Abstract
Aim: Both the ankle brachial index (ABI) and brachial-ankle pulse wave velocity (baPWV) are surrogates for atherosclerosis. In this study, we aimed to evaluate the ability of ABI and baPWV to predict stroke outcome in patients with first-ever non-cardioembolic stroke.
Methods: This study included consecutive patients with first-ever non-cardioembolic stroke admitted within 1 week after onset to Ota Memorial Hospital between January 2011 and December 2013. Baseline characteristics and National Institutes of Health stroke scale scores at admission were noted. ABI and baPWV were evaluated within 5 days of admission. The patients were categorized according to ABI (cut-off 0.9) and baPWV (cut-off 1870 cm/s) determined using the receiver operation curve for poor outcome. Clinical outcomes were defined based on the modified Rankin scale (mRS) scores 3 months after stroke onset as good (0 and 1) or poor (2–6).
Results: A total of 861 patients were available for evaluation. ABI < 0.9 and baPWV > 1870 cm/s were associated with poor outcome in the univariate analysis (p < 0.001 and p < 0.001, respectively). After adjusting for factors that showed differences between groups, ABI < 0.9 was associated with poor outcome. Among patients with ABI ≥ 0.9, higher baPWV showed a slight association with poor outcome after adjustment [odds ratio 1.46 (95% CI 0.95–2.27)].
Conclusion: Our study suggests that the stroke outcome can be predicted using ABI and to an extent using baPWV when ABI ≥ 0.9 in patients with non-cardioembolic stroke.
Keywords: Ankle brachial index, Brachial-ankle pulse wave velocity, Ischemic stroke, Non-cardioembolic stroke, Outcome, Modified Rankin scale
Introduction
Atherothrombosis has been proposed as a composite disease that includes myocardial infarction, non-cardioembolic stroke, and peripheral artery disease. This is reasonable because each of the conditions is based on systemic atherosclerosis. Additionally, peripheral artery disease can be highly complicated with stroke. In the REACH Registry, 8.5% of patients with prior stroke and transient ischemic attack had peripheral artery disease and 23.0% of patients with peripheral artery disease had stroke and transient ischemic attack1). Additionally, 2.6% of patients with peripheral artery disease developed nonfatal stroke and 8.8% died from stroke or myocardial infarction during a 2-year follow-up period2).
Pulse wave velocity (PWV) reflects segmental arterial elasticity. Although carotid-femoral PWV is currently the gold standard measurement method, brachial-ankle PWV (baPWV) is a more convenient and widely available method. It is well correlated with carotid-femoral PWV and indicates central arterial stiffness3, 4). BaPWV is associated with subclinical stage of atherosclerosis and is an independent predictor of future cardiovascular events5, 6). Recently, the ankle brachial index (ABI) and baPWV were reported as predictive factors of stroke outcome7–10). Additionally, there is an inter-influence between atherosis and sclerosis, with ABI indicating arterial atherosis11) and baPWV indicating sclerosis12, 13). However, it has been reported that the accuracy of baPWV in evaluating pathophysiological conditions related to atherosclerosis diminishes when ABI is low14, 15). Therefore, with regard to atherosclerosis pathophysiology, ABI and baPWV are related. Previous studies have not considered the relation between ABI and baPWV in the evaluation of their influence on stroke outcomes.
Aim
In the present study, we aimed to evaluate the ability of ABI and baPWV to predict stroke outcome in patients with first-ever non-cardioembolic stroke. We hypothesized that ABI and baPWV may affect modifiers of each other with their influence on stroke outcome. Additionally, the influence of baPWV on stroke outcome may differ between patients with ABI < 0.9 and those with ABI ≥ 0.9.
Methods
Study Design and Participants
A total of 2413 patients with acute ischemic stroke, without transient ischemic attack, and who were admitted to our Brain Attack Center between January 2011 and December 2013 were considered for inclusion in this study. Among these patients, 597 with cardioembolic stroke, 730 with a prior stroke history, 76 admitted later than 7 days after stroke onset, 92 with tissue plasminogen activator treatment, 142 who received endovascular therapy, 62 who underwent surgical operation, and 546 with premorbid mRS ≥ 2 were excluded from this study. In addition, there were 105 patients without available ABI and baPWV data and 53 patients without 3-month modified Rankin scale (mRS) data. Finally, 861 patients with first-ever acute non-cardioembolic stroke (285 female patients; mean age, 70.2 ± 11.6 years) were available for evaluation in this study (Fig. 1). This study was approved by the institutional review board of Brain Attack Center Ota Memorial Hospital (No. 133) and was performed according to the Ethical Guidelines for Medical and Health Research Involving Human Subject16) based on the Helsinki Declaration of 1964. Because this was a retrospective study, we did not obtain the patient's consent.
Fig. 1.
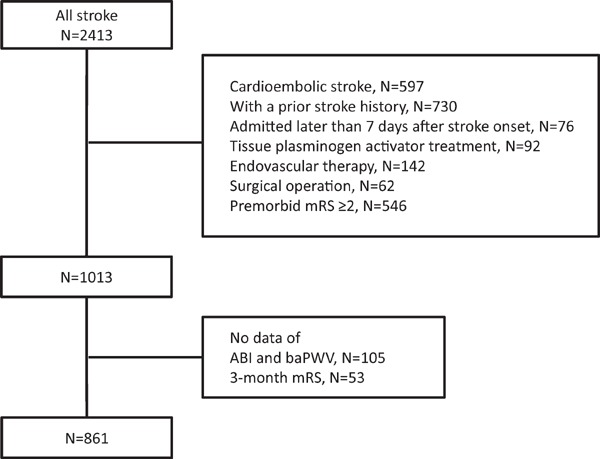
Flow chart of inclusion and exclusion criteria
Diagnosis of Stroke and Date Collection
The final diagnosis of the stroke subtype was made before discharge using echocardiography, brain computed tomography, magnetic resonance imaging, magnetic resonance angiography, and carotid ultrasonography according to the Trial of Org 10172 in Acute Stroke Treatment classification17). Physicians collected detailed data from all patients, including baseline characteristics [age, sex, body mass index (BMI), and drinking and smoking habits], vascular risk factors (hypertension, dyslipidemia, and diabetes mellitus), use of antithrombotic agent prior to the stroke incidence, and neurologic deficits at admission using the National Institutes of Health stroke scale (NIHSS) score. Hypertension was defined as the use of anti-hypertensive medications prior to admission or a confirmed blood pressure ≥ 140/90 mmHg 2 weeks after stroke onset. Diabetes mellitus was defined as an HbA1c value of ≥ 6.5%, fasting blood sugar level ≥ 126 mg/dL, or the use of anti-diabetic medications. Dyslipidemia was defined as a total cholesterol level ≥ 220 mg/dL, low-density lipoprotein cholesterol level ≥ 140 mg/dL, high-density lipoprotein cholesterol level < 40 mg/dL, triglyceride level ≥ 150 mg/dL at admission, or the use of anti-dyslipidemia medications. mRS scores were evaluated 3 months after onset, and patients were categorized into good outcome (mRS score, 0 and 1) or poor outcome (mRS score, 2–6) groups.
Measurements of ABI and baPWV
ABI and baPWV were evaluated within 5 days of hospitalization. Brachial-ankle arterial blood pressures were simultaneously measured using a noninvasive automatic device (model BP-203RPE-III; Nihon Colin, Tokyo, Japan) after a 5-min rest in the supine position. ABI was defined as the ratio of systolic blood pressure in the ankle (dorsalis pedis and posterior tibial arteries) and the higher side of the two brachial arteries. The laterality, which showed a lower ABI, was used for evaluation. baPWV on each side was calculated as the transmission distance divided by the transmission time. The transmission time between the right arm and both ankles was calculated using the waveform. The transmission distance between the right brachium and ankle was automatically calculated according to the height of the patient. baPWV was evaluated on the higher side.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or median (25th and 75th percentiles) for continuous variables and as frequency and percentage for discrete variables. The statistical significance of intergroup differences was assessed using the analysis of variance, Kruskal–Wallis test, or χ2 test, as appropriate. Univariate and multivariate logistic regression analyses were performed to evaluate association of factors with poor outcome, and the odds ratios and 95% confidence intervals were calculated. The receiver operation characteristic curve was used to determine a cut-off baPWV for predicting poor outcome. In multivariate logistic regression analysis, factors that showed intergroup difference in evaluation with p-values < 0.2 in the univariate analysis were used for adjustment. Statistical significance was set at a p value < 0.05. All statistical analyses were performed using JMP 12.0.1 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
ABI was less than 0.9 in 72 (8.4%) patients. The mean baPWV was 2059 ± 491 cm/s. On plotting the values of ABI and baPWV, there were linear associations between these values, separately for low (< 0.9) and high ABI (≥ 0.9) (Fig. 2). Poor outcome 3 months after onset was noted in 254 (29.5%) patients. ABI < 0.9 and baPWV were associated with poor outcome in the univariate analysis (p < 0.001 and p < 0.001, respectively). The cut-off of baPWV for predicting poor outcome was 1870 cm/s.
Fig. 2.
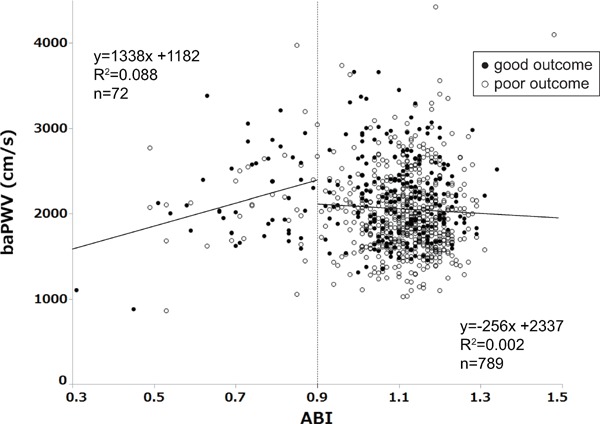
Scatter plot of ABI and baPWV and their regression lines
There are linear associations between these factors, separately for low (< 0.9) and high ABI (≥ 0.9). ABI, ankle brachial index; baPWV, brachial-ankle pulse wave velocity
Patients were categorized into four groups by combination of ABI (ABI < 0.9 or ≥ 0.9) and baPWV (baPWV > 1870 cm/s or ≤ 1870 cm/s). We classified patients with ABI ≥ 0.9 and baPWV ≤ 1870 cm/s as group 1 (n = 316), those with ABI ≥ 0.9 and baPWV > 1870 cm/s as group 2 (n = 473), those with ABI < 0.9 and baPWV ≤ 1870 cm/s as group 3 (n = 21), and those with ABI < 0.9 and baPWV > 1870 cm/s as group 4 (n = 51). Baseline characteristics of the patients are presented in Table 1. Age, BMI, ischemic stroke subtype, NIHSS score on admission, smoking, hypertension, diabetes mellitus, and usage of antithrombotic agent were significantly different among four groups. The NIHSS score was higher in group 4 than in group 1 (P = 0.002). Proportions of poor outcome (3 months mRS, 2–6) were significantly higher in group 3 and 4 (p < 0.001, Fig. 3). With multivariate logistic regression models adjusted with these factors, group 2 showed slightly high odds ratio compared with group 1, although there was no statistical significance. Group 3 and 4 were significantly associated with poor outcome (Table 2).
Table 1. Characteristics among the patients categorized with the ABI and baPWV.
| Group 1 | Group 2 | Group 3 | Group 4 | P-value | |
|---|---|---|---|---|---|
| ABI ≥ 0.9 | ABI ≥ 0.9 | ABI < 0.9 | ABI < 0.9 | ||
| baPWV ≤ 1870 cm/s | baPWV < 1870 cm/s | baPWV ≤ 1870 cm/s | baPWV < 1870 cm/s | ||
| N = 316 | N = 473 | N = 21 | N = 51 | ||
| Age, years | 63.2 ± 11.8 | 74.1 ± 9.1 | 72.5 ± 14.4 | 76.9 ± 8.7 | < 0.001 |
| Female, n (%) | 101 (32.0) | 160 (33.8) | 9 (42.9) | 15 (29.4) | 0.679 |
| BMI | 23.9 ± 3.6 | 23.2 ± 3.4 | 23.3 ± 2.8 | 23.0 ± 3.7 | 0.019 |
| Ischemic stroke | |||||
| Subtype | < 0.001 | ||||
| LAA, n (%) | 76 (24.1) | 127 (26.9) | 11 (52.3) | 27 (53.0) | |
| SVO, n (%) | 129 (40.8) | 166 (35.1) | 4 (19.1) | 10 (19.6) | |
| Other, n (%) | 24 (7.6) | 19 (4.0) | 2 (9.5) | 2 (3.9) | |
| Undetermined, n (%) | 87 (27.5) | 161 (34.0) | 4 (19.1) | 12 (23.5) | |
| NIHSS score on admission | 2 [1–3] | 2 [1–4] | 3 [1–4] | 4 [2–5] | 0.002 |
| (median [IQR]) | |||||
| Drinking, n (%) | 143 (45.4) | 190 (40.6) | 6 (28.6) | 20 (39.2) | 0.310 |
| Smoking, n (%) | 177 (56.0) | 178 (38.4) | 11 (52.4) | 23 (45.1) | < 0.001 |
| Hypertension, n (%) | 211 (69.6) | 374 (81.1) | 16 (76.2) | 47 (92.2) | < 0.001 |
| Dyslipidemia, n (%) | 189 (60.4) | 272 (58.0) | 12 (57.1) | 28 (56.0) | 0.889 |
| Diabetes mellitus, n (%) | 99 (31.5) | 176 (37.5) | 11 (52.4) | 27 (52.9) | 0.008 |
| Antithrombotic agent, n (%) | 23 (7.3) | 60 (12.7) | 5 (23.8) | 13 (25.5) | < 0.001 |
ABI, ankle brachial index; baPWV, brachial-ankle pulse wave velocity; LAA, large artery atherosclerosis; SVO, small vessel occlusion; NIHSS, National Institutes of Health stroke scale; IQR, interquartile raange; 3M mRS, modified Rankin scale 3-month after onset
Fig. 3.
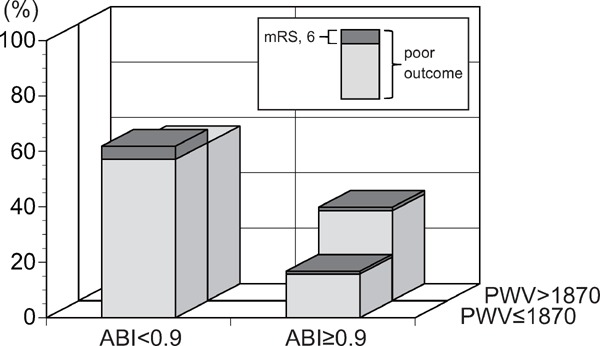
Proportion of patients who showed poor outcome (3 month mRS 2–6) and mortality
Table 2. Odds ratio for poor outcome of groups categorized with ABI and baPWV.
| ABI ≥ 0.9 | ABI < 0.9 | |
|---|---|---|
| baPWV ≤ 1870 | Group 1 | Group 3 |
| N = 316 | N = 21 | |
| 1.0 (reference) | 5.19 (1.85–15.14) | |
| baPWV > 1870 | Group 2 | Group 4 |
| N = 473 | N = 51 | |
| 1.46 (0.95–2.27) | 3.37 (1.63–7.08) |
Odds ratio (95% confidence intervals) was calculated with multivariate logistic regression analysis adjusted with age, body mass index, large artery atherosclerosis, National Institute of Health stroke scale on admission, smoking, hypertension, diabetes mellitus and antithrombotic agent, which associated with poor outcome.
ABI, ankle brachial index; baPWV, brachial-ankle pulse wave velocity.
Discussion
In our study, among patients with first-ever noncardioembolic stroke, ABI < 0.9 and baPWV was associated with the outcome 3 months after stroke onset. The outcome 3 months after stroke onset was poorer in patients with ABI < 0.9 than in those with ABI ≥ 0.9. Among patients with ABI ≥ 0.9, baPWV > 1870 cm/s had some prognostic value of poor outcome in comparison with patients with baPWV ≤ 1870 cm/s.
It has been reported that a low ABI of < 0.9 has a prognostic value for mortality and incidences of coronary artery disease and stroke in the general population18, 19). In our study, 68.1% of the patients with a low ABI showed poor outcome 3 months after the events. A low ABI showed high predictivity for poor outcome 3 months after the events. Kim et al. reported that in patients with acute first-ever stroke, ABI < 0.9 was a predictive factor for mRS 0–2 3 months after stroke onset7). A previous study reported that the mortality rate of patients with ischemic stroke increases when peripheral artery disease is also present20). Some studies showed an association between ABI < 0.9 and an increase in mortality or recurrence of stroke in patients with acute stroke; therefore, poor outcome at 3 months may result from high mortality or recurrence of stroke18, 21–23). In our study, the mortality rate was 4.3% in patients with low ABI and was 1.6% in patients with high ABI. Additionally, the stroke severity at admission was higher in patients with low ABI than in patients with high ABI. Therefore, it may have an influence on poor outcome.
Peripheral artery disease is present in 10.1% patients with cerebrovascular disease24). In the present study, ABI < 0.9 was noted in 8.4% of patients. A recent study showed that 20.1% of patients with acute ischemic stroke or transient ischemic attack were diagnosed with peripheral artery disease on computed tomography angiography, and the proportion of patients with a low ABI was 12.2%25). The criterion of ABI < 0.9 may underestimate the presence of peripheral artery disease, although it is widely used as a surrogate marker for peripheral artery disease. In this study, we did not perform any additional evaluation for peripheral artery disease. Therefore, there may be a higher proportion of patients with peripheral artery disease than those detected with a low ABI.
The findings of our study suggest that a high baPWV is a weak predictive factor for poor outcome when ABI is ≥ 0.9. Our scatter plot of ABI and baPWV and their regression lines suggested that the accuracy of baPWV may diminish when ABI is < 0.9. Our results are supported by the findings of a previous study that reported associations of mortality with ABI and baPWV in patients undergoing dialysis26). In this previous study, a high baPWV predicted mortality only in patients with ABI ≥ 0.9. Kim et al. reported an association between baPWV and the long-term outcome of acute first-ever stroke without consideration of ABI8). They showed that patients with the highest baPWV tertile had a significantly high OR of poor outcome in comparison with patients with the lowest tertile. The proportion of patients with ABI < 0.9 was slightly higher in this previous study (8.8%) than in our study (8.4%). Additionally, the proportion of patients with ABI < 0.9 was lower in their lowest baPWV tertile (< 1755 cm/s, 6.3%) than in our lower baPWV tertile (< 1870 cm/s, 9.7%). Ishizuka K et al. also reported an association between baPWV and 3-month mRS in patients with acute stroke without consideration of ABI9). Their study population was relatively small (n = 327), and the proportion of patients with mRS ≥ 3 was higher (32.1%) than that in our study (16.4%). Details about the proportion of patients with low ABI or PAD were not provided. Moreover, both of the previous reports included a stroke subtype of cardioembolic stroke. These differences might account for the inconsistent results between these previous studies and our study.
Our study indicates that the predictive power of baPWV is weaker than that of ABI for the outcome of non-cardioembolic stroke. There are several explanations for this result. First, baPWV was under estimated when patients had peripheral artery disease as mentioned above. Moreover, baPWV indicates arterial stiffness that increases because of atherosclerosis. On the other hand, ABI indicates stenosis or obstruction of the artery that in turn indicates a progressed stage of atherosclerosis. Therefore, ABI may indicate severe atherosclerosis that has an impact on stroke outcome more directly than baPWV.
Limitations
There are several limitations in this study. First, this study was a single hospital retrospective study. This setting may have caused selection bias in our results. Second, there were significant differences in stroke severity at admission among the groups. In particular patients with ABI < 0.9 had a high NIHSS score at admission. Although we included the NIHSS score for adjustment in the models of multivariate analysis, the difference may have influenced the prediction of stroke outcome with ABI and baPWV.
Conclusion
Our study suggests that the stroke outcome can be predicted using ABI, and to an extent using baPWV when ABI ≥ 0.9 in patients with non-cardioembolic stroke. Further studies evaluating ABI or baPWV for their association with stroke outcome should be designed considering their inter-relationship.
Acknowledgements and Notice of Grant Support
We would like to thank Ms. Tomoko Fukushima from Brain Attack Center Ota Memorial Hospital for her invaluable support with data management.
Conflict of Interest
Dr. Hosomi reports an honorarium from Mochida Pharmaceutical Co., LTD., which is outside the scope of the submitted work. Prof. Matsumoto reports grants from Takeda Pharmaceutical Co., LTD., Sanofi K.K., Mochida Pharmaceutical Co., LTD., Otsuka Pharmaceutical, and Daiichi Sankyo Co., LTD. and honoraria from Sanofi K.K., Bayer Health Care, and Daiichi Sankyo Co., LTD., which are outside the scope of the submitted work. The other authors declare no conflicts of interest.
References
- 1). Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, Goto S, Röther J, Steg PG, Hirsch AT: Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis, 2009; 204: e86-92 [DOI] [PubMed] [Google Scholar]
- 2). Meadows TA, Bhatt DL, Hirsch AT, Creager MA, Califf RM, Ohman EM, Cannon CP, Eagle KA, Alberts MJ, Goto S, Smith SC, Jr, Wilson PW, Watson KE, Steg PG: Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: insights from the reduction of atherothrombosis for continued health (REACH) registry. Am Heart J, 2009; 158: 1038-1045 [DOI] [PubMed] [Google Scholar]
- 3). Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T: Comparison between carotidfemoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hpertens, 2009; 27: 2022-2027 [DOI] [PubMed] [Google Scholar]
- 4). Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, Emoto M, Nishizawa Y: Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb, 2010; 17: 658-665 [DOI] [PubMed] [Google Scholar]
- 5). Torii S, Arima H, Ohkubo T, Fujiyoshi A, Kadota A, Takashima N, Kadowaki S, Hisamatsu T, Saito Y, Miyagawa N, Zaid M, Murakami Y, Abbott RD, Horie M, Miura K, Ueshima H, SESSA Research Group : Association between Pulse Wave Velocity and Coronary Artery Calcification in Japanese men. J Atheroscler Thromb, 2015; 22: 1266-1277 [DOI] [PubMed] [Google Scholar]
- 6). Tomiyama H, Matsumoto C, Shiina K, Yamashina A: Brachial-Ankle PWV: Current Status and Future Directions as a Useful Marker in the Management of Cardiovascular Disease and/or Cardiovascular Risk Factors. J Atheroscler Thromb, 2016; 23: 128-146 [DOI] [PubMed] [Google Scholar]
- 7). Kim J, Lee DH, Cha MJ, Song TJ, Park JH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH: Low ankle-brachial index is an independent predictor of poor functional outcome in acute cerebral infarction. Atherosclerosis, 2012; 224: 113-117 [DOI] [PubMed] [Google Scholar]
- 8). Kim J, Song TJ, Kim EH, Lee KJ, Lee HS, Nam CM, Song D, Nam HS, Kim YD, Heo JH: Brachial-ankle pulse wave velocity for predicting functional outcome in acute stroke. Stroke, 2014; 45: 2305-2310 [DOI] [PubMed] [Google Scholar]
- 9). Ishizuka K, Hoshino T, Shimizu S, Shirai Y, Mizuno S, Toi S, Maruyama K, Uchiyama S, Kitagawa K: Brachialankle pulse wave velocity is associated with 3-month functional prognosis after ischemic stroke. Atherosclerosis, 2016; 255: 1-5 [DOI] [PubMed] [Google Scholar]
- 10). Milionis H, Vemmou A, Ntaios G, Makaritsis K, Koroboki E, Papavasileiou V, Savvari P, Spengos K, Elisaf M, Vemmos K: Ankle-brachial index long-term outcome after first-ever ischaemic stroke. Eur J Neurol, 2013; 20: 1471-1478 [DOI] [PubMed] [Google Scholar]
- 11). Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA: Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol, 1994; 140: 526-534 [DOI] [PubMed] [Google Scholar]
- 12). Lehmann ED: Clinical value of aortic pulse-wave velocity measurement. Lancet, 1999; 354: 528-529 [DOI] [PubMed] [Google Scholar]
- 13). Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI: Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension, 1995; 26: 485-490 [DOI] [PubMed] [Google Scholar]
- 14). Kempczinski RF: Segmental volume plethysmography in the diagnosis of lower extremity arterial occlusive disease. J Cardiovasc Surg (Torino), 1982; 23: 125-129 [PubMed] [Google Scholar]
- 15). Motobe K, Tomiyama H, Koji Y, Yambe M, Gulinisa Z, Arai T, Ichihashi H, Nagae T, Ishimaru S, Yamashina A: Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J, 2005; 69: 55-60 [DOI] [PubMed] [Google Scholar]
- 16).http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf
- 17). Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]
- 18). Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM: Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA, 2008; 300: 197-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Gronewold J, Hermann DM, Lehmann N, Kröger K, Lauterbach K, Berger K, Weimar C, Kälsch HI, Moebus S, Jöckel KH, Bauer M, Erbel R: Ankle-brachial index predicts stroke in the general population in addition to classical risk factors. Atherosclerosis, 2014; 233: 545-550 [DOI] [PubMed] [Google Scholar]
- 20). Koton S, Tanne D, Green MS, Bornstein NM: Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the first national acute stroke Israeli survey (NASIS 2004). Neuroepidemiology, 2010; 34: 90-96 [DOI] [PubMed] [Google Scholar]
- 21). Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D: Anklearm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol, 1999; 19: 538-545 [DOI] [PubMed] [Google Scholar]
- 22). O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB: Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation, 2006; 113: 388-393 [DOI] [PubMed] [Google Scholar]
- 23). Tsivgoulis G, Bogiatzi C, Heliopoulos I, Vadikolias K, Boutati E, Tsakaldimi S, Al-Attas OS, Charalampidis P, Piperidou C, Maltezos E, Papanas N: Low ankle-brachial index predicts early risk of recurrent stroke in patients with acute cerebral ischemia. Atherosclerosis, 2012; 220: 407-412 [DOI] [PubMed] [Google Scholar]
- 24). Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW: International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA, 2006; 295: 180-189 [DOI] [PubMed] [Google Scholar]
- 25). Naito H, Naka H, Kobayashi M, Kanaya Y, Naito K, Kurashige T, Tokinobu H, Matsumoto M: Prevalences of peripheral arterial disease diagnosed by computed tomography angiography in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis, 2016; 25: 1128-1134 [DOI] [PubMed] [Google Scholar]
- 26). Kitahara T, Ono K, Tsuchida A, Kawai H, Shinohara M, Ishii Y, Koyanagi H, Noguchi T, Matsumoto T, Sekihara T, Watanabe Y, Kanai H, Ishida H, Nojima Y: Impact of brachial-ankle pulse wave velocity and ankle-brachial blood pressure index on mortality in hemodialysis patients. Am J Kidney Dis, 2005; 46: 688-696 [DOI] [PubMed] [Google Scholar]


