Abstract
Aim: Sarcopenic obesity (SO) is closely associated with cardiovascular disease (CVD) in elderly women. Increases in body fat and decreases in muscle mass are closely associated with increased carotid intima-media thickness (CIMT). The aim of this study was to examine the influence of a 24-week aerobic and resistance training program on carotid parameters in SO.
Methods: Fifty elderly women (74.1 ± 6.1 years) with SO were randomly divided into an exercise group and a control group. The exercise group performed combined exercise over 24 weeks, consisting of resistance and aerobic training for 50–80 min, 5 times a week. Carotid variables were measured using B-mode ultrasound. The differences in the carotid variables and the relative changes between baseline and after 24 weeks were evaluated.
Results: In the analysis of variance (ANOVA) results, CIMT (p = 0.013), systolic flow velocity (p = 0.007), diastolic flow velocity (p = 0.006), and wall shear rate (p = 0.010) showed significant interactions. In paired t-test results of the exercise group, CIMT significantly decreased (p < 0.01) and systolic flow velocity (p < 0.01), diastolic flow velocity (p < 0.001), and wall shear rate (p < 0.05) significantly increased after 24 weeks.
Conclusion: The 24-week combined exercise effectively decreased CIMT and increased carotid flow velocity and wall shear ratio. Therefore, combined exercise is thought to contribute to the improvement of the risk of CVD in elderly women with SO.
Keywords: Sarcopenic obesity, Combined exercise, Carotid intima-media thickness, Carotid flow velocity
See editorial vol. 24: 1090–1091
Introduction
A combination of sarcopenia and obesity is defined as sarcopenic obesity (SO)1, 2). SO is directly related to increased body fat mass (BFM) and decreased appendicular skeletal muscle mass (ASM) in the elderly1, 2). SO is associated with traditional cardiovascular disease (CVD) risk factors and mortality in the elderly1, 3, 4).
Carotid artery parameters such as carotid artery intima-media thickness (CIMT) and carotid artery flow velocity (FV) are reported to be independent risk factors for CVD5, 6). Increased BFM and decreased ASM are closely associated with increased CIMT7, 8). Moreover, increased BFM is associated with decreased FV as well as CIMT in the elderly7, 9).
Some cross-sectional studies reported that SO is associated with reductions in physical activity, muscle strength, and global physical capacity in elderly adults10, 11). Regular resistance exercise is reported to increase ASM as well as improve muscle strength and cardiorespiratory endurance12, 13). Aerobic exercise is also reported to be effective in improving CVD risk factors, including BFM and cardiorespiratory endurance14). Furthermore, combined exercise (complex resistance and aerobic exercise) has the effect of decreasing BFM and increasing ASM, as well as improving CVD risk factors15, 16).
Previous evidence for exercise and CIMT suggests that exercise training does not affect CIMT in healthy young adults17–19). However, exercise training has numerous beneficial effects in patients with either type 2 diabetes or CAD, and exercise plays an important role in the management of type 2 diabetes and in cardiac rehabilitation20–22). It remains unclear whether regular exercise in elderly women positively modulates CIMT. In addition, the effect of a combined exercise program on CIMT in SO elderly women subjects with the combination of low appendicular skeletal muscle mass and high body fat mass is not yet known, and the association between other carotid artery parameters such as luminal diameter, flow velocity, and function with long-term exercise is not clear.
The aim of the present study was, therefore, to investigate the effect of combined exercise training on CIMT progression in SO elderly women. Identifying effective strategies for physical activity promotion to improve the risks of cardiovascular disease in elderly adults may reduce CVD, making this a key public health issue. We hypothesised that exercise training would reduce the progression of CIMT in elderly women with SO.
Aim
The purpose of this study was to determine the effects of a combined exercise program on carotid artery variables in elderly women with SO.
Methods
Subjects
This study was performed at the Buk-gu Sports Center and Institute of Taekwondo for Health and Culture, Dong-A University, between November 2015 and December 2016. The proposed study is a randomized single-blind controlled community-based trial with a parallel design and a 1:1 allocation ratio. We recruited women aged 65 years or older through recruitment notices in the community centers and health centers for the elderly in Busan City, South Korea. A total of 350 community-dwelling elderly women submitted applications to participate after reading recruitment notices. Inclusion criteria in this study were as follows: SO elderly women (≥ 65 years). Body mass index (BMI) ≥ 25.0 kg/m2 and ASM/weight < 25.1 % were used as the criteria for SO in this study2). Sixty elderly women were selected by the SO criteria after body composition measurement. Our subjects were nonsmokers in the past and present. Ten participants were excluded for the following reasons: history of CVD (n = 1) and medication for hypertension (n = 2), diabetes (n = 1), osteoporosis (n = 1), and arthritis (n = 1) and regular physical activity (n = 4, ≥ 20 min/day and ≥ 3 times/week). Finally, 50 subjects with SO were selected for this study. The 50 participants (aged 65 to 84 years) were divided into a control group (n = 25) and a supervised exercise program group (n = 25) by randomization (Fig. 1 and Table 1).
Fig. 1.
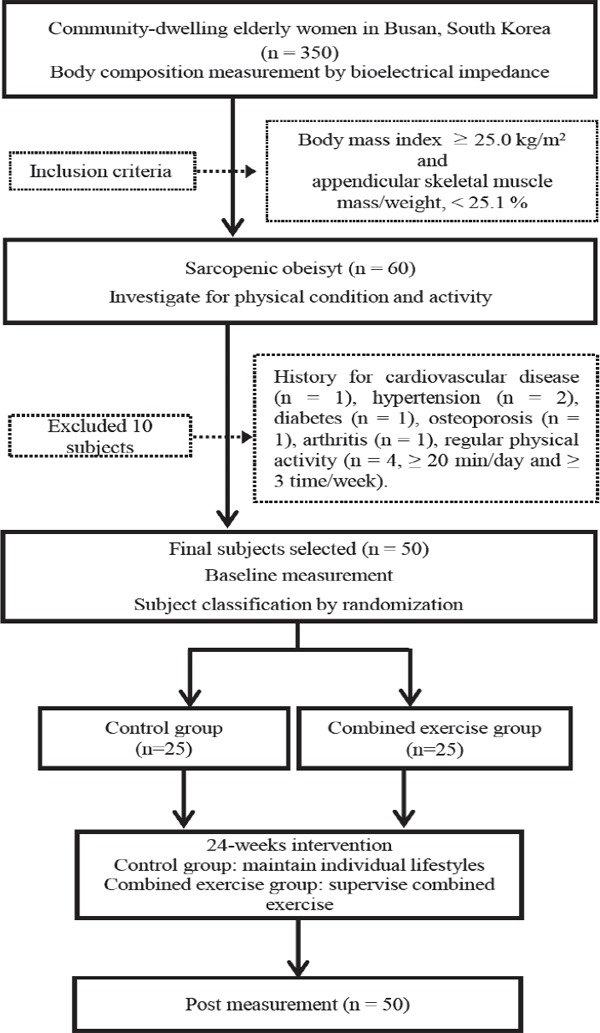
Flow chart of the study
Table 1. The general characteristic of study subjects.
| Control group | Exercise group | |
|---|---|---|
| (n = 25) | (n = 25) | |
| Age (years) | 74.7 ± 5.1 | 73.5 ± 7.1 |
| Height (m) | 1.52 ± 0.05 | 1.51 ± 0.04 |
| Body mass (kg) | 64.0 ± 7.1 | 61.2 ± 5.4 |
| Body mass index (kg/m2) | 27.6 ± 2.0 | 27.0 ± 1.4 |
| Percentage body fat (%) | 40.4 ± 3.6 | 41.0 ± 3.6 |
| Waist circumference (cm) | 90.2 ± 89.4 | 89.4 ± 4.7 |
| ASM (kg) | 15.0 ± 2.0 | 14.1 ± 1.8 |
| ASM/weight (%) | 23.4 ± 1.4 | 23.0 ± 1.5 |
| PA (Mets min/week) | 495.2 ± 157.6 | 500.0 ± 152.9 |
Data are show as mean ± SD; ASM, appendicular skeletal muscle mass; PA, physical activity
In accordance with the ethical standards of the Declaration of Helsinki, informed consent was obtained from all participants after they were provided with a detailed description of the experiment. Ethical approval was obtained from the ethics review board of Dong-A University.
Body Composition and Blood Pressure
After measuring the subjects' height and body mass, their BFM percentage and ASM were measured by bioelectrical impedance analysis (BIA) using a body composition analyzer (Inbody 720, Biospace, Seoul, South Korea). The waist circumference (WC) was measured as the smallest horizontal girth between the costal margin and the iliac crests in the standing position. BMI (kg/m2) and ASM/weight were calculated with their respective formulas. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a mercury sphygmomanometer (HICO, Tokyo, Japan) after the subjects had rested for 10 minutes.
Physical Function and Physical Activity
Physical function was measured using muscle strength (MS), flexibility, walking speed, and aerobic endurance. MS was measured by a hand grip strength dynamometer (TKK-5401, Japan) and the 30-second chair stand-up test. Flexibility was measured with the sit-and-reach test. Maximum walking speed (MWS) was defined as the ratio between distance and time when walking 10 m. Aerobic endurance was measured with the 2-min step test. Physical activity was measured using the Korean short version of the international physical activity questionnaire (IPAQ). The IPAQ is available at https://sites.google.com/site/theipaq/.
Combined Exercise Program
The 24-week combined exercise program intervention consisted of sessions lasting 50–80 min, 5 days per week, of combined aerobic and resistance exercises under the supervision of an exercise specialist. The aerobic and resistance exercises were modified considering the characteristics of our subjects from physical activities recommended for the community-dwelling elderly23).
Resistance exercises were performed with elastic band exercises (Thera - Band, Ohio, USA) for 12 items (elbow flexion, wrist flexion, shoulder flexion, lateral raise, front raise, chest press, reverse flies, side band, dead lift, squat, leg press, ankle plantar flexion), 8–15 repetitions per set (in weeks 1–12, 8–11 repetitions per set; in weeks 13–24, 12–15 repetitions per set), 2–3 sets (1 min rest between sets), 20–30 min per session for 3 days per week. Aerobic exercise involved various walking activities (sideways, backward, and forward walking and slow and fast indoor walking) for 30–50 min per session, 5 days per week, with a rating of perceived exertion (RPE) in the 13–17 range (in weeks 1–12, 13–15 RPE; in weeks 13–24, ≥ 15 RPE). Warm-up and cool-down was performed for 10 minutes before and after the exercise program, respectively. The control group was asked to maintain their usual physical activities during the 24 weeks, and we conducted health and family life education twice during the intervention period.
Blood Analysis
At baseline and after 24 weeks, venous blood samples were collected from both groups from an antecubital vein after an overnight fast (10–12 h). The concentrations of serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were analyzed using an automatic chemical analyzer (Hitachi-7600-110/7170 analyzer, Tokyo, Japan). High-sensitivity C-reactive protein (hs-CRP) level was measured by an automatic biochemical analyzer (Hitachi 747, Tokyo, Japan).
Carotid Artery Examination
CIMT, systolic and diastolic carotid artery luminal diameter (CLD), peak systolic flow velocity (PSV), and end diastolic flow velocity (EDV) were measured by B-mode ultrasound with a 10 MHz probe (LOGIQ 3, GE Healthcare, Wauwatosa, WI, USA). During measurement of the carotid arteries, the subjects lay on their backs in a dark room, heads turned 45 degrees, and the carotid arteries were fully exposed after relaxing for a minimum of 10 minutes; the left carotid artery was then measured by ultrasound. CIMT was measured on the far wall of the distal common carotid artery 1 cm proximal to the carotid bulb. CIMT was defined as the distance from the luminal-intima interface to the medial-adventitial interface. Systolic and diastolic CLD were measured at exactly the same location as CIMT measurement by B-mode ultrasound. CLD was defined as the distance from the intima-lumen interface of the near wall to the lumenintima interface of the far wall. PSV and EDVwere measured by continuous-wave Doppler examination in the carotid artery 1–3 cm proximal to the bifuraction9, 24). The sampling gate was placed at the center of the arterial axis, and FVs were recorded only after the signals stabilized. PSV and EDV were measured in the carotid artery with the insonation angle adjusted between 45° and 60° to the course of the vessel9, 24). Wall shear rate (WSR) was calculated using the Poiseuillian parabolic model of velocity distribution across the arterial lumen on the basis of the assumption of laminar blood flow, according to the following formula25): WSRpeak = 4 × PSV / CLDsystolic
The test-retest coefficient of variation of CIMT, CLD, and PSV were 0.8%, 0.7%, and 0.7%, respectively. The intraclass correlation coefficient for repeated measures of the CIMT, CLD, and PSV were 0.7%, and echocardiographic measurements ranged from 0.6% to 0.9% in our laboratory.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the measurement results are presented as averages and deviation. Student's t-test was used to assess differences in baseline variables. Repeated two-way analysis of variance (ANOVA) was used to compare parameters before and after the 24 weeks of the study. If significant interactions were present, a paired sample t-test with Bonferonni correction was performed to identify differences of within-group factors. Statistical significance was set at p < 0.05.
Results
Random grouping did not result in any significant differences in any variable between the groups at baseline (Table 1). All participants completed the intervention for 24 weeks. The mean combined exercise training attendance was 92 % (86 to 100 %).
Table 2 presents changes in body composition, blood pressure, physical function variables, and blood biochemical markers. In the ANOVA analysis results, BFM percentage (p < 0.001), WC (p = 0.002), left and right MS (p < 0.001), chair stand-up (p = 0.001), sit-and-reach (p = 0.003), MWS (p = 0.001), 2-min step (p < 0.001), SBP (p = 0.008), TC (p = 0.010), and LDL-cholesterol (p = 0.005) had significant interactions between group × time. ASM, DBP, HDL-C, and hs-CRP had no interactions between group × time. In the paired t-test results of the exercise group, BFM percentage (−2.0%), WC (−0.7 cm), SBP (−1.5 mmHg), TC (−3.6 mg/dl), and LDL-cholesterol (−3.7 mg/dl) were significantly decreased, whereas left and right MS (2.5 kg, 3.2 kg), chair stand-up (1.5 repeated), sit-and-reach (1.8 cm), MWS (0.15 m/sec), and 2-min walking (6.0 repeated) were significantly increased. In the control group, left and right MS (−0.6 kg and −0.5 kg), chair stand-up (−1.0 repeated), MWS (−0.03 m/sec), and 2-min step (−1.9 repeated) were significantly decreased.
Table 2. Changes in the body composition, physical function, blood pressure, serum lipid and high-sensitivity C-related protein.
| Control (n = 25) |
Exercise (n = 25) |
F-values | |||
|---|---|---|---|---|---|
| Baseline | 24 week | Baseline | 24week | ||
| Percentage body fat (%) | 40.4 ± 3.6 | 40.8 ± 4.2 | 41.0 ± 3.6 | 39.0 ± 3.9** | 32.45### |
| Waist circumference (cm) | 90.2 ± 4.2 | 90.4 ± 4.1 | 89.4 ± 4.7 | 88.7 ± 5.2 | 10.11## |
| ASM (kg) | 15.0 ± 2.0 | 14.9 ± 1.6 | 14.1 ± 1.8 | 14.5 ± 1.9 | 1.85 |
| Left grip strength (kg) | 22.5 ± 3.1 | 21.9 ± 2.7* | 23.2 ± 2.4 | 25.7 ± 2.6** | 30.42### |
| Right grip strength (kg) | 23.3 ± 2.9 | 22.8 ± 2.8* | 23.7 ± 2.2 | 26.9 ± 2.5*** | 59.50### |
| CSU (repetitions/30 sec) | 16.6 ± 2.9 | 15.6 ± 2.4 | 16.5 ± 3.3 | 18.0 ± 3.9 | 12.81## |
| Sit and reach (cm) | 12.9 ± 4.7 | 12.4 ± 3.9 | 12.9 ± 4.4 | 14.7 ± 4.6 | 9.56## |
| MWS (m/sec) | 1.47 ± 0.21 | 1.43 ± 0.21** | 1.49 ± 0.21 | 1.64 ± 0.21** | 13.57## |
| Two-minute step (repetitions) | 98.5 ± 9.0 | 96.6 ± 10.2* | 98.0 ± 9.2 | 104.2 ± 9.3*** | 54.06### |
| SBP (mmHg) | 128.6 ± 7.3 | 128.9 ± 6.8 | 128.3 ± 6.7 | 126.8 ± 5.0** | 7.68## |
| DBP (mmHg) | 78.2 ± 6.1 | 78.6 ± 4.6 | 78.6 ± 5.7 | 77.8 ± 4.4 | 3.49 |
| Total cholesterol (mg/dl) | 187.7 ± 10.8 | 187.6 ± 9.5 | 188.4 ± 14.5 | 184.8 ± 10.2** | 7.28# |
| Triglyceride (mg/dl) | 116.9 ± 10.7 | 116.2 ± 10.4 | 114.4 ± 11.8 | 115.2 ± 10.7 | 1.67 |
| LDL-cholesterol (mg/dl) | 100.7 ± 14.9 | 102.4 ± 11.8 | 102.1 ± 15.2 | 98.4 ± 9.2 | 8.70## |
| HDL-cholesterol (mg/dl) | 49.0 ± 6.9 | 49.1 ± 5.5 | 49.3 ± 6.2 | 50.3 ± 5.9 | 2.02 |
| High-sensitivity CRP (mg/L) | 0.12 ± 0.13 | 0.12 ± 0.13 | 0.12 ± 0.13 | 0.12 ± 0.12 | 2.16 |
Data are show as mean ± SD. ASM, appendicular skeletal muscle mass; CSU, chair stand up; MWS, maximum walking speed; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low density lipoprotein; HDL, high density lipoprotein; CRP, C-reactive protein;
p < 0.05,
p < 0.01,
p < 0.001, change within the groups,
p < 0.05,
p < 0.01,
p < 0.001, group × time interaction
Table 3 presents changes of carotid artery parameters. In the ANOVA analysis results, CIMT (p = 0.013), PSV (p = 0.007), EDV (p = 0.006), and WSR (p < 0.001) showed significant group × time interactions. CLDs (both systolic and diastolic) had no group × time interactions. In the paired t-test results of the exercise group, CIMT (−0.01 mm) was significantly decreased, whereas PSV (1.4 cm/sec), EDV (0.3 cm/sec), and WSR (10.5 s−1) were significantly increased. However, CLDs (both systolic and diastolic) in the exercise group showed no change nor did any of the carotid artery parameters in the control group.
Table 3. Changes in the carotid artery intima-media thickness, luminal diameter, flow velocity and wall shear rate.
| Control (n = 25) |
Exercise (n = 25) |
F-values | |||
|---|---|---|---|---|---|
| Baseline | 24 week | Baseline | 24week | ||
| CIMT (mm) | 0.67 ± 0.07 | 0.67 ± 0.07 | 0.68 ± 0.08 | 0.67 ± 0.07** | 6.61# |
| Diastolic CLD (cm) | 0.63 ± 0.09 | 0.63 ± 0.09 | 0.63 ± 0.07 | 0.62 ± 0.07 | 0.63 |
| Systolic CLD (cm) | 0.58 ± 0.08 | 0.58 ± 0.07 | 0.59 ± 0.07 | 0.58 ± 0.06 | 2.05 |
| PSV (cm/sec) | 64.2 ± 10.2 | 64.3 ± 9.5 | 66.3 ± 11.3 | 67.7 ± 10.0** | 8.08## |
| EDV (cm/sec) | 19.8 ± 4.9 | 19.8 ± 4.9 | 20.5 ± 4.1 | 20.8 ± 4.1*** | 8.33## |
| Wall shear rate (s−1) | 454.7 ± 110.2 | 452.7 ± 103.3 | 461.3 ± 117.4 | 472.1 ± 104.7* | 8.58## |
Data are show as mean ± SD. CIMT, carotid artery intima-media thickness; CLD, carotid artery luminal diameter; PSV, peak systolic flow velocity; EDV, end diastolic flow velocity;
p < 0.05,
p < 0.01,
p < 0.001, change within the groups;
p < 0.05,
p < 0.01,
p < 0.001, group × time interaction
Discussion
This study examined the effects of a 24-week combined exercise program on carotid artery parameters in elderly women with SO. The main finding of this study is that the 24-week combined exercise program reduced CIMT in elderly women with SO. Additionally, the second finding of our study is that long-term regular exercise increased PSV and WSR in elderly women.
SO is more closely associated with traditional CVD risk factors when compared with either obesity or sarcopenia in the elderly1). Further, SO is more closely associated with increasing CIMT when compared with either obesity or sarcopenia8). Even though previous evidence for exercise and CIMT suggests that exercise training does not affect CIMT in healthy young men and women17–19), two studies of association between exercise training and CIMT demonstrated that aerobic exercise training for 12 or 24 weeks reduced CIMT in overweight and obese adults (−7.8 %) and formerly preeclamptic women (−8.6 %)26, 27). Moreover, one intervention study reported that aerobic exercise for 24 weeks is effective in decreasing the artery wall thickness in a large artery in overweight and obese elderly adults28). In our study, CIMT was decreased (−1.5%) through combined exercise for 24 weeks; however, the effect of exercise training on CIMT was smaller than it was in two previous intervention studies26, 27).
A recent intervention study by Byrkjeland et al. demonstrated that combined exercise training is effective in decreasing CIMT (-0.034 mm) in high-risk individuals with the combination of type 2 diabetes and coronary artery disease (CAD)29). Our study subjects were elderly women with sarcopenic obesity. Obesity and sarcopenia are proven risk factors for CVD and disability in the elderly30, 31). Our study results imply that 6 months of combined exercise is effective in reducing CIMT in elderly women with a combination of low skeletal muscle mass and high body fat mass. Nevertheless, we believe that the effects of exercise on CIMT will appear in limited subjects. Recent reviews on exercise training and atherosclerosis have reported that the reduction in CIMT as a result of exercise training is associated with increased cyclic blood pressure, shear stress, and antioxidant defense and decreased inflammatory processes, vascular tone, and sympathetic nervous system activity32). Another review reported that increased physical activity suppresses the overal CVD risk and hence curtails the progression of carotid atherosclerosis33). The rationale for the association between exercise and CIMT presented in the two reviews is considered to be a potential mechanism for the reduction of CIMT based on combined exercise in this study.
A cross-sectional study34, 35) and a long-term follow-up study9) reported that a large CLD and low FV are closely associated with CVD and stroke in healthy subjects and in patients. Another cross-sectional study reported that CLD in elderly people with high cardiorespiratory function, as evaluated by oxygen uptake, is narrower than it is in those with low cardiorespiratory function36). However, the evidence for the association of CLD and FV with exercise training is very limited. A few interventional studies have reported that shortterm exercise training does not affect the CLD and FV in healthy young men7, 18). In our study, after combined exercise for 24 week, CLDs showed no change; however, PSV (2.1 %), EDV (1.5 %), and WSR (2.3 %) were significantly increased in the exercise group.
Low FV of the carotid artery is associated with BFM and other CVD risk factors7, 37). Low FV and WSR are also associated with accelerated atherosclerosis progression9, 38). Therefore, this study found that regular exercise increases carotid artery FV and WSR in elderly women with SO. The mechanism for changes in the FV in large arteries is not clear. However, several previous studies reported that resting cardiac output is related to middle cerebral artery FV39), and regular exercise improves cardiac function readings such as cardiac output, stroke volume, and ejection fraction40–42). Based on these facts, we think that the improvement of cardiac function such as cardiac output and stroke volume by exercise training contributed to the increase in carotid artery FV. Despite our hypothesis on the mechanism of FV change by exercise training, we have limitations in not investigating cardiac function. In the present study, PSV increased, but the systolic CLD did not change. The change in WSR is determined by changes in PSV and systolic CLD. Therefore, the change in WSR is thought to be due to the increase in PSV. Additionally, the decreasing tendency of systolic CLD may have contributed a little to the increase in WSR. The results of this study suggested that increases in carotid artery FV and WSR by exercise training are considered to have additional benefit for CVD prevention in elderly women subjects with SO. However, more evidence is required to prove a clear association between the carotid FV and regular exercise.
Combined exercise training is effective in improving body composition, physical function, blood pressure, and blood biochemical markers15, 16). Resistance exercise in previous studies used a special exercise machine, requiring a special place15, 16). The resistance exercise in our study, using an elastic band instead of an exercise machine, may be less restrictive, while providing similar effects on body composition and physical function43). Therefore, resistance exercise was performed with an elastic band, and aerobic exercise was performed through walking. Our combined exercise improved the body composition and physical function in elderly women.
Several limitations were inherent in this study. ASM was evaluated by BIA in this study. ASM evaluation by the BIA method has a higher correlation (r2 = 0.95) with dual-energy X-ray (DXA)44), and this method is simple and inexpensive when compared with the DXA method in ASM evaluation. Therefore, BIA was used to evaluate body composition. However, DXA is more widely used in evaluating ASM45). Before interpreting the results, it should be noted that there are certain facts to be considered in this research. The time of the daily physical activities during intervention was not measured not to mention that the diet of either group was not controlled. Moreover, the sample size was small, which is the significant determinant of the research; each factor could have altered the result of the study. Hence, in order to understand the impact of exercise on carotid artery parameters, a bigger sample size and a longer period of trial are needed.
Conclusions
In conclusion, the 24-week combined exercise program effectively decreased CIMT and increased carotid artery FV as well as WSR in elderly women with SO. Therefore, regular combined exercise is thought to contribute to a lower CVD risk in elderly women with SO.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3153).
Disclosure
We have no conflict of interest or financial disclosures to declare in conjunction with the publication of this work.
References
- 1). Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ: Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch Gerontol Geriat, 2013; 56: 270-278 [DOI] [PubMed] [Google Scholar]
- 2). Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC: Sarcopenic obesity: Prevalence and association with metabolic syndrome in the korean longitudinal study on health and aging (klosha). Diabetes Care, 2010; 33: 1652-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY, Park J, Shin KJ, Kim DI, Moon YS: Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens, 2013; 7: 420-425 [DOI] [PubMed] [Google Scholar]
- 4). Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ: Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the national health and nutrition examination survey iii. Eur J Clin Nutr, 2014; 68: 1001-1007 [DOI] [PubMed] [Google Scholar]
- 5). Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB: Carotid-wall intima-media thickness and cardiovascular events. New Engl J Med, 2011; 365: 213-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Bellinazzi VR, Cipolli JA, Pimenta MV, Guimaraes PV, Pio-Magalhaes JA, Coelho OR, Biering-Sorensen T, Matos-Souza JR, Sposito AC, Nadruz W: Carotid flow velocity/diameter ratio is a predictor of cardiovascular events in hypertensive patients. J Hypertens, 2015; 33: 2054-2060 [DOI] [PubMed] [Google Scholar]
- 7). Chuang SY, Bai CH, Chen JR, Yeh WT, Chen HJ, Chiu HC, Shiu RS, Pan WH: Common carotid end-diastolic velocity and intima-media thickness jointly predict ischemic stroke in taiwan. Stroke, 2011; 42: 1338-1344 [DOI] [PubMed] [Google Scholar]
- 8). Kato A, Ishida J, Endo Y, Takita T, Furuhashi M, Maruyama Y, Odamaki M: Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transpl, 2011; 26: 1967-1976 [DOI] [PubMed] [Google Scholar]
- 9). Chuang SY, Bai CH, Cheng HM, Chen JR, Yeh WT, Hsu PF, Liu WL, Pan WH: Common carotid artery end-diastolic velocity is independently associated with future cardiovascular events. Eur J Prev Cardiol, 2016; 23: 116-124 [DOI] [PubMed] [Google Scholar]
- 10). Neto LSS, Karnikowiski MGO, Tavares AB, Lima RM: Association between sarcopenia, sarcopenic obesity, muscle strength and quality of life variables in elderly women. Braz J Phys Ther, 2012; 16: 360-367 [PubMed] [Google Scholar]
- 11). Bouchard DR, Dionne IJ, Brochu M: Sarcopenic/obesity and physical capacity in older men and women: Data from the nutrition as a determinant of successful aging (nuage)-the quebec longitudinal study. Obesity, 2009; 17: 2082-2088 [DOI] [PubMed] [Google Scholar]
- 12). Villareal DT, Holloszy JO: Dhea enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol-Endoc M, 2006; 291: E1003-E1008 [DOI] [PubMed] [Google Scholar]
- 13). Vincent HK, Bourguignon C, Vincent KR: Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity, 2006; 14: 1921-1930 [DOI] [PubMed] [Google Scholar]
- 14). Okura T, Nakata Y, Ohkawara K, Numao S, Katayama Y, Matsuo T, Tanaka K: Effects of aerobic exercise on metabolic syndrome improvement in response to weight reduction. Obesity, 2007; 15: 2478-2484 [DOI] [PubMed] [Google Scholar]
- 15). Tan SJ, Li W, Wang JX: Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J Sport Sci Med, 2012; 11: 495-501 [PMC free article] [PubMed] [Google Scholar]
- 16). Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, Maccora C, Iacobini C, Conti FG, Nicolucci A, Pugliese G, Investigators I: Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the italian diabetes and exercise study (ides). Plos One, 2012; 7: 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H: Unfavorable effects of resistance training on central arterial compliance - a randomized intervention study. Circulation, 2004; 110: 2858-2863 [DOI] [PubMed] [Google Scholar]
- 18). Kawano H, Tanaka H, Miyachi M: Resistance training and arterial compliance: Keeping the benefits while minimizing the stiffening. J Hypertens, 2006; 24: 1753-1759 [DOI] [PubMed] [Google Scholar]
- 19). Okamoto T, Masuhara M, Ikuta K: Effects of eccentric and concentric resistance training on arterial stiffness. J Hum Hypertens, 2006; 20: 348-354 [DOI] [PubMed] [Google Scholar]
- 20). Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ, Med ACS, Assoc AD: Exercise and type 2 diabetes. Med Sci Sport Exer, 2010; 42: 2282-2303 [DOI] [PubMed] [Google Scholar]
- 21). Heran BS, Chen JMH, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS: Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Db Syst Rev, 2011; Ver. 2: 1-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Schuler G, Adams V, Goto Y: Role of exercise in the prevention of cardiovascular disease: Results, mechanisms, and new perspectives. Eur Heart J, 2013; 34: 1790-1799 [DOI] [PubMed] [Google Scholar]
- 23). Bean JF, Vora A, Frontera WR: Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehab, 2004; 85: S31-S42 [DOI] [PubMed] [Google Scholar]
- 24). Ozdemir H, Artas H, Serhatlioglu S, Ogur E: Effects of overweight on luminal diameter, flow velocity and intimamedia thickness of carotid arteries. Diagn Interv Radiol, 2006; 12: 142-146 [PubMed] [Google Scholar]
- 25). Jiang YN, Kohara K, Hiwada K: Association between risk factors for atherosclerosis and mechanical forces in carotid artery. Stroke, 2000; 31: 2319-2324 [DOI] [PubMed] [Google Scholar]
- 26). Feairheller DL, Diaz KM, Kashem MA, Thakkar SR, Veerabhadrappa P, Sturgeon KM, Ling CY, Williamson ST, Kretzschmar J, Lee H, Grimm H, Babbitt DM, Vin C, Fan XX, Crabbe DL, Brown MD: Effects of moderate aerobic exercise training on vascular health and blood pressure in african americans. J Clin Hypertens, 2014; 16: 504-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Scholten RR, Thijssen DJH, Lotgering FK, Hopman MTE, Spaanderman MEA: Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol, 2014; 211. [DOI] [PubMed] [Google Scholar]
- 28). Green DJ, Swart A, Exterkate A, Naylor LH, Black MA, Cable NT, Thijssen DHJ: Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis, 2010; 210: 525-530 [DOI] [PubMed] [Google Scholar]
- 29). Byrkjeland R, Stensaeth KH, Anderssen S, Njerve IU, Arnesen H, Seljeflot I, Solheim S: Effects of exercise training on carotid intima-media thickness in patients with type 2 diabetes and coronary artery disease. Influence of carotid plaques. Cardiovasc Diabetol, 2016; 15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Cho YG, Song HJ, Kim JM, Park KH, Paek YJ, Cho JJ, Caterson I, Kang JG: The estimation of cardiovascular risk factors by body mass index and body fat percentage in korean male adults. Metabolism, 2009; 58: 765-771 [DOI] [PubMed] [Google Scholar]
- 31). Janssen I: Influence of sarcopenia on the development of physical disability: The cardiovascular health study. J Am Geriatr Soc, 2006; 54: 56-62 [DOI] [PubMed] [Google Scholar]
- 32). Thijssen DHJ, Cable NT, Green DJ: Impact of exercise training on arterial wall thickness in humans. Clin Sci, 2012; 122: 311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Kadoglou NP, Iliadis F, Liapis CD: Exercise and carotid atherosclerosis. Eur J Vasc Endovasc Surg, 2008; 35: 264-272 [DOI] [PubMed] [Google Scholar]
- 34). Eigenbrodt ML, Sukhija R, Rose KM, Tracy RE, Couper DJ, Evans GW, Bursac Z, Mehta JL: Common carotid artery wall thickness and external diameter as predictors of prevalent and incident cardiac events in a large population study. Cardiovasc Ultrasound, 2007; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Bai CH, Chen JR, Chiu HC, Pan WH: Lower blood flow velocity, higher resistance index, and larger diameter of extracranial carotid arteries are associated with ischemic stroke independently of carotid atherosclerosis and cardiovascular risk factors. J Clin Ultrasound, 2007; 35: 322-330 [DOI] [PubMed] [Google Scholar]
- 36). Gando Y, Yamamoto K, Kawano H, Murakami H, Ohmori Y, Kawakami R, Sanada K, Higuchi M, Tabata I, Miyachi M: Attenuated age-related carotid arterial remodeling in adults with a high level of cardiorespiratory fitness. J Atheroscler Thromb, 2011; 18: 248-254 [DOI] [PubMed] [Google Scholar]
- 37). Czernichow S, Bertrais S, Oppert JM, Galan P, Blacher J, Ducimetiere P, Hercberg S, Zureik M: Body composition and fat repartition in relation to structure and function of large arteries in middle-aged adults (the su.Vi.Max study). Int J Obesity, 2005; 29: 826-832 [DOI] [PubMed] [Google Scholar]
- 38). Malik J, Kudlicka J, Tuka V, Chytilova E, Adamec J, Rocinova K, Tesar V: Common carotid wall shear stress and carotid atherosclerosis in end-stage renal disease patients. Physiol Res, 2012; 61: 355-361 [DOI] [PubMed] [Google Scholar]
- 39). Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB: The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol-London, 2005; 569: 697-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD: Exercise training in patients with heart failure and preserved ejection fraction meta-analysis of randomized control trials. Circ-Heart Fail, 2015; 8: 33-U73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD: Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation, 2010; 122: 1797-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Alves AJ, Ribeiro F, Goldhammer E, Rivlin Y, Rosenschein U, Viana JL, Duarte JA, Sagiv M, Oliveira J: Exercise training improves diastolic function in heart failure patients. Med Sci Sport Exer, 2012; 44: 776-785 [DOI] [PubMed] [Google Scholar]
- 43). Colado JC, Triplett NT: Effects of a short-term resistance program using elastic bands versus weight machines for sedentary middle-aged women. J Strength Cond Res, 2008; 22: 1441-1448 [DOI] [PubMed] [Google Scholar]
- 44). Kyle UG, Genton L, Hans D, Pichard C: Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (asmm). Clin Nutr, 2003; 22: 537-543 [DOI] [PubMed] [Google Scholar]
- 45). Prado CMM, Wells JCK, Smith SR, Stephan BCM, Siervo M: Sarcopenic obesity: A critical appraisal of the current evidence. Clin Nutr, 2012; 31: 583-601 [DOI] [PubMed] [Google Scholar]


