Abstract
Introduction:
Salt stress is one of the most important abiotic stress factors which severely affect agricultural production. Osmotins and OLPs (osmotin like proteins) are kinds of proteins which were produced during plant adapting to the environmental stress.
Objective:
These proteins were closely related to osmotic regulation and resistance stress. They are widely distributed in plants. Their expression for these genes was induced by salt stress, which played important roles in plants responding to salt stress.
Conclusion:
During salt stress, osmotin can help accumulate proline, and quench reactive oxygen species and free radicals.
Keywords: Salt tolerance, Osmotin, OLPs, Abiotic stress, Proline, Reactive oxygen
1. Introduction
Salt stress is an important factor limiting plant growth and development, and it is also a major abiotic stress factor affecting crop yield. Salt damage is one of the most important adverse circumstances in agricultural production, which seriously restricts the sustainable development of grain production and agriculture [1]. There are more than 20% cultivated land with salinization in the whole world, and the high concentration of salt in the soil causes osmotic stress and ion toxicity, which affects the growth of crops.
Plants under salt and other stresses will produce many stress-responding protein, and osmotin is one of the important. Their expression is induced by salt, and is closely related to salt tolerance. Osmotin in salt stress as osmotic stress protective agent to protect cells from seepage pressure regulating the impact, so as to maintain osmotic balance [2], but its detailed mechanism of salt tolerance is unclear.
2. Characterization and Structure of Osmotin
Singh et al. (1985) analyzed protein composition of tobacco cell treated by different concentrations of NaCl, 26 KD Osmotin increased significantly, 12.3% of total protein of cell can reach, its content and cell resistance was positively rising [3]. The Osmotin protein can be accumulated in the cells without stress and stress [4]. When no stress induced, Osmotin is housekeeping genes involved in cell basic metabolism. In salt adapted tobacco cells, the osmotic proteins were present in two forms, soluble in water soluble I and II soluble in detergent, and their ratio was about 2:3, and the isoelectric point was 8.2 Osmotin shows significant sequence homology at amino acid level with the sweet-tasting protein thaumatin and shares several similar characteristic features like molecular weight, lack of sulfhydral residues, basic pI, disulfide bonds, and high proline content. Osmotin is not sweet in taste and the probable reason may be due to lack of lysine residues that are present [5]. All the groove of osmotin protein contains Glu, Asp, Asp, Asp4 strong acidic amino acid residues, so that it is acidic because of negative charge. But its homologous protein(osmotin like proteins-OLPs)is neutral.
3. Expression of Osmotin During Salt Stress
Osmotins and OPLs are widely existed in the model plant tobacco and Arabidopsis thaliana, most crops, fruits and vegetables. The expression of Osmotin and OPL were induced by salt stress (Table 1). We can see that Osmotin and OPL were induced by salt stress in tobacco, rice, soybean, capsicum, grapes, Atriplex, Bruguiera gymnorrhiza, Populus euphratica [6-19]. Therefore, Osmotin is a kind of stress response protein, and the expression of it is induced by the factors of salt tolerance, and it is related to the salt tolerance of plants. However, the signaling pathways associated with the induction of osmotin by salt stress are not known. Salt tolerance ability is improved in transgenic expression of osmotin and OLPs in various plants including tobacco, soybean, potatoes, strawberries, tomatoes, peppers, when treated with salt stress (Tables 2) [20-27].
Table 1.
Stress response of native plants overexpressing osmotin and OLPs.
| Osmotin/OLP | Native Expression | Expressed During | Reference |
|---|---|---|---|
| Osmotin | Tobacco | Salt stress | [6] |
| OLP | Tobacco | Salt stress | [7] |
| OLP (pA8 and pA9) | Atriplex nummularia | Salt adapted a cells | [8] |
| Osmotin | Tobacco | Salt stress | [9] |
| OLP | Mesembryanthemum crystallinum | Treatment with salt | [10] |
| Osmotin | V. vinifera L. | Salt stress | [11] |
| OLP (GmOLPa) | Glycine max | Sal stress | [12] |
| Osmotin | Rice | Salt stress | [13] |
| Osmotin | Rice | salt stress | [14] |
| OLP (GmOLPa and GmOLPb) | G. max | Salt stress | [15] |
| Osmotin | Bruguiera gymnorhiza | Salt stress | [16] |
| Osmotin | P. euphratica | Salt stress | [17] |
| Osmotin | Anthemis nobilis | Salinity stresses | [18] |
| Osmotin | Tobacco | Salt adapted | [19] |
Table 2.
Stress response of native plants overexpressing osmotin and OLPs.
| Gene | Isolated From | Validated In | Phenotypic Effects of Transgenic Plants | Reference |
|---|---|---|---|---|
| OLP | Tobacco | Potato | Tolerance to salt | [20] |
| Osmotin | Tobacco | Tobacco | Enhanced salt stress | [21] |
| Osmotin | Tobacco | Tobacco | Tolerance to salt | [22] |
| Osmotin | Tobacco | Strawberry | Enhanced tolerance to salt | [23] |
| Osmotin | Tobacco | Tomato | Tolerance to salt and drought | [24] |
| Osmotin | Tobacco | Mulberry | Tolerance against salt stresses | [25] |
| Osmotin | Tobacco | Chili pepper | Enhanced salt tolerance | [26] |
| Osmotin | Tobacco | soybean | Resistance to salinity stress | [27] |
4. Possible Mechanisms of Osmotin under Salt Stress
The acting mechanism of osmotin is not well understood, however, osmotin protects the cells from osmotic shock especially during abiotic stresses by compartmentalization of solutes or by structural or metabolic alterations [28]. Osmotin with the help of cell wall components is involved in permeabilization of membrane and plasmolysed cells become insensitive to osmotin, suggesting that the cell wall components are needed for its activity [29].
Salt stress in plants is mainly caused by Na+, high Na+ concentration can cause osmotic stress, ion toxicity and K+ / Na+ ratio imbalance, resulting in plant metabolic abnormalities to damage to plant cells [1-3, 30]. Osmotin plays important roles in salt stress tolerance by sequestering Na+ ions and compartmentalizing them into vacuoles and intercellular spaces. The association of tobacco osmotin protein with tonoplast [3] and the OLP identified from Mesembryanthemum crystallinum suggests the role of osmotin in the intracellular compartmentation of Na+ ions [30]. But, whether osmotin upregulates sodium-proton antiporter1 (NHX1) gene to sequester Na+ Ions or how it is able to perform this functions is not yet clear. It is also not clear if osmotin has a direct role to play or it stimulates other proteins that are downstream. Transgenics overexpressing osmotin gene exhibited salt tolerance in potato [20], tobacco [28], Triticum aestivum cv. Marvdasht [31], strawberry [23], tomatoes [24], mulberry [25], chili pepper [26], and soybean [27] by retaining chlorophyll, preventing the accumulation of ROS, with an increase in relative water content, proline accumulation, increase in root length, shoot length, plant height, leaf expansion, and improved root growth than controls. Overexpression of OLP lacking short C terminal cDNA also showed such an enhanced salt tolerance in potato [20]. Transgenic mulberry expressing osmotin driven by CaMV35S promoter displayed better tolerance to salt stress than the transgenics containing osmotin under the influence of rd29A promoter, though the rd29A promoter is responsive to dehydration while the CaMV35S promoter is constitutive [25]. Rice transgenic plants expressing OPBP1 showed salt tolerance with enhanced root length and root growth than the untransformed controls [32]. Currently, it is known that there are two main mechanisms for the osmotic regulation proteins.
5. Osmotin Was Tolerant to Salt Stress By Through Antioxidation
Under the stress condition, the antioxidant system of plant is damaged due to the increase of Reactive Oxygen Species (ROS). Under salt stress, the content of hydrogen peroxide in transgenic plants overexpressing osmotin was much lower than that in the control group, which showed that osmotin was helpful to control the accumulation of hydrogen peroxide. Pepper overexpressing osmotin under salt stress, Ascorbate Peroxidase (APX) and Superoxide Enzyme (SOD) increased, release of H2O2 toxicity, but osmotin how to regulate the activity of APX and SOD is not clear. MDA is one of the most important products of membrane lipid peroxidation, which can indirectly determine the degree of damage to the membrane system. In plants, overexpressing osmotin, the decrease of MDA increased the salt stress tolerance [26]. Therefore, plants can neutralize the active oxygen produced by the increase of the ion and the expression of the antioxidant enzyme, thereby increasing the salt tolerance ability of plants.
6. Osmotin Was Tolerant To Salt Stress By Accumulation of Proline
Under abiotic stress conditions, plants can accumulate a certain amount of proline, which can reach more times in some plants compared with control. As a free radical scavenger, it can help the plant to resist salt [33]. Proline can induce cell osmotic adjustment, which does not show any adverse effects on the accumulation in the cytoplasm, but it can eliminate the toxicity of reactive oxygen species and the formation of free radicals [34]. The osmotin can promote the accumulation of osmotic adjustment substances, such as proline and betaine [35], which affect the expression of proline and regulated by both constitutive and inducible promoters. In the potato [20], tobacco [22], tomato [24], mulberry [25], and pepper [26], overexpression of osmotin can lead to increased proline, while in some plants, there was no significant change in the degree of stress [36]. The change of osmotin was closely related to the level of osmotic adjustment protein and the proline content in transgenic plants. Therefore, the plants which overexpressed osmotin protein can enhance the salt tolerance ability through the accumulation of proline.
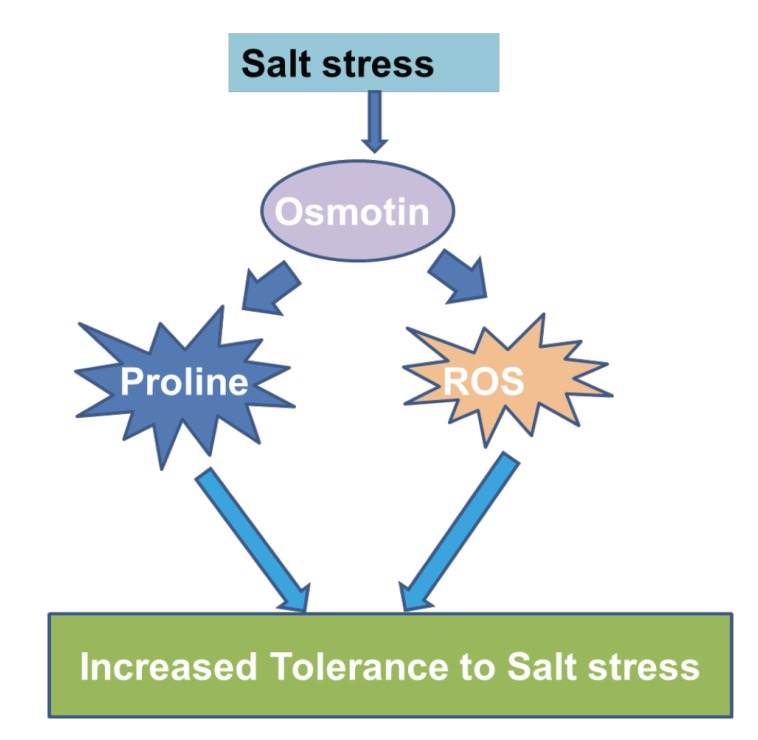
Conclusion
Osmotin, a multifaceted plant protein confers tolerance to abiotic stresses especially salt stress. Transgenic and native expression of osmotin and OLPs was observed in various plants when treated with salt stress. Osmotin was tolerant to salt stress through antioxidation, and accumulation of proline (Fig. 1). Due to the multiple activities of osmotin, it has potential functions in biotic and abiotic stress tolerance in crop plants.
Fig. (1).
Salinity Tolerance Mechanism of Osmotin and Osmotin like proteins.
Consent for Publication
Not applicable.
Acknowledgements
This work was jointly supported by Jiangsu Agricultural Science and Technology Independent Innovation Project [CX (15)1005], Shuangchuang Talent Plan of Jiangsu Province and Jiangsu Natural Science Foundation, China (BK20151364).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ulrich D., Stephan A.B., Tomoaki H., Horie T., Luo W., Xu G., Schroeder J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19(6):371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anil K.S., Hima K.P., Shravan K.G., Mohanalatha C., Kavi K. Osmotin: a plant sentinel and a possible agonist of mammalian adiponectin. Front. Plant Sci. 2015;6:163. doi: 10.3389/fpls.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh N.K., Handa A.K., Hasegawa P.M., Bressan R.A. Proteins associated with adaptation of cultured tobacco cells to NaCl. Plant Physiol. 1985;79:126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N.K., Bracker C.A., Hasegawa P.M., Handa A.K., Buckel S., Hermodson M.A., Pfankoch E., Regnier F.E., Bressan R.A. Characterization of osmotin: a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987;85:529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson M., Valdes-rodriguez S., Blanco-labia A. A possible function for thaumatin and a TMV-induced protein suggested by homology to a maize inhibitor. Nature. 1987;327:432–434. [Google Scholar]
- 6.LaRosa P.C., Singh N.K., Hasegawa P.M., Bressan R.A. Stable NaCl tolerance of tobacco cells is associated with enhanced accumulation of osmotin. Plant Physiol. 1989;91:855–861. doi: 10.1104/pp.91.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda S., Sato F., Ida K., Yamada Y. Nucleotide sequence of a cDNA for osmotin-like protein from cultured tobacco Cells. Plant Physiol. 1991;97:844–846. doi: 10.1104/pp.97.2.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casas A.M., Nelson D.E., Raghothama K.G., Paino D.M., Singh N.K., Bressan R.A., Hasegawa P.M. Expression of osmotin-like genes in the halophyte Atriplex nummularia L. Plant Physiol. 1992;99:329–337. doi: 10.1104/pp.99.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V., Spencer M.E. Nucleotide sequence of an osmotin cDNA from the Nicotiana tabacum cv. White Burley generated by the polymerase chain reaction. Plant Mol. Biol. 1992;18:621–622. doi: 10.1007/BF00040683. [DOI] [PubMed] [Google Scholar]
- 10.Thomas J.C., Bohnert H.J. Salt stress perception and plant growth regulators in the halophyte Mesembryanthemum crystallinum. Plant Physiol. 1993;103:1299–1304. doi: 10.1104/pp.103.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agaoglu Y.S., Ergül A., Aras S. Molecular characterization of salt stress in grapevine cultivars (Vitis vinifera L.) and root stocks. Vitis. 2004;43:107–110. [Google Scholar]
- 12.Onishi M., Tachi H., Kojima T., Takahara H. Molecular cloning and characterization of a novel salt-inducible gene encoding an acidic isoform of PR-5 protein in soybean (Glycine max [L.]Merr.). Plant Physiol. Biochem. 2006;44:574–580. doi: 10.1016/j.plaphy.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N., Matsuoka M., Kitano H., Asano T., Kaku H., Komatsu S. gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (PBZ1) in response to cold stress and pathogen attack. Plant Cell Environ. 2006;29:619–631. doi: 10.1111/j.1365-3040.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang J., Wang H.M., Jiang Y., Wang Q.H., Huang X., Zhang H.S. Stress repressive expression of rice SRZ1and characterization of plant SRZ gene family. Plant Sci. 2008;174:227–235. [Google Scholar]
- 15.Tachi H., Fukuda-Yamada K., Kojima T., Shiraiwa M., Takahara H. Molecular characterization of a novel soybean gene encoding a neutral PR-5 protein induced by high-salt stress. Plant Physiol. Biochem. 2009;47:73–79. doi: 10.1016/j.plaphy.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Tada Y., Kashimura T. Proteomic analysis of salt-responsive proteins in the mangrove plant, Bruguiera gymnorhiza. Plant Cell Physiol. 2009;50:439–446. doi: 10.1093/pcp/pcp002. [DOI] [PubMed] [Google Scholar]
- 17.Brinker M., Brosché M., Vinocur B., Abo-Ogiala A., Fayyaz P., Janz D., Ottow E.A., Cullmann A.D., Saborowski J., Kangasjärvi J., Altman A., Polle A. Linking the salt transcriptome with physiological responses of a salt resistant Populus species as a strategy to identify genes important for stress acclimation. Plant Physiol. 2010;154:1697–1709. doi: 10.1104/pp.110.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siahsar B.A., Sarani S., Allahdoo M. Polypeptide electrophoretic pattern of Matricaria chamomilla and Anthemis nobilis under salt and Fe deficiency stress. Afr. J. Biotechnol. 2011;10:11182–11185. [Google Scholar]
- 19.Trivedi V.R., Chorawala M.R., Shah G.B. Antiatherosclerotic activity of osmotin, an adiponectin agonist in atherogenic diet induced hypertriglyceridemia and hypercholesterolemia in wistar rats. Adv. Res. Pharm. Biol. 2012;2:196–207. [Google Scholar]
- 20.Evers D., Overney S., Simon P., Greppin H., Hausman J.F. Salt tolerance of Solanum tuberosum L. overexpressing an heterologous osmotin-like protein. Biol. Plant. 1999;42:105–112. [Google Scholar]
- 21.Zhang H., Huang Z., Chen B.X., Tian X., Zhang X., Zhang H., Lu X., Huang D., Huang R. The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta. 2004;220:262–270. doi: 10.1007/s00425-004-1347-x. [DOI] [PubMed] [Google Scholar]
- 22.Sokhansanj A., Noori S.A., Niknam V. Comparison of bacterial and plant genes participating in proline biosynthesis with osmotin gene, with respect to enhancing salinity tolerance of transgenic tobacco plants. Russ. J. Plant Physiol. 2006;53:110–115. [Google Scholar]
- 23.Husaini A.M., Abdin M.Z. Overexpression of tobacco osmotin gene leads to salt stress tolerance in strawberry (Fragaria x ananassa Duch.) plants. Indian J. Biotechnol. 2008;7:465–471. [Google Scholar]
- 24.Goel D., Singh A.K., Yadav V., Babbar S.B., Bansal K.C. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.). Protoplasma. 2010;245:133–141. doi: 10.1007/s00709-010-0158-0. [DOI] [PubMed] [Google Scholar]
- 25.Das M., Chauhan H., Chhibbar A., Haq Q.M., Khurana P. High-efficiency transformation and selective tolerance against biotic and abiotic stress in mulberry, Morus indica cv. K2, by constitutive and inducible expression of tobacco osmotin. Transgenic Res. 2011;20:231–246. doi: 10.1007/s11248-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 26.Subramanyam K., Sailaja K.V., Subramanyam K., Rao D.M., Lakshmidevi K. Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L.). Plant Cell Tissue Organ Cult. 2011;105:181–192. [Google Scholar]
- 27.Subramanyam K., Arun M., Mariashibu T.S., Theboral J., Rajesh M., Singh N.K., Manickavasagam M., Ganapathi A. Overexpression of tobacco osmotin (Tbosm) in soybean conferred resistance to salinity stress and fungal infections. Planta. 2012;236:1909–1925. doi: 10.1007/s00425-012-1733-8. [DOI] [PubMed] [Google Scholar]
- 28.Barthakur S., Babu V., Bansal K.C. Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J. Plant Biochem. Biotechnol. 2001;10:31–37. [Google Scholar]
- 29.Abad L.R., D’Urzo M.P., Liu D., Narasimhan M.L., Reuveni M., Zhu J.K., Niua X., Singh N.K., Hasegawaa P.M., Bressan R.A. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996;118:11–23. [Google Scholar]
- 30.Yen H.E., Edward G.E., Grimes H.D. Characterization of salt-responsive 24-Kilodalton glycoprotein in Mesembryanthemum crystallinum. Plant Physiol. 1994;105:1179–1187. doi: 10.1104/pp.105.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noori S.A., Sokhansanj A. Wheat plants containing an osmotin gene show enhanced ability to produce roots at high NaCl concentration. Russ. J. Plant Physiol. 2008;55:256–258. [Google Scholar]
- 32.Chen X., Guo Z. Tobacco OPBP1 enhances salt tolerance and disease resistance of transgenic rice. Int. J. Mol. Sci. 2008;9:2601–2613. doi: 10.3390/ijms9122601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishor P.B., Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- 34.Vinocur B., Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Holmstrom K.O., Somersalo S., Mandal A., Palva T.E., Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000;51:177–185. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- 36.Nanjo T., Kobayashi M., Yoshiba Y., Sanada Y., Wada K., Tsukaya H., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999;18:185–193. doi: 10.1046/j.1365-313x.1999.00438.x. [DOI] [PubMed] [Google Scholar]