Abstract
Introduction: The oral tumor is the sixth most prevalent type of cancer worldwide and the second leading cause of cancer-related mortality. Although chemotherapy and immunotherapy are the main strategies for the treatment of oral cancer, an emergence of inevitable resistance to these treatment modalities is the major drawback that causes recurrence of the disease. Nowadays, probiotics have been suggested as adjunctive and complementary treatment modalities for improving the impacts of chemotherapy and immunotherapy agents. Probiotics, the friendly microflora in our bodies, contribute to the production of useful metabolites with positive effects on the immune system against various diseases such as cancer.
Methods: Lactobacillus plantarum is one of the most important bacteria, which commensally live in the human oral system. In the current study, the impacts of L. plantarum on maintaining oral system health were investigated, and the molecular mechanisms of inhibition of oral cancer KB cells mediated by L. plantarum were evaluated using real-time polymerase chain reaction (PCR) and FACS flow cytometry analyses.
Results: Our findings showed that L. plantarum is effective in the signal transduction of the oral cancer cells through upregulation and downregulation of PTEN and MAPK pathways, respectively.
Conclusion: Based on the biological effects of oral candidate probiotics candidate bacterium L. plantarum on functional expression of PTEN and MAPK pathways, this microorganism seems to play a key role in controlling undesired cancer development in the oral system. Taken all, L. plantarum is proposed as a potential candidate for probiotics cancer therapy.
Keywords: Cell signaling, MAPK, Oral cancer, Probiotics, PTEN, Cancer therapy
Introduction
Despite recent advances in clinical cancer treatment strategies, such as chemotherapy and immunotherapy, the oral cancer is the second leading cause of cancer-related mortality. Accordingly, exploring the new and effective methods for the treatment of oral cancer is highly demanded. Nowadays, probiotics suggested as an adjunctive and complementary strategy to increasing of chemotherapy and immunotherapy efficiency.1,2 Probiotics are the beneficial bacteria and cause the vital positive effects on human’s health. It is believed that probiotics are effective in cancer therapy, in large part because of their positive effects on the immunity system3-5 – they seem to act as key modulators of mucosal immunity against cancer. In addition, their effects on the immune system are rendered by their secondary metabolites, DNA, and cell wall components. Interestingly, probiotics have different effects on normal and cancer cells.6-8 One of the attractive candidates to the probiotics cancer therapy is Lactobacillus plantarum. Accordingly some new studies have established the existence of L. plantarum in the mouths and also their anti-tumor activities.9 More deeply, it was shown that the bacteria released some polysaccharides that could cause anti-tumor activities on the Caco-2, BGC-823, and HT-29 cells.9 Although the beneficial potential of this microorganism has already been recognized, the molecular mechanisms, by which it induce anticancer effect(s), are yet to be fully understood. It is believed that probiotics could modulate the cell signal-transduction pathways. In the current investigation, the main objective was to clarify the molecular mechanism(s) of probiotics in cancer. Thus, we studied the anticancer effects of L. plantarum in the human KB cells as a model for the oral cancer. To uncover the molecular mechanism(s) of this bacterium in the inhibition of cancer, the expression of mitogen-activated protein kinases (MAPK) and phosphatase and tensin homolog (PTEN), which are respectively involved in the initiation and inhibition of cancer, were further investigated.
Materials and Methods
Materials
RPMI1640 medium and Trypsin – EDTA (1X), penicillin, streptomycin, and DAPI (4,6-diamidino- 2-phenylindole) were obtained from Sigma-Aldrich Co. (Poole, UK). Fetal bovine serum (FBS) was from Gibco Life technology, (Paisley, UK). Primers were purchased from Bioneer (Daejeon, Korea). TRIzol® Reagent and MMLV Reverse Transcriptase were from Thermo Fisher Scientific (Waltham, USA). SYBR green PCR Master Mix was purchased from Applied Biosystems (Warrington, UK). Annexin V-FITC apoptosis detection kit was obtained from EMD Chemicals (Gibbstown, USA). Bacterial media, Lactobacillus Mrs Broth, TM 147, was obtained from Titan Biotech Co (New Delhi, India). Primary antibody for PTEN sc-7974 was from Santa Cruz Biotechnology (Dallas, USA).
Microbial strain and molecular identification
Lactobacillus plantarum was isolated from traditional dairy products according to the Ztaliou et al.10 Moreover, bile, acid resistance and antibiotic sensitivities were evaluated. In the end, the bacteria were genotypically identified by 16S rRNA gene sequencing.
Cell culture and treatment
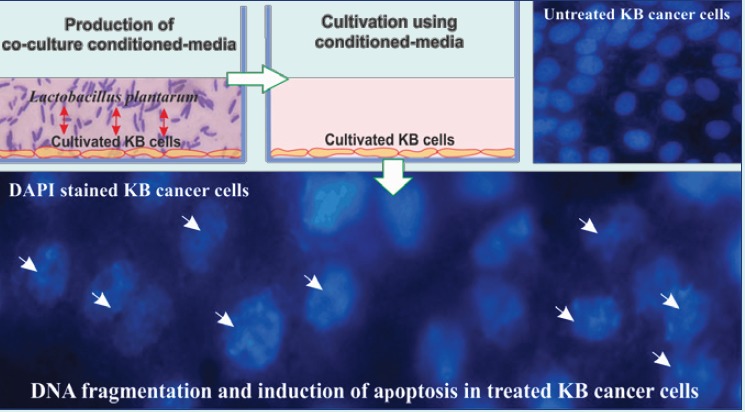
The human KB oral cancer cell line was purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). The KB oral cancer cells were cultured at a seeding density of 1.0×104 cells/cm2 using RPMI 1640 medium containing 10% FBS, penicillin/streptomycin (0.1 μg/μL and 0.1 μg/μL) and incubated in a humidified condition at 5% CO2 and 37°C. At 40%-50% confluency, the cultivated KB cells were treated with the conditioned-media of the co-cultured cells and bacteria for 4-6 hours. To this end, first bacteria were cultured in the MRS broth at 37°C for 24 hours. Then, they were centrifuged at 2800 ×g, and the bacterial pellet was washed with phosphate-buffered saline (PBS). Afterward, 0.5 McFarland concertation of bacteria were co-cultured with KB cells for 4-6 hours. The co-culture conditioned-medium was centrifuged at 2800 ×g, filtered (0.2 µm cut-off). The filtered media were introduced to a different passage of the cultivated KB cells (at 40%-50% confluency) for different time points (2, 6 and 24 hours), The treated cells with the conditioned-media were then subjected to various biological analyses.
DNA ladder assay for detection of apoptotic cells
The treated KB cells with about 70% confluence were collected from the culture plate flask using 0.05% trypsin/EDTA solution. The KB cells genomic DNA was extracted based on a method developed by Rahbar Saadat et al.11
Assessment of mRNA expression by real-time RT-PCR
Total RNA of the cells was extracted by Traizol-based method.12 One microgram of the extracted mRNAs was used for the RT-PCR experiment according to the instruction provided by Thermo Fisher Scientific for MMLV enzyme.
The real-time PCR was performed by utilizing the power SYBR®Green based PCR master mix and running on iQ5 Cycler (Bio-Rad, Hercules, USA). The absolute volume of the reaction was 20 μL. Each well was incorporated with 10 μL of Power SYBR Green PCR Master Mix, 1 μL of cDNA, and 0.25 µM of both forward and reverse primers (Table 1) . The PCR cycling steps were incorporated for 10 minutes at 94°C: 40 cycles of 25 seconds at 94°C for denaturation step, 30 seconds at annealing temperature, and 30 seconds at 72°C for the amplification separately. The GAPDH (house-keeping gene) was used as an internal reference to standardize the Ct of target genes. The obtained Ct were analyzed by Paddle method 2–ΔΔCT. 13
Table 1. The primer sequences using in qPCR .
| Gene name | Tm (oC) | Primer sequence |
| GAPDH | 63 |
F:5'-AAGCTCATTTCCTGGTATGACAACG-3' R: 5'-TCTTCCTCTTGTGCTCTTGCTGG-3' |
| MAPK | 55 |
F: 5-TACCTTATGAGGACTAATCG-3 R: 5-GCTGTGCAGCAACAGTC-3 |
| PTEN | 60 |
F: 5'- TCCCAGTCAGAGGCGCTATG-3' R:5'-CAAACTGAGGATTGCAAGTTC-3' |
Flow cytometry assay
Apoptosis and necrosis in the KB cells under the influence of L. plantarum were evaluated with flow cytometry. The co-cultured cells with L. plantarum were harvested, washed with PBS, re-suspended in the binding buffer and stained with Annexin V-FITC for 15 minutes. Then, the stained cells were incubated at room temperature. Finally, the cells were re-suspended in the PI binding buffer and prepared for the measurement of apoptosis.
DAPI staining
To analyze the morphology of nucleus of the apoptotic cells, 4,6-diamidino- 2-phenylindole (DAPI) method was used. The KB cells were seeded onto the 6-well plate and then condition media added to the cells and incubated for 24 hours. Subsequently, the cells were fixed with 4% paraformaldehyde for 10 minutes. Afterward, the cells were washed with PBS and permeabilized with 0.1% Triton X-100 for 10 minutes. In next step, the cells were washed again with PBS, and then stained with DAPI for 5 minutes. Finally, the morphology of cells was evaluated by invert fluorescence microscope, Olympus IX81equiped with DP72 digital camera (Olympus, Hamburg, Germany).
Results
Molecular identification of probiotics candidate bacteria
The molecular identification of the bacteria was performed by 16S rRNA gene. The sequence was submitted to the NCBI database (accession number: KX499629).
DNA Ladder assay and DAPI staining: detection of DNA fragmentation
The apoptotic cells induced by the bacteria condition media showed DNA fragmentation. Clearly, after DNA extraction and electrophoresis in 2% agarose gel, the ladder of DNA in apoptotic cells was observed. In addition, to analyze the morphology of nucleus of the apoptotic cells, DAPI staining was used. As shown in Fig. 1, no DNA and nucleus fragmentation were observed in the untreated control cells.
Figure 1.
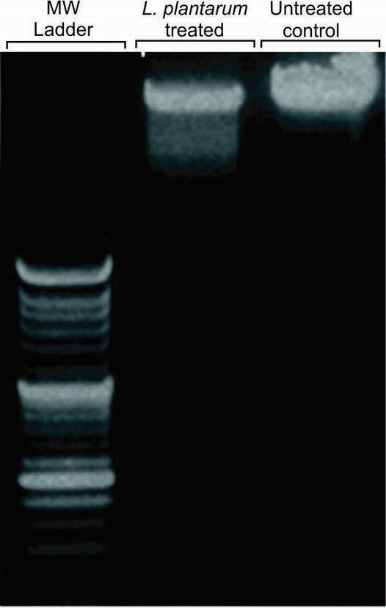
The DNA ladder assay of the KB cells treated with the conditioned-media of KB cells with L. plantarum. Lane 1: 100 bp DNA ladder, Lane 2: DNA extracted from apoptotic KB cells, Lane 3: DNA extracted from the untreated control cells.
MAPK and PTEN genes expression in KB cells
As shown in Fig. 2, the real-time PCR results showed that the mRNA expression of MAPK was decreased in the KB cells treated with the condition-media. Additionally, the mRNA expression level of PTEN was increased in the KB cells treated with the condition-media treatment for 6 hours. The downregulation of MAPK and the upregulation of PTEN were much more significant in the KB cells treated with the conditioned-media for 24 hours.
Figure 2.
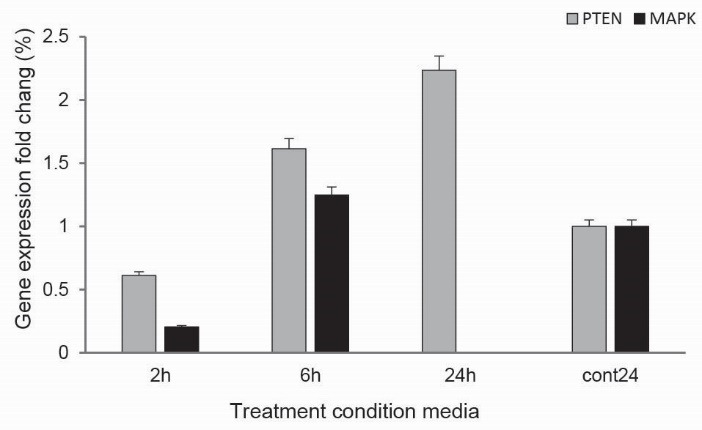
The bacterial effects on the expression of MAPK and PTEN genes.
Flow cytometry assay
Annexin V binds to the membrane phosphatidylserine (PS) on the inner side of the plasma membrane that is expressed on the surface of plasma membrane. Based on the flow cytometry analysis, it was found that about 50.24% apoptosis occurred in the KB cells treated with the conditioned-media for 24 hours (Fig. 3).
Figure 3.
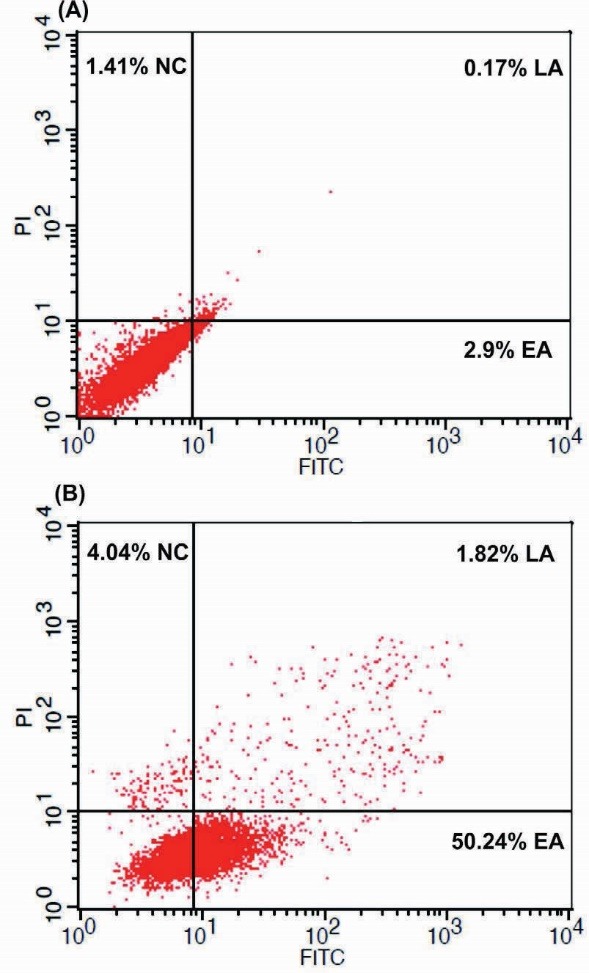
The flow cytometry results of the FITC-Annexin V/PI in the KB cells treated with the bacterial conditioned-media for 24 hours. (A) The untreated control cells. (B) The treated KB cells with bacterial conditioned media for 24 hours. NC: necrosis. EA: early apoptosis. LA: late apoptosis.
DAPI staining
The morphology of nucleus of the treated cells was further analyzed using DAPI staining. As shown in Fig. 4, morphologically, the treated cells with the conditioned-media showed fragmented DNA. Such detrimental effects on the genomic level were also phenotypically manifested (Fig. 5).
Figure 4.
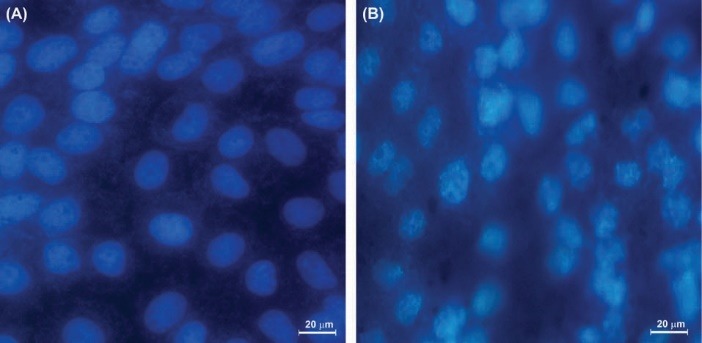
The effect of L. plantarum on KB cells on the integrity of nucleus and induction of apoptosis. The KB cells were treated for 24h with condition media. (A) The untreated control KB cells. (B) The KB cells treated with bacterial condition media for 24 hours.
Figure 5.
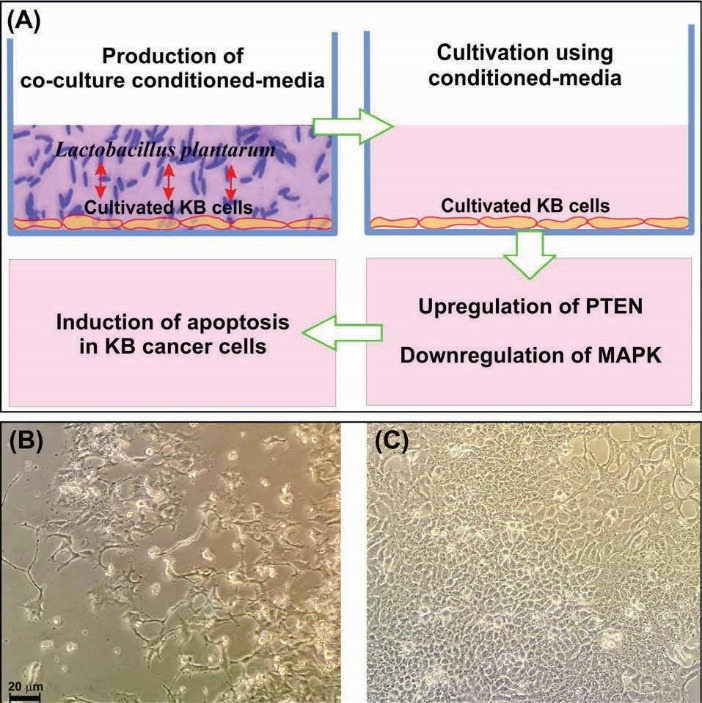
Induction of apoptosis by L. plantarum and KB cells co-culture conditioned medium on the KB cancer cells. (A) Schematic representation of the bacteria co-culture with the KB cells conditioned-media impacts on the regulation of the MAPK and the PTEN/AKT signaling pathways. (B) The light microscopy image of cancer KB cells treated with the conditioned medium of the L. plantarum and KB cells co-culture. (C) The light microscopy image of the untreated of KB cancer cells.
Discussion
Despite recent advances in cancer treatment strategies, the cancers are the second leading cause of mortality worldwide. Probiotics have been suggested as adjunctive and complementary therapies to enhance the effectiveness of the anticancer agents. However, the molecular mechanisms of probiotics effects on the cancer cells are yet to be fully understood. Here, we investigated some molecular mechanisms of L. plantarum, as a mouth-isolated bacterium, on the functional expression of a couple of key genes (i.e., MAPK and PTEN genes) involved in the survival and/or apoptosis signaling pathways in the oral cancer cells. The MAPK is involved in the tumor-inducing pathway, while the PTEN is involved in the cancer inhibition pathway. Further, high level of MAPK expression has been reported in different types of cancers.14 MAPK was shown to play an important role in the modulation of apoptosis as well as the migration and invasiveness of cancer.
It should be noted that this bacterium may commensally exist on the human oral cavity. Thus, we aimed to look at the impacts of such coexisting between this bacterium and the cells. Interestingly, we found that L. plantarum, as probiotics bacterium, was able to reduce the mRNA expression of MAPK and increase the mRNA expression of PTEN (Fig. 2). The relative decrease in the expression of MAPK and increase in the expression of PTEN in the KB cancer cells may be attributed to treatment of the cells with the conditioned-media of KB cells coculture with L. plantarum. These results were confirmed by the flow cytometry analysis (Fig. 3), the DAPI staining experiment (Fig. 4) as well as Western blotting analysis (data not shown). In this respect, activated PTEN by affecting on the signal pathway are probably involved in the inhibition of oral cancer cells. Accordingly, these induced pathways were reported to have positive influence on the processes leading to the cancer cells death.15
Yndestad et al showed that the introduction of PTEN pseudogene in murine breast cancer could increase the functional expressions of PTEN and p53 and AP-2γ, and hence pose an inhibitory impact on the tumor growth.16 Further, PTEN had an inhibitory effect on the activation of AKT whose functional expression favors the survival and progression of cancer cells. Squarize et al reported that inactivation of PTEN in HNSCC cancer cells could result in an increased activity of AKT/PI3K and suppression of p53.17,18 On the other hand, it has been shown that the functional over-expression of PTEN play a protecting role resulting in a significant decrease in tumor growth.19,20 The induction of the PTEN activity was also shown to control aggressive tumors.21 Likewise, our findings revealed that the KB cells treated with the conditioned-media of L. plantarum with the cells could impose a significant increase in the expressions of PTEN as compared to the untreated control cells.
Further, Kavitha et al reported that the inactivation of MAPK pathway induces apoptosis in the cancer cells, in large part because of the inhibition of the NF-κB pathway.22 Sos et al demonstrated that the activation of MAPK pathway could induce the production of oncogenic proteins, affecting on the cancer cells proliferation and apoptosis pathways.23 Our data showed that L. plantarum can significantly reduce the expression of MAPK. In some previous studies, it has been reported that over-expression of MAPK might induce the production of growth factor(s) in the cancer cells, which could favor the growth and progression of cancer cells. In this regard, it should be stated that the induction of angiogenesis and also inhibition of apoptosis appears to be the main impacts of the activation of MAPK pathway on the cancer cells, which may establish strong positive effects on the NF-κB pathway. Therefore, inhibition of MAPK seems to pose a significant negative influence on the tumor growth. Such inhibition might favor the chemotherapy of cancer through sensitizing cancer cells to the anti-cancer drugs, and hence increasing the cancer cells death.24-28
Taken all, based on our findings, the mRNA expression of PTEN in the KB cells was increased after 24 hours treatment with L. plantarum conditioned-media, while the mRNA expression of MAPK was markedly decreased. These results clearly indicate that probiotics, particularly useful commensal bacteria, may play an important role in the initiation/inhibition of oral cancers by slowing down the growth of cancer cells through the regulation of the MAPK and the PTEN/AKT signaling pathways (Fig. 5). Still, we need to conduct thorough experiments to identify the microbial products by which the growth of cancer cells is inhibited. Nevertheless, as this bacterium is able to induce apoptosis in the cancer cells, it/its products may be considered as a complementary treatment modality in the oral cancer therapy. Given that lactobacillus family is largely found in the dairy products (e.g., chees29), their rational use in cancer patients may be beneficial through the integrity of ecological community of commensal microbiota of individuals.30 We have previously reported on the inhibition of cancer cells by the effect of probiotics (Leuconostoc mesenteroides) through modulation of the NF-kappaB/AKT/PTEN/MAPK pathways, and some important onco-microRNAs (miRNA-21 and miRNA-200b).31 Similarly, the results of this current study confirm the impacts of probiotics on the mouth cancer cells. However, probiotics therapy should be done carefully since the imposition of encapsulated non-indigenous probiotics may negatively influence the normal pattern of the commensal microbiome of the body.30
Conclusion
Lactobacillus plantarum, as one of the most important bacteria, commensally lives in the human digestion system. To investigate the impacts of L. plantarum on the inhibition of oral cancer, in the current study, the molecular mechanisms of the induction of apoptosis by this bacterium was studied in the keratin-forming human tumor KB cells. Our findings showed that L. plantarum is able to upregulate and downregulate the PTEN and MAPK pathways, respectively. Based on these findings, this microorganism seems to play a central role in controlling of undesired cancer development in the oral cavity. Conclusively, L. plantarum is proposed as a potential candidate for probiotics therapy of cancer, nevertheless such proposition needs to be thoroughly examined in the relevant animal models prior to its translation into clinical uses.
Ethical approval
There is none to be disclosed.
Competing interests
As one of the contributing authors to this work, YO acts as the Editor-in-Chief of the journal. It should be declared that his contribution to this journal has not influenced the processing procedure.
Acknowledgments
The authors gratefully acknowledge the Research Centre for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences for the financial and technical support (grant No. 94003).
Research Highlights
What is current knowledge?
√ L. plantarum is considered as one of microbiota entities in the human.
√ L. plantarum is one of the most important bacteria commensally live in the human digestion system..
√ Different mechanisms are recruited by L. plantarum that are reflected in human health and diseases.
What is new here?
√ The conditioned-media of L. plantarum co-culture with KB cells can elicit apoptosis in the KB cancer cells inhibiting their growth significantly.
√ L. plantarum is able to upregulate and downregulate the PTEN and MAPK pathways, respectively.
√ L. plantarum imposes inhibitory impacts on the oral cancer.
References
- 1.Shida K, Suzuki T, Kiyoshima-Shibata J, Shimada S, Nanno M. Essential roles of monocytes in stimulating human peripheral blood mononuclear cells with Lactobacillus casei to produce cytokines and augment natural killer cell activity. Clin Vaccine Immunol. 2006;13:997–1003. doi: 10.1128/CVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shida K, Nomoto K. Probiotics as efficient immunopotentiators: translational role in cancer prevention. Indian J Med Res. 2013;138:808–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr. 2011;106:1291–6. doi: 10.1017/S000711451100287X. [DOI] [PubMed] [Google Scholar]
- 4. Food and Agriculture Organization of the United Nations WHO, Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, and Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Probiotics in food: health and nutritional properties and guidelines for evaluation. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties.
- 5.Ferrannini E. Of microbes and men. Diabetes Care. 2015;38:1817–9. doi: 10.2337/dc15-1270. [DOI] [PubMed] [Google Scholar]
- 6.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora 'on the scope'. Br J Clin Pharmacol. 2008;65:453–67. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;67:71–8. doi: 10.1016/j.ijbiomac.2014.02.056. [DOI] [PubMed] [Google Scholar]
- 10.Ztaliou I, Tsakalidou E, Tzanetakis N, Kalantzopoulos G. Lactobacillus plantarum strains isolated from traditional Greek cheese Taxonomic characterization and screening for enzyme activities. Le Lait. 1996;76:209–16. doi: 10.1051/lait:1996318. [DOI] [Google Scholar]
- 11.Rahbar Saadat Y, Saeidi N, Zununi Vahed S, Barzegari A, Barar J. An update to DNA ladder assay for apoptosis detection. Bioimpacts. 2015;5:25–8. doi: 10.15171/bi.2015.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harb Protoc. 2010;2010:pdb prot5439. doi: 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Jo MS, Suh DS, Yoon MS, Shin DH, Lee JH. et al. Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World J Surg Oncol. 2012;10:193. doi: 10.1186/1477-7819-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annunziata CM, Stavnes HT, Kleinberg L, Berner A, Hernandez LF, Birrer MJ. et al. Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer. 2010;116:3276–84. doi: 10.1002/cncr.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yndestad S, Austreid E, Skaftnesmo KO, Lønning PE, Eikesdal HP. Introduction of PTEN pseudogene in murine breast cancer upregulates PTEN, p53 and activating protein 2 gamma and delays tumor growth. Cancer Res. 2015;75:3986. doi: 10.1158/1538-7445.AM2015-3986. [DOI] [Google Scholar]
- 17.Squarize CH, Castilho RM, Santos Pinto D Jr. Immunohistochemical evidence of PTEN in oral squamous cell carcinoma and its correlation with the histological malignancy grading system. J Oral Pathol Med. 2002;31:379–84. doi: 10.1034/j.1600-0714.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- 18.Squarize CH, Castilho RM, Abrahao AC, Molinolo A, Lingen MW, Gutkind JS. PTEN deficiency contributes to the development and progression of head and neck cancer. Neoplasia. 2013;15:461–71. doi: 10.1593/neo.121024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yun J, Poulogiannis G, Brower ET, Klempner S, Cantley LL. The PI3K Pathway in Colorectal Cancers. In: Haigis KM, editor. Molecular Pathogenesis of Colorectal Cancer. New York: Springer; 2013. p. 157-99.
- 20.Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, Feron O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–92. doi: 10.1038/bjc.2013.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saal LH, She Q-B, Gruvberger-Saal SK, Maurer M, Jumppanen M, Su T. et al. PTEN/PI3K oncogenic pathway profiling informs an in vivo synergistic therapeutic model for basal-like breast cancer. Cancer Res. 2013;73:869. doi: 10.1158/1538-7445.AM2013-869. [DOI] [Google Scholar]
- 22.Kavitha K, Kowshik J, Kishore TK, Baba AB, Nagini S. Astaxanthin inhibits NF-kappaB and Wnt/beta-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim Biophys Acta. 2013;1830:4433–44. doi: 10.1016/j.bbagen.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M. et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci U S A. 2009;106:18351–6. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D. et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–83. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 25.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem cell reviews. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 26.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 27.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 28.Kim BW, More SV, Yun YS, Ko HM, Kwak JH, Lee H. et al. A novel synthetic compound MCAP suppresses LPS-induced murine microglial activation in vitro via inhibiting NF-kB and p38 MAPK pathways. Acta Pharmacol Sin. 2016;37:334–43. doi: 10.1038/aps.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mojarad Khanghah S, Ganbarov K. Lactobacillus with probiotic potential from homemade cheese in Azerbijan. Bioimpacts. 2014;4:49–52. doi: 10.5681/bi.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barzegari A, Eslami S, Ghabeli E, Omidi Y. Imposition of encapsulated non-indigenous probiotics into intestine may disturb human core microbiome. Front Microbiol. 2014;5:393. doi: 10.3389/fmicb.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zununi Vahed S, Barzegari A, Rahbar Saadat Y, Goreyshi A, Omidi Y. Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-kappaB/AKT/PTEN/MAPK pathways. Biomed Pharmacother. 2017;94:1094–100. doi: 10.1016/j.biopha.2017.08.033. [DOI] [PubMed] [Google Scholar]


