of lentiviral particles to obtain a multiplicity of infection of 5. After 2 hr of incubation at 37°C, 3 mL of complete media 2X (X-vivo-15, 10% AB serum, and 40 ng/mL IL-2) was added to the cellular suspension. After overnight incubation, cells were washed, resuspended in a complete media at 1 × 106 cells/mL, and passaged every 2 or 3 days.
CARs
Second-generation CD20 CAR was constructed using single-chain antibody fragments derived from antibody clones C230H, hinge and transmembrane regions from CD8A, intracellular domain from TNFRSF9 (CD137), and intracellular domain from CD247 (CD3ζ). The single-chain variable fragment (scFv) is preceded by the sequence encoding the blue fluorescent protein (BFP), which will reflect the expression of the CAR. CD20 CAR was cloned into a commercial lentiviral vector backbone downstream of an EF1a promoter, and concentrated lentiviral vectors were produced by Vectalys (Toulouse, France).
Mutagenesis Analysis
PCR amplifications spanning TRAC or PDCD1 targets or in silico predicted potential off-site targets were performed from gDNA harvested 6 days post-transfection using primers described in the Supplemental Materials and Methods. Purified PCR products were sequenced using the Illumina method (Miseq 2x250 nano V2) or 454 sequencing system (454 Life Sciences). At least 2,065 sequences were obtained per PCR product for Illumina and 1,364 for 454, and sequences were analyzed for the presence of site-specific mutations. The total number of reads, the total number of events detected, and the estimated indel frequencies are summarized in the Supplemental Materials and Methods.
Cytotoxicity Assay
The cytolytic activity of PDCD1 WT or PDCD1 knockout engineered T cells endowed with CD20 CAR was assessed using a flow cytometry-based cytotoxicity assay. In this assay, target cells presenting the CAR target antigen (Jeko or Jeko PD-L1) as well as control cells not presenting the CAR target antigen were labeled with CellTrace carboxyfluorescein succinimidyl (CFSE; Life Technologies). The target cell populations were co-incubated at 37°C at different ratios of engineered effector CAR T cells (effector/target) of 1:20 and 1:10 for 3 days in a final volume of 200 μL of culture medium corresponding to the culture medium of the target cell line. After 3 days, the whole cell population was recovered, washed in PBS, labeled with eFluor 780 viability marker (eBioscience), and target cell viability was assessed by flow cytometry.
Author Contributions
A.-S.G. and A.J. designed and performed the experiments, analyzed data, and wrote the manuscript. A.-S.G. and A.J. contributed equally to this work. V.G., E.D., and J.-M.F. performed experiments. A.D. analyzed the data. P.D. and L.P. supervised the study and wrote the manuscript.
Conflicts of Interest
All coauthors are present Cellectis employees.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and can be found with this article online at https://doi.org/10.1016/j.omtn.2017.10.005.
Contributor Information
Anne-Sophie Gautron, Email: anne-sophie.gautron@cellectis.com.
Philippe Duchateau, Email: philippe.duchateau@cellectis.com.
Supplemental Information
References
- 1.Scharenberg A.M., Duchateau P., Smith J. Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies. Curr. Gene Ther. 2013;13:291–303. doi: 10.2174/15665232113139990026. [DOI] [PubMed] [Google Scholar]
- 2.Gersbach C.A., Perez-Pinera P. Activating human genes with zinc finger proteins, transcription activator-like effectors and CRISPR/Cas9 for gene therapy and regenerative medicine. Expert Opin. Ther. Targets. 2014;18:835–839. doi: 10.1517/14728222.2014.913572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu J., Zhang B., Chen H. Applications of TALENs and CRISPR/Cas9 in human cells and their potentials for gene therapy. Mol. Biotechnol. 2014;56:681–688. doi: 10.1007/s12033-014-9771-z. [DOI] [PubMed] [Google Scholar]
- 4.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A., Qian X., James S.E., Raubitschek A., Forman S.J. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jadus M.R., Natividad J., Mai A., Ouyang Y., Lambrecht N., Szabo S., Ge L., Hoa N., Dacosta-Iyer M.G. Lung cancer: a classic example of tumor escape and progression while providing opportunities for immunological intervention. Clin. Dev. Immunol. 2012;2012:160724. doi: 10.1155/2012/160724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 8.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 11.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pen J.J., Keersmaecker B.D., Heirman C., Corthals J., Liechtenstein T., Escors D., Thielemans K., Breckpot K. Interference with PD-L1/PD-1 co-stimulation during antigen presentation enhances the multifunctionality of antigen-specific T cells. Gene Ther. 2014;21:262–271. doi: 10.1038/gt.2013.80. [DOI] [PubMed] [Google Scholar]
- 15.Menger L., Sledzinska A., Bergerhoff K., Vargas F.A., Smith J., Poirot L., Pule M., Hererro J., Peggs K.S., Quezada S.A. TALEN-mediated inactivation of PD-1 in tumor-reactive lymphocytes promotes intratumoral T-cell persistence and rejection of established tumors. Cancer Res. 2016;76:2087–2093. doi: 10.1158/0008-5472.CAN-15-3352. [DOI] [PubMed] [Google Scholar]
- 16.Ren J., Zhang X., Liu X., Fang C., Jiang S., June C.H., Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirot L., Philip B., Schiffer-Mannioui C., Le Clerre D., Chion-Sotinel I., Derniame S., Potrel P., Bas C., Lemaire L., Galetto R. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 18.Juillerat A., Pessereau C., Dubois G., Guyot V., Maréchal A., Valton J., Daboussi F., Poirot L., Duclert A., Duchateau P. Optimized tuning of TALEN specificity using non-conventional RVDs. Sci. Rep. 2015;5:8150. doi: 10.1038/srep08150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J.C., Zhang L., Xia D.F., Campo J.J., Ankoudinova I.V., Guschin D.Y., Babiarz J.E., Meng X., Hinkley S.J., Lam S.C. Improved specificity of TALE-based genome editing using an expanded RVD repertoire. Nat. Methods. 2015;12:465–471. doi: 10.1038/nmeth.3330. [DOI] [PubMed] [Google Scholar]
- 20.Yang J., Zhang Y., Yuan P., Zhou Y., Cai C., Ren Q., Wen D., Chu C., Qi H., Wei W. Complete decoding of TAL effectors for DNA recognition. Cell Res. 2014;24:628–631. doi: 10.1038/cr.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemnitz J.M., Parry R.V., Nichols K.E., June C.H., Riley J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 22.Juillerat A., Dubois G., Valton J., Thomas S., Stella S., Maréchal A., Langevin S., Benomari N., Bertonati C., Silva G.H. Comprehensive analysis of the specificity of transcription activator-like effector nucleases. Nucleic Acids Res. 2014;42:5390–5402. doi: 10.1093/nar/gku155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meckler J.F., Bhakta M.S., Kim M.S., Ovadia R., Habrian C.H., Zykovich A., Yu A., Lockwood S.H., Morbitzer R., Elsäesser J. Quantitative analysis of TALE-DNA interactions suggests polarity effects. Nucleic Acids Res. 2013;41:4118–4128. doi: 10.1093/nar/gkt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valton J., Guyot V., Marechal A., Filhol J.M., Juillerat A., Duclert A., Duchateau P., Poirot L. A multidrug-resistant engineered CAR T cell for allogeneic combination immunotherapy. Mol. Ther. 2015;23:1507–1518. doi: 10.1038/mt.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing K., Gu B., Zhang P., Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015;16:39. doi: 10.1186/s12865-015-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren J., Liu X., Fang C., Jiang S., June C.H., Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 29.Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 30.Cong L., Zhou R., Kuo Y.C., Cunniff M., Zhang F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat. Commun. 2012;3:968. doi: 10.1038/ncomms1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streubel J., Blücher C., Landgraf A., Boch J. TAL effector RVD specificities and efficiencies. Nat. Biotechnol. 2012;30:593–595. doi: 10.1038/nbt.2304. [DOI] [PubMed] [Google Scholar]
- 32.Deng D., Yan C., Pan X., Mahfouz M., Wang J., Zhu J.K., Shi Y., Yan N. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science. 2012;335:720–723. doi: 10.1126/science.1215670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao H., Wu X., Chai J., Han Z. Crystal structure of a TALE protein reveals an extended N-terminal DNA binding region. Cell Res. 2012;22:1716–1720. doi: 10.1038/cr.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak A.N., Bradley P., Cernadas R.A., Bogdanove A.J., Stoddard B.L. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science. 2012;335:716–719. doi: 10.1126/science.1216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stella S., Molina R., Yefimenko I., Prieto J., Silva G., Bertonati C., Juillerat A., Duchateau P., Montoya G. Structure of the AvrBs3-DNA complex provides new insights into the initial thymine-recognition mechanism. Acta Crystallogr. D Biol. Crystallogr. 2013;69:1707–1716. doi: 10.1107/S0907444913016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flade S., Jasper J., Gieß M., Juhasz M., Dankers A., Kubik G., Koch O., Weinhold E., Summerer D. The N6-position of adenine is a blind spot for TAL-effectors that enables effective binding of methylated and fluorophore-labeled DNA. ACS Chem. Biol. 2017;12:1719–1725. doi: 10.1021/acschembio.7b00324. [DOI] [PubMed] [Google Scholar]
- 37.Valton J., Dupuy A., Daboussi F., Thomas S., Maréchal A., Macmaster R., Melliand K., Juillerat A., Duchateau P. Overcoming transcription activator-like effector (TALE) DNA binding domain sensitivity to cytosine methylation. J. Biol. Chem. 2012;287:38427–38432. doi: 10.1074/jbc.C112.408864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 39.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y., Zha Y., Gajewski T.F. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9:50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beatty G.L. Engineered chimeric antigen receptor-expressing T cells for the treatment of pancreatic ductal adenocarcinoma. OncoImmunology. 2014;3:e28327. doi: 10.4161/onci.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X., Ranganathan R., Jiang S., Fang C., Sun J., Kim S., Newick K., Lo A., June C.H., Zhao Y., Moon E.K. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D.S., Jones D.R., Sadelain M., Adusumilli P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J., Gaj T., Barbas C.F., 3rd Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J. Mol. Biol. 2010;400:96–107. doi: 10.1016/j.jmb.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller J.C., Holmes M.C., Wang J., Guschin D.Y., Lee Y.L., Rupniewski I., Beausejour C.M., Waite A.J., Wang N.S., Kim K.A. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat. Biotechnol. 2007;25:778–785. doi: 10.1038/nbt1319. [DOI] [PubMed] [Google Scholar]
- 46.Szczepek M., Brondani V., Büchel J., Serrano L., Segal D.J., Cathomen T. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat. Biotechnol. 2007;25:786–793. doi: 10.1038/nbt1317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.