See article vol. 24: 1105–1116
Obesity, a major risk factor for the development of diabetes mellitus, cardiovascular diseases and some types of cancer, arises from a chronic positive energy balance that is often due to overeating and a sedentary lifestyle on the background of a genetic and epigenetic vulnerability.
Leptin is an adipocyte hormone that functions as the afferent signal in a negative feedback loop regulating body weight1, 2). The rate of a person's leptin production is related to adiposity, but a large portion of the variability in plasma leptin concentration is independent of the percentage of body fat1, 3, 4). Obese individuals typically have large amounts of leptin; however, their brain usually cannot sense their leptin, resulting in a state termed as leptin resistance3, 4).
Historically, the phenotypes of leptin gene mutation (ob/ob) and leptin receptor mutation (db/db) mice had been described as obesity mutations and abnormalities in numerous behaviors, such as massive hyperphagia, dramatic decrease in locomotor activity, and quite gentle and non-aggressive, and sexually inactive1, 2). Thus, the functional analysis of leptin and leptin receptors2, 5), which are encoded by the db gene, were explored in the central nervous system (CNS). After the initial observation of leptin actions in pancreatic beta cells by Shimabukuro et al.6), its peripheral actions have also attracted researchers' interest. Indeed, enormous progress has been made in defining a set of overlapping neural circuits that control food intake and body weight. However, the mechanism by which leptin controls adiposity, the role of leptin signaling in peripheral tissues and whether most or all of its effects are mediated by CNS, and the physiological and cellular mechanisms by which leptin reduces adipose deposits in fat and other tissues and improves glucose metabolism remain unclear1).
The current issue by Takanashi et al. postulated an interesting notion that hormone-sensitive lipase (HSL), an adipocyte triglyceride lipase, is an efferent effector that confers the effect of leptin on lipolysis as well as on glucose lowering through beyond its anorexic effects7). They also revealed that the role of HSL is more dominant at supra-physiological hyperleptinemia that elicited complete fat loss in lean wild type (WT) mice than at near-physiological hyperleptinemia where the adipose depletion in WT is incomplete. Although the action of leptin in regulating food intake is well known, the molecular mechanisms underlying its adipopenic effect are not fully understood. Chen et al. have previously reported that leptin has an adipopenic effect, at least partly, independent of its anorexic effect8). By using HSL knockout mice and an adenovirally-mediated or osmotic-pump-induced hyperleptinemic model, Takanashi et al. demonstrated that this adipopenic effect of leptin is at least partly mediated by HSL. Unger's laboratory study at the University of Texas Southwestern at Dallas has previously reported that leptin can deplete fat mass through several mechanisms such as via PPARα and its target genes of fatty acid (FA) oxidation9); hence, one can assume that leptin stimulates lipolysis by activating adipocyte lipase(s) to liberate FAs and activates PPARα pathway to oxidize FAs, collectively leading to depletion of fat mass.
Thus, if the precise mechanism by which leptin controls adiposity, either by the role of leptin signaling in CNS or in peripheral tissues, is clarified, then the physiological and cellular mechanisms by which leptin reduces adipose deposits in fat and non-fat tissues can be elucidated. The latter has been considered to be a key phenomenon in obesity-related type 2 diabetes, which is termed as ectopic fat deposition and so-called lipotoxicity10). Thus, new strategies to handle such ectopic fat deposition and its consequences, such as metabolic syndrome, type 2 diabetes mellitus, and non-alcoholic fatty liver or cardiovascular diseases, can be obtained.
Fig. 1.
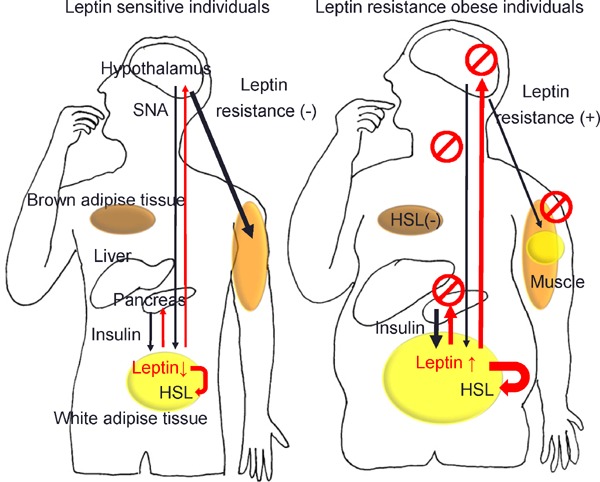
Leptin signals and lipolysis of white adipose tissue in leptin sensitive lean and leptin resistance obese individuals.
(A) In leptin sensitive lean individuals, leptin constrains insulin biosynthesis and secretion in pancreatic β-cells and lower insulin minimizes leptin secretion and regulates lipolysis from adipose tissue via hormone-sensitive lipase (HSL). Leptin maintains hepatic insulin sensitivity via the hepatic branch of the vagus nerve (not shown) and increases glucose uptake in the skeletal muscle, heart, and brown adipose tissue (BAT) via the sympathetic nervous system (black arrows). (B) In leptin resistant obese individuals, effects of leptin, though its plasma levels are increased, is decreased in hypothalamus. Insufficiency of leptin signaling causes hyperinsulinemia in pancreatic β-cells, which lead to subsequent impairment of insulin secretion and diabetes mellitus. Leptin resistance increases productions of leptin in white adipose tissue, but cannot abolish fat via HSL. Combined above mechanisms, leptin resistance instigates ectopic fat disposition in the liver and the skeletal muscle, provoking insulin resistance and chronic inflammation.
Conflicts of Interest
None.
References
- 1). Friedman JM: The function of leptin in nutrition, weight, and physiology. Nutr Rev, 2002; 60: S1-14; discussion S68-84, 85-17 [DOI] [PubMed] [Google Scholar]
- 2). Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI: Identification and expression cloning of a leptin receptor, OB-R. Cell, 1995; 83: 1263-1271 [DOI] [PubMed] [Google Scholar]
- 3). Myers MG, Jr., Leibel RL, Seeley RJ, Schwartz MW: Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab, 2010; 21: 643-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Kalra SP: Circumventing leptin resistance for weight control. Proc Natl Acad Sci U S A, 2001; 98: 4279-4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM: Abnormal splicing of the leptin receptor in diabetic mice. Nature, 1996; 379: 632-635 [DOI] [PubMed] [Google Scholar]
- 6). Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH: Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A, 1997; 94: 4637-4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Takanashi M, Taira Y, Okazaki S, Takase S, Kimura T, Li CC, Xu PF, Noda A, Sakata I, Kumagai H, Ikeda Y, Iizuka Y, Yahagi N, Shimano H, Osuga J-i, Ishibashi S, Kadowaki T, Okazaki H: Role of Hormone-sensitive Lipase in Leptin-Promoted Fat Loss and Glucose Lowering. J Atheroscler Thromb, 2017; 24: 1105-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Chen G, Koyama K, Yuan X, Lee Y, Zhou YT, O'Doherty R, Newgard CB, Unger RH: Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci U S A, 1996; 93: 14795-14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Koyama K, Shimabukuro M, Chen G, Wang MY, Lee Y, Kalra PS, Dube MG, Kalra SP, Newgard CB, Unger RH: Resistance to adenovirally induced hyperleptinemia in rats. Comparison of ventromedial hypothalamic lesions and mutated leptin receptors . J Clin Invest, 1998; 102: 728-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Shimabukuro M: Cardiac adiposity and global cardiometabolic risk: new concept and clinical implication. Circ J, 2009; 73: 27-34 [DOI] [PubMed] [Google Scholar]


