Abstract
Objective:
To provide a comprehensive and latest overview of susceptibility-weighted imaging (SWI) in the application of thrombolysis in acute ischemic stroke, and to update the decision-making effect and clinical value of SWI on identifying stroke patients suitable for thrombolytic therapy and possible benefits and risks followed.
Data Sources:
Literatures referred to this review were collected from PubMed, Medline, and EMBASE published till May 2017, using the search terms including susceptibility-weighted imaging, gradient-echo, T2*, thrombolysis, recombinant tissue plasminogen activator (rt-PA), thrombolytic therapy, and stroke.
Study Selection:
Papers in English or with available English abstracts were considered, with no limitation of study design. References were also identified from the bibliographies of identified articles and the authors’ files.
Results:
SWI is of guiding significance for thrombolytic therapy in stroke patients, it can predict the location and length of thrombus and ischemic penumbra. It is worthy of noting that susceptibility vessel sign (SVS) on SWI can be used to predict recanalization after thrombolytic therapy and whether it is better to implement endovascular thrombolectomy in combination or alone. SWI is sensitive in detecting cerebral microbleed (CMB), and CMB might not be a contraindication for thrombolytic therapy, yet CMBs in multiple foci could possibly be related to intracranial hemorrhage (ICH) after thrombolysis. SVS and CMB on SWI sequence are of instructive value in performing antiplatelet therapy after thrombolytic therapy. Cerebral venous change on SWI is related to lower recanalization rate and poor outcome after thrombolysis.
Conclusions:
It seems that SWI can be applied to guide individualized thrombolytic therapies and assist clinicians in making better decisions by weighing benefits and risks. However, there still exist controversies about the relationship between signs on SWI and thrombolytic therapy.
Keywords: Intracranial Hemorrhage, Outcome, Stroke, Susceptibility-weighted Imaging, Thrombolysis
INTRODUCTION
Ischemic stroke is a predominant stroke type caused by cerebral blood supply interruption, rapidly resulting in energy depletion and cell death.[1] Thrombolysis is a therapeutic approach to restore the blood supply in ischemic area based on the concept of penumbra, which is currently the most promising treatment for acute ischemic stroke (AIS).[2] Clinical trials have demonstrated that intravenous (IV) and intra-arterial thrombolytic therapies improve outcome when administered to select patients with AIS.[3,4] What concern clinicians the most are complications after thrombolysis and whether revascularization can be achieved. It is well established that symptomatic intracranial hemorrhage (sICH) after thrombolysis severely impairs functional recovery and is associated with higher mortality.[5] Determining risk factors for postthrombolysis ICH in individual patients is an essential step in weighing the risks and benefits.[6] Therefore, it is urgent to identify the population who are inclined to develop ICH and whether cerebral blood supply of occluded vessel can be restored after IV thrombolysis.
Thrombolytic therapy is a method to breakdown the blood clot, restore the interrupted blood supply in ischemic area, which includes IV and intra-arterial approaches, the former is more commonly used. Although intra-arterial thrombolysis is of higher recanalization rate, the benefit achieved might be counteracted by the delay of time. Thrombolysis can lead to ischemia-reperfusion injury caused by free radicals, inflammation cytokines, and other factors that are associated with the length of ischemia, collateral circulation, oxygen consumption, et al., which can damage endothelial cell, disrupt blood-brain barrier (BBB), and lead to cerebral hemorrhage. It was suggested that recombinant tissue plasminogen activator (rt-PA) itself increased the BBB disruption as well.[6] Therefore, the risk of ICH after thrombolysis is high and there is no guarantee that recanalization can be achieved and we should be very rigorous when decide to conduct thrombolysis and further combined treatment.
Susceptibility-weighted imaging (SWI), which is originally called blood oxygen level-dependent venographic imaging and can exploit susceptibility differences between tissues and use phase image to detect their differences, is sensitive to detect paramagnetic effect of blood breakdown products. It is a high-resolution, three-dimensional gradient-echo T2* magnetic resonance (MR) technique with enhanced sensitivity for paramagnetic substances, such as blood products, iron, and calcification.[7,8] Moreover, it is characterized by short acquisition times which results in fewer movement artifact and shorter therapeutic delay.[9,10] In AIS, severe reduction of cerebrovascular perfusion and pressure causes increased deoxyhemoglobin to oxyhemoglobin ratio by increasing oxygen extraction fraction.[11,12] As a result, SWI can show asymmetric prominent hypointense vessels potentially from different concentrations of deoxyhemoglobin between ischemic and normal brain area.[13] Furthermore, SWI sequence has the advantage in detecting clot formation and cerebral microbleeding (CMB), cerebral blood perfusion, information that is vital in managing stroke patients,[14,15] especially in thrombolytic therapy which is accompanied with a high risk of ICH and of great significance to acquire blood reperfusion.
CLINICAL APPLICATION OF SUSCEPTIBILITY-WEIGHTED IMAGING FOR THROMBOLYSIS IN ACUTE ISCHEMIC STROKE
The detection of ischemic penumbra
Penumbra is the area surrounding an ischemic event, immediately following the event, blood flow and therefore oxygen transport is reduced locally, leading to hypoxia of the cells near the location of original insult. However, tissues in penumbra area remain viable for several hours after symptom onset, which is the basis of thrombolytic therapy. This can lead to infarction and amplify the original damage from the ischemia over time, which is considerably detected by the mismatch between perfusion reduction and the area already infracted through perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) widely. It was revealed that SWI-DWI mismatch had a similar capability to identify penumbra and predict stroke evolution as PWI-DWI mismatch,[15] which is consistent with a case report that suggested SWI was potential to evaluate the ischemic penumbra and could provide perfusion information comparable with PWI.[16] After cerebral vessel occlusion, different concentrations of paramagnetic deoxyhemoglobin between ischemic and normal brain area can be detected on SWI as asymmetric prominent hypointense vessels. Asymmetric cortical veins can be visualized on SWI after AIS and considered an indicator of salvageable ischemic penumbra.[17,18] It was indicated that the rate of favorable outcome was significantly higher among stroke patients who were treated with thrombolytic therapy with baseline SWI-DWI mismatch, particularly with the presence of reperfusion or recanalization.[19] It may be more logical to move from time-based method to a tissue-based imaging paradigm for patient selection for thrombolytic therapy, because carefully selected patients may still benefit from thrombolytic therapy beyond 4.5 h.[20] It was suggested that the accuracy of SWI-DWI mismatch for predicting favorable outcome was higher than that of perfusion-diffusion mismatch (63% vs. 48.1%) in stroke patients after thrombolysis, and SWI venography may thus provide the oxygen metabolic information about ischemic brain tissue by the noninvasive estimation of blood oxygen level-dependent levels.[19] It was indicated that DWI-SWI mismatch is a good marker for evaluating ischemic penumbra in stroke patients with cerebral infarction, and SWI can detect thrombus in the affected vessels, and may be useful for guiding intra-arterial thrombolytic therapy.[21] Whereas, it was suggested that no significant statistical interaction between the presence of SWI-DWI mismatch and thrombolytic therapy with regard to clinical outcome was found in another study which enrolled 150 patients treated with IV thrombolysis, and SWI-DWI mismatch was identified in 44 (29.3%) of those patients.[22] There is no consensus about the correlation between SWI-DWI mismatch and thrombolytic therapy, but it could still be useful to decide whether to proceed thrombolysis on the basis of predicting penumbra, and further studies and information are needed. SWI can be an ideal alternative to help identify penumbra without contrast agent administration, which is hazardous in a patient with compromised renal function.[14] In consideration of its short acquisition time,[9,10] it might be helpful to decide whether we can conduct thrombolytic therapy beyond the therapeutic time window with the existence of penumbra, which means that there are still viable brain tissues in ischemic area.
The value of cerebral microbleed detected on susceptibility-weighted imaging before thrombolysis
Hemorrhagic transformation (HT) was classified using clinical and imaging criteria as follows: hemorrhagic infarction, parenchymal hemorrhage (PH), and sICH.[23] To date, there are no definite imaging signs that can predict HT with high sensitivity and specificity before thrombolysis, and no consensus is achieved about the precise correlation between CMB detected on SWI and HT. Current guidelines for thrombolytic therapy indicate that a prior history of intracerebral hemorrhage is also a contraindication to thrombolytic therapy.[24] However, these guidelines, referring to clinically manifested prior hemorrhages and hemorrhages diagnosed by head computed tomography (CT), do not address the presence of clinically silent microbleeds that are only detected with advanced MR imaging (MRI) sequences, such as SWI,[25] and it is speculated that HT occurred at remote from ischemic area after thrombolytic therapy may be associated with old microbleeds detected on SWI.[26] Whether preexisting CMBs increase the risk of postthrombolysis ICH and whether their presence is a marker of poor functional outcome are controversial.[6] Several studies have shown that SWI is superior to T2-weighted sequences in detecting CMB,[26,27,28] and it can identify minimal hemorrhages in cerebral infarction and prior asymptomatic CMBs that is undetectable by CT or regular MRI.[29] It was indicated that the presence of CMBs was correlated with sICH, and MRI studies have suggested that CMBs may be a potential marker of a hemorrhage-prone state,[30,31,32] which means that there is a high risk of HT after thrombolysis. Yan et al.[6] discovered that the presence of extensive (≥3) CMBs detected on SWI independently predicted PH and poor outcome, even after adjusting for the baseline National Institutes of Health Stroke Scale (NIHSS) score, age, and baseline infarct volume, and this might be explained by the possibility that CMB is simply a marker of impaired blood-brain barrier. On the contrary, neither the existence of CMBs, their burden, predominant location, nor their presumed pathogenesis influenced the risk for symptomatic or asymptomatic ICH or worsen outcome, whereas a higher CMBs burden marginally increased the risk of ICH outside the infarct.[33] It was mentioned that CMB might be hemosiderin deposits of previous, old bleeding or recent bleeding,[34,35,36] if it was an old clot, the relationship with ICH or functional outcome after thrombolysis might not be as close as a new one. However, we cannot be sure whether the CMB is new or old, maybe further researches are needed to identify the formation time. It was suggested that in stroke patients with CMBs, thrombolytic therapy should not be excluded even with prior use of antiplatelets.[37] Two previous meta-analyses including the same studies of 790 IV thrombolytic patients concluded differently: Shoamanesh et al.[38] found an increased risk for sICH in patients with CMB, whereas Charidimou et al.[39] did not. However, two recent meta-analysis have come to similar conclusions,[40,41] Wang et al.[40] included 11 studies, 2702 AIS patients treated with IV thrombolysis (IVT), and it was concluded that CMB's presence was not significantly associated with the increased risk of early sICH after IVT, whereas CMB's presence increased the risks of 3-month poor functional outcome, PH, and any ICH after IVT. Charidimou et al.[41] found that CMBs are associated with greater sICH risk and poor functional outcome in AIS patients undergoing thrombolytic therapy with the data of 8 studies and 2601 stroke patients treated with IV thrombolysis. Whereas, it was indicated that CMB's presence should not be a contraindication to IVT for AIS patients based on the existing evidence,[40] but thrombolysis may not be a safe choice for stroke patients with the detection of CMBs on SWI, especially extensive CMBs. Turc et al.[42] concluded that there was no relation between CMBs and sICH, neither was poor outcome after adjusting for confounding variables. A recent study discovered that the rates of sICH did not differ between patients with and without 1-10 CMBs, but it was noted that the presence of more than 10 CMBs was correlated with increased risk of sICH, even after adjusting for confounding factors,[43] which was inconsistent with the conclusion the previous study suggested that the risk of sICH and PH was significantly increased in patients who were presented with more than one CMB.[44] It is still controversial about the outcome-predicting role of CMBs, studies relevant to consequences after thrombolytic therapy and previous detection of CMBs are limited, and more studies using SWI to explore the relationship between CMBs and thrombolysis are needed to evaluate the process and relevant consequences.
The application of venous change on susceptibility -weighted imaging for thrombolysis
Venous changes in the affected hemisphere after AIS may play a crucial role in determining clinical outcome.[45,46,47] SWI venography allows clear visualization of cerebral veins.[7] Shortly after vascular occlusion, the uncoupling between oxygen supply and demand in the hypoperfused region leads to an elevated oxygen extraction fraction and subsequent increased level of deoxyhemoglobin in the vessel, which contributes to prominent hypointensity of the draining veins on SWI.[27,48,49] It was found that the presence of ipsilateral prominent thalamostriate vein (IPTSV) on SWI was associated with a lower successful reperfusion rate after adjusting for baseline diastolic blood pressure and onset-to-needle time, in the subgroup of patients without reperfusion, patients with IPTSV had a significantly higher rate of poor outcome compared with those without IPTSV, and IPTSV was not associated with poor outcome in patients with successful reperfusion.[50] Hermier et al.[51] discovered that an abnormal visibility of transcerebral veins within the ischemic area, which was identified as a signal drop within cerebral veins on SWI, was correlated with subsequent of HT in patients with hemispheric ischemic stroke and treated with IV thrombolytic therapy, and it was mentioned that venous blood volume represented 70–80% of the total blood volume,[52] contained higher concentrations of deoxyhemoglobin, the gradient-echo imaging modifications might be mainly related to venous structures.[53] In addition, the maximal T2* signal lowering due to deoxyhemoglobin may be expected within venous structures that drain hypoperfused tissue.[51]
Xu et al.[54] found that brush sign, conspicuity deep medullary vein detected on SWI, which was proved to be associated with lower cerebral blood flow and cerebrovascular reserve in the middle cerebral artery area in moyamoya disease,[55] was independently correlated with HT of stroke patients following IV thrombolysis, which was consistent with the previous finding that BS might predict the development of HT in patients treated with thrombolysis.[56] Zhao et al.[57] conducted a prospective study in which patients were divided into two groups according to the presence of symmetric or asymmetric veins on SWI before thrombolysis, and it was shown that the 90-day mortality of both the groups was zero, and higher good outcome rate was found in patients detected with symmetric venous sign by SWI statistically. The number and level of CMBS were lower but with no statistically significance, and some of the low density signals were inversed after thrombolysis in asymmetric venous sign group, which is believed to be related to the recanalized blood flow.[57]
The association between susceptibility vessel sign on susceptibility-weighted imaging and recanalization
It was hypothesized that the main component in hyperacute thrombi might be oxyhemoglobin, instead of deoxyhemoglobin or hemosiderin, which made the clot invisible to the identification of negative susceptibility vessel sign (SVS).[58] On the contrary, it was found that the negative SVS remained invisible on follow-up SWI in all patients without recanalization, indicating that this sign may not be simply related to the component of oxyhemoglobin,[59] and other undetermined factors may also be involved. It was suggested that higher baseline NIHSS score was associated with poor outcome in patients with positive SVS which was completely or partially disappeared in patients recanalization was achieved.[59] Assouline et al.[60] confirmed that vascular susceptibility signs decreased in size or disappeared after partial or complete recanalization of the occluded artery, and those signs can be seen as soon as 45 min after arterial occlusion and changes with time despite a persisting occlusion. A latest research conducted by Yan et al.[61] discussed the recanalization-predicting role of extensive blooming artifact after thrombolysis, which is the SVS width beyond the lumen, more specifically, the overestimation ratio of thrombus width on SWI.[61] The extent of SVS width beyond the lumen might reflect the content of hemosiderin which exhibits a stronger T2 shortening effect than deoxyhemoglobin, and an extreme overestimation ration might indicated aged thrombus, which may be resistant to thrombolysis;[61] in this scenario, it might be better to implement endovascular thrombectomy instead. Hemosiderin is considered more paramagnetic than deoxyhemoglobin, since the iron-containing cores are more closely packed.[62] As time goes by, the blood clot may contain more hemosiderin that will make larger blooming artifact, but an aging clot with structural reorganization may mask the initial thrombotic resource and thus confuse these results.[62]
Recanalization is the strongest predictor of at-risk tissue rescue and clinical impairment reversal and accordingly is the mainstay of current stroke therapy.[63] Many factors impact the success of recanalization therapy, including clot composition, clot burden, and site of clot impaction.[64,65,66,67] An autopsy study showed that thromboembolic occlusions can be caused by red, white, or mixed thrombi.[68] Red thrombi mainly consist of trapped erythrocytes and fibrin, which form in low-pressure systems, such as cardiac or venous systems, and it is widely accepted that cardiac emboli are mainly composed of red thrombi.[69] White thrombi are predominantly composed of platelet aggregates, which form in areas of high-shear stress, such as the arterial system, and, thus, develop on ruptured plaques,[69] and obstructive arterial thrombi that are platelet rich are resistant to thrombolysis.[70]
The presence of unpaired electrons in deoxyhemoglobin and hemosiderin gives them paramagnetic properties which produce a nonuniform magnetic field and a rapid dephasing of proton spins, resulting in a loss of signal best seen on SWI.[71] Thus, red thrombi in occlusive vessels may be seen as hypointense signals within vascular cisterns on SWI, because of the paramagnetic property of deoxygenated hemoglobin components in trapped red blood cells, which is termed as SVS.[70,72] More proximal and longer clots are harder to treat, leading to a worse outcome as compared to shorter and distal clots.[64,73] SWI enables visualization of thrombotic material in AIS, and it was suggested that clot location and length could be reliably assessed using the SVS, with a high concordance with DSA findings, and not only can SWI show the proximal end of the occluding thrombus, but it can also reveal the length of the clot.[74] It was hypothesized that patients with large clots in proximal vessels might benefit from endovascular interventions,[75] and when using stent retrievers, it is important to deploy the distal tip of the device in the vessel segment distal to the thrombus so as not to shear off parts of the clot during stent retraction, and SWI is helpful for planning this procedure as it can identify the distal thrombus end as well as the curvature of the occluded vessel segment, and length and type of the stent retriever can be selected prior to the intervention on the basis of information from the SWI.[74] A study on middle cerebral artery (MCA) stroke demonstrated that IV thrombolysis had nearly no potential for recanalizing thrombi measuring more than 8 mm in length;[76] on the contrary, thrombus length appears to have no impact on reperfusion success of endovascular therapy;[74] therefore, it might be more appropriate to treat patients who are detected with long thrombus on SWI with endovascular therapy, instead of IV thrombolysis. The sensitivities of SVS on SWI for thrombus differ among studies,[69,72,77,78,79,80] ranging from 34% to 92.6%, and it is believed that differences between these studies are related to the composition, location, and aging of clots associated with stroke etiology.[79] It was revealed that cardioembolic occlusion was associated with higher presence of SVS compared with other stroke subtypes, and SVS might predict cardioembolic stroke and subsequent recanalization. Stroke patients who were present with SVS on SWI might be more sensitive to fibrinolytic therapy.[79] However, there was a statement that recanalization mainly represented delayed spontaneous recanalization in cardioembolic stroke patients, which was a frequent phenomenon in the acute phase of cardioembolic stroke.[81] Therefore, SVS seems to be associated with spontaneous but not fibrinolytic therapy-induced recanalization in cardioembolic stroke.
Kimura et al.[82] conducted a research where patients with internal carotid artery and M1 occlusion were prospectively studied, and it was revealed that patients without the M1 SVS had early recanalization, but none of the patients with the M1 SVS had early recanalization (P = 0.0002), and it was believed that SVS was present in older thrombi, which might be resistant to rt-PA therapy. Hence, it was indicated that M1 SVS on SWI appeared to be a strong predictor for no early recanalization after thrombolysis,[82] the conclusion was consistent with the finding concluded that positive predictive value of M1 SVS for predicting no early recanalization was 89.7%,[83] and it was suggested that in patients with acute stroke within 3 h of onset, M1 SVS might be a sign for combined IV thrombolysis and endovascular therapy or endovascular therapy alone instead of IV thrombolysis as first-line therapy. Kim et al.[84] found that a positive MCA susceptibility sign on initial SWI could be used to predict the immediate effectiveness of intraarterial thrombolysis, but this sign was not associated with a favorable clinical outcome after thrombolysis, and it was discovered that a history of atrial fibrillation was higher in the patients with MCA susceptibility sign, which was accordant with that cardioembolic occlusion was associated with higher presence of SVS.[79] It was indicated that early recanalization was more frequent in patients with cardioembolic stroke compared with other stroke types.[83] Cardioembolic stroke probably represents the stroke subtype with more uniform fibrin-rich clots, given the high binding affinity of rt-PA for fibrin, in fibrin-rich clots, rt-PA penetrates and distributes homogeneously, leading to an entire and rapid clot dissolution, in contrast, in well-organized and platelet-rich clots, penetration and distribution of rt-PA are limited, which may result in nonuniform clot softening and degradation from the outside of the clot, and clot shrinks and moves distally, lodging in smaller arteries.[83]
Legrand et al.[63] conducted a study about clot burden score on SWI, an adaption of the CT angiography-clot burden score, which is assessed by the location and quantity of the SVS using a 10-point scale, and evaluated its correlation of 24-h recanalization and 3-month outcome after IV thrombolysis, and it was suggested that the clot burden but not the presence of any SVS was associated with 24-h recanalization in patients with acute anterior circulation stroke treated with thrombolysis and patients with less thrombus burden were more likely to have favorable functional 3-month outcome after IV thrombolysis. The absence of SVS might be explained by the following reasons: First, MRI characteristics, such as sequence parameters, spatial resolution, and magnetic field strength, all the elements can influence the imaging results, leading to false-negative detection results. Second, time interval between symptom onset and radioexaminiation, hyperacute clot might not be show as hypointense signal on SWI, as the oxyhemoglobin remains the original status, instead of turning into deoxyhemoglobin or hemosiderin. Third, the vessel occlusion is caused by white thrombus consisted of platelets and fibrin, lacking paramagnetic substances. Fourth, spontaneous recanalization of occluded vessel and other undetermined mechanisms involved. Identification of SVS may help guide therapeutic decisions in terms of indicating whether an occlusive clot is susceptible or resistant to fibrinolytic therapy,[85,86] as stated, old, platelet-rich and well-organized thrombi formed under flow conditions have been shown to be more resistant to thrombolysis than fresh, fibrin- and red cell-rich clots formed under conditions of stasis,[87] and it is essential to identify the clot composition and time phase; therefore, we can treat different individuals with the most suitable therapy based on clot characteristics.
The relationship between cerebral microbleed on susceptibility-weighted imaging after thrombolysis and functional outcome
It is still controversial that the impact on functional outcome HT exerts after thrombolytic therapy. It was shown that there was a higher rate of good outcome in patients with asymptomatic hemorrhage after thrombolytic therapy and explained these results by the fact that it could reflect recanalization.[88] Annan et al.[89] concluded that asymptomatic hemorrhage was correlated with a higher poor functional outcome at 3 months, but after adjusting for baseline NIHSS score, it was no longer statistically significant. However, even if HT of ischemic brain tissue after rt-PA thrombolysis as detected by MRI can be asymptomatic, it may impair neurological recovery.[90] Libman et al.[91] concluded that asymptomatic hemorrhage might not benign using CT. On the contrary, Bai et al.[92] revealed that higher CMB incidence was correlated with better clinical improvement in IV thrombolysis-treated super-AIS patients. Evidences about the impact of CMB after thrombolysis are finitude, and SWI is characterized with high sensitivity of CMB or any other types of cerebral hemorrhage for the character of identifying deoxyhemoglobin and hemosiderin; further studies are needed to confirm the predicting role of CMB or asymptomatic hemorrhage after thrombolytic therapy.
The value of susceptibility-weighted imaging in decision-making for combination of antiplatelet therapy
Studies about antiplatelet therapy and the correlation between ICH and outcome after thrombolytic therapy are emerging. It was found that prior antiplatelets use increased neither risk of PH nor adverse functional outcome in patients with CMBs, while in patients who had prior antiplatelets and multiple CMBs (≥3), the HT was higher, but not adverse functional outcome.[37] It was suggested that previous antiplatelet therapy and shorter time from onset to thrombolysis were inversely associated with poor outcome in patients with absence of SVS on SWI in patients treated with thrombolysis.[59] Thromboembolic occlusions can be caused by red, white, or mixed thrombi.[68] White thrombi composed of platelets and fibrin[93] might be shown with lower rate of SVS on SWI, in the consideration of lacking paramagnetic substance comparing to red clot. Absence of SVS on SWI is more likely to indicate white thrombi, which may be easier to grow in a short time and thus is likely to benefit from prior use of antiplatelets agents.[59] In acute coronary syndrome, obstructive arterial thrombi that are platelet rich are resistant to thrombolysis and have an increased tendency to grow reocclusion after initial reperfusion.[70] In these circumstances, an antiplatelet agent used in combination with a thrombolytic agent not only offers the potential for enhancing thrombolysis and reducing the risk of reocclusion, but also permits this to be accomplished with reduced doses of thrombolytics and heparin.[94] Stroke patients who suffer from vascular occlusion by white thrombi are more suitable for antiplatelet therapy whether before or after symptom onset when there are no limitations. On the other hand, the presence of SVS was postulated to represent the red clot containing erythrocytes and some fibrin,[93,95] which is composed of paramagnetic substance, deoxyhemoglobin and hemosiderin; thus, it may be seen as a hypointense signal on SWI.[59] As stated above, the absence of SVS on SWI might indicate white thrombi, but there is no definite conclusion; we should further explore under which circumstance the absence of SVS is a predictor for white thrombi and implent antiplatelet therapy.
Yan et al.[96] allocated 146 patients without sICH on CT after thrombolysis to two group: Group A (n = 72) received antiplatelets 24 h after thrombolytic therapy, regardless of SWI detected hemorrhage; Group B (n = 74) received antiplatelets for patients without SWI-visualised hemorrhage, a higher rate of poor outcome at 90 days was found in patients who received antiplatelets 24 h after thrombolysis with the detection of CMB on SWI, which indicated that CMB might be a potential marker of a hemorrhage-prone state and might develop into HT or PH. It was shown that patients did not receive further antiplatelets with the detection of hemorrhage had a higher rate of favorable outcome; on the contrary, further antiplatelet therapy may lead to worse clinical outcome and may impair patients’ neurological recovery, even CMBs.[96] It was suggested that NIHSS score at 7 days and 14 days after thrombolysis was significantly lower in the patients with the detection of hemorrhage on SWI without continuing antiplatelet therapy, so as a significantly shorter duration of hospitalization and higher favorable outcome rate at 90 days.[97] This indicates that stroke patients who are detected with ICH on SWI, even CMBs, might not be suitable for antiplatelet therapy soon after thrombolysis. As stated above, studies about prior antiplatelets use and signs detected on SWI are limited, either CMB or SVS, hence, more studies should be conducted to clarify the relationships in between. It is believed that patients with negative SVS on SWI can benefit from antiplatelet therapy, but antiplatelet therapy afterwards in stroke patients treated with thrombolysis with the detection of CMBS on SWI are scarce, and conclusions are inconsistent.
CONCLUSION
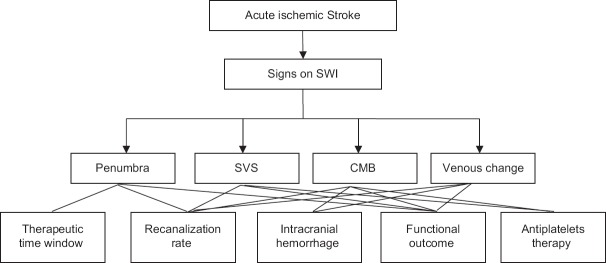
SWI is of critical significance in guiding individualized thrombolytic therapies, but there are controversies about the value of signs detected and their correlations with recanalization, ICH, functional outcome, and antiplatelet therapy in thrombolysis [Figure 1]. Further studies are needed to figure out the authentic relationships and mechanisms involved.
Figure 1.
The undetermined correlations between signs detected on SWI and thrombolytic therapy. SVS: Susceptibility vessel sign; CMB: Cerebral microbleeding; SWI: Susceptibility-weighted imaging.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. doi: 10.1016/S1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 2.Lyden P. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;334:1405–6. [PubMed] [Google Scholar]
- 3.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 5.Strbian D, Sairanen T, Meretoja A, Pitkäniemi J, Putaala J, Salonen O, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. 2011;26(77):341–8. doi: 10.1212/WNL.0b013e3182267b8c. doi: 10.1212/WNL.0b013e3182267b8c. [DOI] [PubMed] [Google Scholar]
- 6.Yan S, Jin X, Zhang X, Zhang S, Liebeskind DS, Lou M. Extensive cerebral microbleeds predict parenchymal haemorrhage and poor outcome after intravenous thrombolysis. J Neurol Neurosurg Psychiatry. 2015;86:1267–72. doi: 10.1136/jnnp-2014-309857. doi: 10.1136/jnnp-2014-309857. [DOI] [PubMed] [Google Scholar]
- 7.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–8. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 8.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–7. doi: 10.1148/radiology.204.1.9205259. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS, Weisskoff RM. Ultra-fast imaging. Magn Reson Imaging. 1991;9:1–37. doi: 10.1016/0730-725x(91)90094-3. [DOI] [PubMed] [Google Scholar]
- 10.Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004;35:1989–94. doi: 10.1161/01.STR.0000133341.74387.96. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Yundt KD, Videen TO, Carpenter DA, Grubb RL, Jr, Powers WJ. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke. 1998;29:754–8. doi: 10.1161/01.str.29.4.754. doi: 10.1161/01.STR.29.4.754. [DOI] [PubMed] [Google Scholar]
- 12.Grubb RL, Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–60. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 13.Kao HW, Tsai FY, Hasso AN. Predicting stroke evolution: Comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol. 2012;22:1397–403. doi: 10.1007/s00330-012-2387-4. doi: 10.1007/s00330-012-2387-4. [DOI] [PubMed] [Google Scholar]
- 14.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: Effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–43. doi: 10.3174/ajnr.A1355. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232–52. doi: 10.3174/ajnr.A1461. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, Luo S, Wang Y, Chen Y, Liu J, Bai J, et al. Use of susceptibility-weighted imaging in assessing ischemic penumbra: A case report. Medicine (Baltimore) 2017;96:e6091. doi: 10.1097/MD.0000000000006091. doi: 10.1097/MD.0000000000006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaitsu Y, Kudo K, Terae S, Yazu R, Ishizaka K, Fujima N, et al. Mapping of cerebral oxygen extraction fraction changes with susceptibility-weighted phase imaging. Radiology. 2011;261:930–6. doi: 10.1148/radiol.11102416. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka M, Okuchi K, Iwamura A, Taoka T, Siesjö BK. A mismatch between the abnormalities in diffusion-and susceptibility-weighted magnetic resonance imaging may represent an acute ischemic penumbra with misery perfusion. J Stroke Cerebrovasc Dis. 2013;22:1428–31. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.009. doi: 10.1016/j.jstrokecerebrovasdis.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Lou M, Chen Z, Wan J, Hu H, Cai X, Shi Z, et al. Susceptibility-diffusion mismatch predicts thrombolytic outcomes: A retrospective cohort study. AJNR Am J Neuroradiol. 2014;35:2061–7. doi: 10.3174/ajnr.A4017. doi: 10.3174/ajnr.A4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bivard A, Spratt N, Levi CR, Parsons MW. Acute stroke thrombolysis: time to dispense with the clock and move to tissue-based decision-making? Expert Rev Cardiovasc Ther. 2011;9:451–61. doi: 10.1586/erc.11.7. doi: 10.1586/erc.11.7. [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol. 2015;42:255–60. doi: 10.1016/j.neurad.2014.07.002. doi: 10.1016/j.neurad.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Payabvash S, Taleb S, Benson JC, Hoffman B, Oswood MC, McKinney AM, et al. Susceptibility-diffusion mismatch in middle cerebral artery territory acute ischemic stroke: Clinical and imaging implications. Acta Radiol. 2017;58:876–82. doi: 10.1177/0284185116675658. doi: 10.1177/0284185116675658. [DOI] [PubMed] [Google Scholar]
- 23.Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–41. doi: 10.1161/01.str.32.2.438. doi: 10.1161/01.STR.32.2.438. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP, Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, et al. Guidelines for thrombolytic therapy for acute stroke: A supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1996;27:1711–8. [PubMed] [Google Scholar]
- 25.Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: An emerging application. Stroke. 2002;33:95–8. doi: 10.1161/hs0102.101792. doi: 10.1161/hs0102.101792. [DOI] [PubMed] [Google Scholar]
- 26.Wycliffe ND, Choe J, Holshouser B, Oyoyo UE, Haacke EM, Kido DK. Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: A retrospective study. J Magn Reson Imaging. 2004;20:372–7. doi: 10.1002/jmri.20130. doi: 10.1002/jmri.20130. [DOI] [PubMed] [Google Scholar]
- 27.Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: A new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009;64:74–83. doi: 10.1016/j.crad.2008.04.022. doi: 10.1016/j.crad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;227:332–9. doi: 10.1148/radiol.2272020176. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 29.Berger C, Fiorelli M, Steiner T, Schäbitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: Asymptomatic or symptomatic? Stroke. 2001;32:1330–5. doi: 10.1161/01.str.32.6.1330. doi: 10.1161/01.STR.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 30.Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, et al. Risk vs.benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255:1679–86. doi: 10.1007/s00415-008-0967-7. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–20. doi: 10.1161/01.STR.0000126807.69758.0e. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004;62:72–6. doi: 10.1212/01.wnl.0000101463.50798.0d. [DOI] [PubMed] [Google Scholar]
- 33.Gratz PP, El-Koussy M, Hsieh K, von Arx S, Mono ML, Heldner MR, et al. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke. 2014;45:1684–8. doi: 10.1161/STROKEAHA.114.004796. doi: 10.1161/STROKEAHA.114.004796. [DOI] [PubMed] [Google Scholar]
- 34.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–42. [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. doi: 10.1016/S1474-4422(09)70013-4. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: Histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32:528–34. doi: 10.1159/000331466. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 37.Yan SQ, Mao YY, Zhong GL, Zhang S, Lou M. Safety of intravenous thrombolysis in cerebral microbleeds patients with prior antiplatelet therapy (in Chinese) J Zhejiang Univ Sci B (Med Sci) 2015;44:618–24. doi: 10.3785/j.issn.1008-9292.2015.11.04. doi: 10.3785/j.issn.1008-9292.2015.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: A systematic review and meta-analysis. Int J Stroke. 2013;8:348–56. doi: 10.1111/j.1747-4949.2012.00869.x. doi: 10.1111/j.1747-4949.2012.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and the risk of intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: Systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:277–80. doi: 10.1136/jnnp-2012-303379. doi: 10.1136/jnnp-2012-303379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Lv Y, Zheng X, Qiu J, Chen HS. The impact of cerebral microbleeds on intracerebral hemorrhage and poor functional outcome of acute ischemic stroke patients treated with intravenous thrombolysis: A systematic review and meta-analysis. J Neurol. 2016;264:1–11. doi: 10.1007/s00415-016-8339-1. doi: 10.1007/s00415-016-8339-1. [DOI] [PubMed] [Google Scholar]
- 41.Charidimou A, Shoamanesh A International META-MICROBLEEDS Initiative. Clinical relevance of microbleeds in acute stroke thrombolysis: Comprehensive meta-analysis. Neurology. 2016;87:1534–41. doi: 10.1212/WNL.0000000000003207. doi: 10.1212/WNL.0000000000003207. [DOI] [PubMed] [Google Scholar]
- 42.Turc G, Sallem A, Moulin S, Tisserand M, Machet A, Edjlali M, et al. Microbleed status and 3-month outcome after intravenous thrombolysis in 717 patients with acute ischemic stroke. Stroke. 2015;46:2458–63. doi: 10.1161/STROKEAHA.115.009290. doi: 10.1161/STROKEAHA.115.009290. [DOI] [PubMed] [Google Scholar]
- 43.Zand R, Tsivgoulis G, Singh M, McCormack M, Goyal N, Ishfaq MF, et al. Cerebral microbleeds and risk of intracerebral hemorrhage post intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2017;26:538–544. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.127. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.127. [DOI] [PubMed] [Google Scholar]
- 44.Dannenberg S, Scheitz JF, Rozanski M, Erdur H, Brunecker P, Werring DJ, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2014;45:2900–5. doi: 10.1161/STROKEAHA.114.006448. doi: 10.1161/STROKEAHA.114.006448. [DOI] [PubMed] [Google Scholar]
- 45.Chen CY, Chen CI, Tsai FY, Tsai PH, Chan WP. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: Prediction of infarct growth and clinical outcome. PLoS One. 2015;10:e0131118. doi: 10.1371/journal.pone.0131118. doi: 10.1371/journal.pone.0131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baik SK, Choi W, Oh SJ, Park KP, Park MG, Yang TI, et al. Change in cortical vessel signs on susceptibility-weighted images after full recanalization in hyperacute ischemic stroke. Cerebrovasc Dis. 2012;34:206–12. doi: 10.1159/000342148. doi: 10.1159/000342148. [DOI] [PubMed] [Google Scholar]
- 47.Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D, et al. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: A novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol. 2014;21:1411–8. doi: 10.1111/ene.12510. doi: 10.1111/ene.12510. [DOI] [PubMed] [Google Scholar]
- 48.Robertson CA, McCabe C, Gallagher L, Lopez-Gonzalez Mdel R, Holmes WM, Condon B, et al. Stroke penumbra defined by an MRI-based oxygen challenge technique: 1. Validation using [14C]2-deoxyglucose autoradiography. J Cereb Blood Flow Metab. 2011;31:1778–87. doi: 10.1038/jcbfm.2011.66. doi: 10.1038/jcbfm.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Crespigny AJ, Wendland MF, Derugin N, Kozniewska E, Moseley ME. Real-time observation of transient focal ischemia and hyperemia in cat brain. Magn Reson Med. 1992;27:391–7. doi: 10.1002/mrm.1910270220. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Zhang S, Chen Q, Ding W, Campbell BCV, Lou M. Ipsilateral prominent thalamostriate vein on susceptibility-weighted imaging predicts poor outcome after intravenous thrombolysis in acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38:875–81. doi: 10.3174/ajnr.A5135. doi: 10.3174/ajnr.A5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermier M, Nighoghossian N, Derex L, Adeleine P, Wiart M, Berthezène Y, et al. Hypointense transcerebral veins at T2*-weighted MRI: A marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab. 2003;23:1362–70. doi: 10.1097/01.WCB.0000091764.61714.79. doi: 10.1097/01.WCB.0000091764.61714.79. [DOI] [PubMed] [Google Scholar]
- 52.Ito H, Kanno I, Iida H, Hatazawa J, Shimosegawa E, Tamura H, et al. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15:111–6. doi: 10.1007/BF02988600. [DOI] [PubMed] [Google Scholar]
- 53.Roussel SA, van Bruggen N, King MD, Gadian DG. Identification of collaterally perfused areas following focal cerebral ischemia in the rat by comparison of gradient echo and diffusion-weighted MRI. J Cereb Blood Flow Metab. 1995;15:578–86. doi: 10.1038/jcbfm.1995.71. doi: 10.1038/jcbfm.1995.71. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Chen ZC, Tang H, Xu MJ, Zhang S, Sun JZ, et al. Signifiance of brush sign on susceptibility-weighted imaging predicts hemorrhagic transformation after intravenous thrombolysis in patients with acute ischemic stroke. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44:625–31. doi: 10.3785/j.issn.1008-9292.2015.11.05. doi: 10.3785/j.issn.1008-9292.2015.11.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horie N, Morikawa M, Nozaki A, Hayashi K, Suyama K, Nagata I. “Brush Sign” on susceptibility-weighted MR imaging indicates the severity of Moyamoya disease. AJNR Am J Neuroradiol. 2011;32:1697–702. doi: 10.3174/ajnr.A2568. doi: 10.3174/ajnr.A2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terasawa Y, Yamamoto N, Morigaki R, Fujita K, Izumi Y, Satomi J, et al. Brush sign on 3-T T2*-weighted MRI as a potential predictor of hemorrhagic transformation after tissue plasminogen activator therapy. Stroke. 2014;45:274–6. doi: 10.1161/STROKEAHA.113.002640. doi: 10.1161/STROKEAHA.113.002640. [DOI] [PubMed] [Google Scholar]
- 57.Zhao G, Sun L, Wang Z, Wang L, Cheng Z, Lei H, et al. Evaluation of the role of susceptibility-weighted imaging in thrombolytic therapy for acute ischemic stroke. J Clin Neurosci. 2017;40:175–9. doi: 10.1016/j.jocn.2017.01.001. doi: 10.1016/j.jocn.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Yan S, Hu H, Shi Z, Zhang X, Zhang S, Liebeskind DS, et al. Morphology of susceptibility vessel sign predicts middle cerebral artery recanalization after intravenous thrombolysis. Stroke. 2014;45:2795–7. doi: 10.1161/STROKEAHA.114.006144. doi: 10.1161/STROKEAHA. [DOI] [PubMed] [Google Scholar]
- 59.Yan S, Liu K, Tong L, Yu Y, Zhang S, Lou M. Different risk factors for poor outcome between patients with positive and negative susceptibility vessel sign. J Neurointerv Surg. 2016;8:1001–5. doi: 10.1136/neurintsurg-2015-011999. doi: 10.1136/neurintsurg-2015-011999. [DOI] [PubMed] [Google Scholar]
- 60.Assouline E, Benziane K, Reizine D, Guichard JP, Pico F, Merland JJ, et al. Intra-arterial thrombus visualized on T2* gradient echo imaging in acute ischemic stroke. Cerebrovasc Dis. 2005;20:6–11. doi: 10.1159/000086120. doi: 10.1159/000086120. [DOI] [PubMed] [Google Scholar]
- 61.Yan S, Chen Q, Zhang X, Xu M, Han Q, Shao A, et al. Extensive blooming artifact predicts no recanalization after intravenous thrombolysis. Eur J Neurol. 2016;23:737–43. doi: 10.1111/ene.12930. doi: 10.1111/ene.12930. [DOI] [PubMed] [Google Scholar]
- 62.Wixom RL, Prutkin L, Munro HN. Hemosiderin: Nature, formation, and significance. Int Rev Exp Pathol. 1980;22:193–225. [PubMed] [Google Scholar]
- 63.Legrand L, Naggara O, Turc G, Mellerio C, Roca P, Calvet D, et al. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke. 2013;44:1878–84. doi: 10.1161/STROKEAHA.113.001026. doi: 10.1161/STROKEAHA.113.001026. [DOI] [PubMed] [Google Scholar]
- 64.Somford DM, Nederkoorn PJ, Rutgers DR, Kappelle LJ, Mali WP, van der Grond J. Proximal and distal hyperattenuating middle cerebral artery signs at CT: Different prognostic implications. Radiology. 2002;223:667–71. doi: 10.1148/radiol.2233011017. doi: 10.1148/radiol.2233011017. [DOI] [PubMed] [Google Scholar]
- 65.Rosenthal ES, Schwamm LH, Roccatagliata L, Coutts SB, Demchuk AM, Schaefer PW, et al. Role of recanalization in acute stroke outcome: Rationale for a CT angiogram-based “benefit of recanalization” model. AJNR Am J Neuroradiol. 2008;29:1471–5. doi: 10.3174/ajnr.A1153. doi: 10.3174/ajnr.A1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–54. doi: 10.1161/01.STR.0000257304.21967.ba. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 67.del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 68.Jörgensen L, Torvik A. Ischaemic cerebrovascular diseases in an autopsy series. I. Prevalence, location and predisposing factors in verified thrombo-embolic occlusions, and their significance in the pathogenesis of cerebral infarction. J Neurol Sci. 1966;3:490–509. doi: 10.1016/0022-510x(66)90004-9. [DOI] [PubMed] [Google Scholar]
- 69.Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379–83. doi: 10.1161/01.STR.0000185932.73486.7a. doi: 10.1161/01.STR.0000185932.73486.7a. [DOI] [PubMed] [Google Scholar]
- 70.Yasuda T, Gold HK, Leinbach RC, Saito T, Guerrero JL, Jang IK, et al. Lysis of plasminogen activator-resistant platelet-rich coronary artery thrombus with combined bolus injection of recombinant tissue-type plasminogen activator and antiplatelet GPIIb/IIIa antibody. J Am Coll Cardiol. 1990;16:1728–35. doi: 10.1016/0735-1097(90)90327-l. [DOI] [PubMed] [Google Scholar]
- 71.Clark RA, Watanabe AT, Bradley WG, Jr, Roberts JD. Acute hematomas: Effects of deoxygenation, hematocrit, and fibrin-clot formation and retraction on T2 shortening. Radiology. 1990;175:201–6. doi: 10.1148/radiology.175.1.2315481. doi: 10.1148/radiology.175.1.2315481. [DOI] [PubMed] [Google Scholar]
- 72.Schellinger PD, Chalela JA, Kang DW, Latour LL, Warach S. Diagnostic and prognostic value of early MR Imaging vessel signs in hyperacute stroke patients imaged <3 hours and treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2005;26:618–24. [PMC free article] [PubMed] [Google Scholar]
- 73.Mattle HP, Arnold M, Georgiadis D, Baumann C, Nedeltchev K, Benninger D, et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke. 2008;39:379–83. doi: 10.1161/STROKEAHA.107.492348. doi: 10.1161/STROKEAHA.107.492348. [DOI] [PubMed] [Google Scholar]
- 74.Weisstanner C, Gratz PP, Schroth G, Verma RK, Köchl A, Jung S, et al. Thrombus imaging in acute stroke: Correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol. 2014;24:1735–41. doi: 10.1007/s00330-014-3200-3. doi: 10.1007/s00330-014-3200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee KY, Han SW, Kim SH, Nam HS, Ahn SW, Kim DJ, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke. 2007;38:192–3. doi: 10.1161/01.STR.0000251788.03914.00. doi: 10.1161/01.STR.0000251788.03914.00. [DOI] [PubMed] [Google Scholar]
- 76.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–7. doi: 10.1161/STROKEAHA.110.609693. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 77.Flacke S, Urbach H, Keller E, Träber F, Hartmann A, Textor J, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: Clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000;215:476–82. doi: 10.1148/radiology.215.2.r00ma09476. doi: 10.1148/radiology.215.2.r00ma09476. [DOI] [PubMed] [Google Scholar]
- 78.Rovira A, Orellana P, Alvarez-Sabín J, Arenillas JF, Aymerich X, Grivé E, et al. Hyperacute ischemic stroke: Middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004;232:466–73. doi: 10.1148/radiol.2322030273. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 79.Radbruch A, Mucke J, Schweser F, Deistung A, Ringleb PA, Ziener CH, et al. Comparison of susceptibility weighted imaging and TOF-angiography for the detection of Thrombi in acute stroke. PLoS One. 2013;8:e63459. doi: 10.1371/journal.pone.0063459. doi: 10.1371/journal.pone.0063459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naggara O, Raymond J, Domingo Ayllon M, Al-Shareef F, Touzé E, Chenoufi M, et al. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013;8:e76727. doi: 10.1371/journal.pone.0076727. doi: 10.1371/journal.pone.0076727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molina CA. Imaging the clot: Does clot appearance predict the efficacy of thrombolysis? Stroke. 2005;36:2333–4. doi: 10.1161/01.STR.0000185933.44619.1b. doi: 10.1161/01.STR.0000185933.44619.1b. [DOI] [PubMed] [Google Scholar]
- 82.Kimura K, Iguchi Y, Shibazaki K, Watanabe M, Iwanaga T, Aoki J. M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke. 2009;40:3130–2. doi: 10.1161/STROKEAHA.109.552588. doi: 10.1161/STROKEAHA.109.552588. [DOI] [PubMed] [Google Scholar]
- 83.Kimura K, Sakamoto Y, Aoki J, Iguchi Y, Shibazaki K, Inoue T. Clinical and MRI predictors of no early recanalization within 1 hour after tissue-type plasminogen activator administration. Stroke. 2011;42:3150–5. doi: 10.1161/STROKEAHA.111.623207. doi: 10.1161/STROKEAHA.111.623207. [DOI] [PubMed] [Google Scholar]
- 84.Kim HS, Lee DH, Choi CG, Kim SJ, Suh DC. Progression of middle cerebral artery susceptibility sign on T2*-weighted images: Its effect on recanalization and clinical outcome after thrombolysis. AJR Am J Roentgenol. 2006;187:W650–7. doi: 10.2214/AJR.05.0447. doi: 10.2214/AJR.05.0447. [DOI] [PubMed] [Google Scholar]
- 85.Blinc A, Keber D, Lahajnar G, Zupancic I, Zorec-Karlovsek M, Demsar F. Magnetic resonance imaging of retracted and nonretracted blood clots during fibrinolysisin vitro. Haemostasis. 1992;22:195–201. doi: 10.1159/000216319. [DOI] [PubMed] [Google Scholar]
- 86.Taber KH, Hayman LA, Herrick RC, Kirkpatrick JB. Importance of clot structure in gradient-echo magnetic resonance imaging of hematoma. J Magn Reson Imaging. 1996;6:878–83. doi: 10.1002/jmri.1880060607. [DOI] [PubMed] [Google Scholar]
- 87.Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004;35:486–90. doi: 10.1161/01.STR.0000110219.67054.BF. doi: 10.1161/01.STR.0000110219.67054.BF. [DOI] [PubMed] [Google Scholar]
- 88.Molina CA, Alvarez-Sabín J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P, et al. Thrombolysis-related hemorrhagic infarction: A marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002;33:1551–6. doi: 10.1161/01.str.0000016323.13456.e5. [DOI] [PubMed] [Google Scholar]
- 89.Annan M, Gaudron M, Cottier JP, Cazals X, Dejobert M, Corcia P, et al. Functional outcome of hemorrhagic transformation after thrombolysis for ischemic stroke: A prospective study. Cerebrovasc Dis Extra. 2015;5:103–6. doi: 10.1159/000440737. doi: 10.1159/000440737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kimura K, Iguchi Y, Shibazaki K, Aoki J, Terasawa Y. Hemorrhagic transformation of ischemic brain tissue after t-PA thrombolysis as detected by MRI may be asymptomatic, but impair neurological recovery. J Neurol Sci. 2008;272:136–42. doi: 10.1016/j.jns.2008.05.012. doi: 10.1016/j.jns.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 91.Libman R, Kwiatkowski T. Asymptomatic hemorrhage after thrombolysis may not be benign: Prognosis by hemorrhage type of the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007;38:75–9. doi: 10.1161/STROKEAHA.107.486456. doi: 10.1161/STROKEAHA.107.486456. [DOI] [PubMed] [Google Scholar]
- 92.Bai Q, Zhao Z, Sui H, Xie X, Chen J, Yang J, et al. Susceptibility -weighted imaging for cerebral microbleed detection in super-acute ischemic stroke patients treated with intravenous thrombolysis. Neurol Res. 2013;35:586–93. doi: 10.1179/1743132813Y.0000000179. doi: 10.1179/1743132813Y.0000000179. [DOI] [PubMed] [Google Scholar]
- 93.Jorgensen L. Experimental platelet and coagulation thrombi.a histological study of arterial and venous thrombi of varying age in untreated and heparinized rabbits. Acta Pathol Microbiol Scand. 1964;62:189–223. doi: 10.1111/apm.1964.62.2.189. [DOI] [PubMed] [Google Scholar]
- 94.Antman EM, Giugliano RP, Gibson CM, McCabe CH, Coussement P, Kleiman NS, et al. Abciximab facilitates the rate and extent of thrombolysis: Results of the thrombolysis in myocardial infarction (TIMI) 14 trial. The TIMI 14 Investigators. Circulation. 1999;99:2720–32. doi: 10.1161/01.cir.99.21.2720. doi: 10.1161/01.CIR.99.21.2720. [DOI] [PubMed] [Google Scholar]
- 95.Flacke S, Urbach H, Keller E, Träber F, Hartmann A, Textor J, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: Clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000;215:476–82. doi: 10.1148/radiology.215.2.r00ma09476. doi: 10.1148/radiology.215.2.r00ma09476. [DOI] [PubMed] [Google Scholar]
- 96.Yan L, Li YD, Li YH, Li MH, Zhao JG, Chen SW. Outcomes of antiplatelet therapy for haemorrhage patients after thrombolysis: A prospective study based on susceptibility-weighted imaging. Radiol Med. 2014;119:175–82. doi: 10.1007/s11547-013-0328-1. doi: 10.1007/s11547-013-0328-1. [DOI] [PubMed] [Google Scholar]
- 97.Lu J, Li YH, Li YD, Li MH, Zhao JG, Chen SW. The clinical value of antiplatelet therapy for patients with hemorrhage after thrombolysis based on susceptibility-weighted imaging: A prospective pilot study. Eur J Radiol. 2012;81:4094–8. doi: 10.1016/j.ejrad.2012.08.002. doi: 10.1016/j.ejrad.2012.08.002. [DOI] [PubMed] [Google Scholar]