Abstract
Background:
Chronic kidney disease (CKD) with moderate-to-severe renal dysfunction usually exhibits an irreversible course, and available treatments for delaying the progression to end-stage renal disease are limited. This study aimed to assess the efficacy and safety of the traditional Chinese medicine, Niaoduqing particles, for delaying renal dysfunction in patients with stage 3b-4 CKD.
Methods:
The present study was a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial. From May 2013 to December 2013, 300 CKD patients with an estimated glomerular filtration rate (eGFR) between 20 and 45 ml·min−1·1.73 m−2, aged 18–70 years were recruited from 22 hospitals in 11 Chinese provinces. Patients were randomized in a 1:1 ratio to either a test group, which was administered Niaoduqing particles 5 g thrice daily and 10 g before bedtime for 24 weeks, or a control group, which was administered a placebo using the same methods. The primary endpoints were changes in baseline serum creatinine (Scr) and eGFR after completion of treatment. The primary endpoints were analyzed using Student's t-test or Wilcoxon's rank-sum test. The present study reported results based on an intention-to-treat (ITT) analysis.
Results:
A total of 292 participants underwent the ITT analysis. At 24 weeks, the median (interquartile range) change in Scr was 1.1 (−13.0–24.1) and 11.7 (−2.6–42.9) μmol/L for the test and control groups, respectively (Z = 2.642, P = 0.008), and the median change in eGFR was −0.2 (−4.3–2.7) and −2.2 (−5.7–0.8) ml·min−1·1.73 m−2, respectively (Z = −2.408, P = 0.016). There were no significant differences in adverse events between the groups.
Conclusions:
Niaoduqing particles safely and effectively delayed CKD progression in patients with stage 3b-4 CKD. This traditional Chinese medicine may be a promising alternative medication for patients with moderate-to-severe renal dysfunction.
Trial Registration:
Chinese Clinical Trial Register, ChiCTR-TRC-12002448; http://www.chictr.org.cn/showproj.aspx?proj=7102.
Keywords: Chronic Kidney Disease, Moderate-to-severe Renal Dysfunction, Niaoduqing Particles, Randomized Controlled Trial, Traditional Chinese Medicine
INTRODUCTION
Chronic kidney disease (CKD) is a global public health challenge. In moderate-to-severe CKD, the estimated glomerular filtration rate (eGFR) is 15–59 ml·min−1·1.73 m−2 and the accelerated decline of renal function is usually irreversible. In 2012, Zhang et al.[1] reported that the prevalence of CKD in China was 10.8%, with the prevalence of stages 3 and 4 CKD being 1.7%; therefore, it is estimated that there are 119.5 million people with CKD and more than 18.8 million people with moderate-to-severe renal dysfunction in China.
In the past two decades, angiotensin-converting enzyme inhibitor (ACEI) and angiotensin II receptor blocker (ARB) have become standard treatment because of their renoprotective effect.[2,3] However, despite undergoing ACEI and ARB treatment, many patients continue to progress to uremia. Combination treatment with ACEIs and ARBs increases the risk of adverse events such as hyperkalemia, decreased renal function, and hypotension.[4] ACEI/ARB + calcium channel blocker conferred no additional renoprotective benefit compared to ACEI/ARB monotherapy.[5] In addition, recent evaluations of new drugs have hardly identified agents that successfully accomplish renoprotective benefit.[6,7,8,9] Therefore, there is an urgent need to find alternative treatments to delay CKD progression.
Niaoduqing particles, based on traditional Chinese medical theory, have been shown to effectively lower serum creatinine (Scr) levels and improve renal blood flow in some small-scale clinical trials.[10,11,12] However, high-level clinical evidence derived from rigorously designed studies is lacking. This study was designed to assess the efficacy and safety of Niaoduqing particles for the treatment of CKD patients with an eGFR of 20–45 ml·min−1·1.73 m−2.
METHODS
Study design and ethical approval
This study was a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial (Chinese Clinical Trial Register, ChiCTR-TRC-12002448). From May 2013 to December 2013, patients with CKD were recruited from 22 hospitals across 11 provinces in China. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Chinese People's Liberation Army General Hospital (No. 2012032-02). Informed written consent was obtained from all patients before their enrollment in this study. Before enrollment, all researchers underwent training regarding the study protocol and passed the evaluation.
Subjects
Patients who met the following inclusion criteria were enrolled: (1) age 18–70 years, any sex; (2) eGFR of 20–45 ml·min−1·1.73 m−2; (3) according to traditional Chinese medicine, patients who had damp filth and deficient spleen or who had spleen deficiency and blood stasis; (4) a blood pressure ≤140/90 mmHg; and (5) who provided signed consent for participation. Patients were excluded for the following reasons: (1) allergy to multiple medications; (2) presence of nephrotic syndrome, lupus nephritis, or other autoimmune diseases; (3) use of Niaoduqing particles within 1 month prior to study enrollment; (4) use of any traditional Chinese medicine containing rhubarb, substances affecting renal function, activated carbon, or adsorbents within 1 month prior to study enrollment or during the trial period; (5) administration of hormone therapy or immunosuppressive agents (including tripterygium glycosides) within 3 months prior to study enrollment or during the trial period; (6) intention to undergo renal replacement therapy; (7) complications from other diseases, including urinary tract infections, noninfectious inflammatory diseases, or recent acute infections; (8) any serious gastrointestinal disease that might affect drug absorption, such as an active ulcer, chronic diarrhea, or previous gastrointestinal surgery; (9) complications from a serious primary disease of the heart, brain, liver, or hematopoietic system; (10) acute urinary tract obstruction requiring surgery; (11) inability to cooperate because of mental health problems; (12) pregnancy or lactation in female patients; (13) history of alcohol or drug abuse; and (14) ongoing participation in another clinical trial. In addition, patients were excluded at the discretion of the researchers for any other reasons including, i.e., a patient who lived far from the sites and would not be available for long-term follow-up, and a patient who agreed to participate in the study, but whose relatives refused.
Standard of traditional Chinese medicine syndrome classification is as follows: (1) the deficiency syndrome: syndromes of Qi Deficiency of Spleen and Kidney: the major symptoms: fatigue, soreness and weakness of waist and knees, and nocturia. The minor symptoms: yellow complexion or shaohua, abdominal fullness and distension, eating less and anorexia, loose stool (having the above 2 major symptoms, or having1 major symptoms and 2 minor symptoms can make a diagnosis). (2) The sthenia syndrome: ① syndromes of dampness turbidity: the major symptoms: nausea, vomiting, and edema. The minor symptoms: sticky in mouth, trapped limbs, nonsmooth diarrhea. ② Syndromes of blood stasis: the major symptoms: fixed lumbago or tingling, purplish or dark purplish lips and tongue with stasis maculae. The minor symptoms: complexion dark, scaly dry skin, numbness of limb (Having the above 1 major symptoms, or 2 minor symptoms can make a diagnosis).
Medication administration
Niaoduqing particles include the following 16 herbs: Rhubarb (dahuang), atractylodes (baizhu), Poria cocos (fuling), Radix Polygonum multiflorum preparata (zhishouwu), Salvia (danshen), Plantain (cheqiancao), Astragalus (huangqi), Cortex mori (sangbaipi), Peony root (baishao), Lanceolata (dangshen), Rhizome of Chuanxiong (chuanxiong), Chrysanthemum (juhua), Sophora flavescens (kushen), Pinellia (jiangbanxia), Bupleurum (chaihu), and Glycyrrhiza (gancao).
Both the Niaoduqing particles and placebo were produced by the Consun Pharmaceutical Group (Guangzhou, Guangdong, China). The appearance and packaging of the Niaoduqing particles and placebo were highly consistent. The placebo was prepared considering the followings: (1) in terms of maintaining a similar color of particles, we selected the same auxiliary material dextrin and natural caramel pigment in the production of the Niaoduqing particles and placebo; (2) spraying the placebo with Niaoduqing particles’ essential oil made them have a similar odor; and (3) the preparation technology and packing for the placebo was the same as for the Niaoduqing particles. Allocation concealment was conducted by the Peking University Clinical Research Institute.
A week after signing the informed consent form and completing the baseline check, each patient was randomly assigned to the Niaoduqing particles group (test group) or the placebo group (control group). The test group was administered 5 g of Niaoduqing particles thrice daily after meals and 10 g before bedtime. Doses were administered with warm water. The control group was administered a placebo with the same mode and frequency of administration. Patients were followed up by professional nephrologists at 8, 16, and 24 weeks.
Study endpoints
The primary endpoints of this study were changes in baseline serum Scr and eGFR after completion of treatment. A central laboratory used an enzymatic method to examine Scr (COBAS Integra 800, Roche Co., Switzerland; Scr, l mg/dl = 88.4 μmol/L) and the Chinese version of the Modification of Diet in Renal Disease equation to measure eGFR (eGFR = 175 × Scr − 1.234 × age − 0.179 × 0.79 [if female]).[13] The secondary endpoints were changes in 24-h urinary protein excretion between baseline and completion of treatment, and Scr doubling or dialysis initiation. Participants were instructed to collect urine over 24 h (from 7:00 a.m. to 7:00 a.m. the next day). A medical flask was used to measure total urine output. After stirring, 10 ml of urine was preserved at −40°C. Concentration was measured using the biuret method (ADVIA 2400 Biochemical Analyser, Siemens, Germany), and 24-h urinary protein excretion was calculated based on multiplying protein concentration by 24-h urine volume. Safety evaluations included assessments of adverse events and laboratory results, including the results of blood, liver function, serum potassium, serum lipids, and blood glucose tests, and blood pressure evaluation. Adverse events and their severity were assessed by professional nephrologists at each visit.
Randomization and blinding
This study used competitive, block randomization. The blocks were assigned competitively among the centers; in other words, if a block was assigned to a center, all the subjects in the block were enrolled in the center. Within each block, participants were randomly assigned to the test group and the control group using a 1:1 ratio. Drugs were dispensed according to the order of enrollment. SAS 9.2 software (SAS Institute, Cary, NC, USA) was used to produce the randomized block. Randomization and blinding were conducted by a statistician who did not analyze data. The participants and researchers were unaware of the treatment allocation.
Sample size and statistical analysis
The present study adopted a superiority trial design. According to previous studies and experts consensus, if compared with baseline, the control group's Scr at 24 weeks increased by 30 ± 24 μmol/L, eGFR decreased by 3.0 ± 1.8 ml·min−1·1.73 m−2, and Cystatin C increased by 0.3 ± 0.2 mg/L, the test group would be 1/3 remission of these primary endpoints. Thus, proposed sample sizes of 122, 69, and 85 patients would be required in each group with a power of 80% and a total significance level of 5% according to Bonferroni correction of the P value (P < 0.017). Assuming a loss to follow-up of 20%, 300 patients were randomly assigned to the test group or to the control group at a 1:1 ratio. PASS 11 software (NCSS LLC, Kaysville, UT, USA) was used to calculate the sample size. Because some centers could not examine cystatin C, this parameter was unevaluated in over 30% of participants. Thus, we did not analyze changes in cystatin C.
Data sets were defined as follows: a full analysis set (FAS) was analyzed for primary endpoints according to intention-to-treat principles, including all patients who were randomized into groups and who took the drugs at least once.[14] In the test group, four participants did not meet our inclusion criteria, while in the control group, three participants did not meet our inclusion criteria, and one participant refused to take the drugs; thus, 146 participants in each group entered the FAS. In FAS, 20 participants in the test group and 24 in the control group did not complete the follow-up; therefore, the per-protocol set (PPS) was composed of 126 participants in the test group and 122 in the control group. We analyzed both FAS and PPS and obtained the similar results. In the manuscript, we reported the results based on FAS. When we analyzed the FAS data, we utilized the last-observation-carried-forward method to fill the missing data. A safety population was composed of patients who took the drug at least once and had accompanying safety data.
Normally distributed quantitative data are described using mean ± standard deviation, nonnormally distributed quantitative data are described using the median (interquartile range), and qualitative data are described using proportions. A t-test or Wilcoxon's rank-sum test was used to compare quantitative data between the groups based on data distribution, and the Chi-square or Fisher's exact tests were used to compare qualitative data. All statistical tests were two-tailed. According to the study protocol, any of the primary endpoints was considered to indicate a statistical difference between groups when the P < 0.017, and P < 0.05 was considered statistically significant regarding baseline characteristics, secondary endpoints, and safety analysis. SAS 9.2 software was used for the statistical analyses.
RESULTS
Baseline characteristics
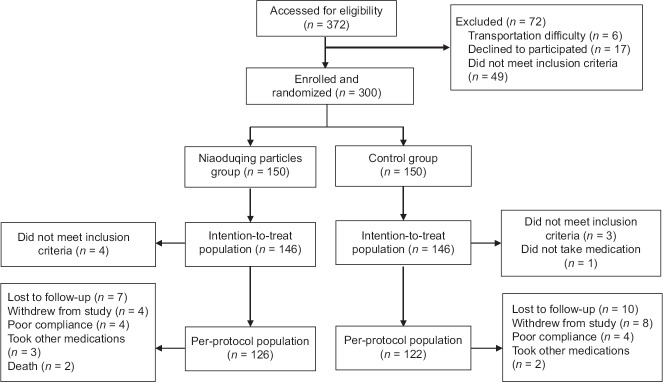
The patient enrollment protocol for the present study is shown in Figure 1. A total of 372 patients were screened for eligibility, and of these, 300 patients were included in this study. Baseline characteristics between the two treatment groups were similar [Table 1]. The median age of patients was 53.2 years in the test group and 52.0 years in the control group. Male patients accounted for 56.9% and 56.2% of the test and control groups, respectively. The median baseline Scr was 191.6 μmol/L in the test group and 185.0 μmol/L in the control group. The median baseline eGFR was 31.0 ml·min−1·1.73 m−2 in the test group and 30.6 ml·min−1·1.73 m−2 in the control group. The median 24-h urinary protein excretion was 1100.0 mg in the test group and 840.0 mg in the control group. The proportion of primary causes of disease, hypertension, diabetes, and cardiovascular disease was not different between both groups (P > 0.05).
Figure 1.
Schematic flow of this study.
Table 1.
Baseline characteristics of the enrolled patients with Stage 3b-4 CKD in the two groups
| Characteristics | Niaoduqing particles (n = 146) | Placebo (n = 146) | Statistic value | P |
|---|---|---|---|---|
| Age (years) | 53.2 (40.8, 60.0) | 52.0 (42.7, 60.4) | 0.069* | 0.945 |
| Male, n (%) | 83 (56.9) | 82 (56.2) | 0.014† | 0.906 |
| BMI (kg/m2) | 24.1 ± 3.6 | 24.5 ± 3.5 | −1.048‡ | 0.857 |
| Scr (µmol/L) | 191.6 (162.0, 223.8) | 185.0 (166.0, 226.0) | −0.083* | 0.933 |
| eGFR (ml·min−1·1.73 m−2) | 31.0 (24.0, 37.1) | 30.6 (24.6, 36.4) | 0.049* | 0.961 |
| 24-h urinary protein excretion (mg) | 1100.0 (370.0, 2130.0) | 840.0 (281.2, 1729.0) | −1.254* | 0.210 |
| SBP (mmHg) | 130.0 (125.0, 134.0) | 130.0 (120.0, 135.0) | −0.471* | 0.638 |
| DBP (mmHg) | 80.0 (75.0, 82.0) | 80.0 (75.0, 85.0) | 0.876* | 0.381 |
| Cause of disease, n (%) | ||||
| Primary glomerular disease | 73 (50.0) | 76 (52.1) | 0.425† | 0.935 |
| Diabetic nephropathy | 18 (12.3) | 16 (11.0) | ||
| Hypertensive renal injury | 14 (9.6) | 16 (11.0) | ||
| Other | 41 (28.1) | 38 (26.0) | ||
| Hypertension, n (%) | 100 (68.5) | 89 (61.0) | 1.815† | 0.178 |
| Diabetes, n (%) | 25 (17.1) | 30 (20.5) | 0.560† | 0.454 |
| Cardiovascular disease, n (%) | 18 (12.3) | 17 (11.6) | 0.032† | 0.857 |
Values are given as the mean ± SD, n (%), or median (Q1, Q3). 1 mmHg = 0.133 kPa. *Wilcoxon's rank-sum test; † Chi-square test; ‡t-test. BMI: Body mass index; DBP: Diastolic blood pressure; eGFR: Estimated glomerular filtration rate; SBP: Systolic blood pressure; Scr: Serum creatinine; SD: Standard deviation; CKD: Chronic kidney disease.
Primary endpoints
Table 2 shows the median levels of Scr and eGFR at 8-week intervals throughout the 24-week treatment period. At baseline and after 24 weeks of treatment, there were no significant differences in median Scr between the groups (P = 0.933 and 0.157, respectively). However, there was a significant difference between the two groups in the change in Scr at 24 weeks compared with the baseline, with the test group increasing by 1.1 (−13.0–24.1) μmol/L and the control group increasing by 11.7 (−2.6–42.9) μmol/L. This difference between the two groups was statistically significant (P = 0.008). At 24 weeks, the median changes in Scr in the test and control groups were 0.51% and 6.40%, respectively (P = 0.010) [Figure 2a].
Table 2.
Changes in Scr and eGFR of the patients with Stage 3b-4 CKD in the two groups
| Variables | Niaoduqing particles (n = 146) | Placebo (n = 146) | Z* | P |
|---|---|---|---|---|
| Scr (μmol/L) | ||||
| 0 week | 191.6 (162.0, 223.8) | 185.0 (166.0, 226.0) | −0.083 | 0.933 |
| 8 weeks | 192.0 (163.8, 234.7) | 192.1 (169.0, 224.4) | 0.267 | 0.790 |
| 16 weeks | 200.0 (164.0, 242.0) | 189.5 (165.50, 229.0) | −0.862 | 0.388 |
| 24 weeks | 198.6 (164.7, 239.6) | 203.1 (172.9, 252.7) | 1.414 | 0.157 |
| ∆Scr | 1.1 (−13.0, 24.1) | 11.7 (−2.6, 42.9) | 2.642 | 0.008 |
| ∆Z | 902.5 | 2136.0 | ||
| ∆P | 0.026 | <0.001 | ||
| eGFR (ml·min−1·1.73 m−2) | ||||
| 0 week | 31.0 (24.0, 37.1) | 30.6 (24.6, 36.4) | 0.049 | 0.961 |
| 8 weeks | 30.4 (23.5, 36.9) | 29.6 (24.3, 36.34 | 0.467 | 0.641 |
| 16 weeks | 30.0 (22.7, 36.5) | 30.1 (23.9, 37.2) | 0.702 | 0.483 |
| 24 weeks | 29.4 (23.5, 35.9) | 28.0 (21.0, 34.3) | −1.735 | 0.083 |
| ∆eGFR | −0.2 (−4.3, 2.7) | −2.2 (−5.7, 0.8) | −2.408 | 0.016 |
| ∆Z | −647.5 | −1943.0 | ||
| ∆P | 0.111 | <0.001 |
Values are given as median (Q1, Q3). *Z statistical value of Wilcoxon's rank-sum test. eGFR: Estimated glomerular filtration rate; Scr: Serum creatinine; CKD: Chronic kidney disease; ∆Scr: Change in Scr between pre- and post-treatment; ∆eGFR: Change in eGFR between pre- and post-treatment; ∆Z: Statistical value of Wilcoxon's rank-sum test, comparison of the changes in Scr or eGFR between pre- and post-treatment; ∆P: Comparison of the changes in Scr or eGFR between pre- and post-treatment.
Figure 2.
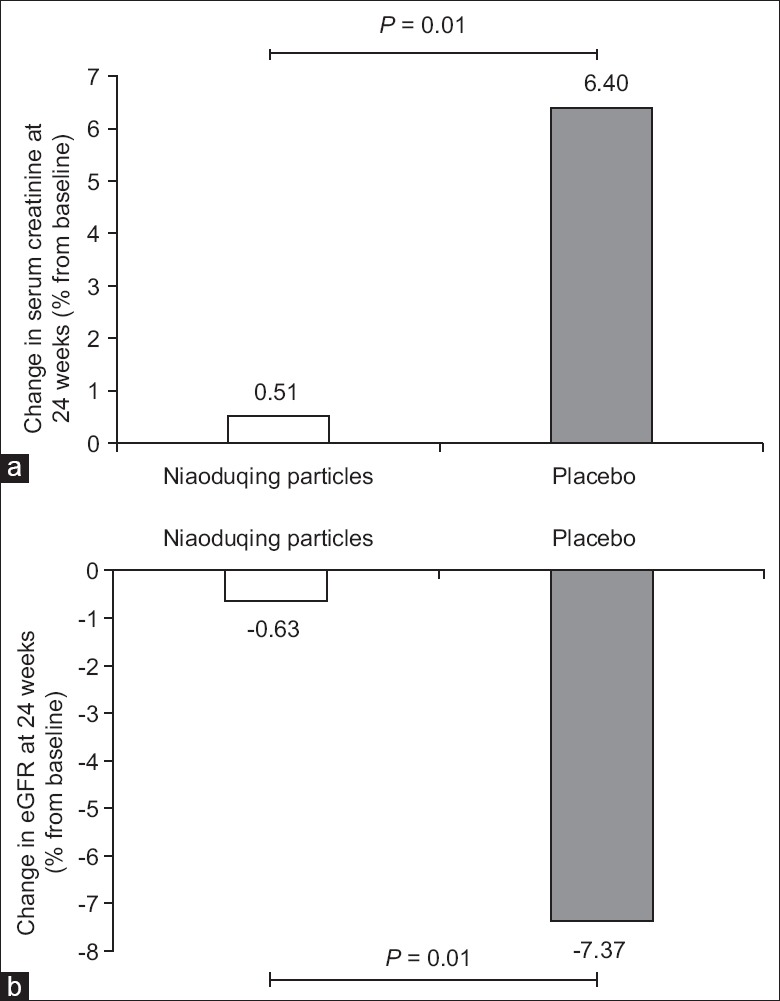
Changes in serum creatinine and estimated glomerular filtration rate in the test (Niaoduqing particles) and control (placebo) groups. (a) Serum creatinine. (b) Estimated glomerular filtration rate (eGFR).
At baseline and after 24 weeks of treatment, there were no significant differences in the median eGFR between the groups (P = 0.961 and 0.083, respectively). However, compared with the baseline, eGFR at 24 weeks in the test group increased by −0.2 (−4.3–2.7) ml·min−1·1.73 m−2, while eGFR at 24 weeks in the control group increased by −2.2 (−5.7–0.8) ml·min−1·1.73 m−2. This difference between the two groups was statistically significant (P = 0.016). At 24 weeks, the median eGFR changes in the test and the control groups were −0.63% and −7.37%, respectively (P = 0.010) [Figure 2b].
Secondary endpoints
At baseline and after 24 weeks of treatment, there were no significant differences in median 24-h urinary protein excretion between the groups (P = 0.210 and 0.409, respectively). Compared with the baseline, 24-h urinary protein excretion at 24 weeks increased by 63.0 (−340.0–900.0) mg in the test group and by 24.6 (−240.0–578.3) mg in the control group. This difference between the two groups was not statistically significant (P = 0.525). Moreover, in the test group, creatinine levels doubled for six patients, and one patient was started on dialysis. In the control group, creatinine levels doubled for four patients and two patients were started on dialysis (P > 0.05).
Safety
There were 13 and 14 cases of adverse events in the test and control groups, respectively (P > 0.05) [Supplementary Table 1]. Two patients in the test group died due to causes unrelated to treatment, including one case of sudden death and one case of pulmonary infection where the patient was not hospitalized. The main adverse events, including liver damage (no case in the test group, three cases in the control group) and elevated serum potassium (two cases each in both groups), showed no significant difference between both groups (P > 0.05).
Supplementary Table 1.
Adverse events
| Items | Niaoduqing particles (n = 146) | Placebo (n = 146) | P |
|---|---|---|---|
| Liver damage | 0 | 3 | 0.122 |
| Elevated serum potassium | 2 | 2 | 1.000 |
| Diarrhea | 3 | 4 | 0.723 |
| Stomach upset | 0 | 2 | 0.247 |
| Pruritus | 1 | 0 | 1.000 |
| Pulmonary infection | 1 | 0 | 1.000 |
| Urinary tract infection | 1 | 1 | 1.000 |
| Composite CV events* | 3 | 2 | 1.000 |
| Death | 2 | 0 | 0.498 |
| Total | 13 | 14 | |
Values were shown as n. CV: Cardiovascular. *Composite CV events included cerebral infarction, myocardial infarction, and heart failure.
DISCUSSION
In the present study, eGFR levels in patients treated with Niaoduqing particles and those treated with a placebo decreased compared to baseline at 24 weeks. However, the decrease in the placebo-treated patients was significant compared with baseline eGFR, whereas the difference in the Niaoduqing-treated patients was not significant, suggesting that Niaoduqing particles treatment successfully delayed renal function decline in patients with CKD.
Treatment options for patients with advanced CKD are lacking, and efficacy and safety trials for drug approvals typically exclude patients with advanced CKD. Depending on the primary disease, GFR can be expected to decline from 2 to 10 ml/min per year in patients with CKD.[15] In patients with diabetic nephropathy, GFR is expected to decline more than 10 ml/min per year.[16] An ideal treatment would be able to restore the decline of GFR in advanced CKD patients to a normal, age-related rate of decline of <1 ml/min per year.[15] In the past 30 years, blood pressure control and the use of renin-angiotensin system (RAS) blockers have played an important role in delaying renal dysfunction. In patients with diabetic nephropathy, RAS blockers and blood pressure maintenance have been reported to reduce GFR decline to 5 to 6 ml/min per year.[2,17,18] Similarly, in patients with nondiabetic nephropathy patients who have significant proteinuria, GFR decline was delayed by 6 to 8 ml/min per year.[19] In the present study, 51% of patients had primary glomerular disease and 12% had diabetic nephropathy. The median decline in eGFR after 24 weeks of Niaoduqing particles treatment was 0.2 ml·min−1·1.73 m−2, which is in line with the normal, age-related eGFR decline rate. However, in the control group, the median eGFR decline after 24 weeks was 2.21 ml·min−1·1.73 m−2, which is consistent with previous studies reporting an annual eGFR decline of approximately 5 ml·min−1·1.73 m−2. These data suggest that Niaoduqing particles successfully delayed renal function decline in patients with CKD 3b-4 and that they would be efficacious for patients with moderate-to-severe renal dysfunction. To exclude the influence of poorly controlled blood pressure on renal function, our study enrolled patients without hypertension or with controlled blood pressures (140/90 mmHg or lower). It is further suggested that the renoprotective effect of Niaoduqing particles is independent of blood pressure control.
The ideological basis of Chinese traditional medicine is the Chinese Taoist philosophy, which proposed that a pair of opposing forces, Yin and Yang, exist in nature. Imbalances of Yin and Yang were thought to bring about disease; however, modern Taoists theorize that internal and external imbalances in the body lead to disease.[20] Most traditional Chinese medicines are mixtures of multiple plants. Chinese researchers have extracted several effective bioactive ingredients from Niaoduqing particles, including emodin, astragaloside, and salvianolic acid A.[21] As the main active ingredient of rhubarb, emodin regulates lipopolysaccharide-induced toll-like receptor 4 and reduces the expression of tumor necrosis factor alpha and interleukin 6, all three being synthesized by renal tubular epithelial cells.[22] Emodin also acts as an anti-inflammatory agent by inhibiting the differentiation and maturation of dendritic cells and increasing the number of regulatory T-cells.[23] Astragaloside is a main active ingredient in traditional Chinese medicine and acts both as an anti-inflammatory and antifibrotic agent,[24,25] while salvianolic acid A has been shown to have cardiovascular protective effects.[26,27] Recent studies have shown that compound Danshen dripping pills, which contain salvianolic acid A, can prevent contrast-induced nephropathy in patients with acute coronary syndrome.[28] In vitro, salvianolic acid A has been shown to inhibit mesangial cell proliferation[29] and to ameliorate the symptoms of doxorubicin-induced nephropathy in vivo.[30]
In China, Niaoduqing particles have been used clinically for more than 20 years and their mechanism of action has been comprehensively investigated. However, before this study, there has been a lack of rigorously designed, randomized-controlled clinical trials providing convincing evidence for the clinical benefits of Niaoduqing particles. The present study focused on a high-risk population with rapid CKD progression to determine if Niaoduqing particles could delay the progression of CKD. The participants in this study were recruited from across China, providing a good representation of the population. In addition, this study population accurately represented the specific subtypes of kidney disease in China and included patients with primary glomerular disease, diabetic kidney disease, and hypertensive renal damage. The proportions of these diseases were consistent with the proportion of diseases leading to hemodialysis treatment in Chinese patients (unpublished data from the Chinese National Renal Data System, http://hd.cnrds.net/hd/).
The present study had several limitations. First, all patients enrolled were Chinese; therefore, further studies are needed to determine the clinical benefits of Niaoduqing particles in other populations. Second, as a compound medication, the mechanisms of action of the active ingredients of Niaoduqing particles need further investigation. Third, although this study showed a delay in eGFR decline with Niaoduqing particles, because of the short study period, a sufficient number of kidney and cardiovascular endpoint events were not observed.
In Conclusion, Niaoduqing particles safely and effectively delayed CKD progression in patients with moderate-to-severe renal insufficiency. Long-term follow-up studies are required to further validate the impact of Niaoduqing particles on kidney and cardiovascular endpoint events.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from the National Key Technology R&D Program (Nos. 2013BAI09B05 and 2015BAI12B06), Key Program of the National Natural Science Foundation of China (No. 81330019), General Program of the National Natural Science Foundation of China (No. 81270794), and the Beijing Science and Technology Project (No. D131100004713003 and No. D171100002817002).
Conflicts of interest
Consun Pharmaceutical Group (Guangzhou, Guangdong, China) provided the Niaoduqing particles and placebo. The Consun Pharmaceutical Group had no effect on the study design, data collection, statistical analysis, and interpretation of results. Prof. Xiang-Mei Chen has no financial or related interest in Consun Pharmaceutical Group.
Acknowledgments
The authors would like to thank Drs. Xiao-Yan Yan and Yong-Pei Yu from the Peking University Clinical Research Institute for statistical support, insightful suggestions, and comments on the study protocol; Drs. Li Zhang, Shu-Wei Duan, Zuo-Xiang Li, and Bo Fu from the Department of Nephrology, Chinese People's Liberation Army General Hospital, for their help with various aspects of the study; the researchers from all 22 research centers, Drs. Ji-Lin Chen, Kai Qu, Yi-Fan Wu, Galiya, Xia-Jing Che, Qin-Tao Chang, Li Wang, Wen-Hu Liu, Xing-Lan Wu, Ling-Yun Zhang, Yan-Hong Huo, Shi-Feng Wu, Wei Shi, Jing-Jing Zhang, Shi-Ren Sun, Ying-Chun Cui, Yan-Fang Yang, Xue-Qiang Xu, Li Wang, and Yu-Wei Gao, for their cooperation, encouragement, and enthusiasm in the clinical trial; and the study participants for their trust and inspiration to the authors.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet. 2012;379:815–22. doi: 10.1016/S0140-6736(12)60033-6. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Feng YH, Fu P. Dual Blockade of the Renin-angiotensin-aldosterone system in type 2 diabetic kidney disease. Chin Med J. 2016;129:81–7. doi: 10.4103/0366-6999.172599. doi: 10.4103/0366-6999.172599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang RS, Cheng YM, Zeng XX, Kim S, Fu P. Renoprotective effect of the combination of renin-angiotensin system inhibitor and calcium channel blocker in patients with hypertension and chronic kidney disease. Chin Med J. 2016;129:562–9. doi: 10.4103/0366-6999.176987. doi: 10.4103/0366-6999.176987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:131–6. doi: 10.1681/ASN.2011030272. doi: 10.1681/ASN.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123–30. doi: 10.1681/ASN.2011040378. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–35. doi: 10.1681/ASN.2009060593. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–503. doi: 10.1056/NEJMoa1306033. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan ZB, Liu J, Yang TC, Liu ZR, Shi XH. A clinical observation of traditional Chinsese medicine Niaoduqing particles in patients with chronic renal failure (in Chinese) Shandong Med J. 1991;31:13–4. [Google Scholar]
- 11.Zhuang YZ, Xie FA, Chen J. Observation of the efficiency and analysis of associated factors of Uremic Clearance Granules (in Chinese) Chin J Integr Tradit West Nephrol. 2003;4:402–3. doi: 10.3969/j.issn.1009-587X.2003.07.013. [Google Scholar]
- 12.Wu HL, Lin HC, Ruan XL, Deng CH, She YP, Fang JA. Observation of the curative efficiency of Uremic Clearance Granules in 118 patients with chronic renal failure (in Chinese) Chin J Integr Tradit West Nephrol. 2004;5:21–4. doi: 10.3969/j.issn.1009-587X.2004.01.008. [Google Scholar]
- 13.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–44. doi: 10.1681/ASN.2006040368. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 14.ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med. 1999;18:1905–42. [PubMed] [Google Scholar]
- 15.Jaber BL, Madias NE. Progression of chronic kidney disease: Can it be prevented or arrested? Am J Med. 2005;118:1323–30. doi: 10.1016/j.amjmed.2005.02.032. doi: 10.1016/j.amjmed.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Parving HH, Smidt UM, Hommel E, Mathiesen ER, Rossing P, Nielsen F, et al. Effective antihypertensive treatment postpones renal insufficiency in diabetic nephropathy. Am J Kidney Dis. 1993;22:188–95. doi: 10.1016/s0272-6386(12)70185-3. doi: 10.1016/S0272-6386(12)70185-3. [DOI] [PubMed] [Google Scholar]
- 17.Parving HH, Hommel E, Smidt UM. Protection of kidney function and decrease in albuminuria by captopril in insulin dependent diabetics with nephropathy. BMJ. 1988;297:1086–91. doi: 10.1136/bmj.297.6656.1086. doi: 10.1136/bmj.297.6656.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parving HH, Hommel E, Damkjaer Nielsen M, Giese J. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. BMJ. 1989;299:533–6. doi: 10.1136/bmj.299.6698.533. doi: 10.1136/bmj.299.6698.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349:1857–63. doi: 10.1016/S0140-6736(96)11445-8. [PubMed] [Google Scholar]
- 20.Zhong Y, Deng Y, Chen Y, Chuang PY, Cijiang He J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013;84:1108–18. doi: 10.1038/ki.2013.276. doi: 10.1038/ki.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng XJ, Wan YG, Wei QX, Chen HL, Shi XM, Huang YR, et al. Overview research of uremic ulearance granule treating chronic renal failure (in Chinese) Zhongguo Zhong Yao Za Zhi. 2013;38:3651–5. doi: 10.4268/cjcmm20132110. [PubMed] [Google Scholar]
- 22.Zhu XL, Wang YJ, Yang Y, Yang RC, Zhu B, Zhang Y, et al. Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol Med Rep. 2012;6:493–500. doi: 10.3892/mmr.2012.960. doi: 10.3892/mmr.2012.960. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Li H, Bu H, Chen H, Tong H, Liu D, et al. Emodin inhibits the differentiation and maturation of dendritic cells and increases the production of regulatory T cells. Int J Mol Med. 2012;29:159–64. doi: 10.3892/ijmm.2011.820. doi: 10.3892/ijmm.2011.820. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH, Zhang XM, et al. Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-ß/Smad signaling pathway in vivo and in vitro. Exp Biol Med (Maywood) 2014;239:1310–24. doi: 10.1177/1535370214532597. doi: 10.1177/1535370214532597. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WJ, Frei B. Astragaloside IV inhibits NF-kappa B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314. doi: 10.1155/2015/274314. doi: 10.1155/2015/274314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Huang F, Zhang S, Leung SW. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. Int J Cardiol. 2012;157:330–40. doi: 10.1016/j.ijcard.2010.12.073. doi: 10.1016/j.ijcard.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 27.Pan H, Li D, Fang F, Chen D, Qi L, Zhang R, et al. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2011;58:535–42. doi: 10.1097/FJC.0b013e31822de355. doi: 10.1097/FJC.0b013e31822de355. [DOI] [PubMed] [Google Scholar]
- 28.Yang R, Chang L, Guo BY, Wang YW, Wang YL, Jin X, et al. Compound danshen dripping pill pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Evid Based Complement Alternat Med. 2014;2014:256268. doi: 10.1155/2014/256268. doi: 10.1155/2014/256268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M, Wang YP, Luo WB, Xuan LJ. Salvianolate inhibits proliferation and endothelin release in cultured rat mesangial cells. Acta Pharmacol Sin. 2001;22:629–33. [PubMed] [Google Scholar]
- 30.Fan HY, Yang MY, Qi D, Zhang ZK, Zhu L, Shang-Guan XX, et al. Salvianolic acid A as a multifunctional agent ameliorates doxorubicin-induced nephropathy in rats. Sci Rep. 2015;5:12273. doi: 10.1038/srep12273. doi: 10.1038/srep12273. [DOI] [PMC free article] [PubMed] [Google Scholar]