Abstract
BACKGROUND
Intestinal alkaline phosphatase (IAP) plays a pivotal role in maintaining gut health and well-being. Oral supplementation with IAP in mice improves gut barrier function and prevents luminal proinflammatory factors from gaining access to the circulation. In this study, we sought to explore the relationship between IAP and tight junction protein (TJP) expression and function.
STUDY DESIGN
The effect of IAP deletion on TJP levels was studied in mouse embryonic fibroblasts (MEFs) generated from IAP-knockout and wild type mice. Regulation of TJPs by IAP was assayed in the human colon cancer Caco-2 and T84 cells by overexpressing the human IAP gene. Tight junction protein levels and localization were measured by using RT q-PCR and antibodies targeting the specific TJPs. Finally, the effect of IAP on inflammation-induced intestinal permeability was measured by in vitro trans-well epithelial electrical resistance (TEER).
RESULTS
Intestinal alkaline phosphatase gene deletion in MEFs resulted in significantly lower levels of ZO-1, ZO-2, and Occludin compared with levels in wild-type control cells; IAP over-expression in Caco-2 and T84 cells resulted in approximate 2-fold increases in the mRNA levels of ZO-1 and ZO-2. The IAP treatment ameliorated lipopolysaccharide-induced increased permeability in the Caco-2 trans-well system. Furthermore, IAP treatment preserved the localization of the ZO-1 and Occludin proteins during inflammation and was also associated with improved epithelial barrier function.
CONCLUSIONS
Intestinal alkaline phosphatase is a major regulator of gut mucosal permeability and appears to work at least partly through improving TJP levels and localization. These data provide a strong foundation to develop IAP as a novel therapy to maintain gut barrier function.
Gut barrier function plays a pivotal role in human health and disease. “Leaky gut,” or the disruption of intestinal integrity, results in the permeation of luminal mediators into the circulation, leading to harmful immune responses and inflammation in various organs.1 An impaired gut mucosal barrier has been implicated to play a causal role in intestinal disease such as Crohn’s and ulcerative colitis, as well as in a variety of other diseases ranging from metabolic syndrome to osteoarthritis, Alzheimer’s, and aging.2–7 In addition, improving gut barrier function has been shown to be beneficial in the recovery of patients suffering from severe trauma, burns, and other conditions associated with critical illness.8 Intestinal permeability to macromolecules is controlled by paracellular tight junction (TJ) formation. Tight junctions are composed of transmembrane and cytosolic proteins, such as claudins, Occludins, and zona occludens.9 Downregulation of tight junction protein (TJP) levels has been implicated in many diseases.1 Furthermore, paracellular permeability is dynamically regulated by altering the TJP localization and TJ integrity.10 Activation of inflammatory pathways such as NF-κB and the resultant increase in cytokine production results in disrupted TJP levels and localization, in turn increasing the passage of intestinal contents to the systemic circulation.11,12 Guo and colleagues13 showed the adverse effects of the gram-negative bacterial proinflammatory factor lipopolysaccharide (LPS), a potent activator of the NF-κB pathway, on the formation of TJs and gut barrier function.
The brush border enzyme intestinal alkaline phosphatase (IAP) is expressed and secreted exclusively in the small-intestinal epithelium.14 The IAP functions as an anti-inflammatory factor, detoxifying a variety of proinflammatory mediators that exist within the gut lumen, including adenosine triphosphate and the toll-like receptor (TLR) ligands: LPS, flagellin, and CpG DNA.15,16 We previously showed that IAP knockout mice have an impaired ability to detoxify luminal LPS and appear to be more susceptible to gut-derived inflammatory conditions.17,18 Interestingly, IAP is down-regulated in settings in which gut barrier dysfunction plays a critical role in the development of diseases such as colitis.19 Furthermore, IAP treatment has been shown to be beneficial in colitis in both humans and mice.17,20 More recently, we showed that IAP levels are decreased in critically ill patients and that IAP supplementation improved the gut barrier function in a relevant mouse model.21 Given the beneficial effects of IAP in regard to intestinal permeability, we speculated that this enzyme might represent a key regulator of TJP levels and TJ formation.
In this study, we show that the IAP deletion lowers intestinal junction protein levels in vitro, similar to our previous findings shown in mice. We demonstrate that IAP upregulates the expression of TJPs in various human colon cell lines. Finally, we show that IAP supplementation improves the barrier function in a Caco-2 trans-well system, likely by preserving TJ formation and integrity.
METHODS
Reagents
Intestinal alkaline phosphatase, LPS (E coli serotype 055:B5), and Ripa buffer were purchased from Sigma-Aldrich, and TRIzol was purchased from Invitrogen. The iScriptReverse Transcription Supermix for RT-qPCR and iQ SYBR Green Supermix Kit were obtained from BIO-RAD, and the Coomassie (Bradford) Blue Protein Assay Kit was from Fisher Scientific. Goat anti-human ZO-1 and rabbit anti-human Occludin were purchased from Santa Cruz Biotechnology, and Alexa Flour secondary antibodies were obtained from Lifetechnology.
Cell culture
Primary mouse embryonic fibroblasts (MEFs) were isolated from day 13.5 wild-type C57BL/6 or Akp3 knockout embryos, passaged at a density of 1 × 106 cells per 10-cm plate every 3 days (reference). The MEFs were maintained in Dulbecco’s modified minimum essential medium (DMEM) supplemented with 55 μM β-mercaptoethanol. Human colon cancer Caco-2 and T84 cells were purchased from American Type Culture Collection and maintained in DMEM or in a 1:1 mixture of Ham’s F12 medium and Dulbecco’s modified Eagle’s medium culture media, respectively. All culture media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, and 1% antibiotic-antimycotic solution obtained from GIBCO.
Plasmid constructs and transfection
Complementary DNA synthesized from RNA extracts from the human colon cancer Caco-2 cell line was used to amplify the human IAP gene by PCR using Q5 High-Fidelity DNA Polymerase (New England Biolabs). The following primers were used: forward, 5′-TTAAGCTTATGCAGGGGCCCTGGGTGCTGCTG-3′; reverse, 5′-TTGGTACCGGGAGCAGCGGACGCCCCCAGCA -3′. The amplified product was digested with HindIII and KpnI, and then inserted into HindIII/KpnI-digested-pDsRed1-N1 vector (Clontech) upstream of the red fluorescent gene and named pDsRed1-IAP. The insert was confirmed using sequencing. The empty pDsRed1-N1 vector was used as a control. Plasmids were transfected into Caco-2 and T84 cells on a 12-well plate using 1 μg/well of pDsRed1-IAP or pDsRed1-Control using Lipofectamine LTX (Invitrogen). Stable transfectant clones expressing pDsRed1-IAP or pDsRed1-Control were selected with 1 mg/mL G418 (InvivoGen). Transfection efficiency was evaluated by real-time polymerase chain reaction (RT-PCR) analysis using the following primers: forward, 5′-GTTCCTGGTGTCCCCACTTC-3′; reverse, 5′GGCACCCCCAACCCATC-3′.
Quantitative real-time polymerase chain reaction
Total RNA from cells was isolated using TRIzol. The iScriptReverse Transcription Supermix for RT-qPCR was used for the generation of cDNA for all samples. Quantitative real-time PCR (qRT-PCR) was performed with a Mastercyclerrealplex instrument (Eppendorf) using the iQ SYBR Green Supermix Kit. Primer sequences are available on request. For each sample, real-time PCR reactions were performed in triplicate and the average threshold cycle (Ct) was calculated. Expression of target-gene mRNA was normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression in MEFs and with ribosomal protein L11 (RPLP11) in Caco-2 and T84 cell lines. Expression levels were calculated using the ΔΔCt method after correcting for differences in PCR efficiencies and were expressed relative to control levels. The average copy number of mRNA expression in control samples was set to 1.0.
Trans-well assay system set-up and intestinal alkaline phosphatase treatment
Trans-well experiments were performed as previously described.22 Caco-2 cells were seeded onto collagen-coated polyethylene terephthalate (PET) filter supports (1 μm pore size) from Corning. Briefly, Caco-2 cells were seeded onto the filter supports at a concentration of 3 × 105 cells/insert and were incubated in cell culture incubator (37°C, 5% CO2). Cells were fed from both sides using DMEM growth media supplemented with 10% heat-inactivated fetal bovine serum. After incubation for up to 3 weeks, Caco-2 cells gradually developed a tight junction monolayer. The integrity of the Caco-2 monolayer was determined by TEER measurements. Permeability experiments were carried out when TEER peak was reached and maintained for 72 hours. To study the effect of IAP on inflammation-induced permeability, LPS 100 ng/mL ± IAP 500 U/mL or IAP vehicle (as a control for IAP) was added to both compartments of the trans-well. Human tumor necrosis factor (TNF)-α 10 ng/mL was used as a positive control. The control group was supplemented with equal amounts of phosphate buffered saline. The growth media was replaced daily with new treatments.
Transepithelial electrical resistance measurements
The integrity of the Caco-2 monolayer was determined by measuring the TEER of the cell monolayer grown on filter supports using Millicell-ERS electrical resistance measuring system (Millipore) using chopstick electrodes. Briefly, the Caco-2 inserts were transferred to a 6-well culture plate with 2 mL medium in the feeding well, and 1 mL in culture inserts. The electrodes were immersed in a way that the shorter electrode was in the insert and the longer electrode was in the outer well. Care was taken to ensure that the electrode did not touch the monolayer. A resistance reading of 200 Ω.cm2 indicated a confluent Caco-2 monolayer with tight junctions, as previously described. The TEER recording was carried out daily for 5 days after the addition of treatments, and TEER values are shown as change ratio to baseline.
Western blot
Lysates from Caco-2 cells were prepared for Western blot analysis, as described elsewhere.23 Blots were probed with antibodies against ZO-1 (Cell Signaling Technology). After incubation with appropriate horseradish-peroxidase conjugated secondary antibodies, membranes were developed using Pierce ECL Plus Substrate (Thermo Scientific).
Immunofluorescence
After 5 days of treatment to Caco-2 trans-wells, the cells were fixed in 4% paraformaldehyde in phosphate buffered saline, then permeabilized in 0.1% Triton X-100 during all incubations. Nonspecific antigens were blocked for 30 minutes in 3% bovine serum albumin. Double labeling was performed sequentially to avoid cross reactions. Anti-ZO-1 and anti-OCLDN primary antibodies diluted in the blocking solution were incubated overnight at 4°C, followed by a 1-hour-30-minute incubation with Alexa Flour secondary antibodies.
Statistical analysis
Statistical significance between 2 groups was tested using the 2-tailed Student’s t-test. Statistical significance between more than 2 groups was tested using 1-way analysis of variance with Tukey’s multiple-comparison post-hoc tests. A value of p < 0.05 was considered statistically significant.
RESULTS
Mouse embryonic fibroblasts from intestinal alkaline phosphatase-knockout mice have lower tight junction protein levels
Down-regulation of TJP levels in the gut results in increased permeation of the harmful intestinal contents into the blood stream. We showed previously that IAP-knockout mice are prone to gut barrier dysfunction, endotoxemia, and sepsis.21,24,25 Furthermore, we previously demonstrated that IAP-knockout mice have lower TJP levels in vivo.21 In order to study the effect of endogenous IAP on TJP levels, we compared the TJP expression levels in MEFs from IAP-knockout and wild type mice in vitro. We found that the absence of IAP resulted in significant decreases in the expression of the junctional proteins, ZO-1, ZO-2, and Occludin (Fig. 1). These results suggest that the endogenous IAP plays an important role in the regulation of junctional protein expression.
Figure 1.
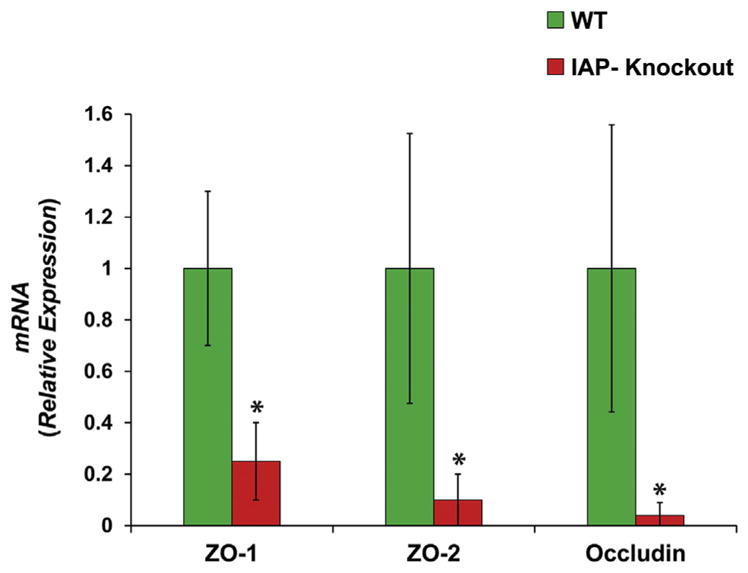
Mouse embryonic fibroblasts (MEFs) from intestinal alkaline phosphatase (IAP)-knockout mice have less tight junction protein levels. The MEFs from wild-type (WT) and IAP-knockout (KO) mice were seeded at a density of 3 × 105 cells per well in 6-well plate (n = 3). At 80% confluence, RNA was isolated using Trizole, and quantitative real-time polymerase chain reaction (qRT-PCR) was performed to determine the levels of tight junction protein expression. Tight junction protein levels in MEFs from IAP-KO mice were compared with those of WT. Values are expressed as mean ± SEM. Statistical significance between the 2 groups was tested using the 2-tailed Student’s t-test. *p < 0.05.
Intestinal alkaline phosphatase abundance up-regulates tight junction protein expression in Caco-2 and T84 cells in vitro
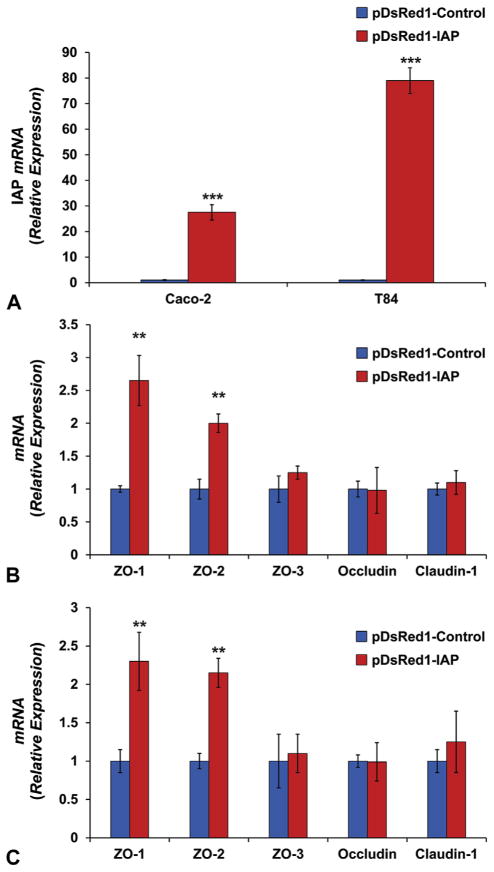
To better understand the effect of the IAP enzyme on TJP levels, we assessed the expression level of TJPs in Caco-2 and T84 cell lines that overexpress the human IAP gene. The results were compared with cells expressing an empty vector as a control. Elevated levels of junctional protein ZO-1 is known to improve the gut barrier function.26 Furthermore, abundance of the ZO-1 protein plays a central role in the TJPs assembly and localization.27 Transfecting human colon cell lines with red fluorescent protein vector with the human IAP gene as an insert resulted in 28- and 79-fold increases in IAP mRNA levels in Caco-2 and T84, respectively (Fig. 2A), indicating that IAP markedly induces ZO-1 and ZO-2 expression in both cell lines (Fig. 2B,C). These effects on ZO-1 and ZO-2 were quite specific in that IAP did not affect the expression of ZO-3, Occludin, or Claudin-1 in the Caco-2 and T84 cells.
Figure 2.
Intestinal alkaline phosphatase (IAP) abundance increases tight junction protein (TJP) levels in the human colon cancer Caco-2 and T84 cell lines. Stably transfected cells with the human IAP gene or with the empty vector were used to study the effect of IAP overexpression on TJP levels. Cells were seeded in 6-well plates in triplicate. After formation of monolayer, changes in mRNA levels were assayed using quantitative real-time polymerase chain reaction (qRT-PCR). Results from IAP overexpressing cells were compared with those from cells transfected with the empty vector: (A) Human IAP relative expression and junctional protein mRNA expression in (B) Caco-2 and (C) T84 cell lines. Values are expressed as mean ± SEM. Statistical significance between the 2 groups was tested using the 2-tailed Student’s t-test. **p < 0.01, ***p < 0.001.
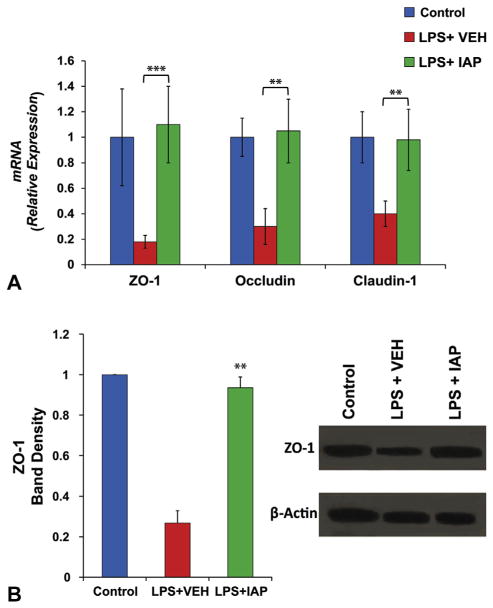
Intestinal alkaline phosphatase supplementation prevented lipopolysaccharide-induced down-regulation of tight junction protein levels in Caco-2 cells in vitro
Activation of inflammatory pathways has been shown to alter the abundance of TJPs and disrupt gut barrier function.10 Furthermore, we showed the beneficial effect of exogenous IAP in preventing gut inflammation and the resulting increased intestinal permeability in mice.21 Therefore, we sought to further elucidate the effect of exogenous IAP supplementation on inflammation-induced down-regulation of TJPs. In this experiment, we measured the mRNA levels of TJPs in Caco-2 cells in vitro after addition of the proinflammatory factor LPS ± IAP. Figure 3A shows that LPS down-regulated the expression levels of ZO-1, Occludin, and Claudin-1 genes and that IAP treatment blocked the LPS effect on TJP expression levels in Caco-2 cells (Fig. 3A). Furthermore, LPS reduced the TJP ZO-1 levels, whereas exogenous IAP preserved the levels of ZO-1 protein (Fig. 3B).
Figure 3.
Exogenous intestinal alkaline phosphatase (IAP) treatment prevents lipopolysaccharide (LPS)-induced decrease in tight junction protein levels. Caco-2 cells were seeded at a density of 3 × 105 cells per well in 6-well plates. After forming the monolayer, around 48 hours, LPS (100 ng/mL) + IAP 500 U or vehicle (VEH) or equal amount of PBS as control were added to the wells in triplicates. Twenty-four hours later, RNA and proteins were isolated for tight junction protein quantification. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to determine the levels of tight junction protein expression. (A) Junctional protein mRNA expression in Caco-2 cells treated with LPS ± IAP. In the same experiment, ZO-1 protein levels were measured by Western blot using antibody against human ZO-1 and normalized the levels of beta-actin in the same samples. (B) Western blot determinations of ZO-1 levels in Caco-2 cells treated with LPS ± IAP. Values are expressed as mean ± SEM. Statistical significance between the groups was tested using 1-way analysis of variance with Tukey’s multiple comparison post-tests. **p < 0.01, ***p < 0.001.
EXOGENOUS INTESTINAL ALKALINE PHOSPHATASE AMELIORATES LIPOPOLYSACCHARIDE-INDUCED BARRIER DYSFUNCTION IN VITRO
Based on the above results, we sought to study the effect of exogenous IAP on the barrier function in vitro by adopting the Caco-2 trans-well system. At a resistance reading of 200 Ω.cm2, LPS 100 ng/mL ± IAP 500 U/mL were added daily to both compartments of the trans-well with fresh growth media in triplicate. The TEER readings were documented daily for 5 days. Figure 4 shows that as expected, LPS caused an increase in permeability, eg, at day 5, LPS addition resulted in a more than 50% reduction in the TEER reading compared with baseline. Furthermore, treatment with exogenous IAP ameliorated the LPS effect on permeability and preserved the barrier function (Fig. 4).
Figure 4.
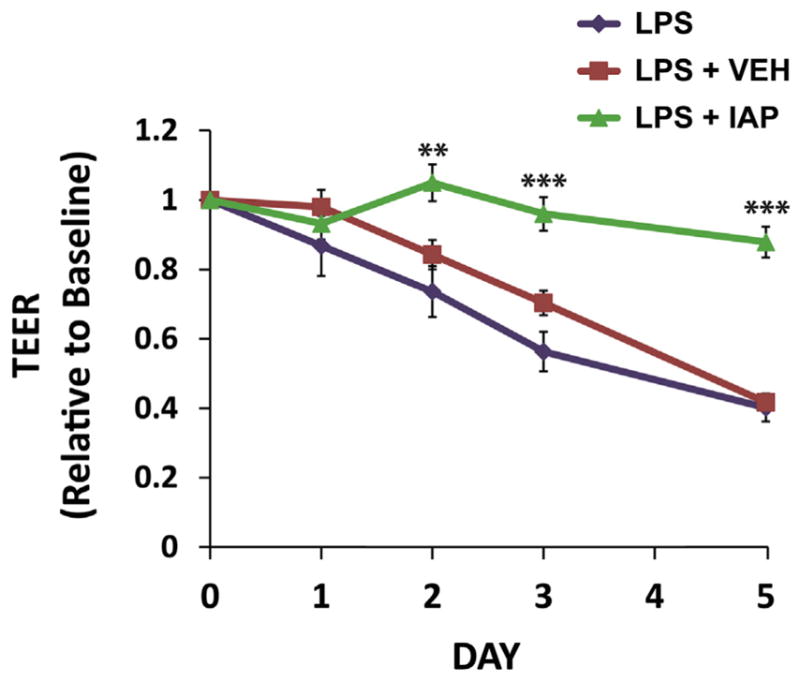
Intestinal alkaline phosphatase (IAP) treatment prevents lipopolysaccharide (LPS)-induced barrier dysfunction in Caco-2 trans-well system. Caco-2 cells were seeded onto the filter supports in the trans-well at a concentration of 3 × 105 cells/insert and were incubated in cell culture incubator (37°C, 5% CO2). At a resistance reading of 200 Ω.cm2 by transepithelial electrical resistance (TEER) in the Caco-2 trans-wells, LPS 100 ng/mL ± IAP 500 U/mL or vehicle (VEH) were added to both compartments of the trans-well with fresh growth media in triplicate daily. Equal amounts of phosphate buffered saline (PBS) were added as a control. The TEER readings were documented daily for 5 days. Values are expressed as mean ± SEM. Statistical significance between the groups was tested using 1-way analysis of variance with Tukey’s multiple comparison post-tests. **p < 0.01, ***p < 0.001.
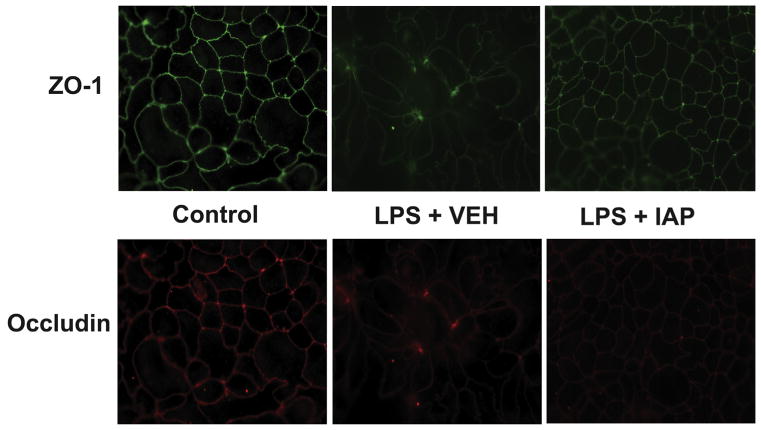
Treatment with intestinal alkaline phosphatase preserved tight junction protein localization after addition of lipopolysaccharide
To further elucidate the mechanism by which the IAP enzyme prevents LPS-induced permeability, we studied the localization of the junctional proteins, ZO-1 and Occludin, after stimulating the Caco-2 cells with LPS ± IAP treatment. It is known that altering ZO-1 or Occludin localization disrupts the formation of tight junctions and gut barrier function.11,28 In this experiment, stimulating monolayer-forming Caco-2 cells with LPS resulted in disruption of ZO-1 and Occludin localization at the cellular junctions compared with the control group (Fig. 5). However, treatment with IAP preserved the localization of ZO-1 and Occludin and the integrity of tight junctions’ formation (Fig. 5).
Figure 5.
Intestinal alkaline phosphatase (IAP) treatment preserve tight junctions’ integrity. Caco-2 cells were seeded onto the filter supports in the trans-well at a concentration of 3 × 105 cells/insert and were incubated in cell culture incubator (37°C, 5% CO2). After forming the monolayer, lipopolysaccharide (LPS) 100 ng/mL ± IAP 500 U/mL or vehicle (VEH) were added to both compartments of the trans-well with fresh growth media in triplicate daily. Equal amounts of phosphate buffered saline (PBS) were added as a control. After 5 days, inserts were removed from wells, and cells were fixed with 4% paraformaldehyde. Localization of ZO-1 and Occludin proteins in the same cells were detected by immunofluorescence using primary antibody against human ZO-1 and Occludin. Secondary antibodies with different fluorescence wavelength were used for the indirect detection of different antigens. (Top) ZO-1 distribution and (bottom) Occludin distribution.
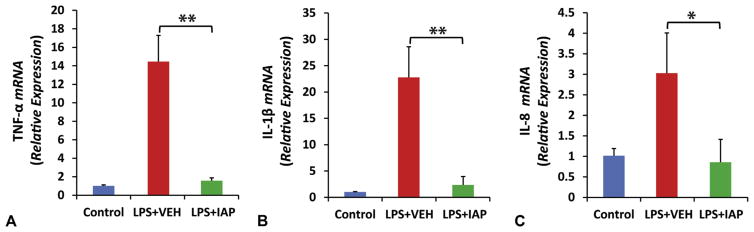
Exogenous intestinal alkaline phosphatase prevents lipopolysaccharide-induced inflammation in Caco-2 cells in vitro
Increased inflammation and cytokine levels play a pivotal role in the pathophysiology of gut barrier dysfunction, more specifically in TJP levels and localization.10 To understand the mechanism by which exogenous IAP affects tight junction integrity, we sought to study the effect of IAP on the inflammatory cytokine levels in Caco-2 cells on LPS stimulation. We measured TNF-α, interleukin (IL)-1β, and IL-8 expression levels in Caco-2 cells after the addition of LPS 100 ng/mL ± IAP 500 U in the growth media in both compartments of the trans-well. Figure 6 shows that stimulating Caco-2 cells with LPS significantly up-regulated the mRNA levels of the proinflammatory cytokines. In contrast, when the LPS was incubated with IAP, activation of inflammatory pathways and the resultant upregulation of cytokines were prevented; TNF-α (14.2 ± 2.8 vs 1.8 ± 0.3 mRNA relative expression; p < 0.001) (Fig. 6A), IL-1β (22.6 ± 5.4 vs 3.0 ± 1.6 mRNA relative expression; p < 0.001) (Fig. 6B), and IL-8 (2.9 ± 0.8 vs 0.8 ± 0.65 mRNA relative expression; p < 0.05) (Fig. 6C).
Figure 6.
Intestinal alkaline phosphatase (IAP) treatment prevents lipopolysaccharide (LPS)-induced cytokine production. Caco-2 cells were seeded onto the filter supports in the trans-well at a concentration of 3 × 105 cells/insert. After forming the monolayer, LPS 100 ng/mL ± IAP 500 U/mL or vehicle (VEH) were added to both compartments of the trans-well with fresh growth media in triplicate daily. After 5 days, RNA was isolated using Trizole and quantitative real-time polymerase chain reaction (qRT-PCR) was performed to determine the levels of inflammatory cytokines: (A) tumor necrosis factor (TNF)-α, (B) interleukin (IL)-1β, and (C) IL-8 expression levels. Values are expressed as mean ± SEM. Statistical significance between the groups was tested using 1-way analysis of variance with Tukey’s multiple comparison post-tests. *p < 0.05, **p < 0.01.
DISCUSSION
The gut mucosa forms a selective barrier that protects the host from intraluminal contents and microbes, while at the same time allowing free movement of nutrients, ions, and water. Intestinal transport of intraluminal contents can occur through 2 major pathways: paracellular diffusion and transcellular transport.9 However, defects of the intestinal barrier and more particularly, the paracellular pathway, can lead to the excessive flux of exogenous harmful macromolecules that play a critical role in the pathogenesis of a spectrum of human diseases, including inflammatory bowel diseases, and could even be related to more systemic diseases such as metabolic syndrome, Alzheimer’s, and aging.2–7 Recently, reinforcing the paracellular pathway has been the target of many therapeutic strategies designed to treat or prevent diseases driven by luminal contents.29 Intestinal paracellular diffusion is regulated by tight junction formation, which is composed of several proteins including Occludin, the claudins, junction adhesion molecule, and the zonula occludens.9 Down-regulation of the junctional protein levels or disruption of their assembly weakens the TJs, in turn, increasing the diffusion of intraluminal antigens and microbes.10
In this study, we focused on the molecular mechanisms regulating gut barrier function, specifically concentrating on the role of the intestinal enzyme IAP in the prevention of the aberrant increase in intestinal permeability to harmful molecules. Intestinal alkaline phosphatase is a brush-border enzyme that is exclusively produced by the enterocytes of the proximal small intestine and is secreted into the intestinal lumen14; its functions include detoxification of LPS and other bacterial products, prevention of gut inflammation, and preservation of the gut microbiotal homeostasis.16,17,30,31 Deletion of IAP in mice results in higher LPS influx to the systemic circulation, and IAP supplementation has been shown to prevent high fat diet-induced intestinal permeability and endotoxemia in mice.18 In humans, IAP levels are known to decrease in clinical settings where gut barrier dysfunction plays a pivotal role, eg, IAP is decreased in patients with inflammatory bowel disease, especially in segments involved with inflammation.19 Pastorelli and colleagues32 showed that the increase in intestinal permeability precedes the development of inflammation in colitis. Interestingly, IAP supplementation has been shown to prevent the development of colitis in both human and mice.17,20 More recently, we showed lower IAP levels in critically ill patients, likely related to the lack of enteral feeding.21 Indeed, administration of parenteral nutrition in ICU patients is associated with increased intestinal permeability and endotoxemia, which can lead to systemic sepsis and multiorgan failure.33 We demonstrated that IAP treatment prevents the increase in intestinal permeability in a mouse starvation model, perhaps through up-regulation of TJP expression.21
This study is the first to use IAP deletion in cells in vitro, and we were able to clearly demonstrate that the absence of IAP results in lower levels of the junctional proteins ZO-1, ZO-2, and Occludin. Similarly, our data indicate that higher IAP levels in human cells are associated with an increased expression of ZO-1 and ZO-2. These findings suggest that the IAP enzyme plays a major role in the expression of TJPs in the cells, similar to our previous data in mice.21 The ZO-1 and ZO-2 are important intracellular TJPs, linking the cell cytoskeleton to the transmembrane TJPs Occludin and claudins, and are required for TJ assembly.10,34 Indeed, ZO-1 depletion in monolayer colon cells is known to destabilize the barrier against large molecules by delaying TJ assembly.35 Furthermore, Occludin knock-down in CaCo-2 cells as well as in ex vivo intestinal murine tissue resulted in an increased permeability for macromolecules.36 These changes in TJP expression could be a direct effect of the IAP enzyme on these gene products, or an indirect effect through the activation or inhibition of 1 or more regulatory pathways. More work is needed to elucidate the exact mechanism by which IAP alters the levels of TJPs.
Although the focus of this study was on the role of IAP on TJP levels in the context of barrier dysfunction, it is thought that TJPs play an important role in other aspects of cellular homeostasis, including proliferation, cell morphology, and cancer development.37 Indeed, we and others have reported on the effects of IAP in cell proliferation and cancer, but further studies will be needed to determine the precise role that IAP plays in these conditions.38,39
Our data are consistent with those in other reports linking inflammation to barrier dysfunction.30 We showed that LPS decreased TEER in Caco-2 monolayer, probably by decreasing the expression of TJPs. Furthermore, our data also demonstrated the effect of LPS on disrupting ZO-1 and Occludin localization to the junctional surface, leading to increased monolayer permeability. In addition, we found that LPS induced higher inflammatory cytokine production including TNF-α, IL-1β, and IL-8 in Caco-2 monolayers. Indeed, recent studies have shown that TNF- α and IL-1β increased intestinal TJ permeability in mice and in monolayer trans-well systems.11,22 In intestinal Caco-2 cells, IL-1β decreases TEER and increases inulin flux, which is, in part, mediated by the decreased expression and redistribution of the transmembrane protein Occludin.22 Although TNF-α was shown to decrease the TEER in the Caco-2 trans-well system, its effect is thought to be mediated by downregulating ZO-1 levels and stability at the junctional surface.11 Given the functions of the IAP enzyme as an intrinsic anti-inflammatory factor and its ability to detoxify LPS, we tested the effect of IAP supplementation on LPS-induced permeability. Our results showed that treatment with IAP prevented LPS-induced barrier dysfunction in vitro, suggesting that IAP ameliorates the effects of LPS on intestinal permeability by preventing the decline in junctional protein expression. Furthermore, IAP preserved the localization and assembly of TJ. Although the IAP effects are likely mediated by preventing LPS-induced inflammation in Caco-2 cells, other mechanisms may also exist, such as direct effects on the junctional proteins.
CONCLUSIONS
Given that IAP has been safely administered to humans without any adverse effects, we suggest that IAP may represent a novel clinical intervention to improve outcomes in diseases driven by gut barrier dysfunction.
Acknowledgments
Support: This study was supported by National Institute of Health grant NIH/NIDDK T32 DK007754 and a grant from The Ellison foundation (RAH).
Abbreviations and Acronyms
- IAP
intestinal alkaline phosphatase
- DMEM
Dulbecco’s modified minimum essential medium
- IL
interleukin
- LPS
lipopolysaccharide
- MEF
mouse embryonic fibroblast
- RT-PCR
real-time polymerase chain reaction
- TEER
transepithelial electrical resistance
- TJ
tight junction
- TJP
tight junction protein
- TNF
tumor necrosis factor
Footnotes
Author Contributions
Study conception and design: Hamarneh, Hodin
Acquisition of data: Liu, Hu, Huo, Zhang, Hamarneh
Analysis and interpretation of data: Liu, Hu, Huo, Zhang, Adiliaghdam, Morrison, Ramirez, Gul, Hamarneh, Hodin
Drafting of manuscript: Hu, Ramirez, Hamarneh, Hodin
Critical revision: Liu, Hu, Huo, Zhang, Adiliaghdam, Morrison, Ramirez, Gul, Hamarneh, Hodin
Disclosure Information: Nothing to disclose.
Presented at the 96th Annual Meeting of the New England Surgical Society, Newport, RI, September 2015.
References
- 1.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 2.Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci. 2012;1258:159–165. doi: 10.1111/j.1749-6632.2012.06612.x. [DOI] [PubMed] [Google Scholar]
- 3.Büning C, Geissler N, Prager M, et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;18:1932–1939. doi: 10.1002/ibd.22909. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira TF, Souza NC, Chiarello PG, et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–740. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 6.Leblhuber F, Geisler S, Steiner K, et al. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J Neural Transm. 2015;122:1319–1322. doi: 10.1007/s00702-015-1381-9. [DOI] [PubMed] [Google Scholar]
- 7.Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov AI. Structure and regulation of intestinal epithelial tight junctions: current concepts and unanswered questions. Adv Exp Med Biol. 2012;763:132–148. doi: 10.1007/978-1-4614-4711-5_6. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1βeta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S, Al-Sadi R, Said HM, et al. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sussman NL, Eliakim R, Rubin D, et al. Intestinal alkaline phosphatase is secreted Bidirectionally from villous enterocytes. Am J Physiol. 1989;257:14–23. doi: 10.1152/ajpgi.1989.257.1.G14. [DOI] [PubMed] [Google Scholar]
- 15.Malo MS, Moaven O, Muhammad N, et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol. 2014;306:826–838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KT, Malo MS, Moss AK, et al. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol. 2010;299:467–475. doi: 10.1152/ajpgi.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasamy S, Nguyen DD, Eston MA, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2011;17:532–542. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaliannan K, Hamarneh SR, Economopoulos KP, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110:7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuin A, Poelstra K, de Jager-Krikken A, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 20.Lukas M, Drastich P, Konecny M, et al. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm Bowel Dis. 2010;16:1180–1186. doi: 10.1002/ibd.21161. [DOI] [PubMed] [Google Scholar]
- 21.Hamarneh SR, Mohamed MM, Economopoulos KP, et al. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann Surg. 2014;260:706–714. doi: 10.1097/SLA.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Sadi RM, Ma TY. IL-1βeta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kourtidis A, Ngok SP, Pulimeno P, et al. Distinct E-cadherin-based complexes regulate cell behaviour through miRNA processing or Src and p120 catenin activity. Nat Cell Biol. 2015;17:1145–1157. doi: 10.1038/ncb3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodin RA, Graham JR, Meng S, et al. Temporal pattern of rat small intestinal gene expression with refeeding. Am J Physiol. 1994;266:83–89. doi: 10.1152/ajpgi.1994.266.1.G83. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RF, Austen WG, Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JM, Van Itallie CM, Peterson MD, et al. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989;109:1047–1056. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen KT, Malo MS, Beasley-Topliffe LK, et al. A role for intestinal alkaline phosphatase in the maintenance of local gut immunity. Dig Dis Sci. 2011;56:1020–1027. doi: 10.1007/s10620-010-1396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malo MS, Alam SN, Mostafa G, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59:1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 32.Pastorelli L, De Salvo C, Mercado JR, et al. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185–190. [PubMed] [Google Scholar]
- 34.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the trans-membrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 35.Umeda K, Matsui T, Nakayama M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 36.Al-Sadi R, Khatib K, Guo S, et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:1054–1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González-Mariscal L, Domínguez-Calderón A, Raya-Sandino A, et al. Tight junctions and the regulation of gene expression. Semin Cell Dev Biol. 2014;36:213–223. doi: 10.1016/j.semcdb.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Shin J, Carr A, Corner GA, et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J Biol Chem. 2015;290:15392. doi: 10.1074/jbc.A114.557546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodin RA, Meng S, Shei A. Differential cloning of novel intestine-specific genes whose expression is altered under conditions of villus atrophy. J Surg Res. 1995;59:115–120. doi: 10.1006/jsre.1995.1141. [DOI] [PubMed] [Google Scholar]