Abstract
The development of new methods to image the onset and progression of thrombosis is an unmet need. Non-invasive molecular imaging techniques targeting specific key structures involved in the formation of thrombosis have demonstrated the ability to detect thrombus in different disease state models and in patients. Due to its high concentration in the thrombus and its essential role in thrombus formation, the detection of fibrin is an attractive strategy for identification of thrombosis. Herein we provide an overview of recent and selected fibrin-targeted probes for molecular imaging of thrombosis by magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), and optical techniques. Emphasis is placed on work that our lab has explored over the last 15 years that has resulted in the progression of the fibrin-binding PET probe [64Cu]FBP8 from preclinical studies into human trials.
Graphical abstract
Herein we provide an overview of peptide-based fibrin-targeted probes developed for molecular imaging of thrombosis by different modalities with particular emphasis for nuclear imaging techniques.
Introduction
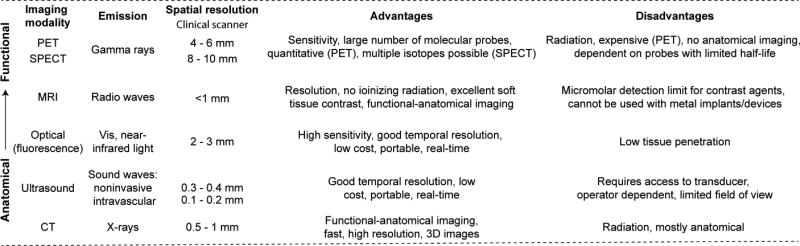
Thrombosis is often the underlying cause of major cardiovascular diseases (CVD), including venous thromboembolism (VTE), ischemic heart disease and ischemic stroke,1,2,3 with the last two accounting for over 20% of deaths worldwide.4 A variety of imaging techniques such as radiography, ultrasonography and angiography by computed tomography (CT) or magnetic resonance (MR) imaging are currently used in clinical cardiology for identifying and monitoring thrombotic disorders.5, 6 These traditional imaging techniques are predominantly based on detecting changes in the anatomy or function of the cardiovascular system (e.g. narrowing of a blood vessel, a filling defect in an angiogram or altered myocardial perfusion).7 Despite their utility, these techniques when used for thrombus detection are still limited. Firstly, multiple factors can give rise to the same alterations in anatomy/function, e.g. vessel narrowing could be resulted from atherosclerosis or from thrombus formation. Secondly, the method used for thrombus detection is dependent on the vascular territory, e.g. Doppler ultrasound is used in the deep veins of the leg, contrast enhanced CT in the lungs and transesophageal echocardiography in the heart. As a result, in diseases where the thrombus location is not clinically apparent, multiple imaging tests and modalities are required. Furthermore, these tests may be contraindicated in some cases, e.g. patients with poor kidney function cannot receive CT contrast and patients with casts at risk of deep vein thrombosis cannot be assessed by means of ultrasounds. In addition, vascular territories such as the aortic arch and pelvic veins are not well imaged by any modality. Finally, these methods do not distinguish between new or old clots, or if they will respond well to thrombolytic therapy. In response to these unmet needs, targeted molecular imaging approaches are being developed to detect specific biological markers and processes that characterise thrombosis.7 It is anticipated that molecular imaging modalities such as single photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI) and optical imaging techniques will increase the reliability of diagnosis and ultimately, improve clinical outcomes (Figure 1). The nuclear imaging techniques SPECT and PET are based on radiolabeling of specific molecular probes with radioisotopes. A major advantage of SPECT and PET over other imaging techniques (e.g. optical and MRI) is the high sensitivity that allows the detection of radiotracers at concentrations as low as 10−10 to 10−12 M, which can enable the detection of a wide range of molecular targets in CVD.8, 9 These nuclear methods, which provide no anatomical imaging, can be coupled with CT or MRI to form hybrid systems such as SPECT/CT, PET/CT and PET/MRI for combined molecular/functional/anatomical imaging.10 For SPECT, more than 80% of radiotracers used in nuclear medicine centres utilise technetium-99m because of its high availability from cost-effective generators, easy incorporation into various compounds and easy development of kit formulations for radiolabeling.11, 12 The predominant isotope used in PET imaging is fluorine-18.13 Other attractive isotopes for medical applications are In-111, I-123, and Tl-201 for SPECT and C-11, N-13, Cu-64, Ga-68 and Zr-89 for PET.14 Although SPECT has the advantages of decreased cost and higher widespread availability, PET offers higher spatial resolution and the ability to perform quantitative imaging that is only possible with SPECT using sophisticated, emerging, algorithms.15 MR contrast agents are also being developed for visualisation of specific in vivo targets. In this case, Gd-based agents, among other MR agents (e.g. iron oxides, Mn-based complexes) exhibit unique physical properties that upon target binding result in signal amplification (e.g. restricted rotational dynamics increases relaxivity).16–18
Figure 1.
Comparative overview for different clinical imaging techniques.
Benefits of MRI include a lack of ionizing radiation, excellent soft tissue contrast, multiple image contrasts, and 3D images with sub-millimeter spatial resolution.16–18 The primary limitation of MRI is the relatively low sensitivity of the contrast agents when compared to nuclear and optical probes (Figure 1).9
Optical-based methods are a clinically emerging approach for molecular imaging that offer high-resolution, good sensitivity, and versatile detection in CVD (Figure 1).19, 20 NIRF imaging (700 – 900 nm) is particularly favorable compared to other wavelengths, as this window presents reduced blood attenuation of light and reduced autofluorescence.19, 20 However, light scattering by tissues limits optical imaging to superficial applications or invasive imaging using a catheter or scope.
In this review we provide an insight into the clinical importance for the development of new approaches to imaging thrombosis. We first provide a brief introduction into the molecular targets that play a key role in thrombosis and have been proposed to detect and interrogate different aspects of thrombosis.21–24 The advantages and disadvantages of these various molecular targets are discussed in terms of their specificity, potential to detect thrombi in different vascular territories and to stratify thrombus based on age or composition (fibrin or collagen rich). We then present selected examples of MRI, PET/SPECT and optical probes based on small peptides developed for fibrin targeted thrombus imaging. We finish by describing our efforts over the last 15 years in the design and application of peptide-based fibrin-binding probes for thrombus imaging. This review does not intend to be a thorough account of the different approaches explored for thrombus imaging, but a personal account, which details our rationale and efforts in this field.
Mechanisms of Thrombus Formation
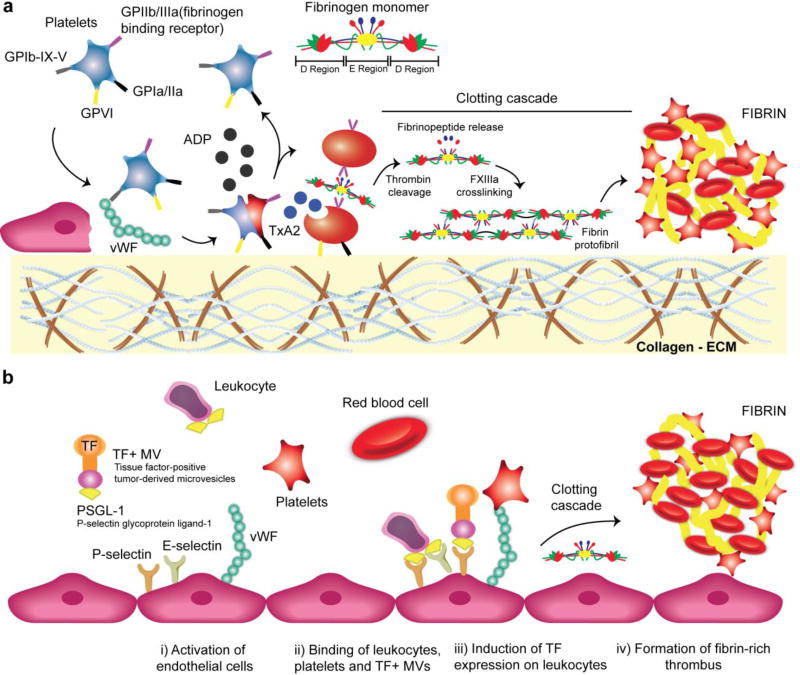
The primary trigger for arterial thrombosis is injury to the vessel wall, often caused either by a mechanical injury or by the rupture of an atherosclerotic plaque.25–28 Vessel injury exposes the subendothelial extracellular matrix (ECM) and initiates rapid platelet recruitment and activation (Figure 2a). The von Willebrand Factor (vWF) and the platelet glycoprotein (GP) receptors Ib-IX-V play a pivotal role in establishing the tethering of platelets to the site of injury where the vWF acts as a bridge between collagen fibrils (type I, III and VI) and platelets (Figure 2a). Indeed, while soluble vWF does not react with platelets to prevent aggregation in the normal circulation, immobilized vWF onto collagen is highly reactive toward flowing platelets.25, 29, 30 After platelet activation, the expression of the clotting factors VII and XI, the recruitment of fibrinogen and conversion of this fibrinogen to fibrin by thrombin culminates in the formation of a complex branched clot network (Figure 2a).25 Venous thrombosis is induced on the surface of intact endothelium, under lower shear stress, by hypoxia or inflammatory stimuli that lead to surface expression of adhesion receptors (Figure 2b).28, 31, 32 These receptors facilitate the binding of circulating leukocytes and microvesicles. Subsequent activation of the leukocytes induces expression of the potent procoagulant protein tissue factor (TF) that triggers thrombosis (Figure 2b). Whereas venous clots are high in red blood cells (RBCs) (so called “red clots”), arterial clots are platelet-rich (so called “white clots”), however, both arterial and venous clots are rich in fibrin.
Figure 2.
Arterial and venous thrombus formation in cardiovascular disease. a. Arterial thrombus: the formation of a platelet aggregate is initiated by the interaction of the platelet surface receptor GPIb-IX-V with vWF. The collagen receptors GPVI and GPIa/IIa (aka integrin α2β1) on platelets then mediate firm adhesion to fibrillar collagen. Adenosine diphosphate (ADP) further promotes platelets to undergo a shape change to release granule contents (e.g. thromboxane A2, TxA2) that allows fibrinogen binding and platelet aggregation to the primary hemostatic plug. Finally, thrombin cleavage and cross-linking between fibrin-fibrin strands by factor XIII results in the formation of a stable fibrin clot.27 b. Venous thrombus: endothelium dysfunction induced by hypoxia and/or inflammation expresses the adhesion proteins P-selectin, E-selectin, and vWF. Next, circulating leukocytes, platelets, and TF+ MVs (tissue factor-positive tumor-derived microvesicles) bind to the activated endothelium. Final step involves expression of TF on activated leukocytes that triggers thrombosis.31
Motivations for Thrombus Imaging
There are several motivations to image thrombosis. First, venous thromboembolism (VTE), which comprises deep-vein thrombosis (DVT) and its complication pulmonary embolism (PE), is a fairly common disease that is associated with reduced survival and substantial health-care costs.2, 33 It is estimated that among people of European ancestry VTE affects from 104 to 183 per 100,000 persons per year.2 DVT can often be diagnosed by compression ultrasonography, but this is not possible in the pelvic veins, in patients with casts, and can be confounded by the presence of vascular scarring. PE is routinely diagnosed by contrast enhanced CT but this cannot be performed in patients with renal insufficiency and is also limited in detection of more distal thrombi.6 DVT and PE often occur together and there is no single technique that can detect both PE and DVT in the same scan session. Moreover current techniques cannot distinguish thrombus composition which can impact therapy. Thus, accurate and early detection of DVT and PE is highly desirable to minimise the risk of complications that can culminate in death if not diagnosed and treated effectively.2, 34 Similarly, since thrombus formation represents the final step in atherosclerosis progression, thrombus imaging may be also helpful to identify patients at high risk for myocardial infarction or stroke. For example, about 30% to 40% of ischemic strokes are "cryptogenic" i.e. the source of thromboembolism is never identified.35 One possible cause of cryptogenic stroke is atherosclerosis in the aortic arch, which is difficult to detect with routine methods.36 Finally, the exact information about the size and location of the thrombi would have an impact in thrombolytic therapy. Without information on the size of the thrombus, the recommended standard dose of intravenous tissue plasminogen activator (tPA) may be insufficient for some patients, resulting in resistance to thrombolysis, and excessive in others, leading to potential haemorrhagic complications.37 Such information would allow patient stratification and help in providing a personalised therapy.
A thrombus-targeted molecular imaging approach could be useful in all of the preceding examples. Depending on the choice of imaging target (e.g. activated platelets), it may be possible to distinguish early forming clots from older constituted thrombi. In PE detection, work by Ginsberg et al. using a 99mTc-radiolabeled antibody fragment targeting the D-dimer regions of fibrin was shown to accurately detect PE in humans.38 In another example target specific probes were shown to be accurate in the assessment of thrombus composition and predict successful therapeutic choices (eg, thrombolysis).39, 40
Molecular Targets for Thrombus Imaging
Direct molecular imaging with probes that target the components involved in thrombus formation offers a noninvasive approach with potential high specificity to expand the diagnostic capabilities of the current imaging methods.21–24 To date several targets have been explored for thrombus imaging such as fibrin, activated platelets and enzymes that are upregulated in the clotting cascade, namely thrombin and factor XIII.41–43
Activated platelets and fibrin both are only present in thrombus and healing wounds and thus represent targets with potentially high specificity for thrombus imaging. In acute thrombosis, activated platelets are cross-linked to fibrinogen and are susceptible to continued thrombus propagation.
One strategy for targeting activated platelets explores the overexpression of the GPIIb/IIIa receptor that is exposed on the membrane surface in very high concentrations (50 000 to 100 000 receptors per platelet).44 Fayad and colleagues along with scientists at Guerbet developed an activated platelet-targeted MRI contrast agent termed P975, comprising a peptide with affinity for the GPIIb/IIIa receptor conjugated to a Gd-DOTA chelate and demonstrated the ability of P975 enhanced MRI to detect platelet-rich thrombi in an animal model.45 Radiolabelling of activated platelets with Apcitide (AcuTec, also known as 99mTc-P280), a 99mTc-labeled RGD-mimicking polypeptide for the GPIIb/IIIa receptors was approved by the United States Food and Drug Administration for SPECT detection of acute DVT in patients.46 Another peptide-based probe targeting the GPIIb/IIIa receptor, is 99mTc-DMP444 that advanced to phase II clinical studies for imaging DVT.47 Both 99mTc-P280 and 99mTc-DMP444, however, showed low sensitivity and specificity for PE due to their low thrombus uptake and prolonged radioactivity accumulation in the blood pool and chest region.48, 49 As such, these compounds have been discontinued.
Other epitopes on the platelet surface have been targeted for thrombus imaging, namely P-selectin, vWF and GPVI however, their specificity for activated platelets varies.43 Targeting P-selectin has been shown to delineate between activated and non-activated platelets. Using fucoidan, a polysaccharidic ligand of P-selectin, labelled with 99mTc, Letourneur et al. showed that the presence of platelet-rich thrombi in rat models of arterial thrombosis could be detected using SPECT imaging.50 Recently, the same group successfully tested ultra-small superparamagnetic iron oxide nanoparticles (USPIO) coated with fucoidan for molecular MRI of intraluminal thrombus.51 P-selectin is not however, specific for platelets and is also expressed on endothelial cells.43
Molecular probes that target the coagulation enzymes thrombin52, 53 and factor XIII54 have shown significant uptake in fresh thrombi, but have not shown any signal enhancement when used to image old thrombi. Such probes have, however, not been widely evaluated in patients and thus the natural history of thrombin and Factor XIII expression in thrombosis is unknown.
Fibrin as a Target
Fibrin-targeting strategies have also been used for detection and monitoring thrombosis. Fibrin is present at high concentrations in both venous and arterial thrombosis providing high sensitivity, but fibrin is not present in circulating blood resulting in potentially high specificity.55 In addition, the fibrin concentration in thrombus is in the hundreds of micromolar,56 making this a target compatible with most imaging modalities. Lastly, fibrin is present in fresh thrombi but when the thrombus becomes aged and organized, the fibrin is replaced by collagen and other fibrotic proteins. Fibrin is also the target of thrombolytic drugs such as tissue plasminogen activator and urokinases, and these drugs are more effective at lysing fibrin-rich clots. Therefore, fibrin imaging offers the opportunity to characterize thrombus composition and guide therapy. A challenge in targeting fibrin is distinguishing it from the precursor fibrinogen, a protein that shares 98% of its structure with fibrin and that is present at high concentrations in the blood (2.5 – 3 mg/mL).57 Specificity for fibrin over fibrinogen has been achieved by using target-selective antibodies and peptides discovered by phage display technologies.
Molecular Imaging Probes for Targeting Fibrin
Thrombus molecular imaging targeting fibrin dates to the 1970’s with gamma scintigraphy approaches using 99mTc- and 125I-labeled fibrinogen.41 Those initial attempts failed to gain clinical relevance due to low clot:blood background, unfavourable pharmacokinetics, and consequently low sensitivity and specificity.41, 58 As an alternative, isotope labelled monoclonal anti-fibrin antibodies were used to visualise thrombi in vivo.41 In a recent example, a deimmunised antibody Fab′ fragment (99mTc-DI-80B3, ThromboView) that targets the D-dimer domains of cross-linked fibrin was shown to detect DVT and acute pulmonary emboli in preclinical experiments and in phase I and II clinical trials.59, 60 Other examples that make use of antibodies to fibrin are summarised in two recent reviews.41, 61 Besides protein based approaches, small cyclic peptides which present high affinity for fibrin and high selectivity over fibrinogen have also been described.41, 42, 61 The potential benefits of small peptides in comparison to antibodies include faster bloodstream clearance and the ability to penetrate into the fibrin mesh, both of which result in improved target-to-background ratios.
Small Peptides for Targeting Fibrin Derived by Phage Display
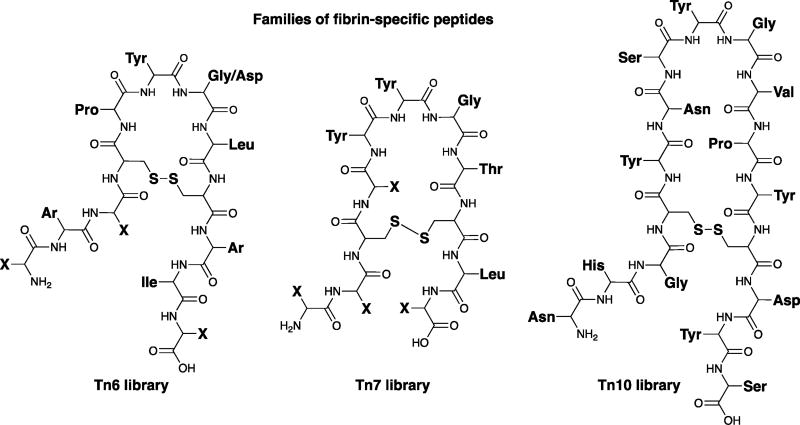
Phage display has proven an important tool for the identification of fibrin-targeting peptides.62–64 Kolodziej et al. identified three families of cyclic peptides that show low micromolar affinity and specificity for fibrin over fibrinogen and other serum proteins (Figure 3).64
Figure 3.
Sequence of fibrin-binding peptides identified by phage display.64
These peptides differ in the size of the central disulphide bridge macrocyclic ring (e.g. Tn6: six amino acids; Tn7: seven amino acids) as well as the nature of the amino acids. Fibrin binding phages were selected by incubating the phage library first with fibrinogen immobilized on biotinylated beads. After removing fibrinogen-binding phages, the depleted phage library was then incubated with fibrin polymerised in a microtiter plate well.64 This process was iterated to identify phage with affinity for fibrin and selectivity for fibrin over fibrinogen. The Tn6 library yielded a consensus sequence of XArXCPY(G/D)LCArIX where Ar is an aromatic amino acid and X can be any amino acid. The Tn7 library yielded the consensus sequence X2CXYYGTCLX where YYGT is strictly conserved along with a preference for polar amino acids (H, D, S, and N) at the lone variable position within the disulfide loop. The Tn10 library gave a single sequence NHGCYNSYGVPYCDYS, which contains a ‘SYGV’ motif that appears to be functionally homologous to the ‘YYGT’ motif of the Tn7 sequence. In all of these peptides the disulphide bridge is absolutely critical for fibrin binding. The peptides bound to fibrin at 2 equivalent sites with Kd in the range of 4.0 to 9.0 µM. Importantly, binding to fibrinogen of these peptides is at least 100-fold weaker.64 The peptides were also shown to bind to the soluble fibrin degradation product DD(E) with similar affinity to that measured for fibrin. Competition studies indicated that the Tn7 and Tn10 sequences bound to the same site on fibrin, while the Tn6 sequence bound to a unique site. Fibrin affinities for Tn6 and Tn7 were further optimized using alanine scanning (Table 1).64 Alanine substitutions showed that the residues P5, Y6, and L8 of the Tn6 peptides exhibit particular sensitivity to substitution. For the Tn7 sequence, a substantial reduction in binding was observed for changes at Y6 and T8, highlighting the critical role of these conserved residues for fibrin binding. Substitution of the other residues flanking the central disulphide bridge macrocyclic ring had little effect on binding. Among all the peptides the Tn10 peptide was shown to be the less tolerant to modification (Table 1).
Table 1.
Structural requirements for Tn6, Tn7, and Tn10 Peptides, as determined by alanine scanning and DD(E) binding (Ki).
| Tn6 1 | Ki (µM) |
Tn6 2 | Ki (µM) |
Tn7 | Ki (µM) |
Tn10 | Ki (µM) |
|---|---|---|---|---|---|---|---|
| WFHCPYDLCHIL | 3.1 | QWECPYGLCWIQ | 2.3 | LPCDYYGTCLD | 2.8 | NHGCYNSYGVPYCDYS | 3.5 |
| AFHCPYDLCHIL | 8.0 | AWECPYGLCWIQ | -- | APCDYYGTCLD | 3.1 | NHGCYDSYGVPYCDYS | >100 |
| WAHCPYDLCHIL | 3.9 | QAECPYGLCWIQ | 4.3 | LACDYYGTCLD | 2.9 | NHGCYNYYGVPYCDYS | 5.9 |
| WFACPYDLCHIL | 3.3 | QWACPYGLCWIQ | 6.2 | LPCAYYGTCLD | 5.9 | NHGCYNYYGTPYCDYS | >500 |
| WFHCAYDLCHIL | 62 | QWECAYGLCWIQ | 32 | LPCDAYGTCLD | 4.5 | NHGCYDYYGTPYCDYS | >500 |
| WFHCPADLCHIL | 16 | QWECPAGLCWIQ | 42 | LPCDYAGTCLD | >100 | ||
| WFHCPYALCHIL | 16 | QWECPYALCWIQ | 7.7 | LPCDYYATCLD | 12 | ||
| WFHCPYDACHIL | 97 | QWECPYGACWIQ | 93 | LPCDYYGACLD | >100 | ||
| WFHCPYDLCAIL | 28 | QWECPYGLCAIQ | 4.3 | LPCDYYGTCAD | 15 | ||
| WFHCPYDLCHAL | 19 | QWECPYGLCWAQ | 17 | LPCDYYGTCLA | 7.3 | ||
| WFHCPYDLCHIA | 2.6 | QWECPYGLCWIA | -- | LPCDYYGVCLD | 28 |
MRI Fibrin-Binding Probes for Thrombus Detection
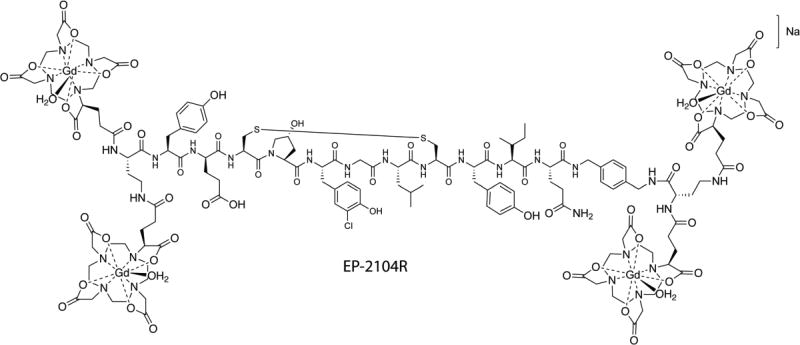
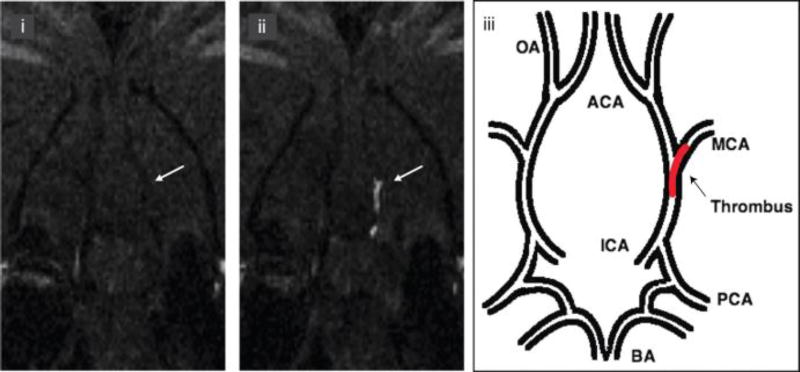
MR probes typically require micromolar concentrations of reporter ion for detection. Fibrinogen circulates at ~7 µM in blood plasma and in thrombus formation the fibrin monomer is amplified to >100 µM. Thus fibrin is amenable to detection with a targeted MRI probe. The Tn765, 66 and Tn667, 68 peptides were modified to include a Gd-chelate as a MRI reporter. In order to provide more robust detection of thrombus, four Gd-chelates were conjugated to the peptide. It was found that derivatives of the peptide with Gd-chelates at both the C- and N-termini not only increased the relaxivity per molecule but also rendered the molecule more stable to degradation by in vivo proteases.66 Owing to higher fibrin affinity, the Tn6 derivatives were explored in more depth. An initial Tn6-based prototype containing four Gd-DTPA moieties (two at the N-terminus and two at the C-terminus) termed EP-1873 had a relaxivity of 84 mM−1s−1 at 1.4 T, 37 °C (21 mM−1s−1 per Gd) and a dissociation constant Kd for fibrin binding of 3.5 µM.67 Using a dose of 2 µmol/kg (compare to 100 µmol/kg for most approved clinical contrast agents), EP-1873 was shown to visualise thrombus with high contrast to noise ratios in a rabbit model of acute thrombosis after atherosclerotic plaque rupture. A further optimised probe, EP-2104R (Figure 4) utilized hydroxyproline, 3-chlorotyrosine, and D-glutamic acid substitutions to improve peptide binding and four Gd-DOTA moieties to improve the stability of molecule with respect to Gd release/deposition.68 The relaxivity of EP-2104R at 1.4 T and 37 °C was 71.4 mM−1s−1 per molecule, approximately 25 times higher than that of Gd-DOTA when compared under the same conditions. EP-2104R was examined in rat, rabbit, and pig models with arterial thrombus,67 venous thrombus,56 pulmonary and cerebral embolism,69, 70 coronary thrombosis71 and atrial thrombus.72 In an embolic stroke model, the occlusive thrombus was clearly evident in the right internal carotid artery, near the origin of middle cerebral artery (MCA) (Figure 5).70 Recently, EP-2104R was used to estimate thrombus composition with respect to fibrin content, and used to identify thrombi that are amenable for thrombolysis.39
Figure 4.
Structure of the Gd-based fibrin-binding probe EP-2104R.
Figure 5.
Intracranial thrombus detected after injection of EP-2104R. i. Precontrast coronal slice from 3-dimensional T1-weighted images at level of the middle cerebral artery (MCA) origin. ii. Postcontrast after injection of EP-2104R clearly identifies the thrombus (arrow) as a bright signal located in the internal carotid artery (ICA) and branching into the MCA. iii. Schematic representation of the location of the thrombus within the cerebral arterial tree. BA, basilar artery; PCA, posterior cerebral artery; ICA, internal carotid artery; OA, olfactory artery; ACA, anterior cerebral artery. Adapted from ref. 70 with permission from American Heart Association, Inc.
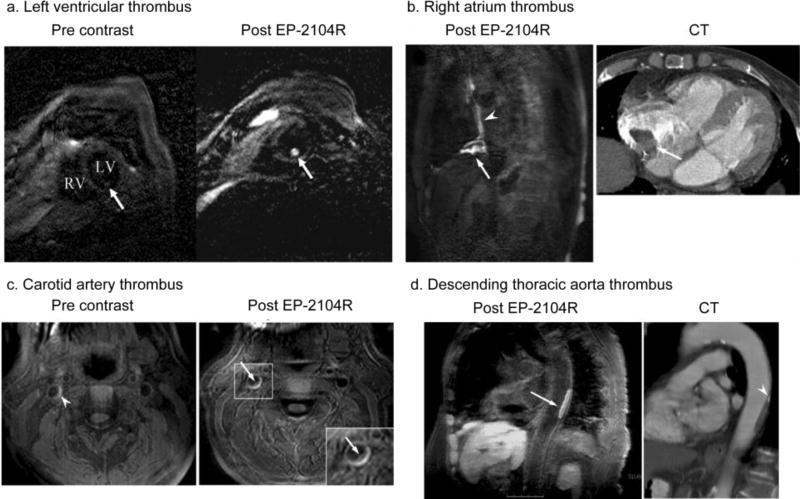
In humans, EP-2104R was evaluated in two phase II trials that enrolled 52 patients with previously confirmed thrombi in the venous, heart or arterial system.73, 74 Using standard clinical 1.5 T scanners thrombi were visualised with high signal amplification and high contrast to noise ratios in the left ventricle (Figure 6a), right atrium (Figure 6b), carotid artery (Figure 6c) and thoracic aorta (Figure 6d).73 MR imaging showed that EP-2104R was able to successfully detect arterial, venous and intracardiac thombosis in humans. These encouraging results demonstrate both the feasibility and potential value of fibrin molecular imaging approaches to detect thrombosis in patients.
Figure 6.
MR imaging of thrombi using EP-2104R in patients. a. molecular MR imaging of left ventricular thrombus. LV Left ventricle, RV right ventricle. b. MRI of thrombus in the right atrium (arrow, clot surface; arrowhead, central venous catheter). CT image shows clot in the right atrium. c. MRI of thrombus in the carotid artery. Signal enhancement at the intraluminal clot surface is seen on post EP-2104R images (arrow and magnification shown as an inset). Pre EP-2104R MR images also show some bright signal of the vessel wall, which may represent intraplaque hemorrhage (arrowhead). d. MRI of thrombus in the descending thoracic aorta (arrow). CT image shows corresponding plaque in the aortic wall. At the cranial end of the plaque a small calcification is also visible (arrowhead). Reprinted from ref. 73 with permission from © European Society of Radiology (2008).
Recent concerns regarding the safety of gadolinium-based probes prompted Gale et al. to prepare a fibrin-targeted probe using Mn(II) chelates for signal generation. They used the same peptide scaffold as EP-2104R but replaced the Gd-DOTA moieties with [Mn(PyC3A)(H2O]− (PyC3A, N-picolyl-N,N′,N′-trans-1,2-cyclohexylenediaminetriacetate). The Mn-fibrin binding probe was rapidly and completely eliminated from rats largely unchanged and enabled thrombus imaging in a rat model of arterial thrombosis with conspicuity equivalent to the EP-2104R probe.75
Optical Probes for Fibrin Detection
Molecular imaging of fibrin in jugular vein thrombus was also investigated by intravital confocal fluorescence microscopy and fluorescence molecular tomography–computed tomography (FMT-CT) using a near-infrared fluorescent derivative of EP-2104R (Figure 7).76
Figure 7.
Molecular imaging of fibrin in jugular vein thrombus by integrated FMT-CT. Arrows indicate a thrombosed left jugular vein. Reprinted from ref. 76 with permission from American College of Cardiology Foundation Copyright © 2012.
Fibrin-targeting nanoparticles are also subject of active investigation for multimodal imaging of thrombosis.77, 78 In one example, ultra-small superparamagnetic iron oxide nanoparticles (CLIO) were conjugated to the peptide from EP-2104R and a near-infrared fluorophore (CyAm7) providing a platform for intravascular ultrasound and fluorescent imaging of fibrin expression in arterial thrombosis. Applications of the CLIO-CyAm7 nanoparticles can also be extended to MR imaging.78
Fibrin Targeting to Detect Activated Platelets
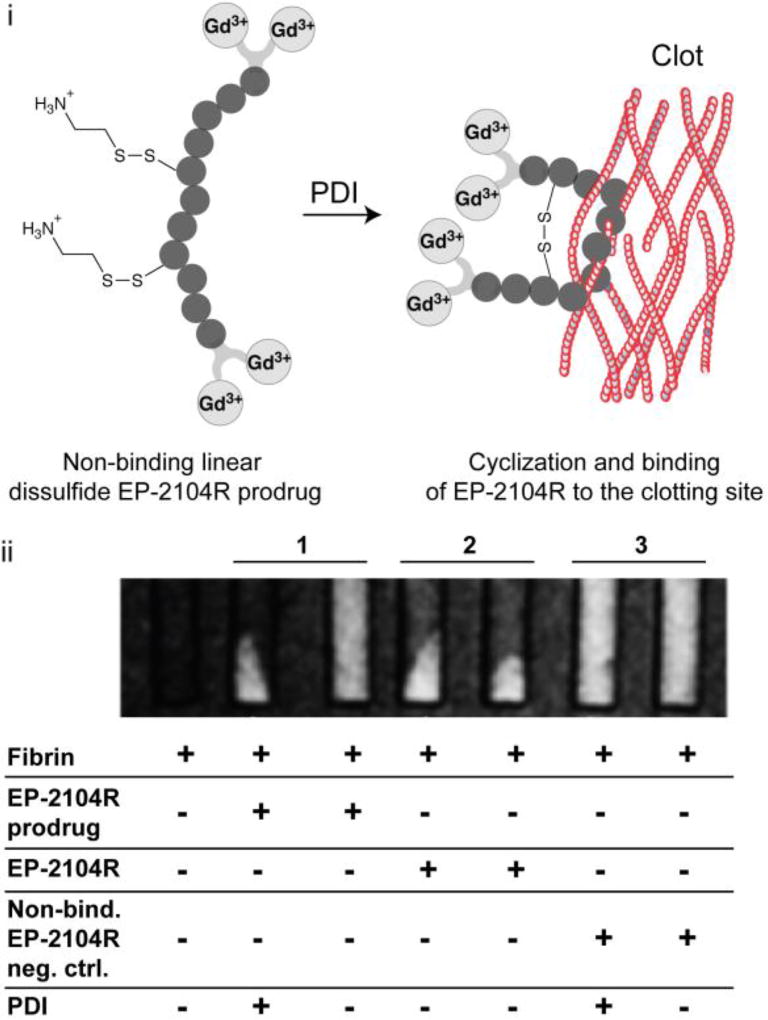
We recently developed a pro-drug approach to image activated platelets and distinguish hyperacute thrombus from older thrombus. When platelets become activated the enzyme protein disulphide isomerase (PDI), is highly expressed on the cell’s surface. In the clotting cascade, PDI catalyses the exchange of disulfide bonds in the integrin α2Bβ3 to result in an active form of α2Bβ3 that allows the platelet to bind fibrinogen. Recognizing that the disulphide bridge in EP-2104R is critical for binding, we prepared a linear analogue of this peptide that contained mixed disulphides (Figure 8a).79 It was hypothesised that in the presence of activated platelets and PDI, the peptide would undergo disulfide isomerization and cyclise, producing a fibrin targeting probe that could bind to newly formed thrombus. The mixed disulfide prodrug was shown to have no appreciable affinity for the soluble fibrin fragment DD(E)79 but in the presence of PDI, the enzyme catalyses the disulfide exchange, re-establishing its fibrin binding properties as illustrated by phantom studies (Figure 8b).79 Control studies with a linear peptide analogue incapable of forming a disulphide bond further demonstrated specificity.79
Figure 8.
PDI activation of the mixed disulfide EP-2104R prodrug. i. Schematic representation of the activation and retention approach for imaging acute thrombosis. ii. T1-weighted images of phantoms at 1.5 T after incubation under different conditions as shown in the figure and after centrifugation to pellet the clots. MR signal is only co-localized with pelleted fibrin clot if PDI is present, while without enzyme MR signal from the prodrug probe is uniformly distributed throughout the solution (column 1). Column 2 and 3 refers to positive (original EP-2104R probe) and negative (linear non-disulfide EP-2104R analogue) controls. Adapted from ref. 79 by permission of John Wiley & Sons, Inc. Copyright © 2014 by John Wiley Sons, Inc.
Bimodal PET-MR Probe for Thrombus Detection
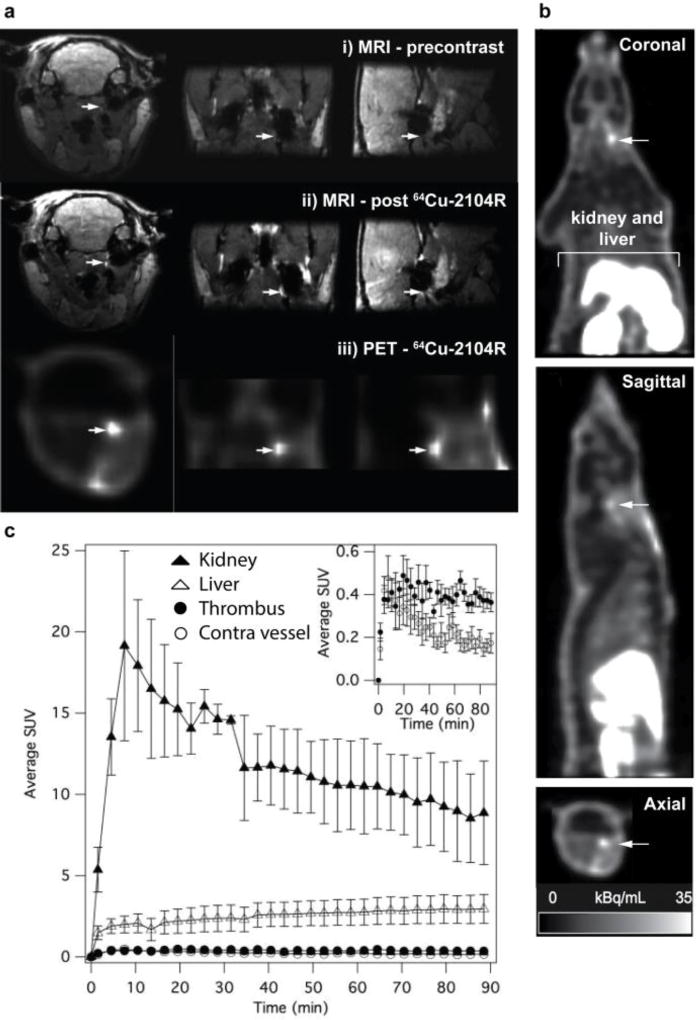
One strength of MRI is the ability to achieve very high spatial resolution, however increasing the resolution results in longer acquisition times and ultra high-resolution imaging is not practical for large fields of view. In order to reduce the acquisition time, we hypothesised that a PET scan could provide a whole body image to indicated where thrombus may be present; then a high resolution MR image could be obtained of that anatomical location. To test this we prepared a dual PET-MR probe by performing a partial exchange of gadolinium for copper-64 in EP-2104R. The feasibility of the 64Cu-EP-2104R (also termed 64Cu-FBP6) to detect thrombus by simultaneous PET-MR imaging was evaluated in a rat embolic stroke model.80 We found that in vivo, thrombus could be clearly identified with both MRI and PET modalities after injection of 64Cu-EP-2104R (Figure 9a). After probe injection, the MRI signal at the thrombus (arrows) increases because the probe binds and the Gd induces T1-shortening. To confidently identify the thrombus, the baseline pre-contrast agent image must be compared with the post injection image to identify regions that enhance. With PET, the image is created solely by the distribution of the probe. The images also illustrate a significant difference in spatial resolution between the two modalities. In this study the MR voxel size was 0.3 × 0.3 × 0.3 mm3 while the PET voxel size was approximately 2.5 × 2.5 × 2.5 mm3. The rat carotid artery is ~0.5 mm in diameter and the MR image reflects the true size of the thrombus while the low spatial resolution in the PET images gives an overestimate of the true thrombus size.
Figure 9.
Bimodal thrombus imaging by PET-MRI. a. PET-MR images showing that64Cu-EP-2104R detects thrombus in the carotid artery. The focal increased signal intensity on PET images (a iii) corresponds to a region that is hyperintense on T1-weighted molecular MR images after probe injection (a ii) but is absent on precontrast MR images (a i). b. PET data for the entire PET field of view showed a hyperintense region in the common carotid artery (arrow) and in the excretory organs. c. Time-activity volume-of-interest curves for the kidney, liver, thrombus, and contralateral vessel. Inset graph compares accumulation in the ipsilateral and contralateral vessels over time. Adapted from ref. 80. Copyright © RSNA, 2011.
This study was also informative with respect to the whole body distribution of the probe. The whole body PET images (Figure 9b), are scaled such that the liver and kidneys are off-scale and illustrate the very low signal observed in the head, neck (apart from the thrombus), and thorax. In fact, the thrombus had the highest concentration of radioactivity, after the liver and kidneys (Figure 9b and 9c). This high specificity for thrombus and low background signal suggested the utility of a PET-only probe for thrombus detection.80 The MRI could also be used to obtain high-resolution images of the blood vessels with fused PET-MR images pinpointing the location of the thrombus within the carotid artery.
PET Probes for Fibrin Detection - in vivo Structure Activity Relationship Study for Probe Optimisation
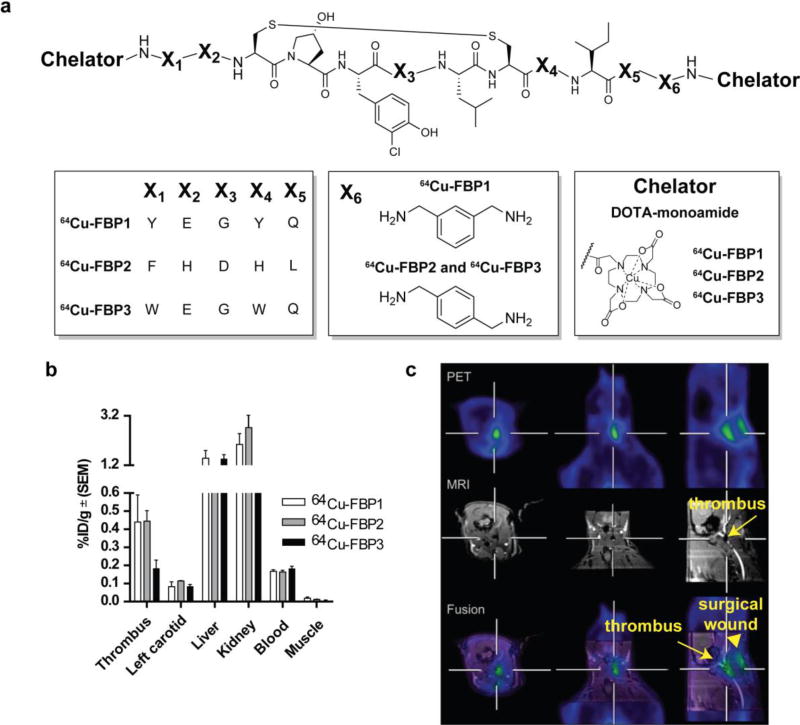
A limitation of the MR probes is the need to acquire two images of the patient, one before injection and one after, in order to observe the contrast induced by the probe. In the human studies, the best post-probe images were observed ≥2 hours post injection. Recently there have been concerns about the safety of gadolinium-based contrast agents and gadolinium deposition. A PET probe would require only a single, post-administration image and the mass dose in PET is very low limiting toxicity concerns to radiation exposure. The promising results observed with the bimodal probe shown in Figure 9 prompted us to develop a PET only probe. During the development of the MR probes we noted that this family of peptides was very sensitive to in vivo protease degradation. Anticipating that the peptide sequence may affect metabolic stability, we prepared three probes based on slightly different peptide sequences that had been previously shown to show high affinity for fibrin (Figure 10). In the development of EP-2104R we had also found that blocking both the N- and C-terminus of the peptide with a hydrophilic metal chelator served to block in vivo protease degradation.66 We functionalised the termini of these peptides with DOTA-monoamide and radiolabelled the compounds with 64Cu (64Cu-FBP1, 64Cu-FBP2, 64Cu-FBP3; Figure 10).81 These compounds were evaluated for binding to fibrin and compared in a rat model of hyperacute arterial thrombosis. In vitro, all three compounds exhibited similar nanomolar affinity (Ki = 290 – 380 nM) to the soluble fibrin degradation product DD(E). The in vivo studies were designed to assess probe uptake into thrombus and also pharmacokinetic and metabolic behaviour.81 Thrombus was induced by isolation and mechanical injury of the common carotid artery. After injury, the probe was injected via the femoral vein and blood was sampled periodically over the course of the study. At 2 hours post injection, the animals were euthanised and the tissues removed for counting. The thrombus-containing carotid artery and contralateral artery were analysed by autoradiography, blood samples were analysed for total radioactivity and for intact probe using a functional fibrin-binding assay and the blood was analysed by radio-HPLC for metabolites. In some cases, simultaneous PET-MRI was performed as well. Excluding the excretory organs, 64Cu-FBP1 and 64Cu-FBP2 showed the highest concentration of radioactivity in the vessel containing the thrombus at 120 min post injection (Figure 10b). The thrombus uptake of 64Cu-FBP1 and 64Cu-FBP2 (0.45 %ID/g) was 3-fold higher than the blood (0.16 %ID/g). Imaging studies with 64Cu-FBP1 and 64Cu-FBP2 demonstrated that both probes could identify thrombus with high conspicuity. However 64Cu-FBP3 showed no selectivity towards clots over blood. Analysis of the blood samples indicated that 64Cu-FBP3 was rapidly metabolised, explaining its inability to detect thrombus, while 64Cu-FBP1 and 64Cu-FBP2 underwent slower metabolism. An unidentified metabolite had a very long circulation time which resulted in a persistently high blood background. Because the three probes all used the same chelator, it was assumed that the faster metabolism of 64Cu-FBP3 was due to degradation of the peptide while the slow clearance of the total 64Cu activity from the blood is likely due to transmetallation of 64Cu to the blood proteins ceruloplasmin and albumin.82 64Cu-FBP2 revealed a slightly better in vivo profile for thrombus imaging based on the highest thrombus:contralateral tissue uptake observed by autoradiography and the lowest uptake in the heart, lungs and liver observed by ex vivo biodistribution, providing a lower background signal.81 Based on these studies, the peptide sequence of 64Cu-FBP2 was used in all subsequent studies.
Figure 10.
a. Structures of 64Cu-FBP1, 64Cu-FBP2, 64Cu-FBP3. b. Biodistribution of 64Cu-FBP1, 64Cu-FBP2, 64Cu-FBP3 (n = 6) in a rat model of arterial thrombosis at 120 min post probe injection. Error bars = standard error of the mean (SEM). c. Hybrid MR-PET imaging of 64Cu-FBP2 in a rat model of arterial thrombosis. The crosshairs show where each plane bisects the site of injury in the right carotid (RC) artery. A gadolinium-based blood pool agent was used to provide positive MR image contrast to the blood vessels and demonstrate an occlusion in the RC (arrow, middle panels). Bottom panel shows fused MR (grayscale) – PET (color) images showing localization of PET signal to the occlusion in the RC. Additional PET activity is seen superficially at the surgical wound (arrow head), due to clotting at the site of tissue injury. Reprinted with permission from ref. 81. Copyright © 2013 by American Chemical Society.
Effect of the Chelator on Thrombus Imaging with 64Cu-FBPx
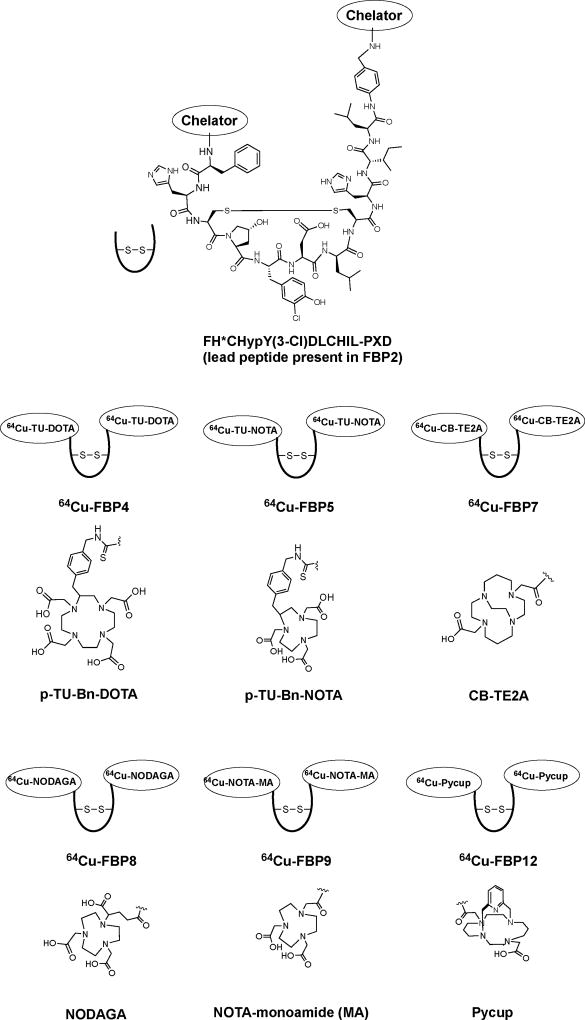
Our initial work with 64Cu-FBP2 showed that the intact probe exhibits fast blood clearance in rats, but transmetallation leads to a high background signal.81 We next wanted to study the effect of the chelator on the metabolic stability and imaging efficacy. We prepared a series of probes with different chelators at the C- and N-termini but maintained the peptide sequence of 64Cu-FBP2. We also compared how the chelators were conjugated to the peptide (by amide or thiourea (TU) conjugation) to assess whether this would also affect metabolic stability and imaging efficacy (Figure 11).
Figure 11.
Schematic representation of the structures of the 64Cu-fibrin-binding probes developed for thrombus imaging. Abbreviations: p-TU-Bn-DOTA, S-2-(4-thioureido)methyl)benzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid; p-TU-Bn-NOTA, 2-S-(4-thioureido)methyl)benzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid; CB-TE2A, 2,2'-(1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diyl) mono amide mono acid; NODAGA, 2-(4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl)pentanedioic amide; NOTA-monoamide, NOTA-MA, 2,2',2''-(1,4,7-triazonane-1,4-diyl)diacetic-acid-7-acetic-amide; Pycup, 1,8-(2,6-pyridinedimethylene)-1,4,8,11-tetraazacyclotetradecane-2-acetic-acid-4-acetic-amide; Hyp, L-4-hydroxyproline; Y(3-Cl), L-3-chlorotyrosine; PXD, para-xylenediamine.
In all cases we measured fibrin affinity in vitro and then assessed thrombus uptake in vivo using the rat carotid artery crush injury model described above. For the rat studies, we performed comprehensive evaluations by dynamic PET imaging, full body biodistribution, and autoradiography of the carotid arteries. Pharmacokinetics and metabolism of the probes were also assessed by blood analysis. We first evaluated the effect of the site of conjugation of the DOTA chelator to the peptide. A known limitation of the DOTA-monoamide chelator for 64Cu labelling is some release of the 64Cu ion in vivo.82 We reasoned that if we could prevent or limit 64Cu release, then the thrombus:background ratio would improve. 64Cu-FBP4 utilises a DOTA chelator conjugated to the peptide via a benzyl thiourea linkage off the cyclen backbone (Figure 11). Unlike the DOTA-monoamide, this bifunctional chelator has all four carboxylate donors available for coordination. However, 64Cu-FBP4 was ineffective for targeting thrombus in vivo having a 3-fold lower thrombus uptake compared to 64Cu-FBP2 and a thrombus:blood ratio less than 1.
The cross-bridged cyclam analogue CB-TE2A was reported to greatly improve the stability of copper complexes,83, 84 and we were able to prepare the CB-TE2A derivative 64Cu-FBP7 (Figure 11).85 64Cu-FBP7 showed a marked improvement compared to 64Cu-FBP2. Thrombus uptake was similar to 64Cu-FBP2 (0.5 %ID/g at 2 h post injection) but the thrombus:blood ratio was much higher (5-fold for 64Cu-FBP7 vs 2.5-fold for 64Cu-FBP2) and this ratio increased with time (15:1 at 5 hours post injection). The high target:background was a result of retention in the thrombus and clearance of the unbound signal. The increased kinetic inertness of the CB-TE2A chelate clearly limited transmetallation in vivo. Limitations of 64Cu-FBP7 were the need for prolonged high heating for radiolabelling, but even then complete reaction could not be achieved after 60 minutes at 95 °C. Therefore, HPLC purification was also required for 64Cu-FBP7 before animal studies could be performed.
We next sought an inert 64Cu-chelate which could be prepared without the need for HPLC purification. Replacement of the ethylene cross-bridge in CB-TE2A with a bridging pyridyl moiety gave the novel “pycup probe” 64Cu-FBP12 (Figure 11). The unconjugated pycup chelator could be completely radiolabelled with 64Cu under milder conditions, 70 °C for 15 minutes. In addition, the chelate was stable in blood plasma for 24 hours at 37 °C and only underwent slow decomplexation under very forcing conditions, e.g. when treated with 5 M HCl at 90 °C (t1/2 = 2.7 hr). In vivo evaluation of 64Cu-FBP12 showed that, while the blood background with 64Cu-FBP12 was lower than that of 64Cu-FBP2 (0.090 vs 0.16 %ID/g, respectively), the clot uptake was also lower (0.38 vs 0.45 %ID/g, respectively). Altogether 64Cu-FBP12 exhibited clot:background values in between those of 64Cu-FBP2 and 64Cu-FBP7. The lower imaging efficacy observed with 64Cu-FBP12 could be traced again to metabolic instability, however this time it was primarily degradation of the peptide (only 50% of the plasma activity at 15 min p.i. was intact probe).
We next turned to NOTA chelators. Radiolabelling of NOTA derivatives with 64Cu is known to be efficient and quantitative. Moreover, there were reports that despite the lability of 64Cu-NOTA under strong acidic conditions, the 64Cu-NOTA chelate was quite resistant to transmetallation in vivo.86 Using commercial chelators we prepared three NOTA derivatives that differed in how the NOTA was conjugated to the peptide (Figure 11). 64Cu-FBP5 used a NOTA conjugated via a benzyl thiourea linkage from the triazacyclononane backbone. For 64Cu-FBP8 the NODAGA ligand was conjugated via a pendant carboxylate derived from glutaric acid. 64Cu-FBP9 had NOTA directly conjugated via a carboxylate as a NOTA-monoamide. For 64Cu-FBP5 and 64Cu-FBP8 there are 3 amine N donors and 3 carboxylic acid O donors for coordination to the 64Cu2+ ion resulting in an overall negative charge of the metal complex (Figure 11). For 64Cu-FBP9, there are 3 amine N donors, 2 carboxylic acid O donors, and potentially a neutral amide O donor resulting in a neutral complex. All three could be radiolabelled quantitatively under mild conditions, pH 5.5, 50 °C, 30 minutes, with no need for HPLC purification.
The in vivo behaviour of each, however, was markedly different. 64Cu-FBP5 showed very poor uptake in thrombus (0.082 %ID/g) and a thrombus:blood ratio less than 1, although it still showed high in vitro affinity to the soluble fibrin fragment DD(E) (Ki = 390 nM, unpublished results). This was similar to what we observed with 64Cu-FBP4 which also used a thioureabenzyl linkage to the chelator (Ki = 370 nM, thrombus uptake = 0.160 %ID/g, unpublished results). On the other hand, the amide bond conjugated NODAGA 64Cu-FBP8 and NOTA-monoamide 64Cu-FBP9 both resulted in high thrombus uptake of about 0.8 %ID/g and high thrombus:background ratios.
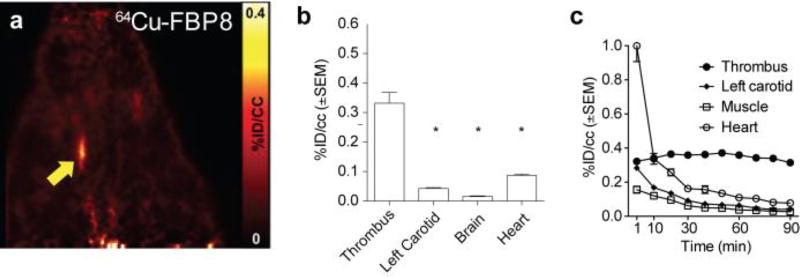
For the best 64Cu probes (64Cu-FBP7, 64Cu-FBP8, 64Cu-FBP9), thrombus could be clearly visualised on PET images from early time points to at least 5 hours post-injection (Figure 12a shows representative results for 64Cu-FBP8 from data collected 30 – 90 min p.i.). The radioactivity was significantly higher in the thrombus compared with the background tissues (contralateral artery, heart, muscle), as shown in Figure 12b. Time-activity curves obtained from the dynamic data showed a steady level of probe uptake at the thrombosed artery, whereas activity in other surrounding tissue decreased over time (Figure 12c). Remarkably, thrombus uptake persists for hours, indicating a slow off-rate of the labelled peptide from the clot and also a slow dissociation of 64Cu from the chelator.
Figure 12.
In vivo evaluation of 64Cu-FBP8. a. Reconstructed PET images for data from 30 – 90 min post injection. b. Corresponding mean PET activity values of thrombus, left carotid, heart, and brain (30 – 90 min p.i.). c. Corresponding time–activity curves of thrombus, left carotid muscle and heart obtained from dynamic PET imaging (*P < 0.0001, vs. thrombus; 1-way ANOVA followed by Tukey post hoc test).
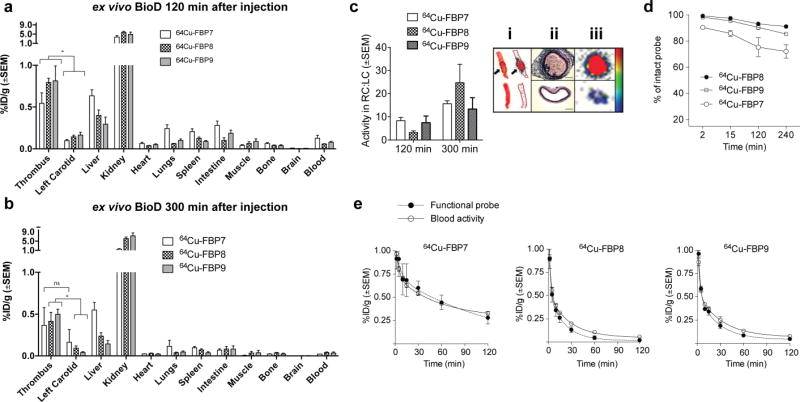
Ex vivo biodistribution studies confirmed the high uptake of 64Cu in the thrombus.85, 87 At 120 min p.i. (Figure 13a), for 64Cu-FBP8 and 64Cu-FBP9 the thrombus had the highest uptake of activity after the kidney, while the CB-TE2A derivative 64Cu-FBP7 had slightly higher activity in the liver compared to thrombus. Both the NOTA derivatives 64Cu-FBP8 and 64Cu-FBP9 showed thrombus:liver ratios greater than 1 at 2 hours and 5 hours p.i.. Importantly, activity in the thrombus persisted for longer than 5 h, while the activity in most other organs decreased with time (Figure 13b). This resulted in a higher thrombus-to-organ ratio at 5 hour p.i. (range of 15 to 20 for thrombus to blood or muscle or bone) when compared to 2 hours p.i. (range of 4 to 8).
Figure 13.
Biodistribution for 64Cu-FBP7, 64Cu-FBP8 and 64Cu-FBP9 at 120 min (a) and 300 min (b) after injection (n = 5 – 7). (c) Mean activity ratios from autoradiography for each probe at 120 and 300 min after injection. Panel c. i shows representative picture and relative autoradiograms of thrombosed carotid arteries showing high uptake at the thrombus site (arrows) but only low activity in the contralateral vessel (left panel). The high activity of the thrombosed vessel (c. iii) corresponds to the intraluminal thrombus and not the vascular tunica, as shown by H & E-stained histological sections. d. Metabolic stability of each probe estimated from HPLC analysis of blood samples (n = 2 – 3/probe). e. Pharmacokinetic data from ex vivo blood analyses. Functional probe was assessed through a fibrin-binding probe assay. (*P < 0.05, ns = not significant, 1-way ANOVA followed by Tukey post hoc test).
Ipsilateral-to-contralateral activity ratios obtained by autoradiography ranged from 4 to 9 at 2 hours p.i. and 15 to 25 at 5 hours p.i. (Figure 13c). Histopathology revealed the presence of mural thrombi and fibrin deposition in the right (ipsilateral) common carotid artery, but not contralaterally (Figure 13c). Analysis of serial blood draws revealed that the new 64Cu probes showed substantially improved metabolic stability compared to the DOTA derivatives, in particular 64Cu-FBP8 and 64Cu-FBP9, as assessed by HPLC analysis of blood plasma (Figure 13d; >85% intact in blood drawn from 0 – 4 h p.i.). Additionally, 64Cu-FBP8 and 64Cu-FBP9 showed a rapid elimination from circulation (Figure 13e, open symbols), however, for 64Cu-FBP7 residual activity was observed in the blood even after 120 min. The concentration of functional fibrin binding probe was similar to the total concentration of activity in the blood (Figure 13e, closed symbols), suggesting minimal decomposition for these 3 probes. The slower blood clearance and the higher liver uptake observed for 64Cu-FBP7 compared to 64Cu-FBP8 or 64Cu-FBP9 is suggestive of increased copper transmetallation with the CB-TE2A chelate. 64Cu-FBP7 was prepared by preparative HPLC which would rule out any injection of unchelated 64Cu. The inferiority of the CB-TE2A derivative relative to the NOTA derivatives was somewhat surprising given the well documented kinetic inertness of the Cu-CB-TE2A chelate, but this underscores how in vivo behaviour can not necessarily be predicted by in vitro physical chemistry measurements.
Extending the Palette of Radionuclides for Thrombus Imaging
Given the strong influence of the chelator and/or linker on thrombus uptake, we anticipated that use of alternative radionuclides would also exhibit different behaviours. Of the other radionuclides that could also be used, 18F has favourable properties for this application. Its 109.8 min half-life is well matched to the anticipated human blood half-life, and also its low β+-energy (0.64 MeV) results in higher-resolution PET images compared to higher energy positron emitters. Another potential radioisotope is 68Ga which has a high positron yield (89% of all disintegrations), a convenient half-life of 68 min, and availability from a 68Ge/68Ga generator which provides access to the isotope without a cyclotron.
Although PET offers superior resolution and absolute quantification, SPECT and scintigraphy are much more established in clinical practice due to the lower costs and availability of multiple radioisotopes, especially 99mTc. 99mTc is also produced from a generator and has a 6 h half-life and a 140 keV gamma emission, within the optimal range for detection. The 111In radionuclide is also used in SPECT imaging although its higher energy gamma rays (171 and 245 keV) and longer half-life (2.8 days) often limit the radiochemical dose resulting in images of lower quality.
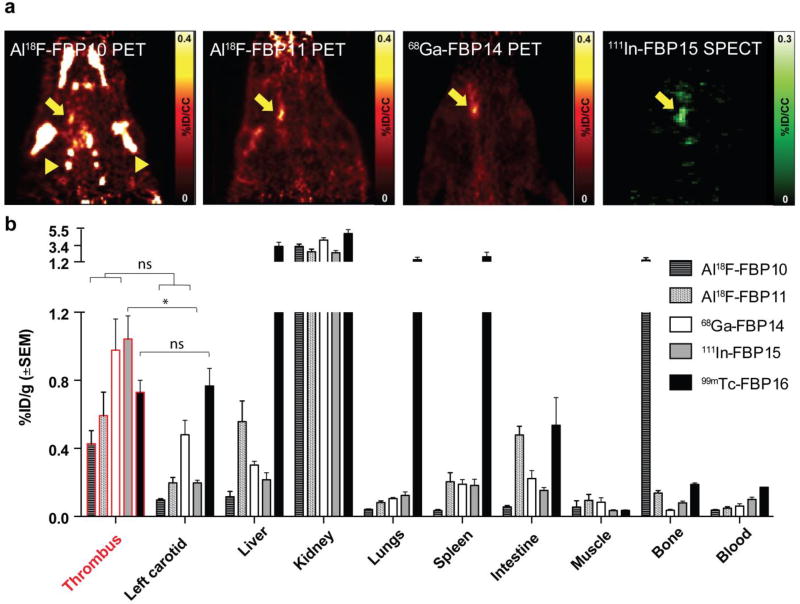
We recognised that several of the precursors developed for the 64Cu-labelled probes could be compatible alternate radionuclides. As such, we used the NODAGA and NOTA-monoamide derivatives to introduce 18F using aluminium fluoride chemistry,88 to give Al18F-FBP10 and Al18F-FBP11, respectively.87 We also used the NODAGA derivative to construct 68Ga-FBP14.89 The DOTA-monoamide conjugate was radiolabelled with In-111 to give 111In-FBP15.89 The choice of the chelators was based on literature describing the superiority of NODAGA and DOTA for stable coordination of 68Ga and 111In in vivo, respectively.90 For 99mTc we synthesised a new precursor using a bifunctional diethylenetriamine ligand (DETA-PA) conjugated to the same fibrin-binding peptide. This precursor allowed radiolabelling with the tricarbonyl precursor fac-[99mTc(CO)3(H2O)3]+ to give 99mTc-FBP16.89 All the probes were evaluated for their affinity for fibrin, imaging profile, metabolic stability, biodistribution and ex vivo autoradiography (Figure 14). Using PET-CT or SPECT-CT imaging, we could readily observe the thrombus with Al18F-FBP11, 68Ga-FBP14, and 111In-FBP15, with high thrombus-to-background ratios (Figure 14a). For Al18F-FBP10 an intense off-target signal was observed in the bone (arrowheads in Figure 14a) with the shoulders, ribs, and jaw all visible. It is well established that free fluoride accumulates in bone and this result suggests that the presence of the additional carboxylate O donor in NODAGA compared to NOTA-monoamide competes with 18F for coordination to aluminium resulting in some dissociation of fluoride. In vivo defluorination from “NODAGA probes” based on the competing coordination mechanism between Al-F and Al-COOH has also been observed by others.91 The NOTA-monoamide derivative Al18F-FBP11 also showed some accumulation in bone although this was more marked at later time points. The 99mTc probe was ineffective to detect the thrombus (Figure 14a). These findings were confirmed by ex vivo γ counting of the harvested tissues (Figure 14b). Al18F-FBP11, 68Ga-FBP14 and 111In-FBP15 accumulated 2 – 4 times more in the thrombosed carotid than in the contralateral vessel. Consistent with this efficacy, Al18F-FBP11, 68Ga-FBP14 and 111In-FBP15 showed a reasonable in vivo stability (>60% intact probe at 1h p.i.). 99mTc-FBP16 showed no thrombus–to–contralateral vessel contrast and high liver (3.2 %ID/g), lung (1.5 %ID/g), and spleen (1.9 %ID/g) accumulation, suggestive of aggregation in vivo.
Figure 14.
In vivo evaluation of the Al18F-FBP10, Al18F-FBP11, 68Ga-FBP14, and 111In-FBP15 probes. a. PET images 30–90 min after injection of Al18F-FBP10, Al18F-FBP11, 68Ga-FBP14, and 111In-FBP15 (n = 3 for Al18F-FBP10 and 6 – 8 for other probes). b. Biodistribution at 120 min (n = 3 – 8). Arrow denotes thrombus and arrowhead bone accumulation. (*P < 0.05, ns = not significant, 1-way ANOVA followed by Tukey post hoc test)
Overall, the rapid blood clearance and low retention in most organs of Al18F-FBP11, 68Ga-FBP14, and 111In-FBP15 suggest translational potential as thrombus imaging agents. Compared with the optimised copper probes, these probes were less stable in vivo, which resulted in higher background radioactivity in the blood and contralateral carotid. It is likely that further improvements could be made by screening different peptide-chelate conjugates with these radioisotopes.
In a study performed by Starmans et al. the fibrin-binding peptide from EP-2104R was radiolabelled with 111In (111In-EPep) and evaluated in a rat model of thrombosis.63 111In-EPep and 111In-FBP15 both had similar thrombus uptake (0.74 %ID/g at 4 h p.i. for 111In-EPep vs 1.04 %ID/g at 3 h p.i. for 111In-FBP15) and comparable accumulation in non-target organs, such as the liver (~0.1 %ID/g at 4 h p.i. for 111In-EPep vs 0.23 %ID/g at 3 h p.i. for 111In-FBP15) and kidneys (~1.6 %ID/g at 4 h p.i. for 111In-EPep vs 2.0 %ID/g at 3 h p.i. for 111In-FBP15).
Specificity of fibrin-binding probes
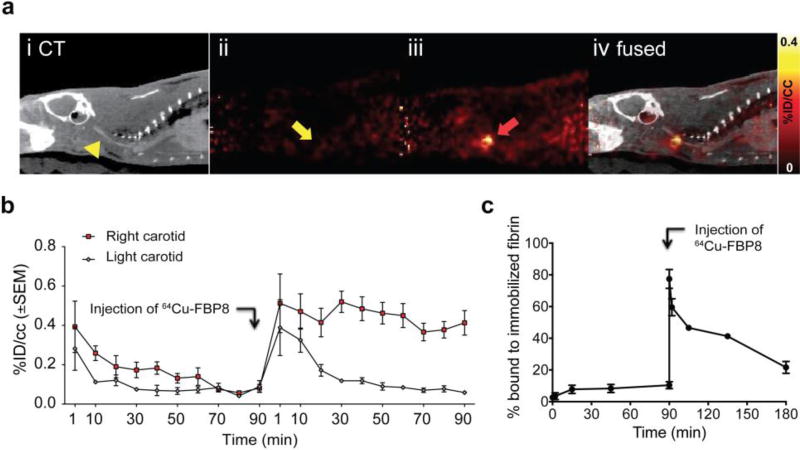
Blocking experiments are commonly used to prove in vivo specificity, particularly in receptor studies where the receptor concentration is in the nanomolar range. In these cases, a large dose of unlabelled probe is administered to saturate the in vivo target, causing a reduction in signal upon introduction of the radiolabelled probe. However, fibrin is present at over 100 µM in thrombus, and the dose required for blocking is implausible. To demonstrate specificity we instead prepared 64Cu-D-Cys-FBP8 as a negative control. 64Cu-D-Cys-FBP8 is an isomer of 64Cu-FBP8 that only differs in the chirality of one cysteine residue, but this modification abolishes the affinity of the peptide for fibrin. The inability of 64Cu-D-Cys-FBP8 to detect thrombus has been confirmed in vivo by PET imaging. In one study, thrombus-containing rats showed no uptake in the thrombus when injected with 64Cu-D-Cys-FBP8 and imaged for 90 minutes (Figure 15a ii). After injected with 64Cu-FBP8, and imaged for a further 90 minutes, a region of high activity was localised to the carotid as observed in both PET (Figure 15a iii, red arrow) and fused PET-CT images (Figure 15a iv). Time–radioactivity curves showed similar enhancement and washout in the ipsilateral and contralateral vessels after injection of 64Cu-D-Cys-FBP8 but after injection of 64Cu-FBP8 the signal in the contralateral vessel decreased over time while the ipsilateral vessel signal remained high (Figure 15b). This indicates that 64Cu-D-Cys-FBP8 is not mechanically trapped in the thrombus whereas 64Cu-FBP8 has high target affinity for fibrin and specifically accumulates in the thrombus. This was further confirmed with blood samples collected over the course of the study and their analysis for fibrin binding (Figure 15c).89
Figure 15.
Specificity of 64Cu-FBP8 for thrombus. a. PET/CT imaging (n = 3) after sequential injection of non-binding 64Cu-D-Cys-FBP8 (aii), followed by injection of its active analog 64Cu-FBP8 (aiii). Thrombus uptake was detected only after injection of 64Cu-FBP8 (red arrow). b. Statistically significant difference in thrombus versus contralateral artery uptake was only observed for 64Cu-FBP8. c. In vitro binding studies of blood plasma incubated with immobilized fibrin showed no binding for 64Cu-D-Cys-FBP8 but significant binding after injection of 64Cu-FBP8 (n = 2). **P < 0.01. ***P < 0.001.
With a validated non-binding probe in hand we designed a multi-modal, multi-isotope imaging experiment to further confirm the high target specificity of the probes for fibrin. We radiolabelled fibrinogen protein with 125I and used this to generate a 125I-labeled thrombus in the carotid artery that could be imaged with SPECT. We then performed energy selective SPECT (125I window and 111In window) after systemic venous injection of 111In-FBP15 followed by 64Cu-D-Cys-FBP8. The SPECT imaging showed co-localisation between 125I and 111In but PET imaging with 64Cu-D-Cys-FBP8 did not reveal any significant uptake at the target site.89 Using a gold nanoparticle CT contrast agent we performed CT angiography to confirm the location of the SPECT signals in the carotid artery. In a complementary study, rats bearing a 125I-labeled thrombus were injected with 111In-FBP15 and then with 68Ga-FBP14. SPECT/PET/CT imaging showed a hot spot corresponding to the 125I, 111In, and 68Ga isotopes on the thrombosed common carotid artery.89
Thrombus Detection, Characterisation, and Treatment Monitoring in preclinical models
After developing an optimised PET probe, we sought to investigate its performance in different settings of thrombosis and to assess whether this probe could be used to stage thrombus or monitor treatment response. Our initial work utilised 64Cu-FBP7, but subsequent studies used 64Cu-FBP8 as this probe provided superior thrombus:background ratios and more facile radiolabelling.
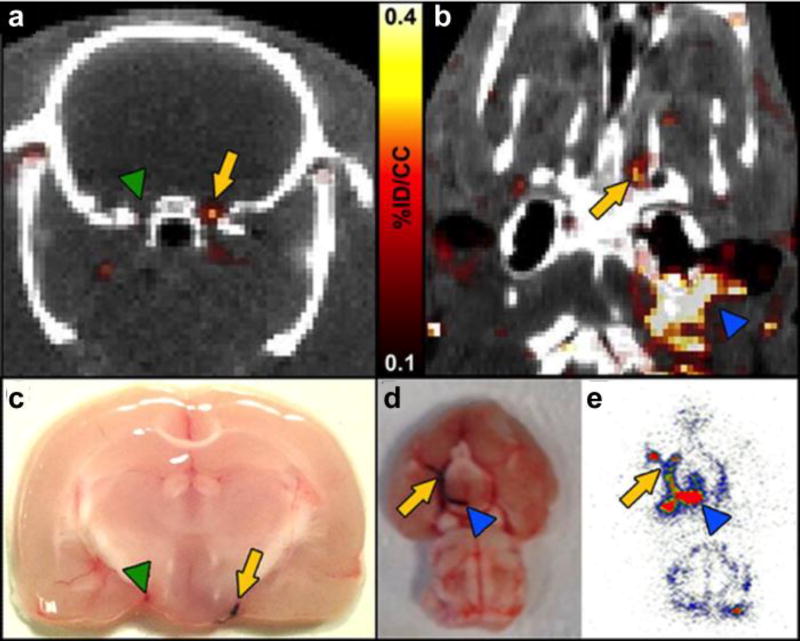
We used 64Cu-FBP7 to image thrombus in the context of embolic stroke.85 We first prepared thrombi by withdrawing blood from a rat and allowing it to clot in polyethylene tubing in the presence of Evans Blue dye. This clot was then reintroduced to the rat via the carotid artery and embolised to the brain. Systemic administration of 64Cu-FBP7 readily detected these emboli as shown in axial (Figure 16a) and coronal (Figure 16b) PET-CT images (yellow arrows) with localisation in the internal carotid/middle cerebral artery (ICA/MCA). In Figure 16b the thrombus extends to the extracranial carotid artery (blue arrowhead). Figure 16c and 16d confirm the presence of the thrombus (stained in blue) in the locations denoted in the PET-CT images in Figures 16a and 16b, respectively. Figure 16e shows an autoradiogram corresponding to the tissue shown in 16d and the image in 16b, and confirms the high signal uptake in the thrombus.
Figure 16.
PET–CT images 64Cu-FBP7 detect occlusive thrombus in both intracranial (a and b) and extracranial (b) arteries (a. arrow denotes internal carotid artery (ICA)/middle cerebral artery (MCA) with thrombus, green arrowhead denotes contralateral ICA/MCA; b. arrow denotes intracranial ICA/MCA, blue arrowhead denotes extracranial ICA). c. and d. Postmortem brains from animals injected with Evans blue-labeled clot (arrow in c denotes ICA/MCA with thrombus, green arrowhead in c denotes contralateral ICA/MCA; arrow in d denotes intracranial ICA/MCA, blue arrowhead in d denotes extracranial ICA). e. Brain autoradiography revealing high activity along the ICA and MCA (arrow denotes intracranial ICA/MCA, blue arrowhead denotes extracranial ICA). Reprinted from ref. 85 with permission. Copyright © 2014 by American Heart Association.
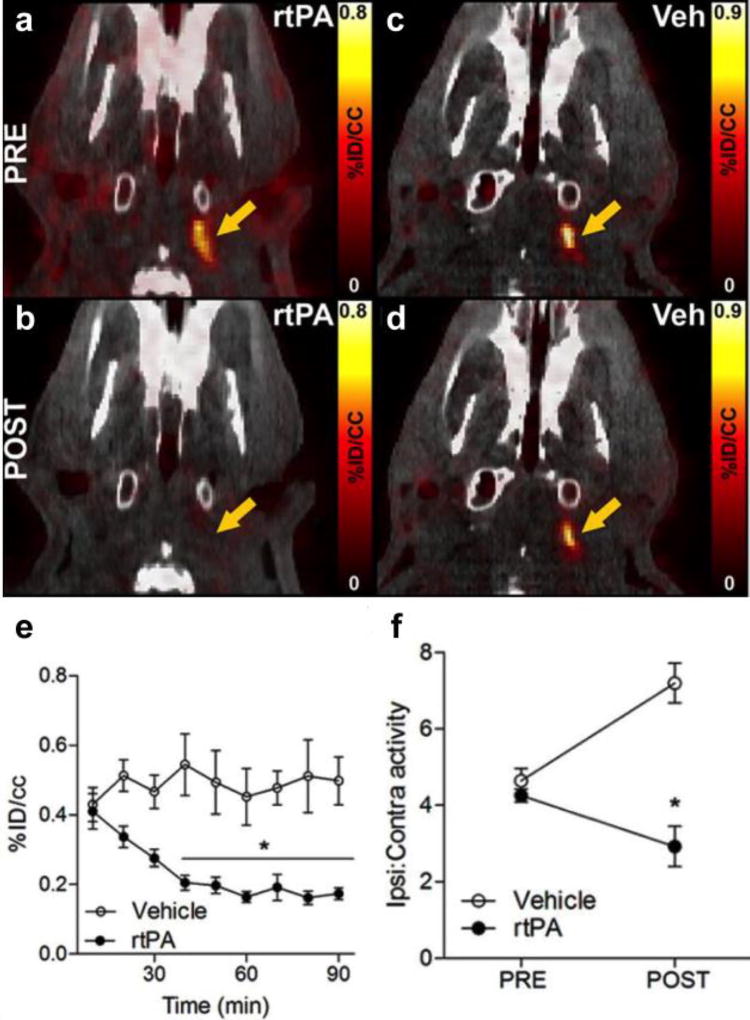
We next examined if 64Cu-FBP7 could be used to monitor thrombolysis by infusion of recombinant tissue plasminogen activator (rtPA). PET/CT imaging with 64Cu-FBP7 allowed clot visualisation as a hyperintense signal in the extracranial ICA (Figure 17a and 17c). After infusion of rtPA treatment this signal was abolished (Figure 17b). For animals infused with saline vehicle (Veh) the signal still remained (Figure 17d). The PET images were reconstructed dynamically to follow the time to clot lysis (Figure 17e). As expected, the ipsilateral-to-contralateral ratio increased over time in saline vehicle injected animals, consistent with a stable signal in the thrombus and increased background clearance with time, while in the rtPA treated animals this ratio decreased with clot lysis (Figure 17f.)
Figure 17.
64Cu-FBP7 enabled in vivo visualization of thrombolysis in extracranial ICA (n = 9 rtPA and n = 7 vehicle). Representative pre (a and c) and post (b and d) PET–CT images at the level of the extracranial ICA (arrow) from rtPA- (a and b) and vehicle-treated animals (c and d). e. Activity at the level of the extracranial ICA showing time-dependent reduction of signal after rtPA treatment (black circles) and steady signal in vehicle-injected rats (white circles). f. Ipsilateral-to-contralateral activity ratios before and after rtPA (black circles) and vehicle (white circles) administration. Error bars represent SEM. *P<0.05. Reprinted from ref. 85 with permission. Copyright © 2014 by American Heart Association.
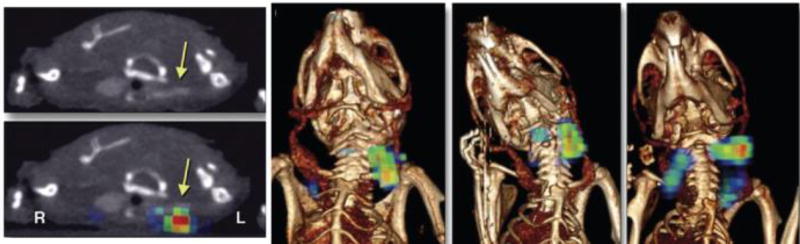
Our initial probe screening studies involved a hyperacute crush injury model of carotid thrombosis. With 64Cu-FBP8 we sought to determine if this probe could detect older arterial thrombi and also venous thrombi. We designed a study where thrombus was induced in the carotid artery and femoral vein by applying a ferric chloride solution to the vessel.40 The site of thrombus (left or right vessel) was randomised and a sham procedure was performed on the contralateral vessel with the exception of applying the ferric chloride solution. 64Cu-FBP8 PET-CT imaging was performed 1, 3, or 7 days after thrombosis to detect thrombus location and to evaluate age-dependent changes in target uptake. Ex vivo biodistribution, autoradiography and histopathology were performed to validate the imaging results. Two blinded readers were able to accurately (97.6% success rate) identify the location of the 42 thrombi in the study.40 In addition, quantitative histopathology also revealed an age-dependent reduction of thrombus fibrin content (P<0.001), which was consistent with the PET results where signal intensity was lower in older thrombi. This study demonstrated the effectiveness of 64Cu-FBP8 in detection of arterial and venous thrombi and showed that PET imaging could provide insight into clot composition.
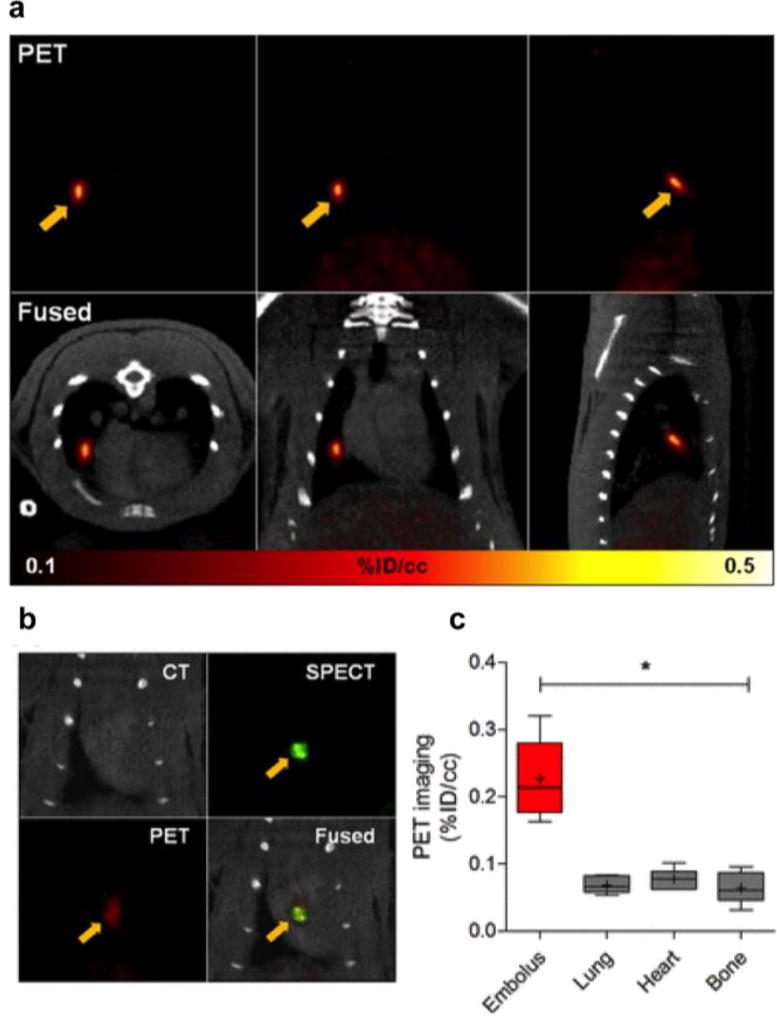
We also demonstrated the feasibility of using 64Cu-FBP8 to detect the source thrombus and the culprit embolus within the same imaging session using a model of venous thromboembolism (Figure 18).92 In a rat model of embolism, 64Cu-FBP8 identified emboli in the lungs (Figure 18a) and cardiac chambers (Figure 18b). An embolus prelabelled with 125I-fibrin was used to verify the location of the embolus by SPECT imaging. 64Cu-FBP8 was then administered intravenously and the PET and SPECT images were compared to confirm that PET imaging with 64Cu-FBP8 could successfully identify the embolus via superimposition of the 64Cu and 125I signals (Figure 18b). PET image quantification showed significant differences in probe uptake between the emboli and the surrounding background tissues (Figure 18c).92
Figure 18.
64Cu-FBP8 PET imaging of lung (a) and cardiac (b) emboli. a. PET-CT images from representative animal injected with 64Cu-FBP8 after induction of pulmonary embolism showing embolus detection in right middle lobe (arrows). b. Representative multimodal PET-SPECT-CT images showing an 125I-embolus detected in cardiac chamber by 64Cu-FBP8. c. PET quantification reveals significant differences in uptake between target embolus and adjacent background tissues. *P < 0.001. Adapted with permission from ref. 92. Copyright (2015) by the Society of Nuclear Medicine and Molecular Imaging, Inc.
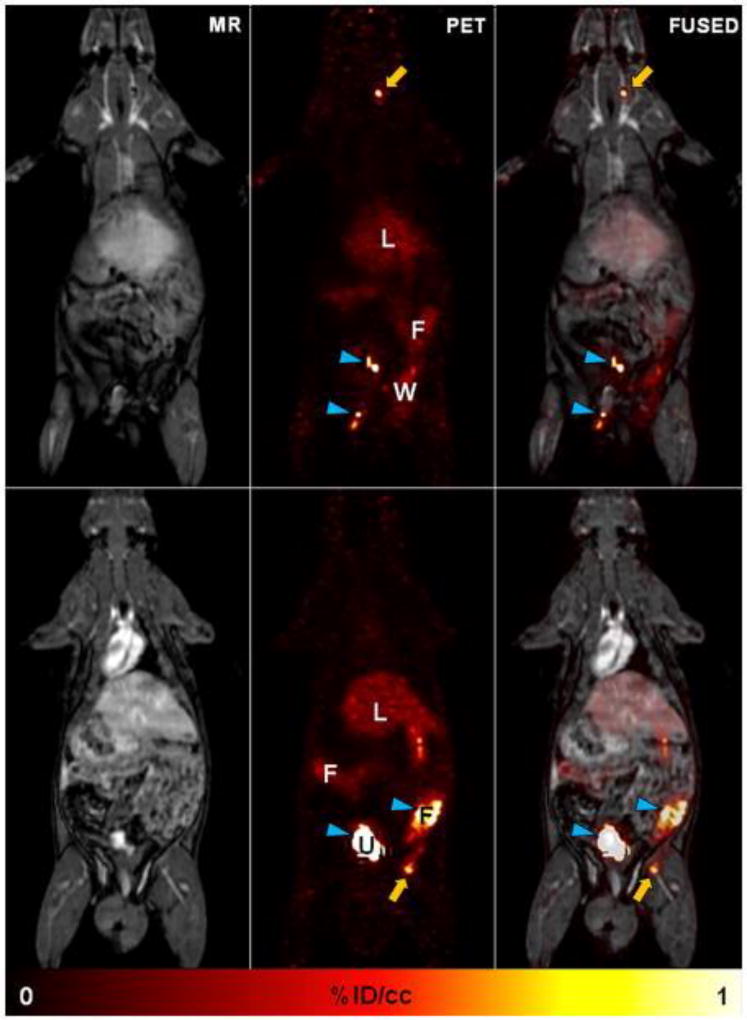
A disadvantage in the current diagnosis of thrombus is the absence of a single-session technique with high sensitivity and specificity that can detect thrombosis in different anatomical locations. In thromboembolism, a time-consuming integrated diagnostic approach involving D-dimer blood tests, compression ultrasound for DVT and CT pulmonary angiography for PE is applied. Similarly, with stroke it is important to find the source of the embolus, but this could arise from the heart, aortic arch, carotid arteries or from a DVT which again requires multiple diagnostic tests. Both diagnosis and treatment planning would benefit from a single method able to identify the location of the embolus (e.g. cerebral or pulmonary artery) as well as the source thrombus (e.g. DVT, aortic arch). This prompted us to test 64Cu-FBP8 in another clinically relevant situation: to prove the ability of 64Cu-FBP8 to detect multisite thrombi using a single whole-body PET scan.40 Rats were imaged for 60 mins in a clinical PET-MR scanner starting 1 hour p.i of 64Cu-FBP8. The location of both arterial and venous thrombi (Figure 19: top and bottom panels respectively) was clearly identified in an extended field of view after a single administration of 64Cu-FBP8. The thrombi were conspicuous and background signal was low. The high thrombus:background ratio is even more impressive considering the severe partial volume signal loss as a result of the approximate 0.5 mm diameter of the rat carotid and the 3 mm resolution of the PET camera.
Figure 19.
64Cu-FBP8-PET/MR whole-body imaging. With a single method 64Cu-FBP8 is able to detect thrombus in the right carotid artery (top) and right femoral vein (bottom). Activity in urine (U) and feces (F) denoted by blue arrowheads; L = liver; W = surgical wound. Reprinted from ref. 40 with permission. Copyright © 2014 by American Heart Association.
Human translation of 64Cu-FBP8
Given the favourable properties of 64Cu-FBP8 in terms of thrombus uptake, background clearance, and demonstrated efficacy in a number of preclinical models, we sought to evaluate this probe in humans. Our rationale for advancing the 64Cu-labeled peptide was based on the preclinical efficacy of 64Cu-FBP8 which provided a thrombus:contralateral vessel ratio that was 2 to 5 times higher for 64Cu-FBP8 than peptides labelled with other radionuclides. Furthermore, unlike F-18 chemistry which requires a nearby cyclotron and multistep synthesis, the production of 64Cu-FBP8 is facile and ideally suited to a kit formulation: 40 minutes at 60 °C in acetate buffer results in complete conversion to product, with no further purification needed. The F-18 probes that we prepared all required multiple steps, chromatography, and the use of organic solvents and as such are not suitable for kit formulation. The 12.7 hour half-life of 64Cu is also a benefit as it is long enough to allow for remote production of 64CuCl2, shipping and 64Cu-FBP8 production, allowing studies to be performed at sites without access to a cyclotron. The longer half-life also enables studies to assess clot lysis where a patient could be injected and imaged, given a lytic drug like alteplase, and then imaged hours later or even the next day without the need for a second injection of the probe. 64Cu-FBP8 is also rapidly eliminated from the body into the urine lowering the concern often faced with longer-lived radionuclide regarding extensive radiation exposure from compounds retained in the body. Radiation exposure, however, was still assessed and detailed biodistribution studies performed on healthy rats (n = 30) at different time points after administration of 64Cu-FBP8.92 The results confirmed that 64Cu-FBP8 has favourable dosimetry properties for human translation with an effective dose of 0.021 mSv/MBq for males and 0.027 mSv/MBq for females. These results also suggest a low risk of radiogenic adverse health effects. 64Cu-FBP8 is currently being tested in healthy volunteers and in patients at the Massachusetts General Hospital (Boston, USA).
Conclusions
Thrombosis is a critical event in CVD such as myocardial infarction, stroke, and pulmonary embolism, which account for considerable morbidity and mortality. Currently detection of thrombus often requires multiple diagnostic techniques that depend on the location of the thrombus. For example, ultrasound is needed for detection of DVT, invasive transesophageal echocardiography (TEE) and MR imaging for cardiac chamber clots, and CT pulmonary angiography for PE. Despite the success of these techniques, there is a need for imaging tests that can identify thrombus anywhere in the body and can be used to image multiple vascular territories. Moreover, current techniques do not provide information on thrombus or embolus composition which may prove invaluable in guiding treatment decisions. The fibrin-specific peptides described in this review enable the development of thrombus specific probes using different imaging modalities. Here we described examples of probes that could detect thrombus by MR, optical, PET, or SPECT imaging. The MR probe EP-2104R was shown to be effective in detection of thrombi in humans. Having EP-2104R as a starting point we optimised a 64Cu-labeled probe that revealed fast renal blood clearance, low background signal, and persistent uptake in thrombus. We anticipate that data with 64Cu-FBP8 in humans will be forthcoming. For clinical applications with 64Cu-FBP8 one could estimate an imaging paradigm where the patient is imaged up to three hours after administration of the probe, allowing ample time for blood clearance. Given the extent of structure-activity data available, it is clear that other probes using a different isotope or imaging reporter could also be developed for human use. The probes Al18F-FBP11, 68Ga-FBP14, and 111In-FBP15, all showed the ability to detect thrombus in rats and we are optimistic that PET or SPECT probes such as these may find clinical utility.
Acknowledgments
PC acknowledges the National Heart Lung and Blood Institute (R01HL109448) and the National Institute for Biomedical Imaging and Bioengineering (R01EB009062) for supporting most of the work presented here. Instrumentation grants S10RR029495, S10OD010650, and S10RR023385 from the National Institutes of Health enabled this work. BLO thanks the EU for the Marie-Sklodowska Curie IEF. We dedicate this review to Professor Isabel Santos for her contributions to the field of nuclear chemistry. BLO would like to personally thank Professor Isabel Santos for her mentorship and for setting a great example as a scientist.
Biographies

Bruno Oliveira received his PhD from the University of Lisbon in 2012 under the guidance of Profs. João Galamba and Isabel Santos. His PhD work involved collaborative visits to the groups of Prof. Roger Alberto (University of Zurich, Switzerland), Prof. Maria João Ramos (University of Oporto, Portugal) and Prof. Francisco Blanco (CNIO, Spain). In 2013 he moved to Boston, USA to work with Prof. Peter Caravan (Massachusetts General Hospital/Harvard Medical School) in the development of PET/SPECT nuclear probes for thrombus detection. Since 2015 he has been working with Dr. Gonçalo Bernardes, University of Cambridge, where he develops new bioorthogonal methods for protein modification and radiolabeling.

Peter Caravan received his BSc from Acadia University and PhD in Chemistry from the University of British Columbia with Professor Chris Orvig. After postdoctoral work with Professor André Merbach at the Université de Lausanne, Dr Caravan joined EPIX Medical in 1998 to develop targeted MR probes including the FDA-approved probe Ablavar. In 2007 he moved to the A. A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH) and Harvard Medical School to establish a translational molecular imaging laboratory. In 2014 he was named Co-Director of the Institute for Innovation in Imaging at MGH. His current research focuses on the development of MR and PET probes and their applications in cardiovascular disease, fibrotic diseases, and cancer.
References
- 1.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:2363. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SP. Arterial thrombosis[mdash]insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality Cardiovascular Molecular Imaging, Part I. Circulation: Cardiovascular Imaging. 2008;1:244–256. doi: 10.1161/CIRCIMAGING.108.824359. [DOI] [PubMed] [Google Scholar]
- 6.Dronkers CE, Klok FA, Huisman MV. Current and future perspectives in imaging of venous thromboembolism. J Thromb Haemost. 2016;14:1696–1710. doi: 10.1111/jth.13403. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 8.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 10.Cherry SR. Multimodality imaging: beyond PET/CT and SPECT/CT. Semin Nucl Med. 2009;39:348–353. doi: 10.1053/j.semnuclmed.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger JR. Pitfalls and Limitations of SPECT, PET, and Therapeutic Radiopharmaceuticals. Semin Nucl Med. 2015;45:470–478. doi: 10.1053/j.semnuclmed.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Khalil MM, Tremoleda JL, Bayomy TB, Gsell W. Molecular SPECT Imaging: An Overview. International Journal of Molecular Imaging. 2011;2011 doi: 10.1155/2011/796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson O, Kiesewetter DO, Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem. 2015;26:1–18. doi: 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland JP, Williamson MJ, Lewis JS. Unconventional nuclides for radiopharmaceuticals. Mol Imaging. 2010;9:1–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey DL, Willowson KP. Quantitative SPECT/CT: SPECT joins PET as a quantitative imaging modality. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41:17–25. doi: 10.1007/s00259-013-2542-4. [DOI] [PubMed] [Google Scholar]
- 16.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chemical Reviews. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 17.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 18.Boros E, Gale EM, Caravan P. MR imaging probes: design and applications. Dalton Trans. 2015;44:4804–4818. doi: 10.1039/c4dt02958e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thukkani AK, Jaffer FA. Intravascular near-infrared fluorescence molecular imaging of atherosclerosis. American Journal of Nuclear Medicine and Molecular Imaging. 2013;3:217–231. [PMC free article] [PubMed] [Google Scholar]
- 20.Taqueti VR, Jaffer FA. High-resolution molecular imaging via intravital microscopy: illuminating vascular biology in vivo. Integrative Biology. 2013;5:278–290. doi: 10.1039/c2ib20194a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrucki LW, Sinusas AJ. PET and SPECT in cardiovascular molecular imaging. Nat Rev Cardiol. 2010;7:38–47. doi: 10.1038/nrcardio.2009.201. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29:983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004;94:433–445. doi: 10.1161/01.RES.0000119321.18573.5A. [DOI] [PubMed] [Google Scholar]
- 24.Shaw SY. Molecular imaging in cardiovascular disease: targets and opportunities. Nat Rev Cardiol. 2009;6:569–579. doi: 10.1038/nrcardio.2009.119. [DOI] [PubMed] [Google Scholar]
- 25.Rivera J, Lozano ML, Navarro-Núñez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 27.Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502–512. doi: 10.1038/nrcardio.2011.91. [DOI] [PubMed] [Google Scholar]
- 28.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thrombosis Research. 2007;120:S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryckaert M, Rosa JP, Denis CV, Lenting PJ. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72:307–326. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackman N. New insights into the mechanisms of venous thrombosis. The Journal of Clinical Investigation. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakefield TW, Myers DD, Henke PK. Mechanisms of Venous Thrombosis and Resolution. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 33.Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29:943–953. doi: 10.1592/phco.29.8.943. [DOI] [PubMed] [Google Scholar]
- 34.Cohen AT, Dobromirski M, Gurwith MM. Managing pulmonary embolism from presentation to extended treatment. Thromb Res. 2014;133:139–148. doi: 10.1016/j.thromres.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Guercini F, Acciarresi M, Agnelli G, Paciaroni M. Cryptogenic stroke: time to determine aetiology. J Thromb Haemost. 2008;6:549–554. doi: 10.1111/j.1538-7836.2008.02903.x. [DOI] [PubMed] [Google Scholar]
- 36.Haeusler KG, Wollboldt C, Bentheim LZ, et al. Feasibility and Diagnostic Value of Cardiovascular Magnetic Resonance Imaging After Acute Ischemic Stroke of Undetermined Origin. Stroke. 2017;48:1241–1247. doi: 10.1161/STROKEAHA.116.016227. [DOI] [PubMed] [Google Scholar]
- 37.Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 38.Morris TA, Marsh JJ, Chiles PG, et al. Single Photon Emission Computed Tomography of Pulmonary Emboli and Venous Thrombi Using Anti–D-Dimer. American Journal of Respiratory and Critical Care Medicine. 2004;169:987–993. doi: 10.1164/rccm.200306-735OC. [DOI] [PubMed] [Google Scholar]
- 39.Andia ME, Saha P, Jenkins J, et al. Fibrin-targeted magnetic resonance imaging allows in vivo quantification of thrombus fibrin content and identifies thrombi amenable for thrombolysis. Arterioscler Thromb Vasc Biol. 2014;34:1193–1198. doi: 10.1161/ATVBAHA.113.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasi F, Oliveira BL, Rietz TA, et al. Multisite Thrombus Imaging and Fibrin Content Estimation With a Single Whole-Body PET Scan in Rats. Arterioscler Thromb Vasc Biol. 2015;35:2114–2121. doi: 10.1161/ATVBAHA.115.306055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houshmand S, Salavati A, Hess S, Ravina M, Alavi A. The role of molecular imaging in diagnosis of deep vein thrombosis. Am J Nucl Med Mol Imaging. 2014;4:406–425. [PMC free article] [PubMed] [Google Scholar]
- 42.Ciesienski KL, Caravan P. Molecular MRI of Thrombosis. Curr Cardiovasc Imaging Rep. 2010;4:77–84. doi: 10.1007/s12410-010-9061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Peter K. Molecular Imaging of Atherothrombotic Diseases: Seeing Is Believing. Arterioscler Thromb Vasc Biol. 2017;37:1029–1040. doi: 10.1161/ATVBAHA.116.306483. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Chakraborty S, Liu S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Imaging Tumors and Thrombosis by SPECT. Theranostics. 2011;1:58–82. doi: 10.7150/thno/v01p0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klink A, Lancelot E, Ballet S, et al. Magnetic resonance molecular imaging of thrombosis in an arachidonic acid mouse model using an activated platelet targeted probe. Arterioscler Thromb Vasc Biol. 2010;30:403–410. doi: 10.1161/ATVBAHA.109.198556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muto P, Lastoria S, Varrella P, et al. Detecting Deep Venous Thrombosis with Technetium-99m-Labeled Synthetic Peptide P280. Journal of Nuclear Medicine. 1995;36:1384–1391. [PubMed] [Google Scholar]
- 47.Klem JA, Schaffer JV, Crane PD, et al. Detection of deep venous thrombosis by DMP 444, a platelet IIb/IIIa antagonist: A preliminary report. Journal of Nuclear Cardiology. 2000;7:359–364. doi: 10.1067/mnc.2000.106967. [DOI] [PubMed] [Google Scholar]
- 48.Fang W, He J, Kim YS, Zhou Y, Liu S. Evaluation of 99mTc-labeled cyclic RGD peptide with a PEG4 linker for thrombosis imaging: comparison with DMP444. Bioconjug Chem. 2011;22:1715–1722. doi: 10.1021/bc2003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taillefer R. Radiolabeled peptides in the detection of deep venous thrombosis. Seminars in Nuclear Medicine. 2001;31:102–123. doi: 10.1053/snuc.2001.21268. [DOI] [PubMed] [Google Scholar]
- 50.Rouzet F, Bachelet-Violette L, Alsac J-M, et al. Radiolabeled Fucoidan as a P-Selectin Targeting Agent for In Vivo Imaging of Platelet-Rich Thrombus and Endothelial Activation. Journal of Nuclear Medicine. 2011;52:1433–1440. doi: 10.2967/jnumed.110.085852. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki M, Bachelet-Violette L, Rouzet F, et al. Ultrasmall superparamagnetic iron oxide nanoparticles coated with fucoidan for molecular MRI of intraluminal thrombus. Nanomedicine (Lond) 2015;10:73–87. doi: 10.2217/nnm.14.51. [DOI] [PubMed] [Google Scholar]
- 52.Jaffer FA, Tung CH, Gerszten RE, Weissleder R. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. Arterioscler Thromb Vasc Biol. 2002;22:1929–1935. doi: 10.1161/01.atv.0000033089.56970.2d. [DOI] [PubMed] [Google Scholar]
- 53.Page MJ, Lourenço AL, David T, et al. Non-invasive imaging and cellular tracking of pulmonary emboli by near-infrared fluorescence and positron-emission tomography. 2015;6:8448. doi: 10.1038/ncomms9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miserus RJ, Herias MV, Prinzen L, et al. Molecular MRI of early thrombus formation using a bimodal alpha2-antiplasmin-based contrast agent. JACC Cardiovasc Imaging. 2009;2:987–996. doi: 10.1016/j.jcmg.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37:e13–e21. doi: 10.1161/ATVBAHA.117.308564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stracke CP, Katoh M, Wiethoff AJ, Parsons EC, Spangenberg P, Spüntrup E. Molecular MRI of Cerebral Venous Sinus Thrombosis Using a New Fibrin-Specific MR Contrast Agent. Stroke. 2007;38:1476–1481. doi: 10.1161/STROKEAHA.106.479998. [DOI] [PubMed] [Google Scholar]
- 57.Standeven KF, Ariëns RAS, Grant PJ. The molecular physiology and pathology of fibrin structure/function. Blood Reviews. 2005;19:275–288. doi: 10.1016/j.blre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Knight LC. Scintigraphic methods for detecting vascular thrombus. J Nucl Med. 1993;34:554–561. [PubMed] [Google Scholar]
- 59.Macfarlane D, Socrates A, Eisenberg P, et al. Imaging of deep venous thrombosis in patients using a radiolabelled anti-D-dimer Fab′ fragment (99mTc-DI-DD3B6/22-80B3): results of a phase I trial. European Journal of Nuclear Medicine and Molecular Imaging. 2009;36:250–259. doi: 10.1007/s00259-008-0934-7. [DOI] [PubMed] [Google Scholar]
- 60.Morris TA, Gerometta M, Yusen RD, et al. Detection of Pulmonary Emboli With 99mTc-Labeled Anti–D-dimer (DI-80B3)Fab′ Fragments (ThromboView) American Journal of Respiratory and Critical Care Medicine. 2011;184:708–714. doi: 10.1164/rccm.201104-0624OC. [DOI] [PubMed] [Google Scholar]
- 61.Lanza GM, Cui G, Schmieder AH, et al. An unmet clinical need: The history of thrombus imaging. Journal of Nuclear Cardiology. 2017:1–12. doi: 10.1007/s12350-017-0942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilch J, Brown DM, Komatsu M, et al. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2800–2804. doi: 10.1073/pnas.0511219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starmans LWE, van Duijnhoven SMJ, Rossin R, et al. Evaluation of 111In-Labeled EPep and FibPep as Tracers for Fibrin SPECT Imaging. Molecular Pharmaceutics. 2013;10:4309–4321. doi: 10.1021/mp400406x. [DOI] [PubMed] [Google Scholar]
- 64.Kolodziej AF, Nair SA, Graham P, et al. Fibrin Specific Peptides Derived by Phage Display: Characterization of Peptides and Conjugates for Imaging. Bioconjugate Chemistry. 2012;23:548–556. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nair SA, Kolodziej AF, Bhole G, Greenfield MT, McMurry TJ, Caravan P. Monovalent and Bivalent Fibrin-specific MRI Contrast Agents for Detection of Thrombus. Angewandte Chemie International Edition. 2008;47:4918–4921. doi: 10.1002/anie.200800563. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Kolodziej AF, Qi J, et al. Effect of Peptide-Chelate Architecture on Metabolic Stability of Peptide-based MRI Contrast Agents. New J Chem. 2010;2010:611–616. doi: 10.1039/b9nj00787c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Botnar RM, Perez AS, Witte S, et al. In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation. 2004;109:2023–2029. doi: 10.1161/01.CIR.0000127034.50006.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Overoye-Chan K, Koerner S, Looby RJ, et al. EP-2104R: A Fibrin-Specific Gadolinium-Based MRI Contrast Agent for Detection of Thrombus. Journal of the American Chemical Society. 2008;130:6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 69.Spuentrup E, Katoh M, Wiethoff AJ, et al. Molecular magnetic resonance imaging of pulmonary emboli with a fibrin-specific contrast agent. Am J Respir Crit Care Med. 2005;172:494–500. doi: 10.1164/rccm.200503-379OC. [DOI] [PubMed] [Google Scholar]
- 70.Uppal R, Ay I, Dai G, Kim YR, Sorensen AG, Caravan P. Molecular MRI of Intracranial Thrombus in a Rat Ischemic Stroke Model. Stroke. 2010;41:1271–1277. doi: 10.1161/STROKEAHA.109.575662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spuentrup E, Buecker A, Katoh M, et al. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation. 2005;111:1377–1382. doi: 10.1161/01.CIR.0000158478.29668.9B. [DOI] [PubMed] [Google Scholar]
- 72.Spuentrup E, Fausten B, Kinzel S, et al. Molecular magnetic resonance imaging of atrial clots in a swine model. Circulation. 2005;112:396–399. doi: 10.1161/CIRCULATIONAHA.104.529941. [DOI] [PubMed] [Google Scholar]
- 73.Spuentrup E, Botnar RM, Wiethoff AJ, et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur Radiol. 2008;18:1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]
- 74.Vymazal J, Spuentrup E, Cardenas-Molina G, et al. Thrombus Imaging With Fibrin-Specific Gadolinium-Based MR Contrast Agent EP-2104R: Results of a Phase II Clinical Study of Feasibility. Investigative radiology. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 75.Gale EM, Atanasova IP, Blasi F, Ay I, Caravan P. A Manganese Alternative to Gadolinium for MRI Contrast. J Am Chem Soc. 2015;137:15548–15557. doi: 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hara T, Bhayana B, Thompson B, et al. Molecular Imaging of Fibrin Deposition in Deep Vein Thrombosis Using Fibrin-Targeted Near-Infrared Fluorescence. JACC: Cardiovascular Imaging. 2012;5:607–615. doi: 10.1016/j.jcmg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein-Merlob AF, Kessinger CW, Erdem SS, et al. Blood Accessibility to Fibrin in Venous Thrombosis is Thrombus Age-Dependent and Predicts Fibrinolytic Efficacy: An In Vivo Fibrin Molecular Imaging Study. Theranostics. 2015;5:1317–1327. doi: 10.7150/thno.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein-Merlob AF, Hara T, McCarthy JR, et al. Atheroma Susceptible to Thrombosis Exhibit Impaired Endothelial Permeability In Vivo as Assessed by Nanoparticle-Based Fluorescence Molecular Imaging. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loving GS, Caravan P. Activation and Retention: A Magnetic Resonance Probe for the Detection of Acute Thrombosis. Angewandte Chemie International Edition. 2014;53:1140–1143. doi: 10.1002/anie.201308607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uppal R, Catana C, Ay I, Benner T, Sorensen AG, Caravan P. Bimodal thrombus imaging: simultaneous PET/MR imaging with a fibrin-targeted dual PET/MR probe--feasibility study in rat model. Radiology. 2011;258:812–820. doi: 10.1148/radiol.10100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ciesienski KL, Yang Y, Ay I, et al. Fibrin-targeted PET probes for the detection of thrombi. Mol Pharm. 2013;10:1100–1110. doi: 10.1021/mp300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boswell CA, Sun X, Niu W, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 83.Cai Z, Anderson CJ. Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. J Labelled Comp Radiopharm. 2014;57:224–230. doi: 10.1002/jlcr.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium and Zirconium for PET and SPECT Imaging of Disease. Chemical Reviews. 2010;110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ay I, Blasi F, Rietz TA, et al. In vivo molecular imaging of thrombosis and thrombolysis using a fibrin-binding positron emission tomographic probe. Circ Cardiovasc Imaging. 2014;7:697–705. doi: 10.1161/CIRCIMAGING.113.001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffman TJ, Smith CJ. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nuclear Medicine and Biology. 2009;36:579–585. doi: 10.1016/j.nucmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Blasi F, Oliveira BL, Rietz TA, et al. Effect of Chelate Type and Radioisotope on the Imaging Efficacy of 4 Fibrin-Specific PET Probes. J Nucl Med. 2014;55:1157–1163. doi: 10.2967/jnumed.113.136275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al(18)F) EJNMMI Research. 2013;3:36–36. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oliveira BL, Blasi F, Rietz TA, Rotile NJ, Day H, Caravan P. Multimodal Molecular Imaging Reveals High Target Uptake and Specificity of 111In- and 68Ga-Labeled Fibrin-Binding Probes for Thrombus Detection in Rats. Journal of Nuclear Medicine. 2015;56:1587–1592. doi: 10.2967/jnumed.115.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roosenburg S, Laverman P, Joosten L, et al. PET and SPECT Imaging of a Radiolabeled Minigastrin Analogue Conjugated with DOTA, NOTA, and NODAGA and Labeled with 64Cu, 68Ga, and 111In. Molecular Pharmaceutics. 2014;11:3930–3937. doi: 10.1021/mp500283k. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Hu X, Liu H, et al. A Comparative Study of Radiolabeled Bombesin Analogs for the PET Imaging of Prostate Cancer. Journal of Nuclear Medicine. 2013;54:2132–2138. doi: 10.2967/jnumed.113.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blasi F, Oliveira BL, Rietz TA, et al. Radiation Dosimetry of the Fibrin-Binding Probe (6)(4)Cu-FBP8 and Its Feasibility for PET Imaging of Deep Vein Thrombosis and Pulmonary Embolism in Rats. J Nucl Med. 2015;56:1088–1093. doi: 10.2967/jnumed.115.157982. [DOI] [PMC free article] [PubMed] [Google Scholar]