Abstract
Introduction
Optimal glycaemic control in women with gestational diabetes mellitus (GDM) reduces maternal and infant morbidity.
Method
A survey was administered to women diagnosed with GDM to explore their views and experiences in achieving optimal glycaemic control.
Results
Sixty women participated. Enablers included being taught to test capillary blood glucose in group settings where the health professional demonstrated this on themselves first (60, 100%); health professionals listening (41, 68%); being reminded to perform blood glucose testing (33, 55%); and being provided healthy meals by friends and family (28, 47%). Barriers included not having information in a woman's first language (33, 55%); being offered unhealthy food (19, 31%); not being believed by health professionals (13, 21%); receiving inconsistent information by health professionals (10, 16%); never being seen twice by the same health professional (8, 13%); and long waiting hours at clinics (7, 11%). Two-thirds of women (37, 62%) reported that food costs were not a barrier, but that they were always or frequently hungry.
Conclusion
Optimising experiences for women with GDM for achieving glycaemic control and overcoming barriers, regardless of glycaemic targets, requires further focus on providing meaningful health literacy and support from health professionals, family, friends, and work colleagues.
1. Introduction
Globally, there are increasing rates of diabetes, including gestational diabetes mellitus (GDM) [1, 2]. The prevalence of GDM varies among populations but probably affects 10–25% of pregnancies [3–5].
Short- and long-term health risks for women with GDM include preeclampsia, induction of labour, caesarean section, and postnatal depression for the women [6–8]. For the baby, health risks include shoulder dystocia, nerve palsy, preterm birth, neonatal hypoglycaemia, respiratory distress syndrome, and the risk of developing obesity and type 2 diabetes (T2DM) in childhood [9–11].
Treatments for women with GDM that maintain glycaemic control within specified targets have a significant impact on short- and long-term health for the woman and her baby [12–14]. Treatments for GDM include dietary and exercise advice alone or combined with pharmacological therapy [15–19].
While some published studies have described women's experiences of developing gestational diabetes [20–25], little is known as to how women feel about achieving their glycaemic treatment targets. This nested study within the TARGET trial (Australian New Zealand Trial Registry: ACTRN12615000282583) aimed to explore women's views and experiences in achieving their recommended glycaemic treatment targets and to identify potential barriers and enablers.
2. Materials and Methods
2.1. Participant Selection
Women diagnosed with GDM were eligible to participate if they had a singleton pregnancy, could communicate in English, had been self-monitoring their capillary blood glucose levels for at least two weeks, and provided written consent. Eligible women were sent an email invitation that included a participant information sheet and consent form. Women could choose to be interviewed face to face, or to be telephoned. Women were aware that the survey was not an assessment of their knowledge about GDM and advised that all their information would be kept confidential.
Hospital sites from two different geographical locations in New Zealand participated. Twenty women, recruited from Canterbury District Health Board (DHB) in the South Island, were using less tight glycaemic treatment targets (fasting blood glucose < 5.5 mmol/L, 1 hour postprandial < 8.0 mmol/L, and 2 hours postprandial < 7.0 mmol/L). Forty women were using tighter glycaemic treatment targets (fasting blood glucose ≤ 5.0 mmol/L, 1 hour postprandial ≤ 7.4 mmol/L, and 2 hours postprandial ≤ 6.7 mmol/L): twenty women recruited from Canterbury DHB in the South Island and twenty women recruited from Counties Manukau DHB in the North Island.
Local hospital policies differed for testing of capillary blood glucose. Canterbury DHB moved from initially less tight targets to tighter glycaemic treatment targets during the survey time. Women were asked to test their capillary blood glucose at one hour postprandial. Counties Manukau DHB was using tighter glycaemic treatment targets during the survey time. Women were asked to test their capillary blood glucose two hours postprandial.
2.2. The Survey
The survey comprised 45 questions. Twenty questions identified participant demographics and twenty-five their views and knowledge of their glycaemic treatment targets. Questions included identifying what had been helpful in learning how to self-monitor blood glucose levels; support received from family, friends, and health professionals; access to written information; costs associated with their GDM management and treatment; and experience of hunger. The survey was piloted with three women following which three questions were modified. There was an opportunity for women to provide additional information. All women answered all the survey questions.
2.3. Analysis
Data analysis was conducted using Pivot Tables in Microsoft Office Excel 2016 calculating frequency and corresponding percentage to describe the responses to the survey questions and included mean and standard deviation for normally distributed data. All analyses were undertaken in Microsoft Office Excel 2016, reporting descriptive statistics for baseline demographics and using simple numeric calculations for survey responses.
2.4. Ethical Approval
The Views Survey was nested within the TARGET trial approved by the New Zealand Health and Disability Ethics committee (HDEC) Ref. 14/NTA/163 and research registration number 1965.
3. Results
3.1. Participants
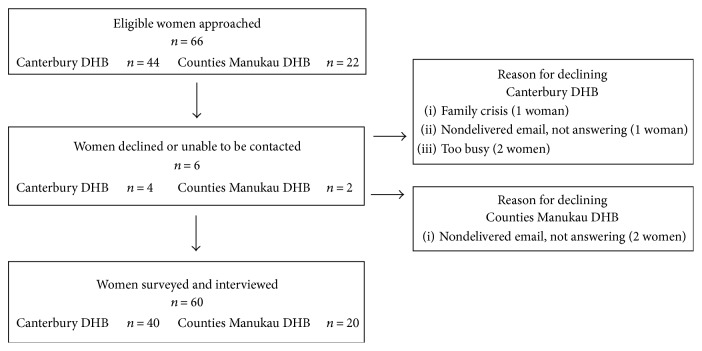
Sixty-six eligible women were approached and sixty women consented to participate in the survey. Six women did not participate because they were too busy, having a family crisis, or not responding to the invitations (Figure 1). Face-to-face surveys were conducted with 34 (57%) women and 26 (43%) of the women chose to be surveyed by telephone. The average age of the participating women was 33 years (standard deviation (SD) ± 4.5). Just under half of the women were primigravid and had a family history of diabetes (27, 45%), and two-thirds were classified as obese or overweight in early pregnancy (39, 65%) (Table 1). Most women were European (24, 40%) followed by Asian ethnicity (22, 37%). Women taking part were evenly distributed across the deprivation index: 18 (30%) women least deprived (levels 1–3) and 19 (32%) women (levels 4–6) and 22 (37%) women most deprived (levels 7–10) (Table 1). The demographics of the participating women are reflective of a cross section of the demographics of New Zealand's pregnant population [26–28] (Table 1).
Figure 1.
Flowchart of recruitment.
Table 1.
Demographic characteristics of women who participated in the survey.
| Characteristics | Women with less tight1 glycaemic treatment targets n = 20 (% or ±20) |
Women with tighter2 glycaemic treatment targets n = 40 (% or ±40) |
Women total n = 60 (% or ±60) |
|---|---|---|---|
| Age (years)4 | 34 (±4.3) | 32 (±4.5) | 33 (±4.5) |
| Primigravida (G1P0)3 | 9 (45) | 18 (45) | 27 (45) |
| BMI category 5,3 | |||
| Normal | 8 (40) | 13 (32.5) | 21 (35) |
| Overweight | 5 (25) | 6 (15) | 11 (18.3) |
| Obese (class I) | 2 (10) | 9 (22.5) | 11 (18.3) |
| Obese (class II) | 2 (10) | 6 (15) | 8 (13.3) |
| Obese (class II) | 3 (15) | 6 (15) | 9 (15) |
| Total obese | 7 (35) | 21 (52.5) | 28 (46.6) |
| Ethnicity 6,3 | |||
| European | 12 (60) | 12 (30) | 24 (40) |
| Māori | — | 6 (15) | 6 (10) |
| Asian | 7 (35) | 15 (37.5) | 22 (36.7) |
| Pacific Peoples | — | 7 (17.5) | 7 (11.6) |
| MELAA | 1 (5) | — | 1 (1.7) |
| Highest educational qualifications after leaving school 7,3 | |||
| (1) No qualification | 1 (5) | 2 (5) | 3 (5) |
| (2) Level 1 certificate | — | 2 (5) | 2 (3.3) |
| (3) Level 2 certificate | 2 (10) | 2 (5) | 4 (6.7) |
| (4) Level 3 certificate | 2 (10) | 4 (10) | 6 (10) |
| (5) Level 4 certificate | — | 4 (10) | 4 (6.7) |
| (6) Level 5 and level 6 diploma | 4 (20) | 9 (22.5) | 13 (21.7) |
| (7) Bachelor degree and level 7 qualification | 8 (40) | 17 (42.5) | 25 (41.6) |
| (8) Postgraduate and honours degree | 1 (5) | — | 1 (1.7) |
| (9) Master's degree | 2 (10) | — | 2 (3.3) |
| NZ deprivation index 8,3 | |||
| 1 (least deprived) | 3 (15) | 5 (12.5) | 8 (13.5) |
| 2 | 2 (10) | 3 (7.5) | 5 (8.4) |
| 3 | 2 (10) | 3 (7.5) | 5 (8.4) |
| 4 | 4 (20) | 6 (15) | 10 (16.7) |
| 5 | 2 (10) | 5 (12.5) | 7 (11.8) |
| 6 | 1 (5) | 1 (1.7) | 2 (3.4) |
| 7 | 2 (10) | 3 (7.5) | 5 (8.5) |
| 8 | 3 (15) | 3 (7.5) | 6 (10) |
| 9 | 1 (5) | 4 (10) | 5 (8.7) |
| 10 (most deprived) | — | 6 (15) | 6 (10) |
| Lead maternity carer (LMC) 9,3 | |||
| Midwife | 19 (95) | 36 (90) | 55 (91.7) |
| Obstetrician | 1 (5) | — | 1 (1.7) |
| Hospital team | — | 4 (10) | 4 (6.7) |
| Gestational age at GDM diagnosis4 (weeks) | 27.7 (±1.9) | 27.9 (±2.0) | 27.8 (±2.0) |
| Time of self-testing capillary blood glucose for (weeks)4 | 7.6 (±2.5) | 6.4 (±2.1) | 6.8 (±2.3) |
| Previous GDM3 | 4 (20) | 6 (15) | 10 (16.7) |
| Previous hypertension3 | 2 (10) | — | 2 (3.3) |
| Current hypertension | — | 3 (7.5) | 3 (5) |
| Family history of hypertension3 | 8 (45) | 16 (40) | 24 (40) |
| Family history of diabetes3 | 7 (35) | 20 (50) | 27 (45) |
| Current smoker3 | — | 3 (7.5) | 3 (15) |
| Current treatment3 | |||
| Diet only | 7 (35) | 11 (27.5) | 18 (30) |
| Insulin and diet | 2 (10) | 11 (27.5) | 13 (21.7) |
| Metformin and diet | 5 (25) | 12 (30) | 17 (28.3) |
| Insulin, metformin, and diet | 6 (30) | 6 (15) | 12 (20) |
1Less tight glycaemic treatment targets for women with GDM: fasting blood glucose < 5.5 mmol/L, 1 hour postprandial < 8.0 mmol/L, and 2 hours postprandial < 7.0 mmol/L; 2tighter glycaemic treatment targets for women with GDM: fasting blood glucose ≤ 5.0 mmol/L, 1 hour postprandial ≤ 7.4 mmol/L, and 2 hours postprandial ≤6.7 mmol/L; 3figures are numbers and percentages; 4figures are mean and standard deviation; 5BMI categories: underweight < 18.50, normal range ≥ 18.55–24.99, overweight ≥ 25.00–29.99, obese (class I) ≥ 30.00–34.99, obese (class II)—severe obese ≥ 35.00–39.99 and obese (class II)—morbid obese ≥ 40.00 (according to WHO and Ministry of Health categories) [44, 45]; 6as categorised by New Zealand government statistics groups for major ethnic groups. MELAA is an acronym for Middle Eastern/Latin American/African (http://www.stats.govt.nz/Census/2013-census/profile-and-summary-reports/infographic-culture-identity.aspx); 7as categorised by New Zealand government statistics groups (http://www.stats.govt.nz/Census/2013-census/profile-and-summary-reports/qstats-education-training/highest-qualification.aspx); 8as categorised by New Zealand 2013 Deprivation Index, University of Otago, Department of Public Health. Deprivation score was unknown for one woman, as her address had no meshblock listed (http://www.otago.ac.nz/wellington/departments/publichealth/research/hirp/otago020194.html); 9a lead maternity carer (LMC) in New Zealand provides lead maternity care (is in charge). This can be either a midwife, obstetrician, or GP (https://www.midwife.org.nz/in-new-zealand/contexts-for-practice).
Women were diagnosed with GDM at a mean of 27.8 ± 2.0-week gestation. At the time of the survey, participants had been checking their daily capillary blood glucose for an average of 6.8 ± 2.3 weeks (Table 1), with just over half checking their blood glucose four times a day (32, 53%) and the other participants six times a day (28, 47%). Ten women (17%) reported having a diagnosis of GDM from a previous pregnancy. Almost a third of women (18, 30%) were treated with diet alone; the remainder received a combination of dietary advice and medications. Thirteen (22%) women were treated with subcutaneous insulin for their GDM, 17 (28%) women metformin, and 12 (20%) women were treated with insulin and metformin (Table 1).
3.2. Views and Experiences about Achieving Recommended Glycaemic Treatment Targets
The majority of women correctly identified their glycaemic treatment targets (59, 98%) and viewed it as very important or important to try to adhere to these targets (Table 2). Documenting the blood glucose results were viewed as less important (56, 93%) compared to viewing adherence to the targets because women knew the results could be downloaded from the glucometer. These findings were similar across participants regardless of their glycaemic treatment targets (Table 2).
Table 2.
Participants views and experiences of capillary blood glucose monitoring.
| Women with less tight glycaemic treatment targets n = 20 (% of 20) |
Women with tighter glycaemic treatment targets n = 40 (% of 40) |
Women total n = 60 (%) |
|
|---|---|---|---|
| Knew their glycaemic treatment targets | 19 (98.3) | 40 (100) | 59 (98.3) |
| Viewed achieving glycaemic treatment targets as very important or important | 20 (100) | 39 (97.5) | 59 (98.3) |
| Viewed documenting blood glucose results as very important or important | 18 (90) | 37 (92.5) | 56 (93.3) |
| Experienced difficult fasting glycaemic treatment target (before breakfast) | 12 (60) | 25 (62.5) | 37 (61.6) |
| Experienced difficult postprandial glycaemic treatment target (after dinner) | 3 (15) | 8 (20) | 11 (18.3) |
Almost two-thirds of women (37, 62%) described achieving their morning fasting glycaemic treatment target as most difficult. These findings were similar across participants, regardless of their recommended glycaemic targets (12 (60%) for less tight targets and 25 (62.5%) for tighter targets) (Table 2). The next most frequent difficulty reported for women to achieve their recommended glycaemic targets was after their evening meal (11, 18%). Again, these findings were similar across participants regardless of their glycaemic treatment targets (Table 2). Almost two-thirds of women (37, 62%) experienced being always or frequently hungry (Table 3).
Table 3.
Enablers identified by women with GDM1.
| Enablers | Women with less tight glycaemic treatment targets n = 20 (% of 20) |
Women with tighter glycaemic treatment targets n = 40 (% of 40) |
Women total N = 60 (%) |
|---|---|---|---|
| Health professional demonstrating on themselves CBGT2 | 20 (100) | 40 (100) | 60 (100) |
| Watching participants perform CBGT2 | 20 (100) | 40 (100) | 60 (100) |
| Group teaching | 11 (55) | 33 (82.5) | 443 (78.5) |
| One to one teaching | 6 (30) | 6 (15) | 123 (21.4) |
| Health professionals listening and explaining | 6 (30) | 35 (87.5) | 41 (68.3) |
| Being ask about their CBGC4 and reminded to do them | 7 (35) | 26 (65) | 33 (55) |
| Others cooking incorporating GDM diet | 11 (55) | 17 (42.5) | 28 (46.6) |
| Using Google | 9 (45) | 13 (32.5) | 22 (36.6) |
| Going for walks/exercising together | 6 (30) | 9 (45) | 15 (25) |
| Less costs | 3 (15) | 5 (12.5) | 8 (13.3) |
1Multiple answers were possible for this part of the survey; 2capillary blood glucose testing; 3results from 56 women; 4capillary blood glucose concentrations.
3.3. Enablers to Achieving Optimal Blood Glucose Control
Participants were asked to identify what helped them when learning to test their capillary blood glucose levels. All 60 (100%) women indicated that having a health professional to demonstrate the collection of capillary blood glucose on themselves and then watch the participant perform it was helpful (Table 3). Fifty-six (93%) women opted to comment further about other factors that they felt were helpful for learning self-monitoring of blood glucose. These related mainly to group or individual teaching. Forty-four (79%) of the women who commented further stated that they found group sessions helpful with some women explaining that they enjoyed talking to other women and recognising that they are not alone living with GDM. A smaller proportion of women (12, 21%) received additional one-to-one teaching sessions and enjoyed them as these enabled them to ask “stupid” questions, they could ask the teacher to slow down when English was their second language, or they felt less as though it was “mass produced” and liked to be treated more as an individual. Over a third of women (22, 37%) identified Google as a helpful tool. It is unclear which websites they visited and in which language.
Support from family, friends, and work colleagues was seen as enabling for achieving glycaemic control. Over half of the women (33, 55%) indicated that they found it helpful to be asked about their capillary blood glucose levels and to be reminded to do them by their partners, children, extended family members, and work colleagues. Having their meals cooked by either their partners or extended family members, who incorporated the GDM diet recommendations, was found to be helpful for nearly half of the women (28, 47%) (Table 4). Comments indicated that this enabled women to eat more vegetables and stopped them from buying confectionary or sugar-sweetened beverages (fizzy drinks). Further comments around supportive provision of food by others included colleagues organising healthy morning teas at work and friends providing healthy food choices for baby showers. While nearly two-thirds of women (37, 62%) indicated that the cost associated with the GDM diagnosis, such as food, petrol, or child care, stayed the same, some women (8, 13%) reported reduced food costs since being diagnosed with GDM as an enabler due to buying fewer take-away meals (fast foods) (Table 3).
Table 4.
Barriers identified by women with GDM.
| Barriers | Women with less tight glycaemic treatment targets n = 20 (% of 20) |
Women with tighter glycaemic treatment targets n = 40 (% of 40) |
Women total n = 60 (%) |
|---|---|---|---|
| Health information available only in English | 8 (40) | 25 (62.5) | 33 (55) |
| Health information in words not visual | 5 (25) | 11 (27.5) | 16 (26.6) |
| Being offered unhealthy food by family, friends, and work colleagues | 5 (25) | 14 (35) | 23 (38.3) |
| Impatient, not being believed, and being judged by health professionals | 7 (35) | 6 (15) | 13 (21.6) |
| Inconsistent information by health professionals | 4 (20) | 6 (15) | 10 (16.6) |
| Never seeing the same health professional twice | 3 (15) | 5 (12.5) | 8 (13.3) |
| Long waiting hours at clinic | 4 (20) | 3 (7.5) | 7 (11.6) |
| Being hungry | 14 (70) | 23 (57.5) | 37 (61.6) |
| Increased costs | 7 (35) | 8 (20) | 15 (25) |
All women attended Diabetes in Pregnancy Services where they saw a range of health professionals. Most women (47, 78%) attended the clinic fortnightly (Table 4). Support from health professionals was valued. Over two-thirds of the women (41, 68%) appreciated that health professionals took time to listen and explain (Table 3). One (1.7%) woman could email the endocrinologist for advice and appreciated their prompt response.
3.4. Barriers to Achieving Optimal Blood Glucose Control
All women received written information about GDM, which explained the importance of healthy eating and its effect on blood glucose levels and how to self-monitor capillary blood glucose levels. Barriers to this written information included feeling overwhelmed with the amount of written material and not being able to read it in their first language. Women requested to receive visual information (16, 27%) rather than words for food choices, food label reading, how to perform the finger pricks for capillary blood glucose collection, and how to give subcutaneous insulin injections (Table 4). Over half of the women (33, 55%) found it difficult that the written information was in English and wanted the health information in their first language for themselves and for their families to better understand what GDM is and what optimal blood glucose control meant (Table 4). Hindi was the language most frequently requested (9, 27% women), followed by Samoan (6, 18% women) and then Chinese and Māori each by 5 (15%) women. This reflects the ethnic diversity of this cohort of women (Table 1).
Over a third of women (23, 38%) reported being offered unhealthy food by family, friends, and work colleagues and their lack of understanding as barriers to achieving optimal glycaemic control.
When engaging with the Diabetes in Pregnancy Services women, just over a fifth of women (13, 22%) reported a judgemental attitude by health professionals, being impatient with them, and being not believed that they had tried their hardest to stay within their recommended glycaemic treatment targets as a barrier. Inconsistent information by health professionals (10, 17%), never seeing the same health professional twice (8, 13%), and long waiting hours at the clinic (7, 12%) were also experienced as difficult (Table 4).
An increased cost for buying more vegetables, fresh fruits, and wholemeal bread was reported as a barrier by a quarter of women (15, 25%) (Table 4).
4. Discussion
In this survey, women with GDM identified enablers and barriers to achieving optimal glycaemic control. While achieving optimal glycaemic control was viewed as important, most women found it difficult to achieve their morning fasting glycaemic treatment targets, experienced hunger, and wanted the health information in their first language or visually displayed. For most women, food costs were not reported as a concern for the family budget. Being taught blood glucose testing in a group setting was considered helpful. Support from health professionals and family, friends, and work colleagues was valued. Barriers reported include long clinic waiting hours; inconsistent advice, judgemental attitudes, impatience, and not being believed by health professionals; and unhealthy food being offered by family members, friends, and work colleagues.
Health care providers recognise that teaching moments can be maximised by incorporating specific adult-learning principles and learning styles into their teaching strategies and providing written information that supports these learning styles [29]. The survey results showed that participants wished to be provided with better visual information and to have written information in their own language. Most women enjoyed group teaching sessions, although some preferred one-to-one sessions.
We found no published studies reporting on the effects of providing visual learning aids for women with gestational diabetes or the impact of having the information in their first language. One mixed-method study [30] among young people with type 1 diabetes (T1DM) in Norway found that a pictorial diary as a mobile phone app covering the topics diet, insulin dosage, physical activity, and pre- and postprandial glucose measurements all led to a change in the participants' applied knowledge about the management of their diabetes. This is an area requiring further research. For information to make sense and motivate behaviour change, it needs to be provided in a language best understood by the women with GDM [23–25, 31]. Women identified Google as a helpful tool. Health professionals need to be aware that women will access information beyond the clinic environment and the quality of this information may vary. Diabetes in Pregnancy Services should consider how they provide health information and the content of their teaching sessions. Health literacy providing clear and relevant health messages has been identified as an effective way to help people manage their own health care [31–34]. It would be challenging for Diabetes in Pregnancy Services to provide the information for women with GDM in all the languages identified through this survey. The solution may be to provide increased visual information which requires little language and/or translate the written information for the languages identified. The use of trained translators has been encouraged, as family members are often unfamiliar with the health care medical terms, may find it difficult talking about sensitive matters, and may have different degrees of English fluency [35].
Achieving adequate fasting blood glucose control prior to breakfast, also known as the dawn phenomenon [36], was identified as a challenge for most women in this survey, regardless of whether their recommended glycaemic targets were identified as less tight or tighter. In the literature, this has been identified previously for people with T1DM and T2DM [37] but we could not find any publications specifically relating to gestational diabetes. Anecdotal evidence through social media indicates that women with GDM do find this control difficult [38]. Various recommendations for achieving glycaemic control include subcutaneous insulin, walking after dinner, restricting carbohydrate intake at dinner time, late protein snack before bed time, and staying hydrated [39] but require further research for women with GDM. Two-thirds of women commented on being hungry. It is unclear from the survey if this relates to women trying to lower their morning fasting blood glucose with eating less at dinner time or eating very low carbohydrate meals. This would benefit from further exploration.
Some women identified barriers regarding health professional's attitude to not achieving adequate glycaemic control. These included judgemental attitude, not being believed when women stated that they were trying their hardest to follow all diet and pharmaceutical recommendations, and seeing a different health professional at each visit receiving inconsistent information. Findings from other qualitative studies reiterate these findings [31, 40, 41] and highlight the importance for health professional to have a woman-centred approach, not only focusing on blood glucose concentrations but investing time to listen, believing what the women say is true, and providing consistent information and continuity of care [41].
Support from family, friends, and work colleagues were appreciated by the women surveyed. These results are consistent with those of other studies [42, 43]. Barrier identification included unhealthy food being offered to them by family members, friends, and work colleagues, indicating a lack of understanding. Diabetes in Pregnancy Services may consider providing opportunity for family and friends to attend information sessions about GDM and its implication or include discussions about effective strategies for difficult situations at clinic appointments.
This study had some limitations. The participants were from two selected areas in New Zealand, and while they were a cross-sectional representation of the demographics of the New Zealand population, this did not include women living in rural or remote areas. The findings may not be generalised as different district health boards provide care for women with GDM through different models of care.
5. Conclusions
This survey identified barriers and enablers for women with GDM in achieving optimal glycaemic control from two different geographical locations in New Zealand. The results provide insights into women's views and experiences with GDM in achieving glycaemic control targets. Two-thirds of women found it difficult to achieve adequate fasting capillary blood glucose control, regardless of their recommended glycaemic targets, and identified the need for better strategies and adequate health professional and family support to manage this difficulty. Barriers for health information and literacy identified that health professionals need to consider using a women-centred and adult-learning style approach, provide visual aids, provide written information in relevant languages, and include extended family members when imparting knowledge on or teaching GDM-related skills. Long clinic waiting hours, inconsistent advice, judgemental attitudes, and not being believed by health professionals require further consideration when providing a health care service for women with GDM. Findings from this survey will be useful for developing strategies for Diabetes in Pregnancy Services to support women with GDM in achieving their glycaemic control.
Acknowledgments
The authors would like to express their sincere thanks to all the women who participated. A special thank you to Jane Yates, research assistant, for the assistance with compiling the demographic information and Samuel Dobson for the Pivot Table assistance. Ruth Martis is a recipient of the University of Auckland senior health research scholarship. The TARGET trial is supported through funding from Health Research Council of New Zealand Grant 14/499. The Liggins Institute provided essential materials to enable the study to be conducted.
Conflicts of Interest
None of the authors have any financial conflict of interests associated with this publication. Caroline A. Crowther and Julie Brown are the lead investigators for the TARGET trial in which this study is nested.
Authors' Contributions
Ruth Martis contributed to the conception and the design of the survey, recruited the women, conducted the survey, entered the data, performed the data analysis, and drafted the article and the revision of the article from coauthors' feedback. Julie Brown and Caroline A. Crowther contributed to the conception and design of the survey, provided advice on the data analysis and data interpretation, commented on all drafts of the manuscript, and approved the final version.
References
- 1.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health. Diabetes in Pregnancy: Quick Reference Guide for Health Professionals on the Screening, Diagnosis and Treatment of Gestational Diabetes in New Zealand. Wellington, New Zealand: Ministry of Health; 2014. [Google Scholar]
- 3.Guariguata L., Linnenkamp U., Beagley J., Whiting D. R., Cho N. H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Research and Clinical Practice. 2014;103(2):176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Kampmann U., Ring Madsen L., Oeskov Skajaa G., Smed Iversen D., Moeller N., Ovesen P. Gestational diabetes: a clinical update. World Journal of Diabetes. 2015;6(8):1065–1072. doi: 10.4239/wjd.v6.i8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Global Report on Diabetes. WK 810. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 6.NICE, National Institute for Health and Clinical Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. NICE Guideline NG3. London, UK: National Institute for Health and Care Excellence; 2015. [PubMed] [Google Scholar]
- 7.HAPO Study Cooperative Research Group, Metzger B. E., Lowe L. P., et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 8.Nicklas J. M., Miller L. J., Zera C. A., Davis R. B., Levkoff S. E., Seely E. W. Factors associated with depressive symptoms in the early postpartum period among women with recent gestational diabetes mellitus. Maternal and Child Health Journal. 2013;17(9):1665–1672. doi: 10.1007/s10995-012-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas A., Morley R., Cole T. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;29(6659):1304–1308. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ornoy A., Reece E. A., Pavlinkova G., Kappen C., Miller R. K. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Research Part C: Embryo Today: Reviews. 2015;105(1):53–72. doi: 10.1002/bdrc.21090. [DOI] [PubMed] [Google Scholar]
- 11.Mitanchez D., Yzydorczyk C., Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World Journal of Diabetes. 2015;6(5):734–743. doi: 10.4239/wjd.v6.i5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poolsup N., Suksomboon N., Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2014;9(3, article e92485) doi: 10.1371/journal.pone.0092485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landon M. B., Spong C. Y., Thom E., et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine. 2009;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowther C. A., Hiller J. E., Moss J. R., McPhee A. J., Jeffries W. S., Robinson J. S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 15.Brown J., Alwan N. A., West J., et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews. 2017;5, article CD011970 doi: 10.1002/14651858.CD011970.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachum Z., Zafran N., Salim R., et al. Glyburide versus metformin and their combination for the treatment of gestational diabetes mellitus: a randomized controlled study. Diabetes Care. 2017;40(2):332–337. doi: 10.2337/dc16-2307. [DOI] [PubMed] [Google Scholar]
- 17.Kalra B., Gupta Y., Singla R., Klara S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. North American Journal of Medical Sciences. 2015;7(1):6–12. doi: 10.4103/1947-2714.150081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu R. J., Hays K. E., Hebert M. F. Gestational diabetes mellitus management with oral hypoglycemic agents. Seminars in Perinatology. 2014;38(8):508–515. doi: 10.1053/j.semperi.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavitha N., De S., Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. The Journal of Obstetrics and Gynecology of India. 2013;63(2):82–87. doi: 10.1007/s13224-012-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons J., Ismail K., Amiel S., Forbes A. Perceptions among women with gestational diabetes. Qualitative Health Research. 2014;24(4):575–585. doi: 10.1177/1049732314524636. [DOI] [PubMed] [Google Scholar]
- 21.Morrison M. K., Lowe J. M., Collins C. E. Australian women’s experiences of living with gestational diabetes. Women and Birth. 2014;27(1):52–57. doi: 10.1016/j.wombi.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Trutnovsky G., Panzitt T., Magnet E., Stern C., Lang U., Dorfer M. Gestational diabetes: women’s concerns, mood state, quality of life and treatment satisfaction. The Journal of Maternal-Fetal & Neonatal Medicine. 2012;25(11):2464–2466. doi: 10.3109/14767058.2012.683900. [DOI] [PubMed] [Google Scholar]
- 23.Hirst J. E., Thach S. T., My An T. D., Forsyth R., Morris J. M., Jeffery H. E. Women with gestational diabetes in Vietnam: a qualitative study to determine attitudes and health behaviours. BMC Pregnancy and Childbirth. 2012;12(1):81–90. doi: 10.1186/1471-2393-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapolla A., Di Cianni G., Di Benedetto A., et al. Quality of life, wishes, and needs in women with gestational diabetes: Italian DAWN pregnancy study. International Journal of Endocrinology. 2012;2012:6. doi: 10.1155/2012/784726.784726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandyopadhyay M., Small R., Davey M., Oats J. J. N., Forster D. A., Aylward A. Lived experience of gestational diabetes mellitus among immigrant South Asian women in Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51:360–364. doi: 10.1111/j.1479-828X.2011.01322.x. [DOI] [PubMed] [Google Scholar]
- 26. http://www.health.govt.nz/new-zealand-health-system/my-dhb/counties-manukau-dhb/population-counties-manukau-dhb. July 2017.
- 27. http://www.health.govt.nz/new-zealand-health-system/my-dhb/canterbury-dhb/population-canterbury-dhb. July 2017.
- 28. http://www.stats.govt.nz/browse_for_stats/population/census_counts/2013CensusUsuallyResidentPopulationCounts_HOTP2013Census.aspx. July 2017.
- 29.Russell S. S. An overview of adult-learning processes. Urologic Nursing. 2006;26(5):349–352, 370. [PubMed] [Google Scholar]
- 30.Frøisland D. H., Årsand E., Skårderud F. Improving diabetes care for young people with type 1 diabetes through visual learning on mobile phones: mixed-methods study. Journal of Medical Internet Research. 2012;14(4):113–125. doi: 10.2196/jmir.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devsam B. U., Bogossian F. E., Peacock A. S. An interpretive review of women’s experiences of gestational diabetes mellitus: proposing a framework to enhance midwifery assessment. Women and Birth. 2013;26(2):e69–e76. doi: 10.1016/j.wombi.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Ministry of Health. A Framework for Health Literacy. Wellington, New Zealand: Ministry of Health; 2015. [Google Scholar]
- 33.Work base Education Trust. Māori Health Literacy Research: Gestational Diabetes Mellitus. Wellington, New Zealand: Ministry of Health; 2014. [Google Scholar]
- 34.Bhavadharini B., Deepa M., Nallaperumal S., Anjana R. M., Mohan V. Knowledge about gestational diabetes mellitus amongst pregnant women in South Tamil Nadu. Journal of Diabetology. 2017;8:22–26. doi: 10.4103/jod.jod_2_17. [DOI] [Google Scholar]
- 35.Gray B. The use of interpreters, chapter 8. In: St. George I. M., editor. Cole’s Medical Practice in New Zealand. 12th. Wellington, New Zealand: Medical Council of New Zealand; 2013. [Google Scholar]
- 36.Carroll M. F., Schade D. S. The dawn phenomenon revisited: implications for diabetes therapy. Endocrine Practice. 2005;11(1):55–64. doi: 10.4158/EP.11.1.55. [DOI] [PubMed] [Google Scholar]
- 37.Porcellati F., Lucidi P., Bolli G. B., Fanelli C. G. Thirty years of research on the Dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care. 2013;36(12):3860–3862. doi: 10.2337/dc13-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. http://www.gestationaldiabetes.co.uk/high-fasting-levels/
- 39.Sheehan J. P. Fasting hyperglycemia: etiology, diagnosis, and treatment. Diabetes Technology & Therapeutics. 2004;6(4):525–533. doi: 10.1089/1520915041705910. [DOI] [PubMed] [Google Scholar]
- 40.Fahy K. What is woman-centred care and why does it matter? Women and Birth. 2012;25:149–151. doi: 10.1016/j.wombi.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Janes R., Titchener J., Pere J., Pere R. Understanding barriers to glycaemic control from the patient’s perspective. Journal of Primary Health Care. 2013;5(2):114–122. [PubMed] [Google Scholar]
- 42.Miller T. A., DiMatteo M. R. Importance of family/social support and impact on adherence to diabetic therapy. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2013;6:421–426. doi: 10.2147/DMSO.S36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayberry L. S., Osborn C. Y. Family support, medication adherence and glycemic control among adults with type 2 diabetes. Diabetes Care. 2012;35:1239–1245. doi: 10.2337/dc11-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization (WHO) Obesity: Preventing and Managing the Global Epidemic, Report of a WHO Consultation. Geneva, Switzerland: WHO Technical Report Series 894; 2000. [PubMed] [Google Scholar]
- 45.Ministry of Health. Understanding Excess Body Weight: New Zealand Health Survey. Wellington, New Zealand: Ministry of Health; 2015. [Google Scholar]