Abstract
Changes in functional connectivity of cortical networks have been observed in resting-state EEG studies in healthy aging as well as preclinical and clinical stages of AD. Little information, however, exists on associations between EEG connectivity and cortical amyloid load in people with subjective memory complaints. Here, we determined the association of global cortical amyloid load, as measured by florbetapir-PET, with functional connectivity based on the phase-lag index of resting state EEG data for alpha and beta frequency bands in 318 cognitively normal individuals aged 70–85 years with subjective memory complaints from the INSIGHT-preAD cohort. Within the entire group we did not find any significant associations between global amyloid load and phase-lag index in any frequency band. Assessing exclusively the subgroup of amyloid-positive participants, we found enhancement of functional connectivity with higher global amyloid load in the alpha and a reduction in the beta frequency bands. In the amyloid-negative participants, higher amyloid load was associated with lower connectivity in the low alpha band. However, these correlations failed to reach significance after controlling for multiple comparisons. The absence of a strong amyloid effect on functional connectivity may represent a selection effect, where individuals remain in the cognitively normal group only if amyloid accumulation does not impair cortical functional connectivity.
Keywords: Preclinical Alzheimer's disease, Cortical amyloid load, Functional connectivity, EEG, PET
Highlights
-
•
A confirmatory study in subjective memory complainers (SMC) is presented.
-
•
Amyloid is not associated with functional connectivity in SMC.
-
•
Effects of amyloid on cognition are independent of functional connectivity in SMC.
-
•
Functional connectivity changes may follow amyloid load with a temporal delay.
1. Introduction
Evidence from combined functional MRI and amyloid-sensitive PET studies suggests that the cortical accumulation of amyloid may lead to reduced functional connectivity in cognitively unimpaired older people, including but not restricted to the default mode network (Drzezga et al., 2011, Lim et al., 2014).
Due to its high temporal resolution EEG provides information on synchronization of neuronal activity, a potential constitutive mechanism of functional network integration (Fries, 2015). Different to fMRI EEG provides a high temporal resolution across different frequency bands, however, with smaller spatial resolution. So far, only one previous study determined associations between EEG measures of functional connectivity and amyloid status (based on CSF examination) in cognitively normal older people (Stomrud et al., 2010). In a previous study in MCI, 142 CSF amyloid positive patients had more global slowing of oscillatory brain activity than 101 amyloid negative patients, indicated by a lower relative alpha and beta power (Gouw et al., 2017). Recent developments in computational analysis of EEG signals, including connectivity measures such as coherence or phase lag and graph analysis characteristics in combination with data driven machine learning classification have shown promising discrimination between at risk groups for Alzheimer's disease (AD) (for example homozygote ApoE4 carriers) and healthy people (Cuesta et al., 2015), however, based on small samples. Only few studies with small sample sizes have so far assessed EEG alterations, including functional connectivity measures, in people with subjective memory complaints (SMC) (Babiloni et al., 2010, Prichep, 2007) a potential at risk group for cognitive decline of the AD type (Jessen et al., 2014).
Here, we investigated the association of cortical amyloid load, measured by florbetapir-PET, with interhemispheric functional connectivity based on the phase-lag index of resting state EEG data (Stam et al., 2007) in a large sample of 318 people with SMC from the INSIGHT-preAD cohort. We decided to use functional connectivity rather than local power as primary outcome based on previous evidence from fMRI studies on the negative effect of amyloid on functional connectivity in cognitively healthy people (Hedden et al., 2009), and we selected the phase-lag index as primary outcome for functional connectivity due to its lower sensitivity towards volume conduction effects ((Cohen, 2014), page 346) (that may lead to spurious connectivity effects) compared with more widely used measures such as coherence. We hypothesized that high amyloid load would be associated with decreased functional connectivity in the alpha and beta frequencies and that a possible association between amyloid load and cognitive function would partly be mediated by the effects of amyloid load on EEG functional connectivity. Determining the associations of EEG based functional connectivity with amyloid load in potentially preclinical stages of AD may give access to a low-invasive, widely available and inexpensive biomarker of AD.
2. Participants and methods
2.1. Participants
Participants were recruited in the INSIGHT-preAD study, a mono-centric academic university based cohort derived from the Institute for Memory and Alzheimer's Disease (IM2A) at the Pitié-Salpêtrière University Hospital in Paris, France, with the objective to investigate the earliest preclinical stages of AD and its development including influencing factors and markers of progression (Dubois et al., in preparation).
The INSIGHT-preAD study currently includes baseline data of 318 cognitively normal Caucasian individuals from the Paris area, between 70 and 85 years old, with subjective memory complaints and with defined brain amyloid status. The study aims at 7-years of follow-up. Demographic, cognitive, functional, nutritional, biological, genetic, genomic, imaging, electrophysiological and other assessments were performed at baseline. Subjective memory complaints were confirmed by an affirmative answer to both of the following questions: (i) “Are you complaining about your memory?”, and (ii) “Is it a regular complaint which lasts more than 6 months?”.
Each participant had a total recall at the Free and Cued Selective Reminding Test in the normal range (mean 46.1 ± 2.0).
Written informed consent was provided by all participants. The study was approved by the local Institutional Review Board, and has been conducted in accord with the Helsinki Declaration of 1975.
2.2. Cognitive testing
A comprehensive neuropsychological battery was administered to all participants of the INSIGHT-preAD cohort including the Mini-Mental State Examination (MMSE) (Folstein et al., 1975, Kalafat et al., 2003), the Digit span (forward and backward) (Wechsler, 1997) and the Free and Cue Selective Reminding Tests (Buschke, 1984); Letter and Category Verbal Fluency test(Benton, 1968), the Rey-Osterrieth Complex Figure Copy (Fastenau et al., 1999); and the Trail Making Test (Tombaugh, 2004). To reduce the number of comparisons, we a priori decided to explore associations of amyloid load and structural covariance only with measures of global cognition, episodic memory and executive function, respectively. Therefore, we selected performance in the MMSE, the delayed recall of the Free and Cued Selective Reminding Test (FCSRT), and the ratio of the TMT-B to TMT-A performance (time TMT-B divided by time TMT-A) (Drane et al., 2002) as measure of executive function (Arbuthnott and Frank, 2000).
2.3. MRI acquisition
MRI acquisitions of the brain were conducted using a 3 Tesla scanner with parallel imaging capabilities (Siemens Magnetom Verio, Siemens Medical Solutions, Erlangen, Germany). The scanner used a quadrature detection head coil with 12 channels (transmit-receive circularly polarized CP-head coil).
For the anatomical study, 3D TurboFLASH sequences were performed (orientation sagittal; repetition time 2300 ms; echo time 2.98 ms; inversion time 900 ms; flip angle 9°; 176 slices; slice thickness 1 mm; field of view 256 ∗ 240 mm; matrix 256 ∗ 240; bandwidth 240 Hz/Px).
2.4. PET acquisition
All florbetapir-PET scans were acquired in a single session on a Philips Gemini GXL CT-PET scanner 50 (± 5) minutes after injection of approximately 370 MBq (333–407 MBq) of Florbetapir. PET acquisition consisted of 3 × 5 min frames, a 128 × 128 acquisition matrix and a voxel size of 2 × 2 × 2 mm3. Images were then reconstructed using iterative LOR-RAMLA algorithm (10 iterations), with a smooth post-reconstruction filter. All corrections (attenuation, scatter, and random coincidence) were integrated in the reconstruction. Lastly, frames were realigned, averaged and quality-checked by the CATI team (http://cati-neuroimaging.com).
2.5. EEG acquisition
The neurophysiological EEG data were recorded using a high-density 256 channel EGI system (Electrical Geodesics Inc., USA) with a sampling rate of 250 Hz. The electrodes used are sponge-based in order to have a quick application time (10–20 min) necessary due to the elderly population. The impedances of all electrodes were kept below 50 kΩ (Ferree et al., 2001). During the recording, patients were instructed to keep awake and relaxed, with their eyes closed in a quiet room. 60 s of eyes-closed resting-state were selected for analysis.
2.6. PET processing
Reconstructed PET images were analyzed with a pipeline developed by the CATI, a neuroimaging platform funded by the French Plan Alzheimer (http://cati-neuroimaging.com). Structural MRI images were co-registered to Florbetapir-PET images using SPM8 with visual inspection to detect any co-registration errors. Using inverse deformation fields and matrix transformation from MRI data processing, composite cortical ROIs (left and right precuneus, posterior and anterior cingulate, parietal, temporal and orbitofrontal cortex) derived from (Clark et al., 2012) and a reference region (in pons and whole cerebellum) were placed in the individual native PET space. After correcting for partial volume effect with the RBV-sGTM method (Thomas et al., 2011), parametric PET images were created for each individual, by dividing each voxel with the mean activity extracted from the reference region. Finally, standard uptake value ratios (SUVR) were calculated by averaging the mean activity of all cortical ROIs in the individual PET native space.
The SUVR threshold to determine amyloid positivity was extracted performing a linear correlation between the above method and the one used by (Joshi et al., 2015) using PET images of normal controls, MCI and AD patients from the IMAP cohort (Besson et al., 2015). Indeed, several previous studies have shown that positivity cutoffs could be reliably converted between tracers and processing methods, using the linear association across subjects (Landau et al., 2013, Villemagne et al., 2012). The positivity threshold of 1.10 associated with Joshi et al.'s (2015) method was defined as the confidence limit for the upper 5% of the SUVR distribution based on 2 groups of respectively 10 and 11 healthy young controls (age interval 38–52 years) and corresponded to a value of 0.88 with our method. Thus all INSIGHT subjects with a SUVR above 0.88 were considered as amyloid positive.
2.7. EEG processing
As a first step we selected 70 electrodes according to the international 10–10 system. Then EEG continuous data from these channels were high-pass filtered at 1 Hz to compensate for the low-frequency ‘drift’ of the signal know to be spatiotemporally non-stationary (Winkler et al., 2015). Sinusoidal line noise artifacts were removed using a phase-invariant method (CleanLine, Mullen T. NITRC: CleanLine: Tool/Resource Info, 2012). Then, bad channels were identified and interpolated using the Artifact Subspace Reconstruction scheme (ASR) (Kothe and Makeig, 2013), which is a critical step particularly before average referencing. After average referencing, the continuous data was cleaned from eye and muscular artifacts using an automatic classification of artifactual independent component analysis (ICA) activations method (MARA) (Winkler et al., 2014). The pre-processing pipeline was performed with a mixture of EEGlab plugins and in-house Matlab code.
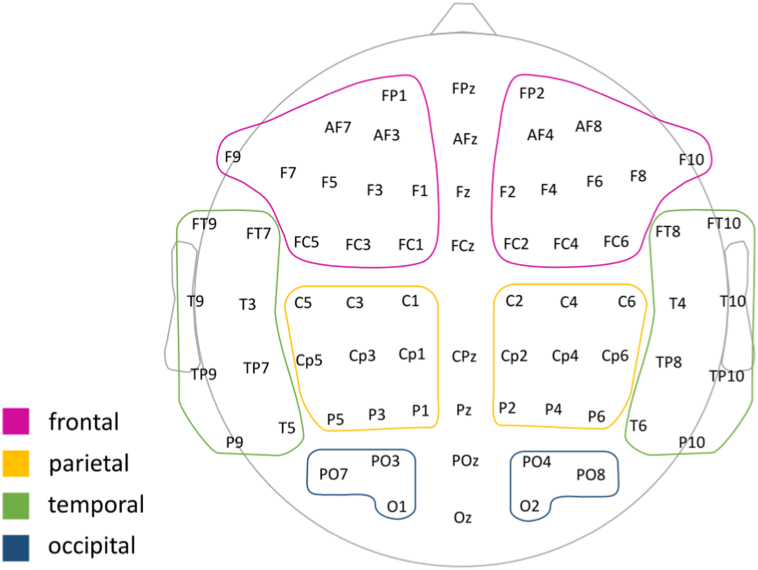
Next, we computed the relative power spectra for each subject, as the ratio between the absolute power in a single band and the summed power of 6 frequency bands defined as: delta (2–3.9 Hz), theta (4–7.3 Hz), low alpha (7.5–9.75 Hz), high alpha (10.25–12.5 Hz), beta (13–30 Hz) and gamma (31–49 Hz). Higher frequencies were not included because this fast activity cannot reliably be distinguished from muscle artifacts. The mean relative power was computed within the electrodes selected to compose 8 different hemispheric regions: left Frontal (FP1, AF3, AF7, F1, F3, F5, F7, F9, FC1, FC3, FC5), left Parietal (C1, C3, C5, CP1, CP3, CP5, P1, P3, P5), left Temporal (FT7, FT9, T3, T5, T9, Tp7, TP9, P9), left Occipital (O1, PO3, PO7), right Front (FP2, AF4, AF8, F2, F4, F6, F8, F10, FC2, FC4, FC6), right Parietal (C2, C4, C6, Cp2, CP4, CP6, P2, P4, P6), right Temporal (FT8, FT10, T4, T6, T10, TP8, TP10, P10), right Occipital (O2, PO4, PO8), excluding electrodes commonly affected by muscular and ocular artifacts at the mid-line (see Fig. 1).
Fig. 1.
Definition of large cortical regions for EEG PLI averaging.
The labels show the locations of the reduced 70 EEG electrodes that correspond to the 10–20 system. Colored boundaries indicate the proximal lobar region under the electrodes used as reference to group the electrodes. PLI values obtained between each pair of electrodes were averaged according to this ROI definition. The illustration is shown from the top of the head with the front pointing upwards. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The phase lag index (PLI) (Stam et al., 2007), which evaluates the asymmetry of the time-distribution of phase differences between pairs of channels, was used as an index of functional connectivity. Previous studies have shown that the PLI is less influenced by the effects of volume conduction and active reference electrodes than traditional measures like coherence (Stam et al., 2007). We computed the instantaneous phases using the Hilbert transform. Then, for each subject the PLI was computed for all electrode pair combinations as the mean of data epochs of 4 s length. To obtain the mean regional PLI connectivity patterns we combined the electrodes from the eight above mentioned regions. The connectivity patterns were finally defined as connectivity for the average of all PLIs within single scalp regions (left/right Frontal, Parietal, Temporal, Occipital); and for the average of PLI values between all pairs of inter-hermispheric and intra-hemispherical regions.
2.8. Statistical analyses
Partial correlations were computed to assess the relationship between the EEG markers and amyloid load or any of the measures of global cognition, episodic memory and executive function, respectively, while accounting for the effect of age, gender, and education. Correlations were considered significant only when the FDR-adjusted p value was lower than 0.05, using the Benjamini and Hochberg (1995) correction. Associations between ApoE4 allele frequency (binarized into no ApoE4 vs. at last one ApoE4 allele) with amyloid load and EEG markers were assessed using partial correlation accounting for the effect of age, gender, and education. Mediation analysis was conducted following Baron and Kenny's (1986) approach. Analyses were performed using R implemented in RStudio, Version 0.99.903.
3. Results
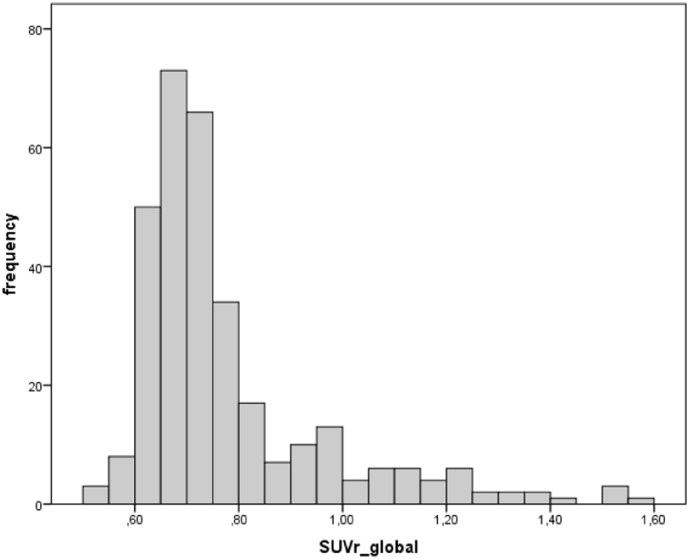
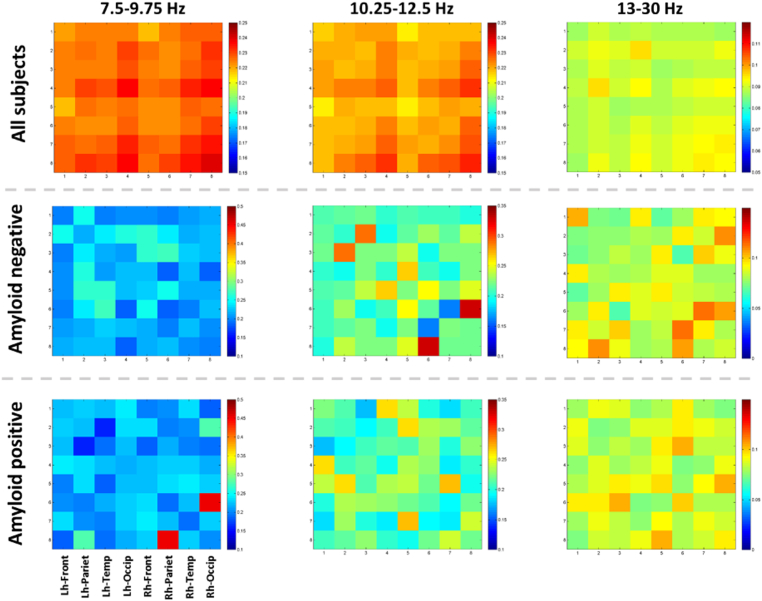
Demographic characteristics, including cognitive performance and ApoE genotype, split according to global amyloid status, are shown in Table 1. Of the 318 participants, 63 had a global amyloid load above the positivity threshold. The frequency distribution of global SUVR values is shown in Fig. 2. Within the entire group we did not find any significant associations between global amyloid load and phase-lag index in any frequency band (including low alpha, high alpha and beta), neither at adjusted nor unadjusted p-values. As shown in Fig. 3, the phase-lag index values showed small to modest cross-correlations across the 8 regions, suggesting no major effect of volume conduction on these measures. The absence of significant associations between global amyloid and EEG connectivity was confirmed when we used the more traditional measures of coherence as well as power in the low and high alpha and beta frequency bands (data not shown). In addition, when we used posterior cingulate amyloid load as regressor instead of global amyloid load, we did not find significant effects of amyloid on PLI connectivity at an FDR-corrected level of significance of p < 0.05 either.
Table 1.
Participants' demographics.
| Amyloid-negative | Amyloid-positive | |
|---|---|---|
| Age [years] (SD), min-maxa | 75.9 (3.5) | 76.7 (3.5) |
| 70–85 | 70–85 | |
| Sex (female/male)b | 164/91 | 40/23 |
| Education (no to primary education vs. secondary education or higher)c | 64/191 | 21/42 |
| MMSE (SD), min–maxd | 28.7 (1.0) | 28.4 (0.9) |
| 27–30 | 27–30 | |
| FCSRT-DR (SD), min–maxe | 12.0 (2.2) | 11.4 (2.5) |
| 6–16 | 6–16 | |
| TMT-B/TMT-A (SD), min–maxd | 2.0 (0.7) | 2.3 (0.9) |
| 0.8–6.0 | 0.9–5.8 | |
| ApoE (2/2, 2/3, 2/4, 3/3, 3/4, 4/4) [Percent]f | 0.4, 15.3, 0.8, 71.4, 12.2, 0 | 0, 7.9, 3.2, 52.4, 31.7, 4.8 |
FCSRT-DR: delayed recall of the Free and Cued Selective Reminding Test.
TMT-B/TMT-A: time TMT-B divided by time TMT-A.
No significant difference between groups, Student's t = − 1.7, 316 df, p = 0.10.
No significant difference between groups, Chi2 = 0.015, 1 df, p = 0.9.
No significant difference between groups, Chi2 = 1.7, 1 df, p = 0.19.
Significantly different between groups, Mann-Whitney-U test, p < 0.02.
Not significantly different between groups, Mann-Whitney-U test, p = 0.12.
Significantly different between groups, Chi2 = 31.4, 5 df, p < 0.001.
Fig. 2.
Histogram of SUVR global values.
Histogram of SUVR global values from florbetapir-PET.
Fig. 3.
Connectivity matrices of PLI values at different frequency bands.
From top to bottom:
Mean correlation across all subjects (N = 318).
Mean correlation across amyloid negative subjects (N = 255).
Mean correlation across amyloid positive subjects (N = 63).
The color bars show the correlation coefficients.
Lh-Frontal – PLI within left hemispheric frontal leads.
Rh-Frontal - PLI within right hemispheric frontal leads. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
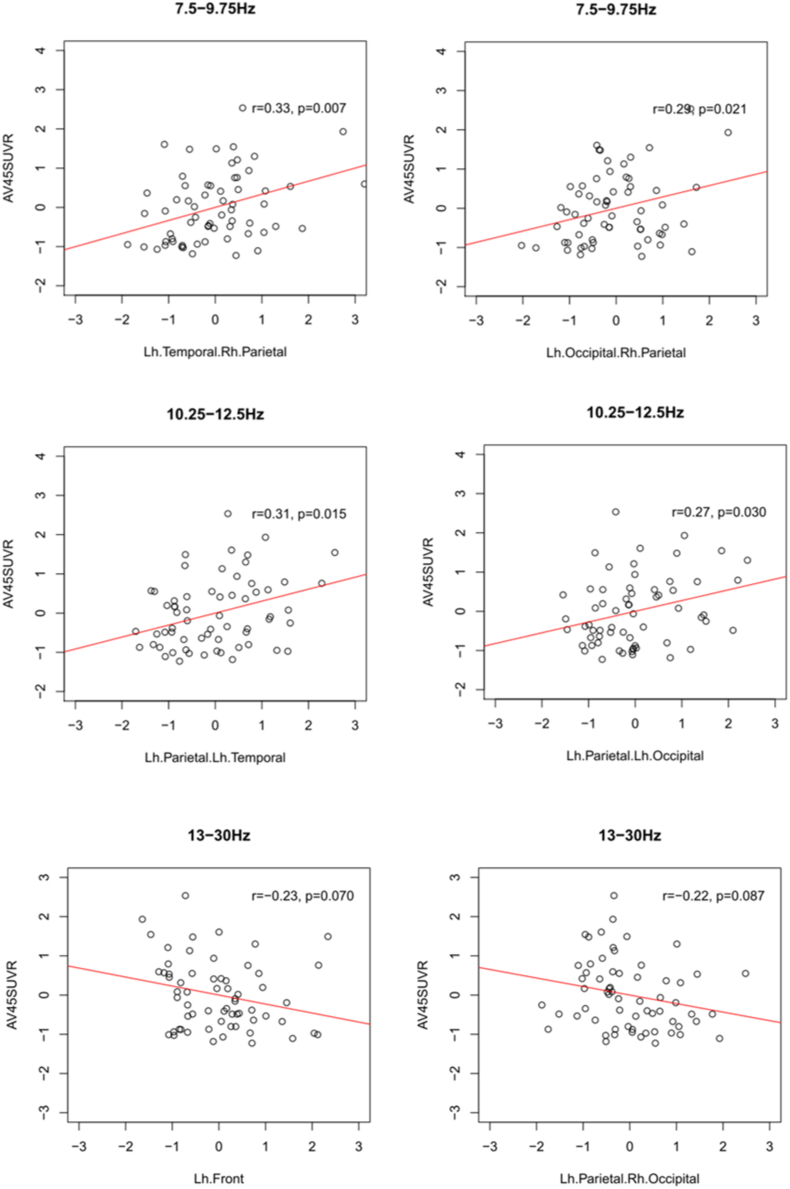
When we only assessed the subgroup with an SUVR value > 0.88, indicating significant cortical amyloid load, adjusted p-values showed no significant associations between global amyloid load and phase-lag index in any frequency band. Only with unadjusted p-values, we found significant positive correlations of global amyloid load with left temporal and right parietal as well as left occipital and right parietal PLI in the low alpha band, and with left parietal and left temporal as well as left parietal and left occipital PLI for the high alpha band; in addition, unadjusted p-values revealed significant negative associations between global amyloid load and the left frontal PLI as well as the left parietal to right occipital PLI in the beta band (Fig. 4).
Fig. 4.
Correlations between PLI and global SUVR in the high amyloid subgroup.
Scatter plots of the EEG PLI values by global amyloid load (AV45-SUVR) for the subsample of participants with a global amyloid load above a SUVR threshold of 0.88. Regression lines represent the linear least square fit. The correlation coefficients r represent the partial correlations controlling for age, sex and education; the associated p-values are uncorrected for multiple comparisons.
In the amyloid negative cases (SUVR value < 0.88), we found no significant associations at FDR corrected p-values. At an uncorrected level of significance, we found a significant negative correlation of global amyloid load with left parietal (r = − 0.15, p < 0.015), right frontal (r = − 0.12, p = 0.05), left frontal to left parietal (r = − 0.13, p < 0.04), and left frontal to right frontal (r = − 0.13, p = 0.04) PLI in the low alpha band.
Across all cases, the presence of at least one ApoE4 allele was significantly associated with global amyloid load (partial r = 0.27, 313 df, p < 0.001), but not with the PLI in any region of the three frequency bands.
Global amyloid level was associated with MMSE score (partial correlation = − 0.12, 313 df, p < 0.04), delayed free recall performance (partial correlation = − 0.13, 313 df, p < 0.025), and the TMT-B to TMT-A ratio (partial correlation = − 0.14, 313 df, p < 0.02). When we assessed correlations of EEG PLI values and cognitive scores, we found only one significant association at an unadjusted level of significance across all cases in the low alpha band with small effect size (partial correlation = − 0.12), and within the amyloid positive cases only one in the alpha and four in the beta band with moderate effect sizes (partial correlation = 0.27 to 0.33) (Supplementary Table 1); none of these effects, however, survived FDR correction (p > 0.15). Following up these negative findings, we also determined expected associations between regional EEG PLI values and cognitive scores, including all available cognitive scales. As shown in Supplementary Table 2, we expected that frontal PLI values would be associated with verbal fluency and working memory performance, temporal PLI values with free and delayed recall, and parietal and occipital PLI with visuoconstructive performance (Rey figure tests) and visual attention (TMT-A, TMT-B and TMT-B to TMT-A ratio). We found, however, no significant correlations that survived FDR correction (Supplementary Table 1). Following Baron and Kenny's (1986) steps for mediation analysis, the lack of a significant effect of EEG connectivity on the cognitive outcomes indicates that EEG connectivity is not functioning as a mediator for the effect of amyloid on the cognitive outcomes.
4. Discussion
We determined the association of the EEG based PLI as a measure of functional cortical connectivity with global amyloid load in a cohort of cognitively normally performing older people with SMC. Against our primary hypothesis, we did not find an association between global amyloid load and EEG connectivity both when considering the entire range of SUVR values and in the subgroup with supra-threshold amyloid accumulation. Only at an unadjusted level of significance, we found higher cortical amyloid load with higher functional connectivity in the low and high alpha band, and higher amyloid load with lower functional connectivity in the beta band in people with a supra-threshold cortical amyloid accumulation.
Resting state fMRI studies in healthy controls reported decline as well as increase of local functional connectivity in cognitively normal people with higher amyloid load (Drzezga et al., 2011, Elman et al., 2016, Myers et al., 2014). In contrast to previous studies in healthy controls, our sample of SMC cases was relatively large and statistically well powered for the detection of even small effect sizes, limiting the risk of false negative findings. The absence of an association of EEG connectivity with amyloid load agrees with findings from a previous CSF study in 33 cognitively normal people who showed no association between amyloid load and EEG mean peak frequency and relative power (Stomrud et al., 2010). It differs, however, from a study in 243 MCI patients that showed reduced alpha and beta frequency oscillations in CSF amyloid positive compared with amyloid negative cases (Gouw et al., 2017). Different to this previous study, however, our cases had no cognitive impairments in psychometric tests, suggesting an earlier stage of amyloid pathology.
The commonly used dichotomous distinction between amyloid negative/positive categories based on a threshold applied to the global cortical amyloid-PET signal aims to demarcate significant global amyloid burden, but does not necessarily mean that amyloid-negative individuals are completely free of amyloid (Thal et al., 2015, Villeneuve et al., 2015). In fact, according to neuropathological studies the great majority of individuals in the considered age range of our study show at least neocortical amyloid load of Thal phase 1, even when cognitively unimpaired at last clinical evaluation (Murray et al., 2015). There is no neurobiological reason, why people slightly below the global threshold should fundamentally differ from people slightly above this threshold. Indeed, when we assessed regional amyloid load in an independent study on 179 cognitively healthy people from the ADNI cohort, we found a pattern of regional amyloid load increases that followed a systematic staging pattern with a continuous decline in CSF Aβ42 levels and delayed recall performance across progressing amyloid stages, even in healthy people below the global amyloid load threshold (Grothe et al., 2017). These data suggest that amyloid load below a global threshold may still be neurobiologically meaningful and serve as rational for regressing functional connectivity on amyloid load across the entire range of global amyloid values.
The small risk of false negative findings is supported by the only few significant effects even with unadjusted p-values when separating the sample into amyloid positive and negative subgroups. These unadjusted effects may be spurious, given the large number of comparisons and the identification of such effects only in one (PLI) of three metrics employed (PLI, coherence, and power). Since to our knowledge this is the first study on the associations between EEG connectivity and cortical amyloid load using PET, these unadjusted effects can only serve to generate, rather than confirm a hypothesis on associations with amyloid load. If confirmed, these findings would imply a complex picture. In people with no increase of global amyloid, higher amyloid load was associated with lower connectivity in the low alpha frequency band in the frontal and fronto-parietal regions. Albeit a lower connectivity with higher amyloid would agree with the notion that amyloid impairs functional connectivity (Hedden et al., 2009), this straight-forward explanation does not fully agree with the findings in the high amyloid load subgroup. Here, we found that in people with increased overall amyloid accumulation, indicating the presence of preclinical AD according to IWG-2 criteria (Dubois et al., in preparation), higher amyloid load was associated with higher functional connectivity in the alpha band. A negative effect of amyloid load was only found on connectivity in the beta band. Considered separately, the direction of such effects would agree with a near infrared spectroscopy study, where EEG alpha band activity was suggested to be associated with metabolic deactivation (Moosmann et al., 2003), and a simultaneous EEG-fMRI, where higher beta band power was found associated with higher default mode network BOLD signal (Neuner et al., 2014). Inferring from these previous findings, increased alpha connectivity and decreased beta band connectivity would reflect deactivation of attention related networks as well as disinhibition of inhibitory neuronal networks, respectively, in predominantly long distance connections; our findings would suggest that such mechanisms may be associated with increased amyloid load in cognitively normally performing older people with SMC above a threshold of increased overall amyloid. We would, however, be very reluctant to accept such an hypothesis unless replicated in an independent sample given the contradictory findings between the amyloid negative and amyloid-positive subgroups, the low effect sizes observed in the present study, and the fact that the previous studies (Moosmann et al., 2003, Neuner et al., 2014) included young healthy people, limiting the comparability of findings. Such effect, if confirmed, could be explained along two directions, with functional connectivity decline (in the beta-band) down-stream of amyloid load build-up, but also with amyloid load as consequence of higher functional activation (in the alpha band), as cortical hub regions with high functional activity have been suggested to be at higher risk for amyloid accumulation (de Haan et al., 2012). The direction of effects cannot be resolved based on cross-sectional data.
The absence of a strong amyloid effect on functional connectivity as measured using resting state EEG in SMC cases may represent a selection effect, where people will remain in the cognitively normal group only if supra-threshold amyloid accumulation does not impair cortical functional connectivity. The analysis of longitudinal outcomes, which will be possible with the second wave of the INSIGHT-preAD cohort, will allow the investigation of EEG connectivity changes over time in people with higher amyloid levels, corresponding to a time lag for the amyloid effect on neuronal function. Such effect has previously been described in a longitudinal study where the regional hypometabolism as determined using FDG-PET was associated with the baseline distribution of amyloid accumulation as determined using amyloid sensitive 11C-PIB-PET only at follow-up but not yet at baseline in a group of 20 AD dementia patients (Forster et al., 2012). Similarly to our findings, default mode network connectivity in resting state fMRI was not associated with global and network specific amyloid load as determined using 11C-PIB-PET in 18 healthy controls (Adriaanse et al., 2014). The heterogeneity of amyloid effects on functional connectivity in previous studies is likely related to the regionally differing size and direction of effects within and between neuronal networks as well as the small sample sizes.
Global amyloid load was significantly associated with all three preselected cognitive domains, including global cognition, delayed free recall, and executive function. The effect of amyloid on cognition was independent of EEG connectivity, falsifying our second hypothesis. Only few of the EEG connectivity measures were associated with the preselected cognitive scores, all with small effect size (Pearson's r < 0.15), and none surviving multiple comparison correction. Associations between resting state EEG connectivity measures and cognitive scores in aging and dementia have typically been studied across diagnostic groups, (Babiloni et al., 2010, Lee et al., 2010) likely confounding domain specific associations with global effects of disease severity. When assessing cognitively normal older people alone, one study described associations between resting state MEG activity and cognitive performance measures that did, however, not survive multiple comparison correction (Leirer et al., 2011). Thus, the absence of significant associations between EEG connectivity and cognitive scores within our group of cognitively normally performing older people agrees with this previous report.
A limitation of our study is the lack of a group of cognitively healthy controls without subjective memory complaints. This would have allowed studying the interaction of amyloid by subjective memory complainer status on functional connectivity. Another critical point is the multitude of EEG comparisons arising from the use of several brain regions and frequency bands. We already restricted the number of comparisons by focusing on alpha and beta band frequencies and collapsing the 70 selected EEG channels into 8 larger regions. Still, we ended up with 36 within and between regions connectivity measures per frequency band. To deal with this, we employed FDR correction (Benjamini and Hochberg, 1995) that had been shown to be more powerful than family wise error rate (Benjamini and Hochberg, 1995). Focusing on effect size estimates, however, it is reassuring that when assessing only the expected associations between regional connectivity and cognitive score, we found at most small effect sizes throughout (Supplementary Table 2), underscoring the absence of a convincing association between EEG connectivity metrics and cognitive scores in these cognitively normally performing people. The interpretation of our findings is hampered by the fact that only few previous data exist on the association between amyloid load and EEG measures of functional connectivity in prodromal AD patients and cognitively normal older people (Gouw et al., 2017, Stomrud et al., 2010).
In summary our data do not show an association between cortical amyloid load and EEG based functional connectivity in the alpha and the beta band frequencies in a large cohort of cases with SMCs, including 63 subjects fulfilling biomarker criteria of preclinical at risk for AD (Dubois et al., in preparation). This finding suggests that functional effects of cortical amyloid load that previously have been shown in similar cohorts using fMRI (Drzezga et al., 2011, Mormino et al., 2011) and FDG-PET (Kljajevic et al., 2014) cannot be replicated by EEG recording using the PLI as measure of functional connectivity. However, it has to be noted that mixed findings have also been reported for the association between amyloid load and activity changes in fMRI (Adriaanse et al., 2014, Lim et al., 2014) or FDG-PET (Altmann et al., 2015, Ossenkoppele et al., 2014), indicating a complex and probably non-linear relationship between amyloid deposition and brain functional connectivity in the course of AD pathogenesis (Sorg and Grothe, 2015). Direct multimodal comparisons of functional markers within the same cohort will be necessary to establish the sensitivity of EEG for amyloid-related alterations of neuronal function compared with these functional imaging modalities. Future studies will also need to determine associations between amyloid load and EEG-based measures of functional connectivity in prodromal stages of AD, where the potentially lower sensitivity of EEG may at least partly be off-set by the stronger amyloid effect in a more advanced stage of disease.
Potential conflicts of interest
Drs. Teipel, Bakardjian, Cavedo, Weschke, Grothe, Dyrba, Gonzalez-Escamilla, and Potier report no biomedical financial interests or potential conflicts of interest.
Dr. Dubois has received consultant fees from Lilly, Boehringer Ingelheim and has received grants from Roche for his institution.
Dr. Hampel serves as Senior Associate Editor for the Journal Alzheimer's & Dementia; he has been a scientific consultant and/or speaker and/or attended scientific advisory boards of Axovant, Anavex, Eli Lilly and company, GE Healthcare, Cytox Ltd., Jung Diagnostics GmbH, Roche, Biogen Idec, Takeda-Zinfandel, Oryzon Genomics, Qynapse, Merck, Sharp & Dohme (MSD); and he receives research support from the Association for Alzheimer Research (Paris), Pierre and Marie Curie University (Paris), Pfizer & Avid (paid to institution); and he has patents, but receives no royalties.
Dr. Habert reports having received honoraria from Lilly, PIRAMAL and GE as a speaker.
Funding
The INSIGHT-preAD study was promoted by INSERM in collaboration with ICM, IHU-A-ICM and Pfizer and has received a support within the “Investissement d'Avenir” (ANR-10-AIHU-06). The study was promoted in collaboration with the “CHU de Bordeaux” (coordination CIC EC7), the promoter of Memento cohort, funded by the Foundation Plan-Alzheimer. The study was further supported by AVID/Lilly.
This research publication benefited from the support of the Program “PHOENIX” led by the UPMC Foundation and sponsored by la Fondation pour la Recherche our Alzheimer.
HH is supported by the AXA Research Fund, the “Fondation Université Pierre et Marie Curie” and the “Fondation pour la Recherche sur Alzheimer”, Paris, France. Ce travail a bénéficié d'une aide de l'Etat “Investissements d'avenir” ANR-10-IAIHU-06. The research leading to these results has received funding from the program “Investissements d'avenir” ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Agence Institut Hospitalo-Universitaire-6).
INSIGHT-preAD Study Group
Audrain C, Auffret A, Bakardjian H, Baldacci F, Batrancourt B, Benakki I, Benali H, Bertin H, Bertrand A, Boukadida L, Cacciamani F, Causse V, Cavedo E, Cherif Touil S, Chiesa PA, Colliot O, Dalla Barba G, Depaulis M, Dos Santos A, Dubois B, Dubois M, Epelbaum S, Fontaine B, Francisque H, Gagliardi G, Genin A, Genthon R, Glasman P, Gombert F, Habert MO, Hampel H, Hewa H, Houot M, Jungalee N, Kas A, Kilani M, La Corte V, Le Roy F, Lehericy S, Letondor C, Levy M, Lista S, Lowrey M, Ly J, Makiese O, Masetti I, Mendes A, Metzinger C, Michon A, Mochel F, Nait Arab R, Nyasse F, Perrin C, Poirier F, Poisson C, Potier MC, Ratovohery S, Revillon M, Rojkova K, Santos-Andrade K, Schindler R, Servera MC, Seux L, Simon V, Skovronsky D, Thiebaut M, Uspenskaya O, Vlaincu M.
INSIGHT-preAD Scientific Committee Members
Dubois B, Hampel H, Bakardjian H, Colliot O, Habert MO, Lamari F, Mochel F, Potier MC, Thiebaut de Schotten M.
Acknowledgements
CONTRIBUTORS TO THE ALZHEIMER PRECISION MEDICINE INITIATIVE – WORKING GROUP (APMI–WG): Babiloni C, Baldacci F, Benda N, Black KL, Bokde ALW, Bonuccelli U, Broich K, Bun RS, Cacciola F, Castrillo J†, Cavedo E, Ceravolo R, Chiesa PA, Colliot O, Coman CM, Corvol JC, Dubois B, Duggento A, Durrleman S, Escott-Price V, Ferretti MT, Frank RA, Garaci F, George N, Habert MO, Hampel H, Herholz K, Koronyo Y, Koronyo-Hamaoui M, Lamari F, Lista S, Nisticò R, Nyasse-Messene F, O'Bryant SE, Ritchie C, Rojkova K, Rossi S, Santarnecchi E, Sporns O, Toschi N, Verdooner SR, Vergallo A, Younesi E.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.10.031.
Appendix A. Supplementary data
Supplementary tables
References
- Adriaanse S.M., Sanz-Arigita E.J., Binnewijzend M.A., Ossenkoppele R., Tolboom N., van Assema D.M., Wink A.M., Boellaard R., Yaqub M., Windhorst A.D., van der Flier W.M., Scheltens P., Lammertsma A.A., Rombouts S.A., Barkhof F., van Berckel B.N. Amyloid and its association with default network integrity in Alzheimer's disease. Hum. Brain Mapp. 2014;35:779–791. doi: 10.1002/hbm.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A., Ng B., Landau S.M., Jagust W.J., Greicius M.D., Alzheimer's Disease Neuroimaging, I Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain. 2015;138:3734–3746. doi: 10.1093/brain/awv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott K., Frank J. Trail Making Test, Part B as a measure of executive control: validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Babiloni C., Visser P.J., Frisoni G., De Deyn P.P., Bresciani L., Jelic V., Nagels G., Rodriguez G., Rossini P.M., Vecchio F., Colombo D., Verhey F., Wahlund L.O., Nobili F. Cortical sources of resting EEG rhythms in mild cognitive impairment and subjective memory complaint. Neurobiol. Aging. 2010;31:1787–1798. doi: 10.1016/j.neurobiolaging.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Benton A.L. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mezenge F., Egret S., Landeau B., Barre L., Abbas A., Ibazizene M., de La Sayette V., Desgranges B., Eustache F., Chetelat G. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J. Neurosci. 2015;35:10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. J. Clin. Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Coleman R.E., Doraiswamy P.M., Fleisher A.S., Reiman E.M., Sabbagh M.N., Sadowsky C.H., Schneider J.A., Arora A., Carpenter A.P., Flitter M.L., Joshi A.D., Krautkramer M.J., Lu M., Mintun M.A., Skovronsky D.M., Group A.-A.S. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- Cohen M.X. Theory and Practice. MIT PRess; Cambridge, Mass. USA: 2014. Analyzing neural time series data. [Google Scholar]
- Cuesta P., Garces P., Castellanos N.P., Lopez M.E., Aurtenetxe S., Bajo R., Pineda-Pardo J.A., Bruna R., Marin A.G., Delgado M., Barabash A., Ancin I., Cabranes J.A., Fernandez A., Del Pozo F., Sancho M., Marcos A., Nakamura A., Maestu F. Influence of the APOE epsilon4 allele and mild cognitive impairment diagnosis in the disruption of the MEG resting state functional connectivity in sources space. J. Alzheimers Dis. 2015;44:493–505. doi: 10.3233/JAD-141872. [DOI] [PubMed] [Google Scholar]
- Drane D.J., Yuspeh R.L., Huthwaite J.S., Klingler L.K. Demographic characteristics and normative observations for derived-Trail Making Test indices. Neuropsychiatry Neuropsychol. Behav. Neurol. 2002;15:39–43. [PubMed] [Google Scholar]
- Drzezga A., Becker J.A., Van Dijk K.R., Sreenivasan A., Talukdar T., Sullivan C., Schultz A.P., Sepulcre J., Putcha D., Greve D., Johnson K.A., Sperling R.A. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134:1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B.; Feldman H.H.; Jacova C.; Hampel H.; Molinuevo J.L.; Blennow K.; DeKosky S.T.; Gauthier S.; Selkoe D.; Bateman R.; Cappa S.; Crutch S.; Engelborghs S.; Frisoni G.B.; Fox N.C.; Galasko D.; Habert M.O.; Jicha G.A.; Nordberg A.; Pasquier F.; Rabinovici G.; Robert P.; Rowe C.; Salloway S.; Sarazin M.; Epelbaum S.; de Souza L.C.; Vellas B.; Visser P.J.; Schneider L.; Stern Y.; Scheltens P.; Cummings J.L., Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 13.614–629. (in preparation) [DOI] [PubMed]
- Elman J.A., Madison C.M., Baker S.L., Vogel J.W., Marks S.M., Crowley S., O'Neil J.P., Jagust W.J. Effects of beta-amyloid on resting state functional connectivity within and between networks reflect known patterns of regional vulnerability. Cereb. Cortex. 2016;26:695–707. doi: 10.1093/cercor/bhu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastenau P.S., Denburg N.L., Hufford B.J. Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. Clin. Neuropsychol. 1999;13:30–47. doi: 10.1076/clin.13.1.30.1976. [DOI] [PubMed] [Google Scholar]
- Ferree T.C., Luu P., Russell G.S., Tucker D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental-state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forster S., Grimmer T., Miederer I., Henriksen G., Yousefi B.H., Graner P., Wester H.J., Forstl H., Kurz A., Dickerson B.C., Bartenstein P., Drzezga A. Regional expansion of hypometabolism in Alzheimer's disease follows amyloid deposition with temporal delay. Biol. Psychiatry. 2012;71:792–797. doi: 10.1016/j.biopsych.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw A.A., Alsema A.M., Tijms B.M., Borta A., Scheltens P., Stam C.J., van der Flier W.M. EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiol. Aging. 2017;57:133–142. doi: 10.1016/j.neurobiolaging.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Grothe M.J., Barthel H., Sepulcre J., Dyrba M., Sabri O., Teipel S.J. In-vivo staging of regional amyloid deposition. Neurology. 2017 doi: 10.1212/WNL.0000000000004643. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W., Mott K., van Straaten E.C., Scheltens P., Stam C.J. Activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput. Biol. 2012;8 doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T., Van Dijk K.R., Becker J.A., Mehta A., Sperling R.A., Johnson K.A., Buckner R.L. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G., Dubois B., Dufouil C., Ellis K.A., van der Flier W.M., Glodzik L., van Harten A.C., de Leon M.J., McHugh P., Mielke M.M., Molinuevo J.L., Mosconi L., Osorio R.S., Perrotin A., Petersen R.C., Rabin L.A., Rami L., Reisberg B., Rentz D.M., Sachdev P.S., de la Sayette V., Saykin A.J., Scheltens P., Shulman M.B., Slavin M.J., Sperling R.A., Stewart R., Uspenskaya O., Vellas B., Visser P.J., Wagner M., Subjective Cognitive Decline Initiative Working G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A.D., Pontecorvo M.J., Lu M., Skovronsky D.M., Mintun M.A., Devous M.D., Sr. A semiautomated method for quantification of F 18 florbetapir PET images. J. Nucl. Med. 2015;56:1736–1741. doi: 10.2967/jnumed.114.153494. [DOI] [PubMed] [Google Scholar]
- Kalafat M., Hugonot-Diener L., Poitrenaud J. The Mini Mental State (MMS): French standardization and normative data. Rev. Neuropsychol. 2003;13:209–236. [Google Scholar]
- Kljajevic V., Grothe M.J., Ewers M., Teipel S., Alzheimer's Disease Neuroimaging, I Distinct pattern of hypometabolism and atrophy in preclinical and predementia Alzheimer's disease. Neurobiol. Aging. 2014;35:1973–1981. doi: 10.1016/j.neurobiolaging.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Kothe C.A., Makeig S. BCILAB: a platform for brain-computer interface development. J. Neural Eng. 2013;10 doi: 10.1088/1741-2560/10/5/056014. [DOI] [PubMed] [Google Scholar]
- Landau S.M., Breault C., Joshi A.D., Pontecorvo M., Mathis C.A., Jagust W.J., Mintun M.A., Alzheimer's Disease Neuroimaging, I Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J. Nucl. Med. 2013;54:70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Park Y.M., Kim D.W., Im C.H. Global synchronization index as a biological correlate of cognitive decline in Alzheimer's disease. Neurosci. Res. 2010;66:333–339. doi: 10.1016/j.neures.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Leirer V.M., Wienbruch C., Kolassa S., Schlee W., Elbert T., Kolassa I.T. Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav. 2011;5:222–228. doi: 10.1007/s11682-011-9126-3. [DOI] [PubMed] [Google Scholar]
- Lim H.K., Nebes R., Snitz B., Cohen A., Mathis C., Price J., Weissfeld L., Klunk W., Aizenstein H.J. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137:3327–3338. doi: 10.1093/brain/awu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M., Ritter P., Krastel I., Brink A., Thees S., Blankenburg F., Taskin B., Obrig H., Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Mormino E.C., Smiljic A., Hayenga A.O., Onami S.H., Greicius M.D., Rabinovici G.D., Janabi M., Baker S.L., Yen I.V., Madison C.M., Miller B.L., Jagust W.J. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex. 2011;21:2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M.E., Lowe V.J., Graff-Radford N.R., Liesinger A.M., Cannon A., Przybelski S.A., Rawal B., Parisi J.E., Petersen R.C., Kantarci K., Ross O.A., Duara R., Knopman D.S., Jack C.R., Jr., Dickson D.W. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138:1370–1381. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N., Pasquini L., Gottler J., Grimmer T., Koch K., Ortner M., Neitzel J., Muhlau M., Forster S., Kurz A., Forstl H., Zimmer C., Wohlschlager A.M., Riedl V., Drzezga A., Sorg C. Within-patient correspondence of amyloid-beta and intrinsic network connectivity in Alzheimer's disease. Brain. 2014;137:2052–2064. doi: 10.1093/brain/awu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I., Arrubla J., Werner C.J., Hitz K., Boers F., Kawohl W., Shah N.J. The default mode network and EEG regional spectral power: a simultaneous fMRI-EEG study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Madison C., Oh H., Wirth M., van Berckel B.N., Jagust W.J. Is verbal episodic memory in elderly with amyloid deposits preserved through altered neuronal function? Cereb. Cortex. 2014;24:2210–2218. doi: 10.1093/cercor/bht076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichep L.S. Quantitative EEG and electromagnetic brain imaging in aging and in the evolution of dementia. Ann. N. Y. Acad. Sci. 2007;1097:156–167. doi: 10.1196/annals.1379.008. [DOI] [PubMed] [Google Scholar]
- Sorg C., Grothe M.J. The complex link between amyloid and neuronal dysfunction in Alzheimer's disease. Brain. 2015;138:3472–3475. doi: 10.1093/brain/awv302. [DOI] [PubMed] [Google Scholar]
- Stam C.J., Nolte G., Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E., Hansson O., Minthon L., Blennow K., Rosen I., Londos E. Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiol. Aging. 2010;31:215–223. doi: 10.1016/j.neurobiolaging.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Thal D.R., Beach T.G., Zanette M., Heurling K., Chakrabarty A., Ismail A., Smith A.P., Buckley C. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer's disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11:975–985. doi: 10.1016/j.jalz.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Thomas B.A., Erlandsson K., Modat M., Thurfjell L., Vandenberghe R., Ourselin S., Hutton B.F. The importance of appropriate partial volume correction for PET quantification in Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1104–1119. doi: 10.1007/s00259-011-1745-9. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N. Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Villemagne V.L., Mulligan R.S., Pejoska S., Ong K., Jones G., O'Keefe G., Chan J.G., Young K., Tochon-Danguy H., Masters C.L., Rowe C.C. Comparison of 11C-PiB and 18F-florbetaben for Abeta imaging in ageing and Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:983–989. doi: 10.1007/s00259-012-2088-x. [DOI] [PubMed] [Google Scholar]
- Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M., La Joie R., Arthur-Bentil S.K., Vogel J.W., Marks S.M., Lehmann M., Rosen H.J., Reed B., Olichney J., Boxer A.L., Miller B.L., Borys E., Jin L.W., Huang E.J., Grinberg L.T., DeCarli C., Seeley W.W., Jagust W. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Weschsler Memory Scale-III. [Google Scholar]
- Winkler I., Brandl S., Horn F., Waldburger E., Allefeld C., Tangermann M. Robust artifactual independent component classification for BCI practitioners. J. Neural Eng. 2014;11 doi: 10.1088/1741-2560/11/3/035013. [DOI] [PubMed] [Google Scholar]
- Winkler I., Debener S., Muller K.R., Tangermann M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Annual international conference of the IEEE engineering in medicine and biology. Society. 2015:4101–4105. doi: 10.1109/EMBC.2015.7319296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables