Abstract
PET using the amino-acid O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) is gaining increasing interest for brain tumour management. Semi-quantitative analysis of tracer uptake in brain tumours is based on the standardized uptake value (SUV) and the tumour-to-brain ratio (TBR). The aim of this study was to explore physiological factors that might influence the relationship of SUV of 18F-FET uptake in various brain areas, and thus affect quantification of 18F-FET uptake in brain tumours. Negative 18F-FET PET scans of 107 subjects, showing an inconspicuous brain distribution of 18F-FET, were evaluated retrospectively. Whole-brain quantitative analysis with Statistical Parametric Mapping (SPM) using parametric SUV PET images, and volumes of interest (VOIs) analysis with fronto-parietal, temporal, occipital, and cerebellar SUV background areas were performed to study the effect of age, gender, height, weight, injected activity, body mass index (BMI), and body surface area (BSA). After multivariate analysis, female gender and high BMI were found to be two independent factors associated with increased SUV of 18F-FET uptake in the brain. In women, SUVmean of 18F-FET uptake in the brain was 23% higher than in men (p < 0.01). SUVmean of 18F-FET uptake in the brain was positively correlated with BMI (r = 0.29; p < 0.01). The influence of these factors on SUV of 18F-FET was similar in all brain areas. In conclusion, SUV of 18F-FET in the normal brain is influenced by gender and weakly by BMI, but changes are similar in all brain areas.
Keywords: 18F-FET PET, Glioma, Quantitative analysis, SUV, Gender, BMI
Highlights
-
•
SUVmean of 18F-FET in the normal brain is influenced by gender.
-
•
SUVmean of 18F-FET in the normal brain is weekly influenced by BMI.
-
•
The influence of these factors on SUV of 18F-FET is similar in all brain areas.
1. Introduction
The 18F labelled amino acid O-(2-[18F]-fluoroethyl)-l-tyrosine (18F-FET) can be produced with high efficiency. Its favourable properties such as high in vivo stability, high tumour to background contrast, and tissue specific tracer kinetics make it an ideal tracer for brain tumour assessment (Langen et al., 2017). Accordingly, positron emission tomography (PET) using 18F-FET is gaining increasing clinical interest, and has been recently recommended by the Response Assessment in Neuro-Oncology (RANO) working group as an additional tool in the diagnosis of brain tumours (Albert et al., 2016). In detail, many studies investigated the potential of this radiotracer in differential diagnosis and grading of brain tumours (Jansen et al., 2012, Pöpperl et al., 2007, Rapp et al., 2013), delineation of tumour extent (Pauleit et al., 2005, Stockhammer et al., 2008, Mehrkens et al., 2008, Misch et al., 2015), treatment monitoring (Galldiks et al., 2012, Galldiks et al., 2013), and prognostication (Jansen et al., 2015).
A few studies have tried to evaluate 18F-FET uptake by pharmacokinetic modelling but without clinical gain. Any advantage over simpler approaches to characterize 18F-FET uptake has not been reported (Langen et al., 2017). Today, semi-quantitative analysis using the standardized uptake value (SUV) is the standard approach to determine 18F-FET uptake in brain tumours. SUV is calculated by dividing the radioactivity (kBq/ml) in the tissue by the radioactivity injected per gram of body weight (Langen et al., 2017). Generally, 18F-FET uptake in brain tumours is expressed as maximum and mean SUV (SUVmax and SUVmean) and a number of studies have used SUV as a direct measure to quantify 18F-FET uptake in the tumour (Calcagni et al., 2011, Pöpperl et al., 2004, Pöpperl et al., 2006, Pöpperl et al., 2007). Moreover, this parameter has been applied to delineate the target volume for radiotherapy planning using an SUV of 3–5 or a fraction of SUVmax (Rickhey et al., 2008, Vees et al., 2009, Weber et al., 2008). In the majority of studies, however, tracer uptake in the tumour was quantified by the ratio of SUVmax or SUVmean in the tumour to the SUVmean in a background region in the contralateral hemisphere expressed as maximal and mean tumour-to-brain ratios (TBRmax and TBRmean) (Jansen et al., 2012, Jansen et al., 2015, Mehrkens et al., 2008, Misch et al., 2015, Pauleit et al., 2005, Pöpperl et al., 2007, Rapp et al., 2013, Stockhammer et al., 2008). This method is based on the assumption that 18F-FET uptake in the normal brain is relatively homogeneous although there is some discussion about the optimal positioning of the region of interest in the normal brain (Unterrainer et al., 2017). Nevertheless, it cannot be excluded that physiological variations of SUVmean of 18F-FET uptake in background could influence TBRs and the results of semi-quantitative analysis.
It is well-known that age and gender influence cerebral metabolism as measured for example by PET using the glucose analogue 18F-fluorodeoxyglucose (18F-FDG) (Hsieh et al., 2012, Kakimoto et al., 2016, Van Der Gucht et al., 2015, Yoshizawa et al., 2014). Although amino acid transport in the brain as reflected by 18F-FET uptake is not directly associated with metabolic processes (Langen et al., 2017), a recent study suggest that epileptic neural activity may lead to temporarily increased 18F-FET uptake (Hutterer et al., 2017). Furthermore, it has been reported that pharmacotherapy with dexamethasone increases 18F-FET uptake in the normal brain tissue leading to a reduction of the TBR (Stegmayr et al., 2016).
The aim of this study was to assess the effect of various physiological factors upon 18F-FET uptake in the normal brain tissue. For this purpose, 18F-FET uptake in the brain was evaluated in a large cohort of subjects with an inconspicuous distribution of 18F-FET and which were rated as “negative scans” with respect to brain tumour diagnosis. Indeed, brains with strong abnormalities in radiotracer uptake are not compatible with whole-brain SPM analysis. SUVmean of 18F-FET in the brains of this cohort was correlated with different parameters such as age, gender, weight, height, injected dose, body mass index (BMI), and body surface area (BSA).
2. Materials and methods
2.1. Subjects
Out of a series of patients with suspicious brain lesions who were admitted for 18F-FET PET to the Institute of Neuroscience and Medicine in Jülich (Germany) from February 2010 to April 2017, 107 scans which revealed no abnormality in 18F-FET PET and which had been rated in the medical reports as “negative scans” with respect to tumour diagnosis, were retrospectively identified by two experienced nuclear physicians (AV and GS). Negative scans were defined as showing 18F-FET uptake in the suspected lesion area (identified by corresponding MR images) to be indistinguishable from that of the normal brain within a TBRmax range of 0.9–1.1, which is in line with the expectable background SUV changes (± 8%) in the brain for this radiopharmaceutical (Unterrainer et al., 2017). Other inclusion criteria for further data evaluation were age > 18 years, no pre-treatment with radio- or chemotherapy or received therapy with dexamethasone, and complete coverage of the brain by the PET acquisition which was essential for data analysis. In the majority of patients (91), no histological examination was performed and there was no evidence for an aggressive tumour. In 16 patients, however, a biopsy was performed owing to the conspicuous MRI despite negative FET PET. Histopathology yielded an astrocytoma WHO grade II in four cases, astrocytoma WHO grade III in six cases, oligoastrocytoma WHO grade II in two cases, oligoastrocytoma WHO grade III in one case and oligodendroglioma WHO grade II in one case. In two cases, histology revealed no evidence of tumour. For each subject, data of age, gender, weight and height were collected. Subsequently, BMI [kg/m2] was calculated by dividing the weight [kg] by the square of the height [m2] (Gorber et al., 2007). BSA [m2] was calculated using weight and height and the Gehan and George formula (Gehan and George, 1970).
Subjects included in the present study were from 18 to 84 years old (45.4 ± 16.5 years old, 40 women, 41 hybrid PET/MR scans). Detailed characteristics of subjects are available in Table 1. No difference in age, type of PET scanner, injected dose, or BMI was noticed between women and men. However, weight, height, and BSA were higher in men than in women (p = 0.01). All subjects gave written informed consent for the investigations and the local ethics committee approved the evaluation of retrospectively collected data. Moreover, all subjects from our institution are informed that their medical data can be rendered anonymously and used for scientific purposes prior to providing consent.
Table 1.
Characteristics of subjects.
| Women n = 40 |
Men n = 67 |
p-Value⁎ | |
|---|---|---|---|
| Age | 44.1 ± 13.9 | 46.2 ± 17.9 | 0.51 |
| Hybrid PET/MR scans | 19 (48%) | 22 (33%) | 0.13 |
| Injected dose [MBq/kg] | 2.8 ± 0.3 | 2.7 ± 0.3 | 0.10 |
| Weight [kg] | 78.9 ± 17.8 | 87.2 ± 12.7 | 0.01⁎ |
| Height [cm] | 167.5 ± 6.9 | 179.4 ± 7.2 | 0.01⁎ |
| BMI [kg/m2] | 28.2 ± 6.4 | 27.1 ± 3.6 | 0.33 |
| BSA [m2] | 1.9 ± 0.2 | 2.1 ± 0.2 | 0.01⁎ |
BMI: body mass index.
BSA: body surface area.
p-Value for comparison between women and men.
2.2. 18F-FET PET acquisition
The amino acid 18F-FET was produced and applied as described previously (Galldiks et al., 2012). According to the German guidelines for brain tumour imaging using labelled amino acid analogues, all subjects fasted for at least 12 h before PET scanning (Langen et al., 2011). Dynamic PET studies were acquired up to 50 min after intravenous injection of 2.8 ± 0.3 MBq of 18F-FET/kg of body weight. PET imaging was performed either on an ECAT Exact HR + PET scanner in 3-dimensional mode (Siemens Medical Systems) (axial field of view, 15.5 cm; image resolution, 6 mm) or simultaneously with MR imaging using a BrainPET insert. The BrainPET is a compact cylinder that fits in the bore of the Magnetom Trio MR scanner (axial field of view, 19.2 cm; optimum image resolution, 3 mm) (Herzog et al., 2011). Iterative reconstruction parameters were 16 subsets, 6 iterations using the OSEM algorithm for ECAT Exact HR + PET scanner and 2 subsets, 32 iterations using the OP-OSEM algorithm provided by the manufacturer for the BrainPET, with correction for random, scattered coincidences, and dead time for both systems. Attenuation correction for the ECAT Exact HR + PET scan was based on a transmission scan, and for the BrainPET scan on a template-based approach. This attenuation map template was obtained from 8 different subjects recorded with the ECAT Exact HR + PET (Siemens Medical Systems) and which is spatially co-registered to the MPRAGE scan of the individual patient using SPM (Filss et al., 2014, Herzog et al., 2011, Kops et al., 2014). Sixty-six subjects (48.2 ± 16.6 years, 21 women, 32%) had their examination on the ECAT PET, 41 (40.9 ± 15.4 years, 19 women, 46%) in the PET/MR system (p = 0.03 for the comparison of age between two groups, non-significant for the comparison of gender). For the evaluation of 18F-FET uptake, summed PET images over the period of 20–40 min post-injection were used.
2.3. SPM analysis
Whole-brain statistical analysis was performed at voxel-level using SPM8 software (Wellcome Department of Imaging Neuroscience, University College, London, UK). PET images were spatially normalized to an in-house adaptive template derived from MR and 18F-FET PET images of 14 scans performed in the hybrid PET/MR system (Gispert et al., 2003). Briefly, the in-house PET template was built on the basis of previous MRI-aided spatial normalization of 14 subjects (39.8 ± 15.6 years old, 12 women). PET scans of each subject were initially co-registered to its corresponding MRI using the co-registration algorithm included in the SPM8 package. After this step, the MR images of the subjects were normalized to the Montreal National Institute (MNI) MRI template, also using the algorithm provided with SPM8, and the resulting deformation field was applied to the PET scans. The final template was built by averaging these normalized PET images and applying a 3-dimensional Gaussian filter (FWHM 8 mm3). The dimensions of the resulting voxels were 2 mm3. This original adaptive 18F-FET PET template is available in Fig. 1A and in Supplementary materials. Then, initial PET images were normalized to this in-house adaptive template, using the algorithm provided by SPM8 and thereafter, normalized PET images were smoothed with a 3-dimensional Gaussian filter (FWHM 8 mm3).
Fig. 1.
Slice sections of A, original 18F-FET PET template calculated from images of 18F-FET PET negative scans of 14 patients (39.8 ± 15.6 years old, 12 women) and volumes of interest defined for the background uptake of 18F-FET in the fronto-parietal (B), the temporal (C), the occipital (D), and the cerebellar (E) areas.
In order to obtain parametric SUV PET images, normalized PET images were divided by the value of the injected dose for each subject [MBq/g] according to the definition of SUV value:
where r is the radioactivity concentration [MBq/ml] within a volume of interest (VOI), in the present study the overall brain area, a′ is the decay-corrected amount of injected 18F-FET [MBq], the decay-correction being constant in this current study because all subjects performed their scans at the same time after injection, and w is the weight of the subject [g], which is used as surrogate for a distribution volume of the tracer. SUVs used in this study are dimensionless under the assumption that 1 ml of tissue is equivalent to 1 g of body weight (W. A. Weber et al., 2008). Finally, a correction for the voxel size was applied by dividing each image by 8 (voxel size of resulting images is 8 mm3) in order to obtain a radioactivity concentration in 1 ml.
Statistical analysis was performed with a linear model of regression analysis for age, BMI, and BSA effects, with gender, and type of PET as nuisance covariates. Analysis of variance (ANOVA)-test comparisons were performed for: i) gender with age, BMI, BSA, and type of PET as covariates, and ii) type of PET with age, gender, BMI, and BSA as covariates. SPM (T) maps were obtained at a threshold (voxel-level significance) of p < 0.001 uncorrected, but corrected for cluster volume as recommended to avoid type II errors (Lieberman and Cunningham, 2009). The anatomical localization of the most significant voxels was then identified using MNI coordinates.
In order to extract the quantitative values of SUVmean of 18F-FET, several Volumes of Interest (VOIs) were applied at individual level using Marsbar® (Marseille, France), including a large crescent shape fronto-parietal area placed on the semi-oval centre as recommended (Unterrainer et al., 2017), but also in the temporal, occipital, and cerebellar areas. Afterwards, it was evaluated whether the SUVmean of 18F-FET in these brain areas is differently influenced by various factors. These VOIs are illustrated in Fig. 1B, C, D, and E.
2.4. Statistical analysis
Quantitative variables are expressed as mean ± standard deviation, and categorical variables as percentage. t-Tests were performed for the comparison of means between two quantitative variables with normal distribution and Chi-square tests for comparison between two categorical variables. Pearson coefficients were used for correlation analysis. A linear regression model was applied for multivariate analysis. A p < 0.05 was determined as significant. Statistical analysis based upon SPM is mentioned above.
3. Results
3.1. SPM analysis
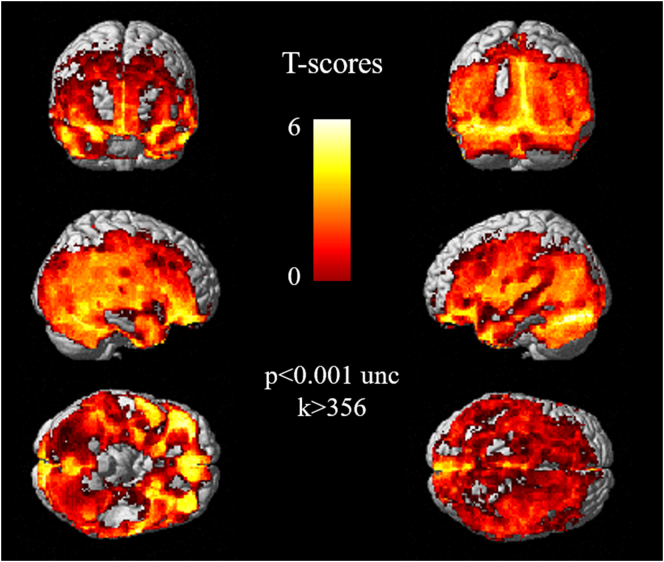
SUV of 18F-FET was higher in women than in men in diffuse cortical areas involving frontal, parietal, temporal, occipital lobes, and cerebellum (p < 0.001 uncorrected, k > 356) (Fig. 2).
Fig. 2.
Anatomical localization of areas of increased SUV of 18F-FET in women in comparison with men, projected onto 3D volume rendering. SUV of 18F-FET was higher in women than in men in diffuse cortical areas including frontal, parietal, temporal, and occipital lobes, as well as the cerebellum (p < 0.001 uncorrected, k > 356).
Moreover, BMI was positively correlated with higher SUV of 18F-FET in diffuse cortical areas involving frontal, parietal, temporal, occipital lobes and cerebellum (p < 0.001 uncorrected, k > 332) (Fig. 3). However, the extent of findings was lower than for gender effects (277 vs. 818 cm3).
Fig. 3.
Anatomical localization of areas of increased SUV of 18F-FET related to Body Mass Index (BMI), projected onto 3D volume rendering. BMI was positively correlated with higher SUV of 18F-FET in diffuse cortical areas including frontal, parietal, temporal, and occipital lobes, as well as the cerebellum (p < 0.001 uncorrected, k > 332).
No statistical significant finding was observed for the effects of age, BSA and type of PET.
3.2. Marsbar analysis and influence on SUVmean VOI
An univariate analysis was calculated for effects of age, gender, type of PET, weight, height, injected activity, BMI, and BSA on SUVmean VOI. Gender, height, and BMI significantly influenced SUVmean of 18F-FET in the fronto-parietal VOI. In detail, SUVmean of 18F-FET in women was 23% higher than in men (p < 0.01) as shown in box-plots in Fig. 4. Moreover, SUVmean of 18F-FET was weakly positively correlated with BMI, in overall population and for women (r = 0.29, 0.33 p < 0.04) but not in men (p = 0.14) as illustrated in Fig. 5, and negatively correlated with height, in overall population and in women (r = − 0.40 and − 0.32 respectively; p < 0.05) but not in men (p = 0.95). In addition, SUVmean of 18F-FET was 12% higher in obese subjects (n = 25, defined as BMI ≥ 30 kg/m2, (Gorber et al., 2007)) than in subjects with normal weight (1.42 ± 0.31 vs. 1.27 ± 0.26, p = 0.02).
Fig. 4.
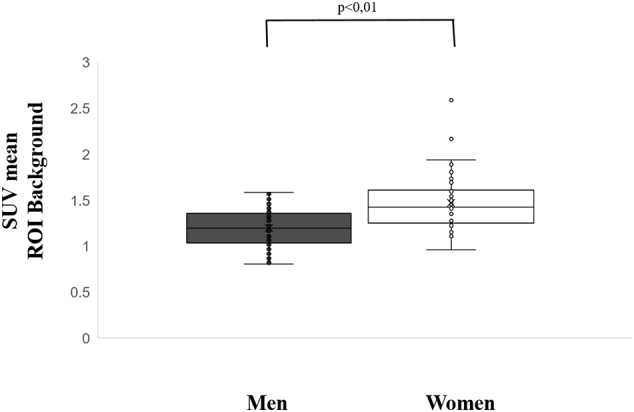
Box-plots of SUVmean of 18F-FET in women and men within a fronto-parietal VOI, used as background region. Women had higher SUVmean of 18F-FET than men (1.47 ± 0.31 vs. 1.20 ± 0.20, p < 0.01).
Fig. 5.
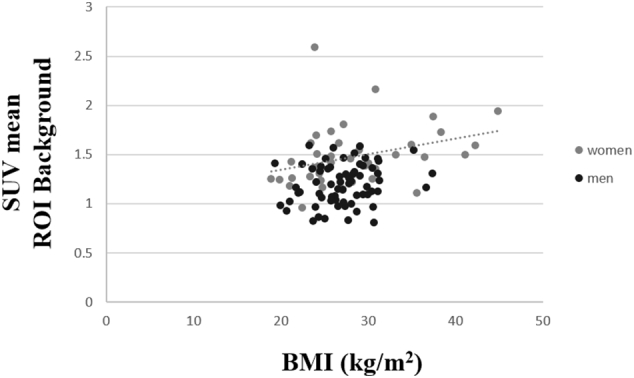
Correlation curve between SUVmean of 18F-FET within a fronto-parietal VOI, used as background region, and Body Mass Index (kg/m2). SUVmean of 18F-FET was positively correlated to BMI for women (r = 0.33 p < 0.04).
In multivariate analysis using a linear regression model with all factors mentioned above and taking into account inter-correlated parameters, only gender and BMI remained as significant parameters influencing SUVmean of 18F-FET in the brain tissue VOI (SUVmean = 0.257 × female gender + 0.014 × BMI + 1.080; p < 0.01).
For the evaluation of regional 18F-FET distribution, the previously defined VOIs (temporal, occipital, and cerebellar) were influenced by gender, height, and BMI in the same way as the fronto-parietal VOI (increase of 24% of SUVmean of 18F-FET in women in comparison to men, and a correlation coefficient with height and BMI of − 0.40 and 0.31 for the temporal VOI, 22%, − 0.41 and 0.37 for the occipital VOI, and 26%, − 0.39 and 0.33 for the cerebellar VOI, p < 0.01). Thus, the influence of physiological factors on SUV of 18F-FET was similar in all brain areas.
4. Discussion
This study shows that female gender and weakly high BMI are two independent factors leading to significantly increased SUVmean of 18F-FET in the entire brain.
These relationships have not been previously known and it seems important to discuss the influence of these parameters on the quantification of 18F-FET uptake in brain tumours. An effect of gender has also been observed for other PET tracers such as 18F-FDG showing diffusely higher cerebral glucose metabolism in females than in males (Willis et al., 2002, Yoshizawa et al., 2014). Earlier studies have reported that cerebral glucose metabolism in women is 19 to 26% higher than in men which corresponds to the proportion of higher 18F-FET uptake in the current study (Baxter et al., 1987, Yoshii et al., 1988). A possible explanation for this finding may be a higher cerebral blood flow in female patients (Yoshizawa et al., 2014) which could be linked to effect of hormones, especially estrogen, on cerebral metabolism (Reiman et al., 1996). It was speculated that these differences in gender are applicable to different PET tracers (Niven et al., 2001).
The second factor in the present study that influenced SUVmean of 18F-FET weakly is BMI. We assume that differences in BMI are associated with a differential whole-body distribution of 18F-FET. Whole-body 18F-FET PET scans have shown that there is noticeable tracer accumulation in the skeletal muscles and the heart with about 40% of the total injected dose activity accumulated in muscles (Pauleit et al., 2003). In contrast, low uptake was observed in adipose tissue. It is well known that obese patients have a greater loss of muscle mass compared to patients with BMI < 30 kg·m− 2 (Dickerson, 2005, Port and Apovian, 2010). Moreover, there is a greater tendency to develop sarcopenia in these patients, which is related to increased age and intramuscular fat infiltration (Thornell, 2011), and overweight in elderly people induces impaired protection mechanisms of skeletal muscle, leading to a decrease of muscle mass (Potes et al., 2017). Thus, in obese patients the lower relative muscle mass would increase the amount of injected tracer which is available to be delivered to the brain, leading to a higher SUVmean of 18F-FET in the brain. This hypothesis could also partly explain the higher SUVmean of 18F-FET in the brain of women for whom the ratio of lean to fat mass is lower than in men (Ulbrich et al., 2017). It has to be considered, however, that both parameters were independent in multivariate analysis and the weak correlation between SUVmean of 18F-FET and BMI could not alone explain the significant difference between SUVmean of 18F-FET in women and in men.
If higher SUVmean of 18F-FET in women and patients with high BMI would have been caused by differences in cerebral blood flow or in whole-body distribution, SUV of 18F-FET uptake in the normal brain and in brain tumours would increase to the same extent. This finding would make the use of SUVmax and SUVmean as a direct measure to quantify 18F-FET uptake a source of error and could influence the delineation of the target volume for radiotherapy planning of brain tumours as used by some authors (Rickhey et al., 2008, Vees et al., 2009, Weber et al., 2008).
If, in the other case, the increase of SUVmean caused by gender - or more moderately by BMI - affects only 18F-FET uptake in areas of background VOI without influencing tumour uptake, the TBR in brain tumours would be decreased in these patient groups.
Another major finding of this study is that the influence of physiological factors on SUV of 18F-FET is similar in all brain areas. Thus, 18F-FET distribution in the brain appears to be highly stable, which suggests that background regions in different brain areas are equally valid for the evaluation of TBR. Nevertheless, intra- and inter-reader variability regarding the assessment of the background reference remains an important issue. Recently, it has been shown that background activity assessment using a crescent-shaped volume of interest in the contralateral hemisphere including white and grey matter allows minimization of both intra- and inter-reader variability and might facilitate comprehensive methodological standardization of amino acid PET, which is of interest in the light of future technical guidelines (Unterrainer et al., 2017). Moreover, a standardization of the reference region is needed, while absolute values of SUV of 18F-FET could differ between areas even if they are influenced in a similar way by external factors.
The following limitations need to be discussed. Firstly, subjects included in this study cannot be considered as healthy subjects since all showed brain abnormalities in MRI. For ethical reasons, however, it would be difficult to investigate such a large number of healthy subjects by 18F-FET PET. In addition, each scan was carefully checked before inclusion to ascertain its negative state with regard to 18F-FET uptake. Secondly, scans were performed on two different PET systems. However, the quantitative analysis did not show an effect of type of PET scanner on SUV of 18F-FET uptake and this parameter was included as a covariate in our statistical model. Finally, a whole-brain quantitative analysis has been performed although the areas with suspicious brain lesions as identified on MRI have not been excluded from the VOI analyses. The brain lesions areas were nevertheless indistinguishable from that of the normal brain, as reported in the Materials and methods section and were differently located for each individual subjects, making it almost unlikely to influence overall results of group analysis.
5. Conclusion
In summary, SUVmean of 18F-FET in the normal brain is influenced by gender and weakly by BMI. We suggest that these findings are partly caused by different whole-body distribution of 18F-FET depending on the muscle mass but other gender specific factors dominate. The influence of these factors on SUV of 18F-FET is similar in all brain areas. However, although 18F-FET distribution in the normal brain appears to be highly stable, a standardization of the reference region is necessary for comparison of data between different centres.
Funding
None.
Conflict of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.11.005.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- Albert N.L., Weller M., Suchorska B., Galldiks N., Soffietti R., Kim M.M., la Fougère C., Pope W., Law I., Arbizu J., Chamberlain M.C., Vogelbaum M., Ellingson B.M., Tonn J.C. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncol. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter L.R., Mazziotta J.C., Phelps M.E., Selin C.E., Guze B.H., Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21:237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Calcagni M.L., Galli G., Giordano A., Taralli S., Anile C., Niesen A., Baum R.P. Dynamic O-(2-[18F]fluoroethyl)-l-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin. Nucl. Med. 2011;36:841–847. doi: 10.1097/RLU.0b013e3182291b40. [DOI] [PubMed] [Google Scholar]
- Dickerson R.N. Hypocaloric feeding of obese patients in the intensive care unit. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:189–196. doi: 10.1097/00075197-200503000-00014. [DOI] [PubMed] [Google Scholar]
- Filss C.P., Galldiks N., Stoffels G., Sabel M., Wittsack H.J., Turowski B., Antoch G., Zhang K., Fink G.R., Coenen H.H., Shah N.J., Herzog H., Langen K.-J. Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014;55:540–545. doi: 10.2967/jnumed.113.129007. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Langen K.-J., Holy R., Pinkawa M., Stoffels G., Nolte K.W., Kaiser H.J., Filss C.P., Fink G.R., Coenen H.H., Eble M.J., Piroth M.D. Assessment of treatment response in patients with glioblastoma using O-(2-18F-Fluoroethyl)-l-tyrosine PET in comparison to MRI. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012 doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Rapp M., Stoffels G., Fink G.R., Shah N.J., Coenen H.H., Sabel M., Langen K.-J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-l-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:22–33. doi: 10.1007/s00259-012-2251-4. [DOI] [PubMed] [Google Scholar]
- Gehan E.A., George S.L. Estimation of human body surface area from height and weight. Cancer Chemother. Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- Gispert J.D., Pascau J., Reig S., Martínez-Lázaro R., Molina V., García-Barreno P., Desco M. Influence of the normalization template on the outcome of statistical parametric mapping of PET scans. NeuroImage. 2003;19:601–612. doi: 10.1016/s1053-8119(03)00072-7. [DOI] [PubMed] [Google Scholar]
- Gorber S.C., Tremblay M., Moher D., Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes. Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Herzog H., Langen K.-J., Weirich C., Rota Kops E., Kaffanke J., Tellmann L., Scheins J., Neuner I., Stoffels G., Fischer K., Caldeira L., Coenen H.H., Shah N.J. High resolution BrainPET combined with simultaneous MRI. Nukl. Nucl. Med. 2011;50:74–82. doi: 10.3413/Nukmed-0347-10-09. [DOI] [PubMed] [Google Scholar]
- Hsieh T.-C., Lin W.-Y., Ding H.-J., Sun S.-S., Wu Y.-C., Yen K.-Y., Kao C.-H. Sex- and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J. Neuroimaging Off. J. Am. Soc. Neuroimaging. 2012;22:21–27. doi: 10.1111/j.1552-6569.2010.00543.x. [DOI] [PubMed] [Google Scholar]
- Hutterer M., Ebner Y., Riemenschneider M.J., Willuweit A., McCoy M., Egger B., Schröder M., Wendl C., Hellwig D., Grosse J., Menhart K., Proescholdt M., Fritsch B., Urbach H., Stockhammer G., Roelcke U., Galldiks N., Meyer P.T., Langen K.-J., Hau P., Trinka E. Epileptic activity increases cerebral amino acid transport assessed by 18F-Fluoroethyl-l-tyrosine amino acid PET: a potential brain tumor mimic. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017;58:129–137. doi: 10.2967/jnumed.116.176610. [DOI] [PubMed] [Google Scholar]
- Jansen N.L., Graute V., Armbruster L., Suchorska B., Lutz J., Eigenbrod S., Cumming P., Bartenstein P., Tonn J.-C., Kreth F.W., la Fougère C. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1021–1029. doi: 10.1007/s00259-012-2109-9. [DOI] [PubMed] [Google Scholar]
- Jansen N.L., Suchorska B., Wenter V., Schmid-Tannwald C., Todica A., Eigenbrod S., Niyazi M., Tonn J.-C., Bartenstein P., Kreth F.-W., la Fougère C. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015;56:9–15. doi: 10.2967/jnumed.114.144675. [DOI] [PubMed] [Google Scholar]
- Kakimoto A., Ito S., Okada H., Nishizawa S., Minoshima S., Ouchi Y. Age-related sex-specific changes in brain metabolism and morphology. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016;57:221–225. doi: 10.2967/jnumed.115.166439. [DOI] [PubMed] [Google Scholar]
- Kops E.R., Herzog H., Shah N.J. Comparison template-based with CT-based attenuation correction for hybrid MR/PET scanners. EJNMMI Phys. 2014;1:A47. doi: 10.1186/2197-7364-1-S1-A47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen K.-J., Bartenstein P., Boecker H., Brust P., Coenen H.H., Drzezga A., Grünwald F., Krause B.J., Kuwert T., Sabri O., Tatsch K., Weber W.A., Schreckenberger M. German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids. Nukl. Nucl. Med. 2011;50:167–173. doi: 10.3413/nuk-2011041. [DOI] [PubMed] [Google Scholar]
- Langen K.-J., Stoffels G., Filß C., Heinzel A., Stegmayr C., Lohmann P., Willuweit A., Neumaier B., Mottaghy F.M., Galldiks N. Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[(18)F]fluoroethyl)-l-tyrosine (FET) Methods San Diego Calif. 2017 doi: 10.1016/j.ymeth.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrkens J.H., Pöpperl G., Rachinger W., Herms J., Seelos K., Tatsch K., Tonn J.C., Kreth F.W. The positive predictive value of O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J. Neuro-Oncol. 2008;88:27–35. doi: 10.1007/s11060-008-9526-4. [DOI] [PubMed] [Google Scholar]
- Misch M., Guggemos A., Driever P.H., Koch A., Grosse F., Steffen I.G., Plotkin M., Thomale U.-W. (18)F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2015;31:261–267. doi: 10.1007/s00381-014-2552-y. [DOI] [PubMed] [Google Scholar]
- Niven E., Thompson M., Nahmias C. Absorbed dose to the adult male and female brain from 18F-fluorodeoxyglucose. Health Phys. 2001;80:62–66. doi: 10.1097/00004032-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Pauleit D., Floeth F., Herzog H., Hamacher K., Tellmann L., Müller H.-W., Coenen H.H., Langen K.-J. Whole-body distribution and dosimetry of O-(2-[18F]fluoroethyl)-l-tyrosine. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:519–524. doi: 10.1007/s00259-003-1118-0. [DOI] [PubMed] [Google Scholar]
- Pauleit D., Floeth F., Hamacher K., Riemenschneider M.J., Reifenberger G., Müller H.-W., Zilles K., Coenen H.H., Langen K.-J. O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain J. Neurol. 2005;128:678–687. doi: 10.1093/brain/awh399. [DOI] [PubMed] [Google Scholar]
- Pöpperl G., Götz C., Rachinger W., Gildehaus F.-J., Tonn J.-C., Tatsch K. Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1464–1470. doi: 10.1007/s00259-004-1590-1. [DOI] [PubMed] [Google Scholar]
- Pöpperl G., Kreth F.W., Herms J., Koch W., Mehrkens J.H., Gildehaus F.J., Kretzschmar H.A., Tonn J.C., Tatsch K. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006;47:393–403. [PubMed] [Google Scholar]
- Pöpperl G., Kreth F.W., Mehrkens J.H., Herms J., Seelos K., Koch W., Gildehaus F.J., Kretzschmar H.A., Tonn J.C., Tatsch K. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1933–1942. doi: 10.1007/s00259-007-0534-y. [DOI] [PubMed] [Google Scholar]
- Port A.M., Apovian C. Metabolic support of the obese intensive care unit patient: a current perspective. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:184–191. doi: 10.1097/MCO.0b013e328335f1e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potes Y., de Luxán-Delgado B., Rodriguez-González S., Guimarães M.R.M., Solano J.J., Fernández-Fernández M., Bermúdez M., Boga J.A., Vega-Naredo I., Coto-Montes A. Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic. Biol. Med. 2017;110:31–41. doi: 10.1016/j.freeradbiomed.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Rapp M., Heinzel A., Galldiks N., Stoffels G., Felsberg J., Ewelt C., Sabel M., Steiger H.J., Reifenberger G., Beez T., Coenen H.H., Floeth F.W., Langen K.-J. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013;54:229–235. doi: 10.2967/jnumed.112.109603. [DOI] [PubMed] [Google Scholar]
- Reiman E.M., Armstrong S.M., Matt K.S., Mattox J.H. The application of positron emission tomography to the study of the normal menstrual cycle. Hum. Reprod. Oxf. Engl. 1996;11:2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- Rickhey M., Koelbl O., Eilles C., Bogner L. A biologically adapted dose-escalation approach, demonstrated for 18F-FET-PET in brain tumors. Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft Al. 2008;184:536–542. doi: 10.1007/s00066-008-1883-6. [DOI] [PubMed] [Google Scholar]
- Stegmayr C., Schöneck M., Oliveira D., Willuweit A., Filss C., Galldiks N., Shah N.J., Coenen H.H., Langen K.-J. Reproducibility of O-(2-(18)F-fluoroethyl)-l-tyrosine uptake kinetics in brain tumors and influence of corticoid therapy: an experimental study in rat gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1115–1123. doi: 10.1007/s00259-015-3274-4. [DOI] [PubMed] [Google Scholar]
- Stockhammer F., Plotkin M., Amthauer H., van Landeghem F.K.H., Woiciechowsky C. Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J. Neuro-Oncol. 2008;88:205–210. doi: 10.1007/s11060-008-9551-3. [DOI] [PubMed] [Google Scholar]
- Thornell L.-E. Sarcopenic obesity: satellite cells in the aging muscle. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:22–27. doi: 10.1097/MCO.0b013e3283412260. [DOI] [PubMed] [Google Scholar]
- Ulbrich E.J., Nanz D., Leinhard O.D., Marcon M., Fischer M.A. Whole-body adipose tissue and lean muscle volumes and their distribution across gender and age: MR-derived normative values in a normal-weight Swiss population. Magn. Reson. Med. 2017 doi: 10.1002/mrm.26676. [DOI] [PubMed] [Google Scholar]
- Unterrainer M., Vettermann F., Brendel M., Holzgreve A., Lifschitz M., Z?hringer M., Suchorska B., Wenter V., Illigens B.M., Bartenstein P., Albert N.L. Towards standardization of 18F-FET PET imaging: do we need a consistent method of background activity assessment? EJNMMI Res. 2017;7 doi: 10.1186/s13550-017-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Gucht A., Verger A., Guedj E., Malandain G., Hossu G., Yagdigul Y., Roch V., Poussier S., Maillard L., Karcher G., Marie P.-Y. Age-related changes in FDG brain uptake are more accurately assessed when applying an adaptive template to the SPM method of voxel-based quantitative analysis. Ann. Nucl. Med. 2015;29:921–928. doi: 10.1007/s12149-015-1022-2. [DOI] [PubMed] [Google Scholar]
- Vees H., Senthamizhchelvan S., Miralbell R., Weber D.C., Ratib O., Zaidi H. Assessment of various strategies for 18F-FET PET-guided delineation of target volumes in high-grade glioma patients. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:182–193. doi: 10.1007/s00259-008-0943-6. [DOI] [PubMed] [Google Scholar]
- Weber W.A., Grosu A.L., Czernin J. Technology insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat. Clin. Pract. Oncol. 2008;5:160–170. doi: 10.1038/ncponc1041. [DOI] [PubMed] [Google Scholar]
- Weber D.C., Zilli T., Buchegger F., Casanova N., Haller G., Rouzaud M., Nouet P., Dipasquale G., Ratib O., Zaidi H., Vees H., Miralbell R. [(18)F]Fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma. Radiat. Oncol. Lond. Engl. 2008;3:44. doi: 10.1186/1748-717X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis M.W., Ketter T.A., Kimbrell T.A., George M.S., Herscovitch P., Danielson A.L., Benson B.E., Post R.M. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. 2002;114:23–37. doi: 10.1016/s0925-4927(01)00126-3. [DOI] [PubMed] [Google Scholar]
- Yoshii F., Barker W.W., Chang J.Y., Loewenstein D., Apicella A., Smith D., Boothe T., Ginsberg M.D., Pascal S., Duara R. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1988;8:654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- Yoshizawa H., Gazes Y., Stern Y., Miyata Y., Uchiyama S. Characterizing the normative profile of 18F-FDG PET brain imaging: sex difference, aging effect, and cognitive reserve. Psychiatry Res. 2014;221:78–85. doi: 10.1016/j.pscychresns.2013.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2