Abstract
The peroxisome-proliferator-activated-receptor-γ (PPARγ) is a member of the nuclear receptor superfamily that plays a critical role in diverse biological processes, including adipogenesis, lipid metabolism, and placental development. To study the activity of PPARγ, we constructed two new reporter genes: a fluorescent GFP-tagged histone-2B (PPRE-H2B-eGFP) and a secreted nanoluciferase (PPRE-pNL1.3[secNluc]). This study demonstrates their usage to monitor PPARγ activity in different cell types and screen for PPARγ's potential ligands.
1. Introduction
PPARγ is an isoform of the PPAR subset of nuclear receptors that also includes PPARα and PPARβ. They all bind to DNA as heterodimers with retinoid X receptors (RXR). The heterodimers activate the expression of their target genes by binding to peroxisome proliferator response elements (PPREs), which are composed of direct tandem repeats of a consensus sequence spaced by a single nucleotide. Such PPREs are found in genes that are involved in lipid metabolism and homeostasis [1]. PPARγ is a ligand-activated transcription factor that is involved in embryonic development [2, 3], lipid metabolism [4, 5], insulin resistance [6], inflammation [6], immune response, and differentiation of several tissues, including placenta and trophoblast differentiations [2, 7]. The ligand-binding domain (LBD) of PPARγ has a larger and more accessible tertiary structure than that of many other nuclear receptors, enabling the binding of a wide spectrum of ligands [8].
Within the last two decades, PPARγ has become a focus of attention as a transcription factor implicated in metabolic syndrome [9]. Indeed, PPARγ has important roles in pathologies, such as obesity [10], cardiovascular diseases [11, 12], type 2 diabetes [10], atherosclerosis [13], or lipodystrophy [14] because it has lipophilic compounds as ligands: fatty acids and their derivatives, for instance. Thiazolidinediones (TZDs) are synthetic ligands (potent activators of PPARγ) that have been used in treating type 2 diabetes but were finally withdrawn due to critical cardiovascular diseases and bladder cancers as side effects [15]. It is thus of general interest to identify new PPARγ-modulators with less or no side effects.
PPARs have a relatively high basal activity [16]. To date, two approaches have been described to investigate the activity of PPARγ, based on (i) cell transfection with a nonsecreted luciferase reporter gene (transiently in cells of interest [17] or stably using cell lines [18]) and (ii) a humanized in vivo reporter model (Xenopus laevis [19]).
Historically, the luciferase (Luc) gene has been successfully used for many years (PPREx3-TK-luc, PPRE-Luc, e.g., [20, 21]), but to measure Luc expression the cells need to be lysed so that a different well is needed at each time point of the assay.
More recently, the green fluorescent protein (GFP or eGFP) has become the reporter of choice for in vitro time lapse imaging and in vivo analysis [19]. This inert reporter is excited by UV light (395 nm) and emits in green light (509 nm) making it easier to visualize using fluorescence microscopy.
Given the importance of PPARγ as a key regulator in several tissues, it is essential to develop a new method to quantify and monitor the activity of PPARγ in any cell types. While PPARγ transcriptional nonsecreted luciferase reporters do exist [20, 21], nuclear-localized fluorescent and secreted luciferase reporters suitable for live imaging (and cell sorting) or quantification without cell lysis are lacking. These tools will present an advantage especially when the number of cells is limited (e.g., primary cultures), or to perform a time course. Furthermore, at the end of the assay, cells can be used for additional experiments (RT-qPCR, western blot).
The current work describes the construction and evaluation of two novel reporters, one based on GFP (PPRE-H2B-eGFP) and the other on a secreted luciferase (PPRE-pNL1.3[secNluc]). To quantify the transcriptional responses elicited from these reporters, we used the well-characterized agonist (GW1929) and antagonist (GW9662) of PPARγ in human trophoblasts, where PPARγ is activated during their differentiation [22]. The exchange and endocrine tissue of the human placenta is the syncytiotrophoblast (ST). It is renewed all along pregnancy by fusion with the underlying villous cytotrophoblast (VCT). This fusion process can be studied in vitro, using human primary cultures of VCT. The functionality of the ST is assessed by the measurement of hCG secretion in the supernatant [23].
Our results verified the benefits of these 2 reporters in evaluating different PPARγ ligands taking into account different cell specificities.
2. Materials and Methods
2.1. Ethical Statement
The study was performed according to the Declaration of Helsinki. Placentas were obtained with the patients' written informed consent. The protocol was approved by the local ethics committee (CPP 2015-mai-13909). Placental tissues were obtained from women with uncomplicated pregnancy undergoing normal Cesarean section at the Cochin Port-Royal, Antony and Montsouris maternity units (Paris, France).
2.2. Cell Culture
Villous cytotrophoblasts (VCT) were isolated from human term placentas as previously described [24–26]. Briefly, several cubic millimeters of the basal plate surface were removed sharply. Villous tissue was gently scraped free from vessels and connective tissue using forceps. After washing thoroughly two times with DMEM and once in 1x Ca2+, Mg2+-free HBSS, the tissue was cut into small pieces. About 15 grams of minced tissues was digested one time in 50 ml of a filtered digestion enzyme medium (containing 500 mg of trypsin powder (Difco), 17.5 ml of fat-free milk, 250 μl DNase I (50 U/ml), 250 μl 0.1 M MgCl2, and 250 μl 0.1 M CaCl2 in 250 ml warm 1x Ca2+, Mg2+-free HBSS) at 37°C for 30 min for a first cycle in a shaking incubator (70 rpm). Then the tissues were incubated with 30 ml of the digestion medium for 10 mins for up to 5 cycles in same conditions. Enzymatic degradation was monitored under light microscopy and stopped by filtering into 50 ml tubes containing 5% FBS. The mix was filtered through a 40 μm strainer (Thermo Fisher Scientific) to remove contaminating tissue debris. Cell suspensions were collected and centrifuged at 1200 rpm for 10 min at room temperature. Cell pellets were suspended in 3 ml of DMEM, layered on the top of a preformed Percoll gradient (60%, 50%, 45%, 35%, 30%, 20%, and 10%) and centrifuged at 2500 rpm at room temperature without braking for 20 minutes. The layer between the 45% and 35% of Percoll containing trophoblast cells was collected, suspended in DMEM, and centrifuged at 1200 rpm at room temperature for 10 min. The resulting cell pellet was suspended in complete DMEM (1% glutamine, 1% PS, and 10% FCS) and the cells were counted using a TC20™ Automated Cell Counter (Biorad). The cells were seeded in triplets onto either a sterile 24-well plate (250.000/well, Corning) for nanoluciferase luciferase assay (PPRE-pNL1.3 transfection) or sterile 12-well cell culture-treated plastic slides (40.000/well, Ibidi) for fluorescent experiments (immunofluorescence and PPRE-H2B-eGFP transfection).
2.3. Plasmid Constructs
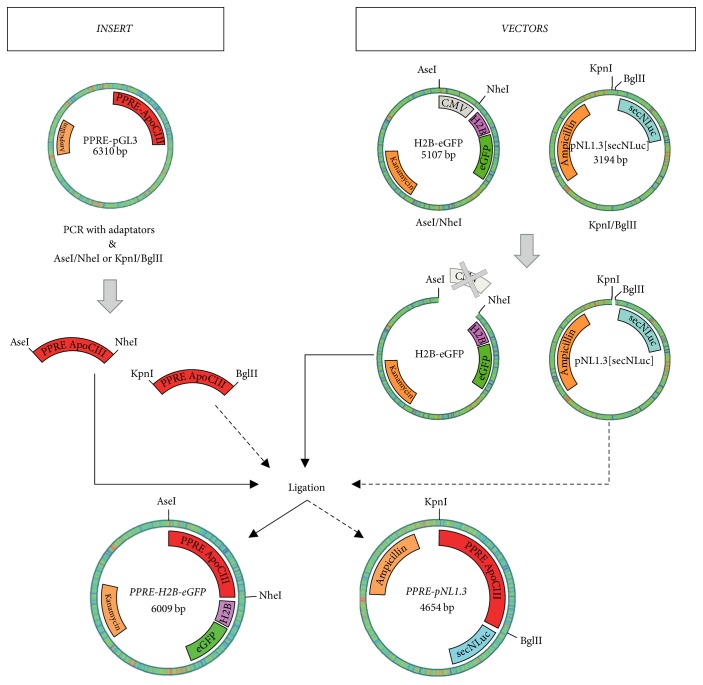
The PPRE-H2B-eGFP and PPRE-pNL1.3 were constructed by a PCR-based method using appropriate primers (Table 1). The peroxisome proliferator response element (PPRE) sequence was amplified by PCR using the PPRE(ApoCIII)-pGL3 construct as a template. The amplified fragments were subcloned into the KpnI/BglII sites of the secreted NanoLuc® luciferase reporter vector, pNL1.3[secNluc] (Promega Corporation) and of AseI/NheI sites of the H2B-eGFP plasmid (Addgene #11680 [27]). The general structure of the plasmids is shown in Figure 1. The integrity of reporter plasmid sequences was confirmed by DNA sequencing. Plasmid DNA was prepared for transfection using the Qiagen Plasmid Mini and Midi Kits.
Table 1.
Primers used to amplify DNA fragments by PCR for vector construction, confirmation, and generation of cloned fragments.
| Primer name | Sequence |
|---|---|
| PPRE-AseI | ATTAATAATGCCTGCAGGTCAATTCTG |
| PPRE-NheI | GCTAGCGATCGCAGATCCTCTAGAGTCC |
| PPRE-KpnI | CGGGGTACCATGCCTGCAGGTCAATTCTG |
| PPRE-BglII | GAAGATCTGATCGCAGATCCTCTAGAGTCC |
| pNL_F | AGGCTGTCCCCAGTGCAAGT |
| pNL_R | CGGATTGCCAAGCTTGGC |
| H2B_F | CACCATGCCAGAGCCAG |
| H2B_R | CTTAGCGCTGGTGTACTTG |
| GFP_F | CATGGTCCTGCTGGAGTTCGTG |
| GFP_R | CGTCGCCGTCCAGCTCGACCAG |
Restriction enzyme sites added to primer 5′-ends are underlined.
Figure 1.
Schematic diagram of PPRE-H2B-eGFP and PPRE-pNL1.3 plasmid constructions.
2.4. Treatments and PPRE-H2B-eGFP and PPRE-pNL1.3[secNluc] Transfections
The cells were treated with 1 μM GW1929 (agonist, #ab142213, Abcam) or 1 μM GW9662 (antagonist, #ab141125, Abcam) diluted in a culture medium containing 1% glutamine and 10% FCS (without PS) for 2 h. Before transfections, cells were washed and then incubated with Opti-MEM I medium without serum (Gibco; 200 μl/12-well slide or 500 μl/24-well plate). For transient transfection with PPRE-H2B-eGFP, VCT were transfected in 12-well cell culture-treated plastic slides (Ibidi) using Lipofectamine 3000 (Invitrogen) following the manufacturer's protocol. Briefly, plasmid DNA and Lipofectamine were separately diluted in Opti-MEM I medium without serum (Gibco). Then, DNA was combined with the Lipofectamine mixture and incubated for 5 minutes at room temperature. Finally, the DNA-Lipofectamine complexes were added dropwise to each well (25 μl/well). For transient transfection with PPRE-pNL1.3 or pNL1.3 basic secreted luciferase reporter as control, VCT were transfected in 24-well plates (Corning) using Lipofectamine 3000 (Invitrogen) following the manufacturer's protocol. Briefly, plasmid DNA and Lipofectamine were separately diluted in Opti-MEM I medium without serum (Gibco). Then, DNA was combined with the Lipofectamine mixture and incubated for 5 minutes at room temperature. Finally the DNA-Lipofectamine complexes were added dropwise to each well (50 μl/well). Four hours later, the transfected cells were washed and treated again with 1 μM GW1929 or 1 μM GW9662 for 48 h.
2.5. Immunofluorescence and Image Analysis
After 72 h of culture, cells were fixed in 4% PFA for 20 minutes at room temperature, washed in PBS, and permeabilized in 0.5% Triton X-100 in PBS for 30 minutes. Then, cells were blocked in 5% BSA IgG free and 0.1% Tween-20 in PBS for 1 h at room temperature and (i) for PPARγ expression, cells were incubated with primary PPARγ antibody (E-8 from Santa Cruz, 1-100) in blocking solution; (ii) for PPRE-H2B-eGFP transfected cells, only Alexa Fluor® 555 Phalloidin and DAPI labeling were performed. The next day, cells were rinsed three times with 0.1% Tween-20 in PBS (PBST), then the staining was revealed with VectaFluor™ Excel R.T.U. Antibody Kit, DyLight® 488, Anti-Mouse IgG (DK-2488, Vector Laboratories) according to the manufacturer's instructions. After three washes with PBST, cells were incubated with Alexa Fluor 555 Phalloidin (Molecular Probes, 1/200 in PBST) for 1 h, in the dark at room temperature, then washed three times in PBST, and counterstained with DAPI for 10 mins at room temperature. Finally slides were mounted with Fluoromount-G (Molecular Probes) and stored at 4°C. Confocal microscopy images (obtained with a Leica spinning-disk microscope equipped with a Plan Apo 63X/1.4 oil objective and a CoolSnap HQ2 CCD camera) were processed with ImageJ (National Institutes of Health, https://imagej.nih.gov/ij/) or Icy (Institut Pasteur, http://icy.bioimageanalysis.org/).
2.6. Western Blotting
After 72 h of culture, total cell extracts were prepared using NP40 Cell Lysis Buffer (Invitrogen). Protein samples were resolved by SDS-PAGE and immunoblotted with antibodies to GFP (1 μg/ml, Sigma) and actin (0.2 μg/ml, Sigma-Aldrich). After incubation with appropriate Alexa Fluor-conjugated secondary antibody (680 or 800 conjugate, Molecular Probes) blots were revealed by using Odyssey infrared fluorescent system (Li-Cor).
2.7. Nanoluciferase Assay
VCT transfected with PPRE-pNL1.3 or basic pNL1.3 were cultured in a 24-well plate for 72 hours at 37°C under the following 48 h treatments: 1 μM GW1929 or 1 μM GW9662. After 24 hours of treatment, 50 or 100 μl of each cell supernatant was dispensed into the wells of a 96-well plate (#3610, Corning) and then frozen at −20°C. After 48 hours of treatment, the 96-well plate was thawed at room temperature and 50 or 100 μl of each cell supernatant was dispensed into the wells of the same 96-well plate. The amount of secreted NanoLuc luciferase activity was determined using the Nano-Glo® Luciferase Assay (Promega Corporation) based on manufacturer's instructions. Luminescence in each well was then measured by using an EnSpire Multimode plate reader (Perkin Elmer).
2.8. Statistics
Three independent experiments were performed for each assay (n = 3). For PPRE-H2B-eGFP experiments, a minimum of 100 nuclei were analyzed per condition. For quantification, fluorescence signals were integrated over the entire nucleus. For PPRE-pNL1.3[secNluc] experiments, each condition was run in triplicate. Each luminescence reading was normalized to the corresponding nontransfected cell control. The data are expressed as the mean ± SD of the indicated number. Statistical analysis (paired t-test) was performed using the GraphPad Prism 6 software. Results were considered significant if p value < 0.05.
3. Results
3.1. PPARγ Is Expressed in Villous Cytotrophoblasts (VCT) and Highly Expressed in Syncytiotrophoblasts (ST) and Plays a Role in Trophoblast Differentiation
Immunostaining, with PPARγ antibody in primary trophoblast cells, showed that PPARγ is expressed in VCT and ST, with a higher expression in ST (see Supplementary Figure S1(A) in the Supplementary Material available online at https://doi.org/10.1155/2017/6139107). Furthermore, treatment with 1 μM GW1929 (PPARγ agonist) significantly increased fusion index whereas VCT treated with 1 μM GW9662 (PPARγ antagonist) significantly decreased cell fusion. This indicates that an increase in PPARγ activity leads to an increase in ST formation, whereas a decrease in PPARγ activity leads to a decrease in ST formation (Supplementary Figure S1(B)). Not only this but also treatment with 1 μM GW1929 significantly increased hCG secretion whereas VCT treated with 1 μM GW9662 significantly decreased hCG secretion. This shows that an increase in PPARγ activity leads to an increase in hCG secretion, whereas a decrease in PPARγ activity leads to a decrease in hCG secretion (Supplementary Figure S1(C)). In total, an increase of PPARγ activity in VCT will increase cell fusion leading to more formation of multinuclear ST and so higher levels of secreted hCG.
3.2. PPRE-H2B-eGFP Transfection of VCT: A New Model to Visualize the Activity of PPARγ
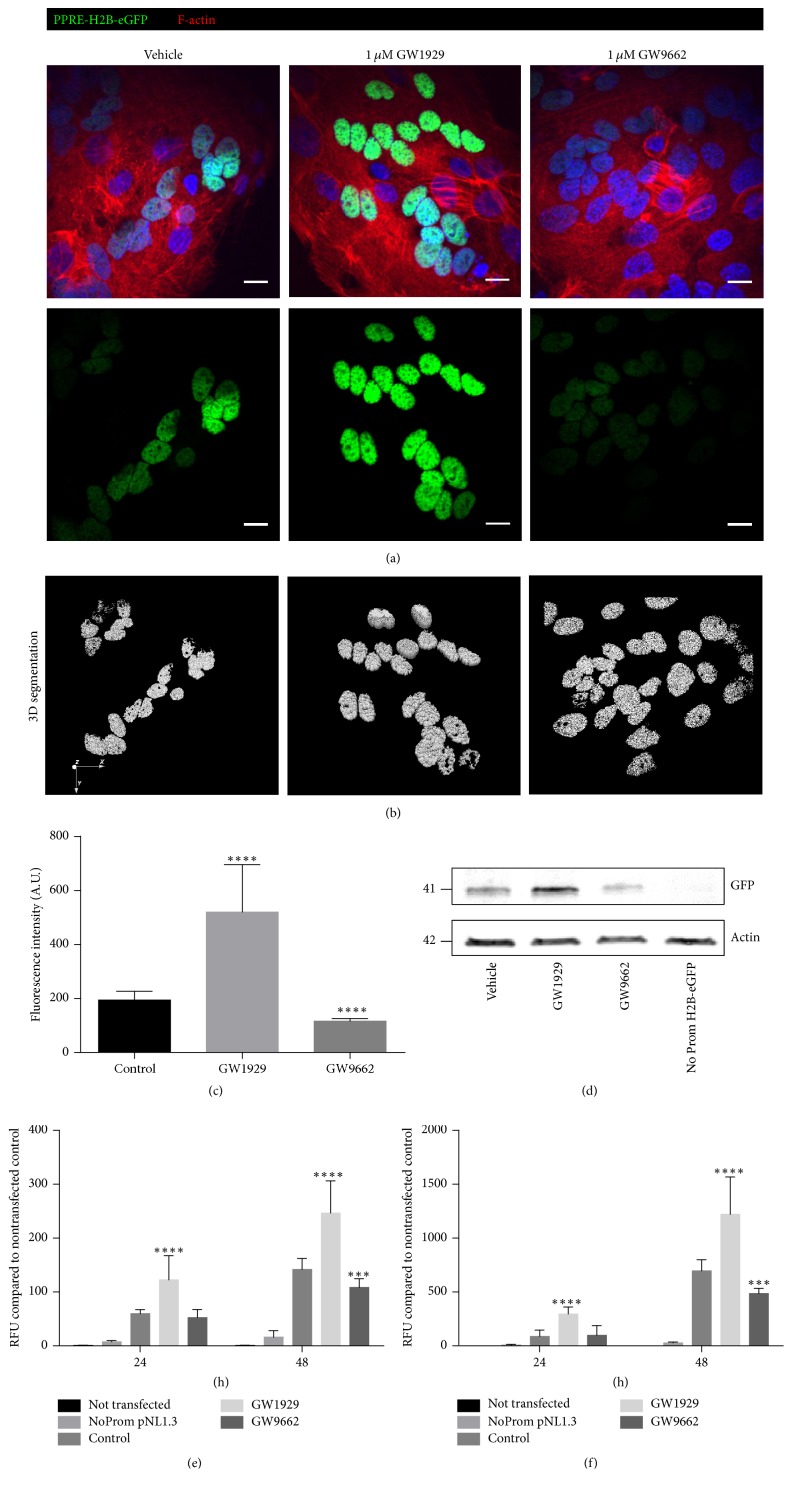
The transfection of primary trophoblasts with the newly constructed plasmid PPRE-H2B-eGFP showed a normal activity (normal intensity) of PPARγ in the nuclei of untreated cells (Figure 2). This activity increased in the cells treated with 1 μM GW1929 (PPARγ agonist), whereas it decreased in cells treated with 1 μM GW9662 (PPARγ antagonist; Figure 2(a)). The 3D segmentation was used to quantify the signal while defining the nuclei boundaries (Figure 2(b)). The graphical representation showed a significant increase of the fluorescence intensity (by about 2.5-fold) in cells treated with 1 μM GW1929, while there is a significant decrease (by about 2-fold) in cells treated with 1 μM GW9662 (Figure 2(c)). Confirmed by western blotting (Figure 2(d)), this illustrates that the plasmid PPRE-H2B-eGFP is of great help to study the activity of PPARγ in primary trophoblasts through visualizing methods.
Figure 2.
PPRE-H2B-eGFP and PPRE-pNL1.3[secNluc] transfections of VCT: new models to visualize and quantify the activity of PPARγ. (a) Merged staining of PPRE-H2B-eGFP (green), cell shape using F-actin (red), and nuclei using DAPI (blue) in 48 h treated VCT with 1 μM GW1929 (agonist of PPARγ) or 1 μM GW9662 (PPARγ antagonist) compared to control (vehicle). 3D nuclei segmentation (b) and quantification (c) of PPRE-H2B-eGFP fluorescence intensity using Icy software. (d) PPRE-H2B-eGFP protein levels were assessed using western blotting. (e-f) Spectrophotometry reading of luciferase expression for 50 μl (e) or 100 μl (f) of supernatant from 24- and 48-hour PPRE-pNL1.3 transfected and 1 μM GW1929 or 1 μM GW9662 treated VCT compared to nontransfected cells (control). Values are represented as mean ± SD; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 versus vehicle control (n = 3).
3.3. PPRE-pNL1.3[secNluc] Transfection of VCT: A New Model to Study the Activity of PPARγ without Cell Lysis
The other method that we used to study the activity of PPARγ in primary trophoblasts was to transfect VCT with the new PPRE-pNL1.3 plasmid, so that the expression of PPARγ leads to the secretion into the supernatant of transfected cells of the luciferase protein. Spectrophotometry reading for 50 μl of supernatant from 24 and 48 hours of PPRE-pNL1.3 transfected/GW treated VCT showed an increase in luminescence intensity with 1 μM GW1929 (PPARγ agonist) and a decrease in luminescence intensity with 1 μM GW9662 (PPARγ antagonist) compared to nontransfected cells with more relevant results in cells that were treated for 48 hours (Figure 2(e)). On the other hand, spectrophotometry reading for 100 μl of supernatant from 24- and 48-hour PPRE-pNL1.3 transfected/GW treated VCT showed an increase in luminescence intensity with 1 μM GW1929 (PPARγ agonist) and a decrease in luminescence intensity with 1 μM GW9662 (PPARγ antagonist) compared to nontransfected cells with more relevant results in cells treated for 48 hours (Figure 2(f)). Altogether these results showed that the PPRE-pNL1.3 is another great tool to study PPARγ activity in primary trophoblasts without cells lysis, so that they can be further used for other experiments.
3.4. PPRE-H2B-eGFP Model Works in Different Types of Cells
To confirm that PPRE-H2B-eGFP worked on other cell types, an immunostaining with a PPARγ antibody was performed after transfection on human or bovine cells available in the laboratory, namely, human trophoblast primary cells, human BEWO trophoblast cell line, human placental mesenchymal cells, or bovine extraembryonic mesoderm cells (bXMCs), was done. Immunocytofluorescence data evidenced that the four cell types expressed PPARγ and that PPARγ transcriptional activity was detectable in their nuclei due to the fluorescence of translated eGFP reporter gene (Supplementary Figure S2).
4. Discussion
In brief, the current work demonstrated the validity of two novel PPRE reporter systems for the in vitro study of PPARγ activity. On the one hand, our novel assay using a secreted nanoluciferase is highly sensitive requiring only a small amount of culture medium to evaluate changes in luciferase activity over time. On the other hand, our GFP reporter can be used to monitor PPARγ activity through live imaging. Moreover, these tools can easily be adapted to high-throughput screening of compounds (96-well or 384-well plates) which could identify agonist or antagonist effects on PPARγ activity on the cells of interest.
Supplementary Material
Figure S1: PPARγ: expression and role during trophoblast differentiation (fusion, hCG secretion). A) PPARγ in villous cytotrophoblast (VCT) and syncytiotrophoblast (ST). To the left- merged immunostaining in VCT and ST for PPARγ expression using PPARγ antibody (green), cell shape using F-actin (red) and nuclei using DAPI (blue). To the right- Immunostaining showing the differential expression of PPARγ between VCT and ST. PPARγ is highly expressed in ST. B) Fusion index of 48h-treated VCT with 1µM GW1929 (agonist of PPARγ) or 1µM GW9662 (PPARγ antagonist) compared to control (vehicle). C) hCG secretion of 48h-treated VCT with 1µM GW1929 (agonist of PPARγ) or 1µM GW9662 (PPARγ antagonist) compared to control (vehicle). Values are represented as mean ±S.D; ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001 versus vehicle control (n=5).
Figure S2: PPRE-H2B-eGFP works in different cell types and species. Left panels- PPARγ is expressed in human primary cells (VCT/ST), BEWO, and mesenchyme, as well as in bovine extra-embryonic mesoderm cells. Displayed are the merged immunostaining for PPARγ expression using PPARγ antibody (green), cell shape using F-actin (red) and nuclei using DAPI (blue). Middle panels- only PPARγ expression (green). Left panels- PPARγ activity, PPRE-H2B-eGFP (green) and nuclei (Hoechst, blue).
Acknowledgments
The authors are grateful to Stéphane Mandard (INSERM U866, University of Bourgogne, France) and Promega Corporation for providing the PPRE-pGL3 and pNL1.3 plasmids, respectively. The authors also thank the Cochin Institute Imaging facility. This work was supported by ANSES (PhthalatPreG project).
Data Access
The PPRE-H2B-eGFP and PPRE-pNL1.3[secNluc] plasmids described in this report have been submitted for inclusion in the nonprofit plasmid repository Addgene (https://www.addgene.org/) and will be available as Plasmid #84393 and Plasmid #84394, respectively.
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Reddy J. K., Goel S. K., Nemali M. R., et al. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proceedings of the National Acadamy of Sciences of the United States of America. 1986;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barak Y., Nelson M. C., Ong E. S., et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/S1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 3.Nadra K., Quignodon L., Sardella C., et al. PPARγ in placental angiogenesis. Endocrinology. 2010;151(10):4969–4981. doi: 10.1210/en.2010-0131. [DOI] [PubMed] [Google Scholar]
- 4.Latruffe N., Vamecq J. Peroxisome proliferators and peroxisome proliferator activated receptors (PPARs) as regulators of lipid metabolism. Biochimie. 1997;79(2-3):81–94. doi: 10.1016/S0300-9084(97)81496-4. [DOI] [PubMed] [Google Scholar]
- 5.Schoonjans K., Staels B., Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochimica et Biophysica Acta—Lipids and Lipid Metabolism. 1996;1302(2):93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 6.Giannini S., Serio M., Galli A. Pleiotropic effects of thiazolidinediones: Taking a look beyond antidiabetic activity. Journal of Endocrinological Investigation. 2004;27(10):982–991. doi: 10.1007/BF03347546. [DOI] [PubMed] [Google Scholar]
- 7.Tarrade A., Schoonjans K., Guibourdenche J., et al. PPARγ/RXRα heterodimers are involved in human CGβ synthesis and human trophoblast differentiation. Endocrinology. 2001;142(10):4504–4514. doi: 10.1210/en.142.10.4504. doi: 10.1210/en.142.10.4504. [DOI] [PubMed] [Google Scholar]
- 8.Krey G., Braissant O., L'Horset F., et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. doi: 10.1210/me.11.6.779. [DOI] [PubMed] [Google Scholar]
- 9.Semple R. K., Chatterjee V. K. K., O'Rahilly S. PPARγ and human metabolic disease. The Journal of Clinical Investigation. 2006;116(3):581–589. doi: 10.1172/jci28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross B., Pawlak M., Lefebvre P., Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nature Reviews Endocrinology. 2016;13(1):36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 11.Abushouk A. I., El-Husseny M. W., Bahbah E. I., et al. Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomedicine & Pharmacotherapy. 2017;95:692–700. doi: 10.1016/j.biopha.2017.08.083. [DOI] [PubMed] [Google Scholar]
- 12.Kvandova M., Majzunova M., Dovinova I. The role of PPARgamma in cardiovascular diseases. Physiol Res. 2016;65(Supplement 3):S343–S363. doi: 10.33549/physiolres.933439. [DOI] [PubMed] [Google Scholar]
- 13.Wang N., Yin R., Liu Y., Mao G., Xi F. Role of peroxisome proliferator-activated receptor-γ in atherosclerosis: an update. Circulation Journal. 2011;75(3):528–535. doi: 10.1253/circj.CJ-11-0060. [DOI] [PubMed] [Google Scholar]
- 14.Astapova O., Leff T. PPARγ mutations, lipodystrophy and diabetes. Hormone Molecular Biology and Clinical Investigation. 2014;20(2):63–70. doi: 10.1515/hmbci-2014-0033. [DOI] [PubMed] [Google Scholar]
- 15.Graham D. J., Ouellet-Hellstrom R., Macurdy T. E., et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. Journal of the American Medical Association. 2010;304(4):411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 16.Molnar F., Matilainen M., Carlberg C. Structural determinants of the agonist-independent association of human peroxisome proliferator-activated receptors with coactivators. The Journal of Biological Chemistry. 2005;280(28):26543–26556. doi: 10.1074/jbc.M502463200. [DOI] [PubMed] [Google Scholar]
- 17.Ma J.-J., Zhang T., Fang N., et al. Establishment of a cell-based drug screening model for identifying agonists of human peroxisome proliferator-activated receptor gamma (PPARγ) Journal of Pharmacy and Pharmacology. 2012;64(5):719–726. doi: 10.1111/j.2042-7158.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 18.Gijsbers L., Man H.-Y., Kloet S. K., et al. Stable reporter cell lines for peroxisome proliferator-activated receptor γ (PPARγ)-mediated modulation of gene expression. Analytical Biochemistry. 2011;414(1):77–83. doi: 10.1016/j.ab.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Punzon I., Latapie V., Le Mével S., et al. Towards a humanized PPARγ reporter system for in vivo screening of obesogens. Molecular and Cellular Endocrinology. 2013;374(1-2):1–9. doi: 10.1016/j.mce.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Schulman I. G., Shao G., Heyman R. A. Transactivation by retinoid X receptor-peroxisome proliferator-activated receptor γ (PPARγ) heterodimers: Intermolecular synergy requires only the PPRAγ hormone-dependent activation function. Molecular and Cellular Biology. 1998;18(6):3483–3494. doi: 10.1128/MCB.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q., Seltmann H., Zouboulis C. C., Konger R. L. Involvement of PPARγ in oxidative stress-mediated prostaglandin E2 production in SZ95 human sebaceous gland cells. Journal of Investigative Dermatology. 2006;126(1):42–48. doi: 10.1038/sj.jid.5700028. [DOI] [PubMed] [Google Scholar]
- 22.Tarrade A., Schoonjans K., Pavan L., et al. PPARγ/RXRα heterodimers control human trophoblast invasion. The Journal of Clinical Endocrinology & Metabolism. 2001;86(10):5017–5024. doi: 10.1210/jc.86.10.5017. [DOI] [PubMed] [Google Scholar]
- 23.Cocquebert M., Berndt S., Segond N., et al. Comparative expression of hCG β-genes in human trophoblast from early and late first-trimester placentas. American Journal of Physiology-Endocrinology and Metabolism. 2012;303(8):E950–E958. doi: 10.1152/ajpendo.00087.2012. [DOI] [PubMed] [Google Scholar]
- 24.Alsat E., Mirlesse V., Fondacci C., Dodeur M., Evain-Brion D. Parathyroid hormone increases epidermal growth factor receptors in cultured human trophoblastic cells from early and term placenta. The Journal of Clinical Endocrinology & Metabolism. 1991;73(2):288–295. doi: 10.1210/jcem-73-2-288. [DOI] [PubMed] [Google Scholar]
- 25.Degrelle S. A., Gerbaud P., Leconte L., Ferreira F., Pidoux G. Annexin-A5 organized in 2D-network at the plasmalemma eases human trophoblast fusion. Scientific Reports. 2017;7 doi: 10.1038/srep42173.42173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frendo J.-L., Cronier L., Bertin G., et al. Involvement of connexin 43 in human trophoblast cell fusion and differentiation. Journal of Cell Science. 2003;116(16):3413–3421. doi: 10.1242/jcs.00648. [DOI] [PubMed] [Google Scholar]
- 27.Kanda T., Sullivan K. F., Wahl G. M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Current Biology. 1998;8(7):377–385. doi: 10.1016/S0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: PPARγ: expression and role during trophoblast differentiation (fusion, hCG secretion). A) PPARγ in villous cytotrophoblast (VCT) and syncytiotrophoblast (ST). To the left- merged immunostaining in VCT and ST for PPARγ expression using PPARγ antibody (green), cell shape using F-actin (red) and nuclei using DAPI (blue). To the right- Immunostaining showing the differential expression of PPARγ between VCT and ST. PPARγ is highly expressed in ST. B) Fusion index of 48h-treated VCT with 1µM GW1929 (agonist of PPARγ) or 1µM GW9662 (PPARγ antagonist) compared to control (vehicle). C) hCG secretion of 48h-treated VCT with 1µM GW1929 (agonist of PPARγ) or 1µM GW9662 (PPARγ antagonist) compared to control (vehicle). Values are represented as mean ±S.D; ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001 versus vehicle control (n=5).
Figure S2: PPRE-H2B-eGFP works in different cell types and species. Left panels- PPARγ is expressed in human primary cells (VCT/ST), BEWO, and mesenchyme, as well as in bovine extra-embryonic mesoderm cells. Displayed are the merged immunostaining for PPARγ expression using PPARγ antibody (green), cell shape using F-actin (red) and nuclei using DAPI (blue). Middle panels- only PPARγ expression (green). Left panels- PPARγ activity, PPRE-H2B-eGFP (green) and nuclei (Hoechst, blue).