Abstract
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replicate during acute infection in lymphocytes of the gastrointestinal tract, before disseminating systemically. Localized replication and associated loss of gut-resident CD4+ T cells occur regardless of the portal of entry of the virus (e.g., intravenous vs. rectal). Thus, HIV and SIV are tropic for gut tissue, and their pathogenesis requires the special environment of the intestine. T helper 17 (Th17) cells are important contributors to microbial defense in the gut that are vulnerable to HIV infection and whose loss is associated with translocation of microbial products to the systemic circulation, leading to chronic immune activation and disease progression. Interleukin (IL)-21 promotes differentiation and survival of Th17 cells and stimulates CD8+ T cell function. By promoting Th17 cell survival, IL-21 could limit bacterial translocation and immune activation in the setting of acute or rebounding HIV/SIV disease. In this study, we tested the effect of recombinant IL-21-IgFc treatment, given at the time of infection, on SIVmac251 infection. We found that rIL-21-IgFc decreases immune activation and maintains effective antiviral responses by CD8+ T cells in blood, but this maintenance is not associated with lower viral loads. rIL-21-IgFc treatment also did not generally support Th17 cell populations, but Th17 cells remained strongly and independently associated with control of plasma viremia. For example, the single animal exhibiting greatest control over viremia in our study also manifested the highest levels of IL-21 in plasma, Th17 cell maintenance in blood, and Th17 cells in intestinal tissue. These findings provide rationale for further exploration of IL-21 treatment as a support for host CD8+ T cell responses in HIV cure strategies.
Keywords: : interleukin-21, Th17, rhesus macaques, SIV, immune activation, cure
Introduction
Progressive human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections are characterized by rapid and profound depletion of CD4+ T cells from the gastrointestinal mucosa, as well as structural deterioration of the gut epithelium associated with microbial translocation.1–4 T helper 17 (Th17) cells are preferentially depleted during pathogenic infections, with more severe depletion being associated with increased permeability of the intestinal epithelium, immune activation, and disease progression.5,6 We previously reported that greater frequencies of circulating and gut-resident Th17 cells present at the time of SIV infection are associated with lower viral loads and more polyfunctional CD4+ T cells.7
Interleukin-21 (IL-21) is a pleiotropic cytokine that is produced primarily by natural killer T cells and CD4+ T cells, particularly T follicular helper cells and Th17 cells.8,9 IL-21 exerts its effects on a broad range of cell types. Given its breadth of immunomodulatory targets, IL-21 and its receptor are attractive targets for therapeutic manipulation (reviewed in ref.10). IL-21 has previously been administered to chronically SIV-infected antiretroviral therapy (ART)-naive macaques, resulting in improved natural killer cell and T cell cytotoxicity, as well as greater SIV-specific antibody production.11 In another trial of IL-21 treatment in early SIV infection, preservation of intestinal Th17 cells was observed in association with reduced levels of intestinal T cell proliferation, microbial translocation, and systemic activation/inflammation.12 These trials indicate that IL-21 may strengthen beneficial immune responses while limiting generalized immune activation induced by chronic lentiviral replication.
We hypothesized that the most important role of IL-21 in HIV/SIV infection might be to maintain Th17 cell populations by promoting Th17 cell differentiation, resulting in a more moderate disease course similar to that observed in animals having naturally high levels of Th17 cells.7 To test this hypothesis, we infected 12 rhesus macaques (RMs) with SIVmac251 and treated four starting at the time of infection with a recombinant rhesus IL-21-immunoglobulin Fc fusion protein (rIL-21-IgFc), maintaining the treatment for 22 weeks. Treated animals manifested impressive changes in the immune system, including larger effector-memory T cell compartments and enhanced virus-specific CD8+ T cells, but surprisingly these changes were not associated with diminished plasma viral loads. Moreover, although the treatment did not have a profound effect on Th17 cell frequency, administration of rIL-21-IgFc did result in reduced CD4+ T cell activation and fewer “exhausted” T cells expressing the inhibitory receptor PD-1. Our findings suggest that IL-21 therapy may have greatest utility in cure strategies requiring a contribution from effective host T cell responses.
Materials and Methods
Animal care
This study was performed under strict compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Established policies of the Institutional Animal Care and Use Committee (IACUC) of the University of California, Davis were followed for sample collections, housing, and medical care. The study was performed at the California National Primate Research Center (CNPRC), which is one of seven centers supported by the NIH, Office of the Director (OD), and is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The CNPRC houses nonhuman primates, most of which are RMs (Macaca mulatta), in outdoor corrals or indoor cages. All animals were negative for HIV-2 (serology), SIV (serology), type D retrovirus (serology and polymerase chain reaction), and simian T cell lymphotropic virus type 1 (serology). All monkeys were of Indian origin, except for animal no. 41714, which was 1/8 Chinese. SIV-infected animals were housed in the infectious housing unit in pairs of two animals per cage.
Production and testing of rhesus rIL-21-IgFc
Rhesus rIL-21-IgFc (IL-21) fusion protein was provided by Dr. Kenneth Rogers and generated as previously described by the Resource for Nonhuman Primate Immune Reagents of Emory University.12 Briefly, IL-21 was produced in the Drosophila S2 system as a fusion protein between rMamuIL-21 and a macaque IgG2 Fc mutated to prevent binding to complement or Fc receptors, similar to a previously reported PD-1-IgFc.13 rIL-21-IgFc was purified to greater than 95% by protein G sepharose affinity chromatography, dialyzed against phosphate-buffered saline, and tested for sterility and the potential presence of residual endotoxin.
Animals and SIV infection
Twelve RMs were included in the study (Table 1) and infected intrarectally with 1,000 TCID50 of an SIVmac251 stock propagated in rhesus peripheral blood mononuclear cells (PBMCs) (“6/04” stock; day 0).14 All animals were infected after this single challenge. Starting from day 0, four animals were treated with 21 weekly doses (subcutaneous) of 0.1 mg/kg (104 U of activity/kg) of rIL-21-IgFc (IL-21).11 The remaining eight RMs remained untreated and were used as SIV-infected controls. Peripheral blood sample collections were performed before rIL-21-IgFc administration at day 0 (pretreatment baseline) and at weeks 1, 2, 4, 8, 12, and 16 after treatment. Lymph node sample collections were performed at weeks 8 and 16 after treatment, and ileum sample collections were performed at necropsy.
Table 1.
Study Groups
| Group | Number | Age (years) | Weight (kg) | Sex (males:females) |
|---|---|---|---|---|
| IL-21-IgFc | 4 | 1.49 ± 0.06 (1.4–1.54) | 2.54 ± 0.2 (2.36–2.75) | 2:2 |
| Controls | 8 | 1.48 ± 0.04 (1.42–1.56) | 2.74 ± 0.13 (2.55–2.90) | 3:5 |
Data shown as mean ± standard deviation (range).
Flow cytometric analysis
PBMCs were stained using standard methods in four panels containing Pacific Blue (PacBlue)-conjugated anti-CD3, Alexa700-conjugated anti-CD3, PE-Cy7-conjugated anti-CD25, PE-conjugated anti-CD38, PE-Cy7-conjugated anti-HLA-DR, APC-conjugated anti-CCR5, FITC-conjugated anti-PD-1 (BD Biosciences); Qdot655-conjugated anti-CD4, PE-Cy5.5-conjugated anti-CD8 (Invitrogen); PE-Texas Red® (ECD)-conjugated anti-CD45RA, PE-conjugated anti-CD127, ECD-conjugated anti-HLA-DR (Beckman Coulter); and APC-Cy7-conjugated anti-CD27 (BioLegend). A stain reagent for dead cells (Invitrogen Aqua LIVE/DEAD Fixable Dead Cell Stain) was included in all panels. Cells were washed and fixed in phosphate-buffered saline containing 1% paraformaldehyde or permeabilized using a FOXP3 Fix/Perm Kit (BioLegend), according to the manufacturer's instructions, intracellularly stained with Alexa 488-conjugated anti-Ki67 (BD Biosciences) and PacBlue-conjugated anti-FOXP3, and fixed. All sample data were then acquired on LSR II flow cytometers. Data analysis was performed using FlowJo.
Intracellular cytokine staining
Frequencies of cytokine-producing cells were determined by intracellular flow cytometry analysis after in vitro stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin or with overlapping peptides from SIV p27 protein. One million PBMCs were incubated for 16 h at 37°C with peptides (1 μg/ml) and anti-CD28 + anti-CD49d co-stimulation (2 μg/ml of BD Biosciences FastImmune) or with PMA (50 ng/ml) and ionomycin (1 μg/ml) in complete RPMI-1640 medium containing GolgiPlug (5 μg/ml). After incubation, cells were washed and stained with PacBlue-conjugated anti-CD3, Qdot655-conjugated anti-CD4, and PE-Cy5.5-conjugated anti-CD8. Cells were then washed, permeabilized using a Cytofix/Cytoperm Kit (BD Biosciences), intracellularly stained with PE-Cy7-conjugated anti-IFNγ, Alexa 700-conjugated anti-tumor necrosis factor alpha (TNF-α), PE-conjugated anti-IL-17 (eBioscience), and FITC-conjugated anti-IL-4 (BioLegend), washed, fixed, and acquired.
IL-21 in vitro immune activation assay
PBMCs from animals before infection were stimulated with PMA and ionomycin in the presence or absence of rIL-21-IgFc (10 ng/ml equivalent to 1 U/ml).
Plasma levels of endogenously produced IL-21
The concentration of IL-21 in EDTA plasma was determined using the Human IL-21 ELISA MAX Deluxe Set (BioLegend) in accordance with the manufacturer's instructions. rIL-21-IgFc was provided by Dr. Kenneth Rogers (Emory University) in lyophilized 50-μg vials, which were resuspended in Assay Diluent A from the ELISA Kit to 35 μg/ml and stored at 4°C before dilution. In one alternate assay, the capture and detection antibodies were replaced with 50 ng/ml of mouse anti-human IL-21 (clone J148–1134) and biotinylated mouse anti-human IL-21 (clone I76–539), respectively. All samples were analyzed in duplicate using the average of the OD values to calculate concentrations. The minimum detection limit for IL-21 was determined to be 16 pg/ml. Sensitivity for rIL-21-IgFc was 100- to 1,000-fold lower than expected based on known concentrations in the standard curve; thus, assay results reflect endogenously produced cytokine.
Statistical analysis
Statistical analysis was performed in the R programming environment. Wilcoxon rank-sum tests were used to identify immune cell subsets found to be significantly different between the two experimental groups. To assess baseline immunophenotypic profile, we used the ade4 package to perform principle component analysis based on data from baseline samples.
Results
IL-21-IgFc treatment moderates T cell activation in early SIV infection
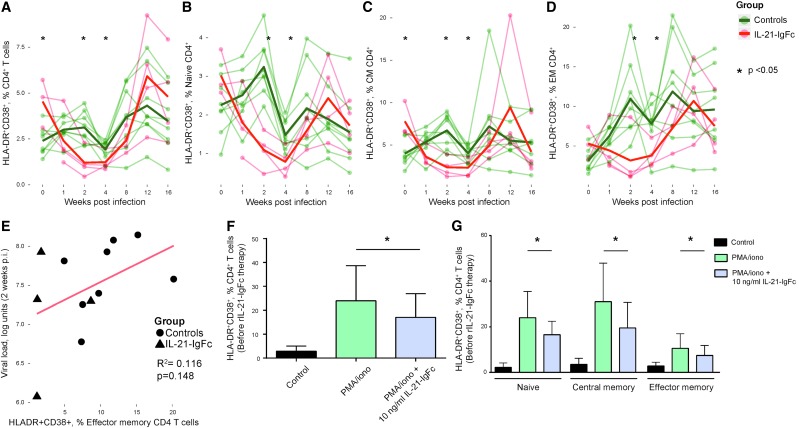
Th17 cells are thought to contribute to maintenance of gut mucosal integrity, whose failure has been linked to chronic T cell activation in HIV-infected people. Interestingly, IL-21–treated animals showed an early (at 2 and 4 weeks postinfection) and significant reduction of CD4+ T cell activation (HLA-DR+CD38+; Fig. 1A), which was evident in all CD4+ T cell maturation subsets (TN, TCM, TEM; Fig. 1B–D). Interestingly, at 2 weeks postinfection, there was a trend to lower viral loads in association with reduced activation (Fig. 1E).
FIG. 1.
rIL-21-IgFc treatment temporarily reduces T cell activation. (A–D) Longitudinal representation of the frequency of CD38+HLA-DR+ cells among total, naive, central memory, and effector memory CD4+ T cells. (E) Positive trend between plasma viremia and the CD38+HLA-DR+ phenotype among effector memory CD4+ T cells. (F) Frequency of CD38+HLA-DR+ cells among total CD4+ T cells under conditions of no stimulation (Control), PMA/ionomycin stimulation (PMA/iono), and PMA/ionomycin stimulation +10 ng/ml rIL-21-IgFc (PMA/ionomycin + IL-21-IgFc). (G) Frequency of CD38+HLA-DR+ cells among naive, central memory, and effector memory CD4+ T cells under conditions of no stimulation, PMA/ionomycin stimulation, or PMA/ionomycin with 10 ng/ml rIL-21-IgFc. PMA, phorbol 12-myristate 13-acetate.
To test if IL-21 signaling made a direct contribution to reduced T cell activation, PBMCs from animals before infection were stimulated with PMA and ionomycin in the presence or absence of rIL-21-IgFc. A concentration of 10 ng/ml was sufficient to reduce CD4+ T cell activation (HLA-DR+CD38+) among total CD4+ T cells (Fig. 1F) and in all CD4+ T cell maturation subsets (Fig. 1G). No effects were observed among CD8+ T cells or their maturation subsets, in vivo or in vitro (data not shown).
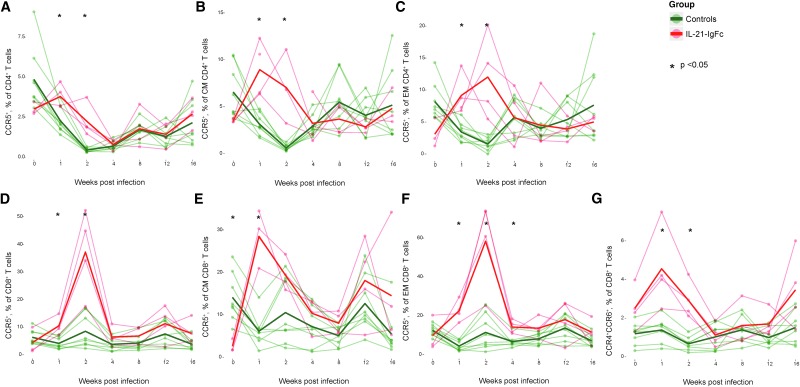
IL-21-IgFc–treated animals transiently upregulate the CCR5 chemokine receptor
Due to the importance of the CCR5 chemokine receptor in HIV/SIV transmission and dissemination, we evaluated expression levels on CD4+ (Fig. 2A) and CD8+ T cells (Fig. 2B), as well as on central and effector memory subsets (Fig. 2C–F, for CD4+ and CD8+ cells, respectively). The receptor was dramatically upregulated in IL-21–treated animals soon after SIV infection, reaching the maximum value 2 weeks after infection. Exposure of PBMCs to IL-21-IgFc in vitro, however, had no effect on CCR5 expression (data not shown), strongly suggesting that the in vivo effect is indirect. Others have noted the upregulation of CCR5, CXCR6, and CXCR3 receptors after IL-21 therapy.15 Although we did not evaluate CXCR6 or CXCR3, we did observe transient upregulation of the chemokine receptors, CCR4 and CCR6 (Fig. 2G).
FIG. 2.
IL-21-IgFc–treated animals transiently upregulate the CCR5 chemokine receptor. (A–C) Longitudinal representation of CCR5+ expression among total CD4+, central memory, and effector memory T cells. (D–F) Longitudinal representation of CCR5+ expression among total CD8+, central memory, and effector memory T cells. (G) Longitudinal representation of the CCR4+CCR6+ phenotype among total CD8+ T cells.
IL-21-IgFc supports both CD4+ and CD8+ T cell effector function
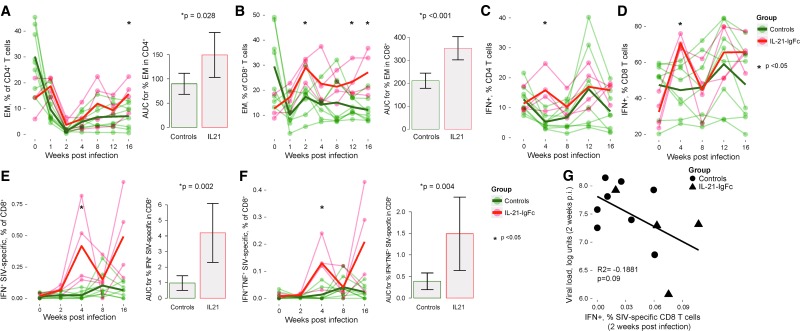
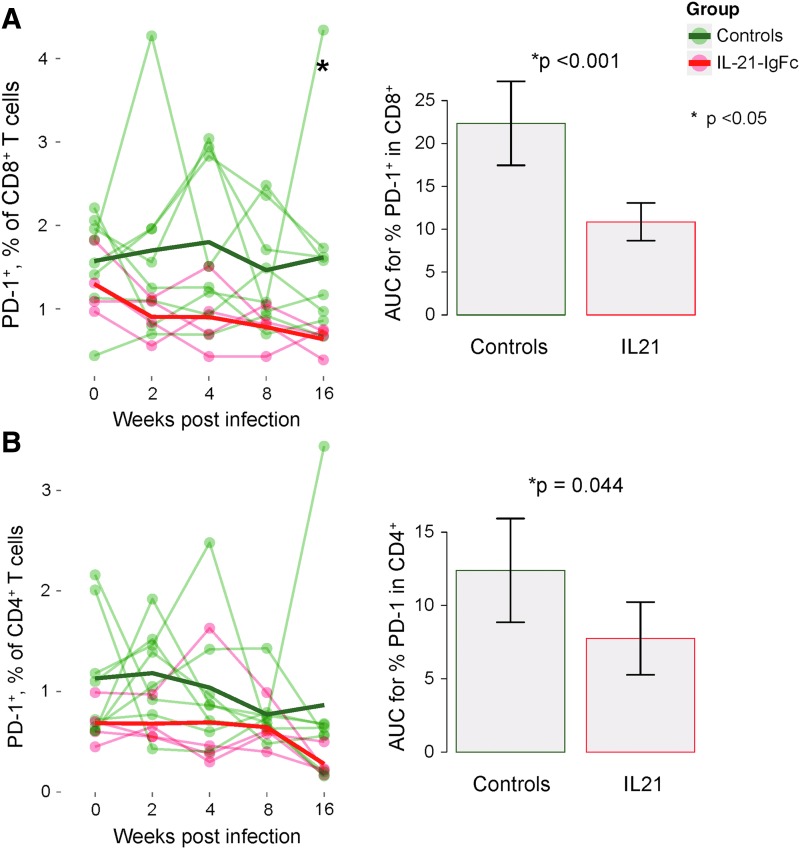
IL-21-IgFc administration resulted in expansion of both CD4+ (Fig. 3A, left) and CD8+ (Fig. 3B, left) effector memory T cells, which remained at higher frequency until 16 weeks after infection. Analysis of the area under curve (AUC) demonstrated significantly greater frequencies of both subsets over time (Fig. 3A, B, right, respectively). We then evaluated the capacity for expression of TNF-α and IFN-γ in both total and SIV-specific CD4+ and CD8+ T cells. Following rIL-21-IgFc treatment, significant increases were noted at 4 weeks after infection for total CD4+ and CD8+ T cells capable of expressing IFN-γ (Fig. 3C, D). rIL-21-IgFc administration was also associated with higher frequencies of SIV-specific CD8+ T cells expressing IFN-γ and/or TNF-α starting at 4 weeks after infection and continuing until the end of the experiment (Fig. 3E, F, left). AUCs demonstrated significantly greater frequencies of both populations over time (Fig. 3E, F, right, respectively). Importantly, the levels of SIV-specific CD8+ T cells producing IFN-γ at 2 weeks postinfection were inversely associated (although not significantly) with the viral loads reached at the same time point (Fig. 3G), suggesting the possibility that SIV-specific CD8+ T cells limited viral replication. The effects of IL-21-IgFc on SIV-specific CD4+ T cells were limited; however, the frequencies of SIV-specific CD4+ T cells detected were very low in all animals (data not shown). In keeping with this finding of overall increased effector function, IL-21-IgFc–treated animals demonstrated significantly lower frequencies of “exhausted” CD8+ and CD4+ T cells expressing the inhibitory receptor PD-1 (Fig. 4A, B).
FIG. 3.
IL-21-IgFc supports both CD4+ and CD8+ T cell effector function. (A) Longitudinal representation of the frequency of effector memory CD4+ T cells (left) and areas under the curve for effector memory CD4+ T cells in control and IL-21-IgFc–treated animals (right). (B) Longitudinal representation of the frequency of effector memory CD8+ T cells (left) and areas under the curve for effector memory CD8+ T cells (right). (C, D) Longitudinal representation of the frequency of IFN-γ–producing T cells among total CD4+ and CD8+ subsets. (E) Longitudinal frequency of IFN-γ–producing, SIV-specific CD8+ T cells (left) and area under the curve for such cells (right). (F) Longitudinal representation of IFN-γ- and TNF-α–producing, SIV-specific CD8+ T cells (left) and area under the curve for such cells (right). (G) Inverse trend between IFN-γ–producing, SIV-specific CD8+ T cells and plasma viral loads at 2 weeks postinfection. SIV, simian immunodeficiency virus; TNF-α, tumor necrosis factor alpha.
FIG. 4.
IL-21 administration protects T cells from exhaustion. (A) Longitudinal representation of PD-1 expression among CD8+ T cells (left) and area under the curve for PD-1+CD8+ T cells (right). (B) Longitudinal representation of the PD-1 expression on CD4+ T cells (left) and area under the curve for the PD-1+ CD4+ T cells (right).
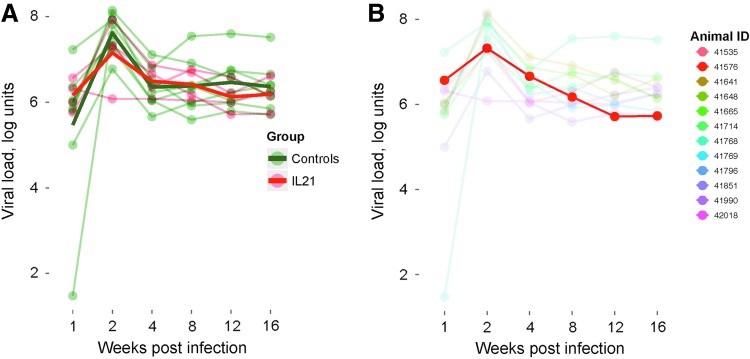
IL-21-IgFc treatment does not affect viral load
Viral loads in the entire cohort followed a similar longitudinal profile, with peak viremia achieved 2 weeks after IV challenge and evidence for post-peak control in all RMs. As shown in Figure 5A (individual traces in lighter color and mean values for experimental groups in bold), rIL-21-IgFc treatment had no consistent effect on viral load. Of note, one rIL-21-IgFc–treated animal (no. 41576) experienced approximately average peak viremia, but subsequently achieved the lowest plasma viral load of the entire cohort by week 16 (red trace in Fig. 5B). As will be explained below, this animal proved to have the highest average level of endogenous IL-21 production.
FIG. 5.
Effect of rIL-21-IgFc treatment on viral load. (A) Longitudinal plasma viral loads in the two experimental groups, rIL-21-IgFc treated and controls. (B) Longitudinal viral loads, with traces for all animals faded except that for animal no. 41576.
Control of SIV viral load is dependent on Th17 cell development before infection
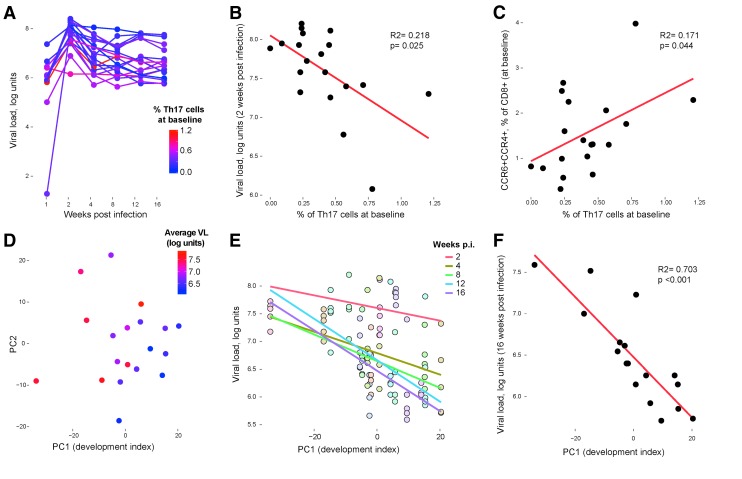
We previously found that host immune system composition before SIV infection, especially Th17 cell development, has a strong influence on the course of disease after infection.7 In this study, Th17 cell populations present at baseline (i.e., before infection) were again associated with control over virus (Fig. 6A, B, IL-17- and IFN-γ–producing cells). This relationship was most striking 2 weeks after infection (peak viral load; Fig. 6B), but was true throughout the first 16 weeks. Variability in Th17 cells was associated with similar overarching variability in multiple immunologic parameters tested at baseline, for example, with CD8+ T cells expressing the chemokine receptors, CCR4 and CCR6 (Fig. 6C). Many of these variable cell populations were previously identified in a study of dam-reared versus nursery-reared animals that underwent strikingly variable immune development.16 To illustrate these trends, we selected immune cell populations (CD4+ effector memory T cells, CD8+ effector memory T cells expressing Ki-67, CD4+ and CD8+ T cells expressing combinations of CCR4 and CCR6, and CD4+ and CD8+ T cells expressing IL17 and IFN-γ) associated with dam versus nursery rearing and plotted subject animals along the first two principal components (Fig. 6D). After color-coding the plotted points according to the eventual average viral load (red for highest viral load; Fig. 6D), it is apparent that development of these immune cells before infection is associated with control over virus throughout the experiment. This observation can be demonstrated statistically by interpreting the first component as a “development index” and showing that this index has a significant association with lower viral loads at subsequent time points (Fig. 6E, F).
FIG. 6.
SIV viral load is dependent on Th17 cell development before infection. (A) Longitudinal plasma viral load levels, after color-coding the plotted points according to the baseline frequency of Th17 cells coexpressing IFN-γ (red for highest frequency of Th17 levels at baseline). (B) Correlation between the frequency of baseline IFN-γ+ Th17 cells and plasma viremia at week 2 postinfection. (C) Correlation between the frequency of IFN-γ+ Th17 cells and the frequency of CD8+ T cells expressing both CCR4 and CCR6 at baseline. (D) Baseline immune cell populations associated with dam versus nursery rearing plotted along the first two principal components after color-coding the plotted points according to the eventual average viral load (red for highest viral load). (E) Correlation between baseline first principal component axis (“development index”) and viral loads at weeks 2, 4, 8, 12, and 16 postinfection (each colored line represents a best fit for one postinfection time point, e.g., the purple fitted line represents the association between viral loads at 16 weeks postinfection development indices for each animal). (F) Correlation between baseline first principal component axis (“development index”) and viral loads at week 16. Th17, T helper 17.
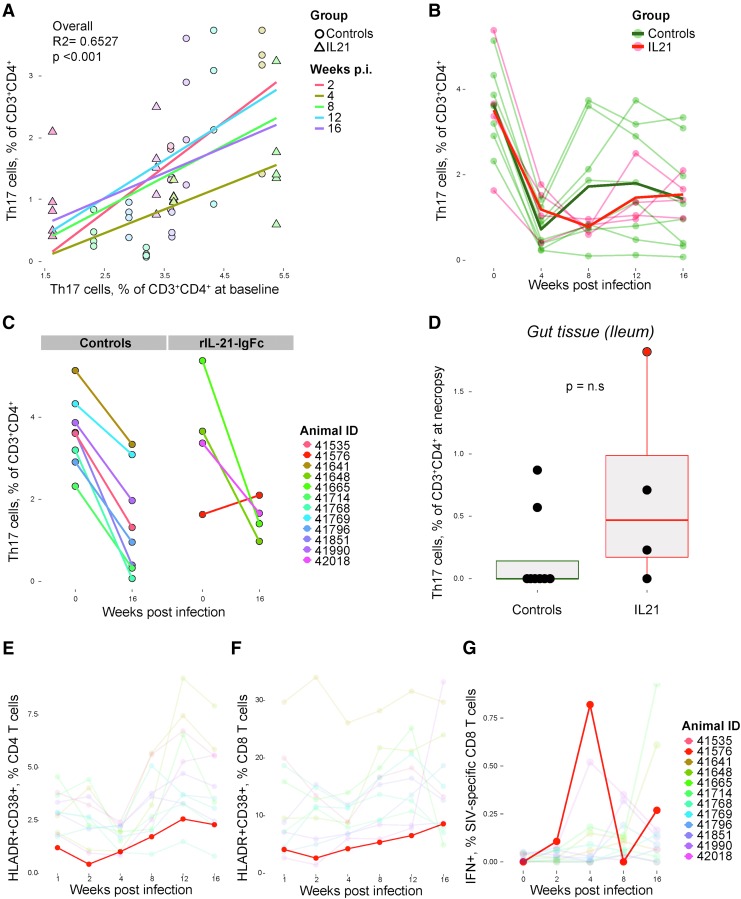
Th17 cell loss in SIV infection is profound in animals with few Th17 cells before infection and this loss is not prevented by treatment with rIL-21-IgFc
We and others demonstrated that both SIV and HIV infections lead to Th17 cell depletion and that the magnitude of depletion depends on the frequency of Th17 cells present before infection.7 That is, all animals experience a similar loss of Th17 cells in percentage terms, so that animals with more Th17 cells experience greater losses but nonetheless retain their position as high expressers relative to other animals. IL-21 contributes to the functional differentiation and survival of Th17 cells17,18; therefore, we evaluated the effects of rIL-21-IgFc treatment on the frequency of Th17 cells throughout infection. First, we again observed that preinfection Th17 cell levels are positively associated with the levels of Th17 cells at all time points after infection, indicating that animals with more Th17 cells preserve those cells despite SIV-mediated depletion (Fig. 7A). In addition, we observed that both rIL-21-IgFc–treated animals and control animals experienced similar degrees of Th17 cell depletion (Fig. 7B, with Th17 cell nadir at 4–8 weeks postinfection). The overall loss of Th17 cells over the first 16 weeks of infection was similar in both groups (Fig. 7C), although one rIL-21-IgFc–treated animal, no. 41576, was unique in regaining all Th17 cells by 16 weeks (Fig. 7C, right). This animal had also the lowest viral load by 16 weeks postinfection (Fig. 5B), retained the highest Th17 cell levels in the gut at necropsy (Fig. 7D), maintained low T cell activation levels (Fig. 7E, F), manifested high frequencies of SIV-specific CD8+ T cells (Fig. 7G), and demonstrated the most robust expression of endogenous IL-21 (Fig. 8).
FIG. 7.
Th17 cell loss in SIV infection is profound in individuals with few Th17 cells before infection despite rIL-21-IgFc treatment. (A) Correlation between the frequency of Th17 cells at baseline and frequency at weeks 2, 4, 8, 12, and 16 postinfection (each colored line represents a best fit for one postinfection time point). (B) Longitudinal plot of Th17 cell frequencies in rIL-21-IgFc–treated and control groups. (C) Changes in Th17 cell frequencies between baseline and week 16 in controls (left panels) and rIL-21-IgFc–treated animals (right panels). (D) Intestinal (ileal) Th17 cell frequencies measured at necropsy. (E) Longitudinal frequencies of the CD38+HLA-DR+ phenotype among circulating CD4+ T cells, with traces for all animals faded except that for 41576. (F) Longitudinal frequencies of the CD38+HLA-DR+ phenotype among circulating CD8+ T cells, with traces for all animals faded except that for 41576. (G) Longitudinal representation of IFN-γ production among SIV-specific CD8+ T cells, with traces for all animals faded except that for 41576.
FIG. 8.
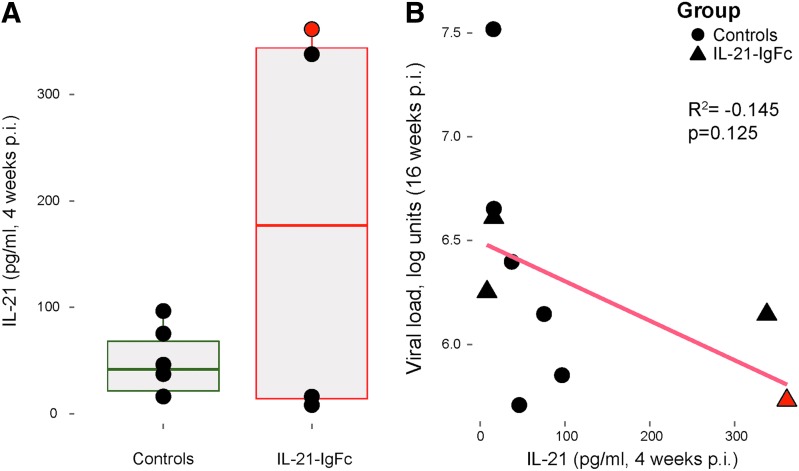
Plasma levels of circulating host IL-21. (A) Host IL-21 plasma levels in control and rIL-21-IgFc–treated rhesus macaques. (B) Inverse correlation between plasma viremia and IL-21 plasma levels at week 16.
Given the apparently modest effects of rIL-21-IgFc on both viral load and Th17 cells, we next measured plasma levels of circulating host IL-21, to determine if drug administration may have been undermined by compensatory reductions in endogenous production. We found no evidence for a systematic change in IL-21 among the four treated animals (Fig. 8A); however, two of the four animals manifested unusually high and sustained IL-21 production (at baseline and 4 weeks postinfection). One of these animals, no. 41576, was shown in Figure 7C and D to regain its baseline level of Th17 cells and maintain the highest levels of intestinal Th17 cells at necropsy, respectively, suggesting the possibility that sufficiently high doses of IL-21 might indeed affect Th17 cell frequency. We observed nonsignificant association between endogenous IL-21 and viral loads at 16 weeks postinfection, suggesting that both IL-17- and IL-21–producing cells may influence host control of infection (Fig. 8B).
Discussion
CD4+ T cells are the major producers of IL-21 and are important targets of HIV and SIV.19,20 Given the roles of IL-21 in long-term maintenance of functional CD8+ T cells,21,22 cytotoxic function of CD8+ T cells and natural killer (NK) cells,11,21–24 as well as in differentiation and expansion of Th17 cells,17,18,25 lower IL-21 availability may contribute to compromised immune responses in HIV/SIV infections. IL-21 administration during early SIV infection (2 weeks postinfection) was associated with preserved intestinal Th17 cells and limited microbial translocation.12 Based on our previous data,7 we hypothesized that Th17 cell function is important at the time of infection and that rIL-21-IgFc treatment might prevent acute Th17 cell depletion, supporting IL-17 and IL-21 production and resulting in a moderate disease course similar to that observed in animals having naturally high levels of Th17 cells.7
Although we did not observe uniform support of Th17 cell populations in rIL-21-IgFc–treated animals, our data suggest that the goal of Th17 cell maintenance remains important. One treated animal achieved complete Th17 cell maintenance, likely due to robust endogenous IL-21 production. Protection and maintenance of Th17 cells in this animal were associated with reduced T cell activation, improved CD8+ T cell function, and lower viral load. More generally, we showed that rIL-21-IgFc administration in acute SIV infection is associated with rapid (1 week postinfection) reduction in CD4+ T cell activation, rapid increases of CD4+ and CD8+ T cells expressing CCR5, maintenance of both CD4+ and CD8+ effector memory compartments, increased IFN-γ production by total and SIV-specific CD8+ T cells, and downregulation of PD-1 receptors among both CD4+ and CD8+ T cells. The potential long-term clinical benefit of such effects remains to be evaluated.
Depletion of intestinal Th17 cells is associated with mucosal immune dysfunction and immune activation in pathogenic HIV/SIV infections.6 We previously reported that circulating and gut-resident Th17 cells present at the time of SIV infection are associated with lower viral loads.7 We again found in our present study that a higher frequency of Th17 cells present before infection is predictive of lower viral loads at early and later time points; indeed, in this study the correlation was more strongly significant throughout infection (Fig. 6B). Furthermore, a broader collection of baseline immune characteristics, previously identified as different between dam-reared and nursery-reared animals,16 was found to be strongly associated with control over virus replication at later time points. As others have reported, plasma viral load did not differ significantly between rIL-21-IgFc–treated and control RMs.11,12,19 Of note, our cohort was not genotyped for major histocompatibility complex (MHC) class I or TRIM5α alleles, both of which may have affected viral loads but are not known to have an independent effect on T cell activation, homing molecule expression, exhaustion markers, Th17 cells, or IL-21 expression.
Consistent with a mechanistic link between preservation of the intestinal epithelial barrier and limited immune activation, Pallikkuth et al. found that IL-21–treated animals manifested both limited microbial translocation and reduced soluble markers of inflammation, although not reduced T cell activation.12 Similarly, Micci et al. showed that IL-21 supplementation of antiretroviral therapy reduced residual inflammation and virus persistence.26 Using rIL-21-IgFc treatment at the beginning of infection, however, we observed a significant reduction in CD4+ T cell activation, which was maintained until 8 weeks postinfection. As others have suggested, and consistent with altered CCR5 chemokine receptor expression, IL-21 therapy may support migratory properties of CD4+ and CD8+ T cells and could therefore facilitate the recruitment of effector cells to the site of inflammation, resulting in anatomic redistribution of lymphocytes.15 While a reduction in viral loads was not observed, the mechanism by which a reduction of immune activation can limit virus persistence may be very complex.26 Alternatively, or in addition, IL-21 may have a direct effect: CD4+ T cell activation was reduced when PBMCs were cultured in vitro in the presence of rIL-21-IgFc.
IL-21 also regulates the function and cytotoxic potential of CD8+ T cells,21–24 which are compromised in HIV and SIV infections. Our results and others19 show unequivocally that IL-21–treated animals experience durable expansion of CD8+ T cells with favorable markers of effector function. In our study, reduced PD-1 expression was coupled with expansion of CD4+ and CD8+ T cells able to produce cytokine in response to nonspecific or SIV-specific stimulation. Furthermore, 2 weeks after infection animals with more frequent IFN-γ–producing CD8+ T cells manifested a trend to lower viral load peaks. Similarly, studies investigating the role of IL-21 in immune responses to lymphocytic choriomeningitis virus (LCMV) have shown that chronic LCMV infection in the absence of IL-21 results in severe functional exhaustion.27
As mentioned above, IL-21 has been implicated in the differentiation and maintenance of Th17 cells.17,18,25 Our results show no significant effects of rIL-21-Ig-Fc treatment on peripheral, intestinal, and/or lymphoid Th17 cells. Interestingly, however, one animal (rIL-21-IgFc treated) showed a complete maintenance of its Th17 cell compartment in the ileum at necropsy and in blood by 16 weeks postinfection. This animal seems to have benefited from rIL-21-IgFc treatment and/or from a high level of endogenous IL-21 expression. The rationale for this suggestion is fivefold. First, although starting with the lowest frequency of Th17 cells in the treatment group, only this animal experienced a maintenance of those cells by the end of the study. Second, this animal showed very low T cell activation throughout the experiment. Third, plasma IL-21 levels were found to be the highest in this animal, which might be a fortuitous coincidence or a result of effective therapy in this individual. Fourth, this animal manifested the highest SIV-specific responses (IFN-γ–producing CD8+ T cells) at early time points. Finally, by the end of the study this animal presented the lowest viral load.
In conclusion, rIL-21-IgFc treatment in SIV-infected RMs given at the time of infection reduces immune activation and maintains effective antiviral responses by CD8+ T cells, but these beneficial effects are not associated with durable control over viral load. We again observed powerful effects of preexisting host immune characteristics on later disease course. For this reason, we suggest that the effects of a treatment such as rIL-21-IgFc must be assessed against the expected outcome for the drug's recipient, that is, our models must account for development of immune cells at baseline. Finally, while we think it is apparent from results of multiple studies that IL-21 therapy is unlikely to play a direct role in viral load suppression, this treatment may be important for HIV cure strategies that rely on adequate T cell function. By including concomitant antiretroviral (ARV) administration to fully control virus replication, such strategies might benefit from or even potentiate the immune-restoration effects of IL-21. Possibly, IL-21 therapy can support CD8+ T cell function without the known side effects of checkpoint inhibitors such as anti-PD-1 antibody.28 Such side effects are unlikely to be tolerated in drug regimens meant to cure people who are fundamentally healthy.29
Acknowledgments
The authors thank Shakuntala Aswani for technical assistance. The authors thank Joyce Lee, veterinary staff, Colony Research staff, Clinical Laboratories, and Pathology for expert technical assistance at CNPRC. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award no. K23AI081540 to D.J.H.O.C., by the Bill and Melinda Gates Foundation under a “Grand Challenges Exploration” award (no. 52094) to D.J.H.O.C., by the National Center for Research Resources (P51RR000169) and subsequently the Office of Research Infrastructure Programs/OD (P51OD011107) through their funding of the California National Primate Research Center and by the California HIV/AIDS Research Foundation under award F13-D-312 to G.M.L. The authors also thank Dr. Kenneth Rogers and the Resource for Nonhuman Primate Immune Reagents at Emory (recently relocated to the New Iberia Research Center at the University of Louisiana at Lafayette) for provision of rMamuIL-21-IgFc, as well as ORIP/OD grant 2R24 OD10947 to F.V.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. : Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009;199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. : Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010;6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. : Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 2012;5:646–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. : Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008;112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, et al. : Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog 2009;5:e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartigan-O'Connor DJ, Abel K, Van Rompay KK, Kanwar B, McCune JM: SIV replication in the infected rhesus macaque is limited by the size of the preexisting TH17 cell compartment. Science translational medicine 2012;4:136ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. : Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000;408:57–63 [DOI] [PubMed] [Google Scholar]
- 9.Spolski R, Leonard WJ: Interleukin-21: Basic biology and implications for cancer and autoimmunity. Ann Rev Immunol 2008;26:57–79 [DOI] [PubMed] [Google Scholar]
- 10.Spolski R, Leonard WJ: Interleukin-21: A double-edged sword with therapeutic potential. Nat Rev Drug Discov 2014;13:379–395 [DOI] [PubMed] [Google Scholar]
- 11.Pallikkuth S, Rogers K, Villinger F, Dosterii M, Vaccari M, Franchini G, et al. : Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine 2011;29:9229–9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. : Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog 2013;9:e1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onlamoon N, Rogers K, Mayne AE, Pattanapanyasat K, Mori K, Villinger F, et al. : Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection. Immunology 2008;124:277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marthas ML, Van Rompay KKA, Abbott Z, Earl P, Buonocore-Buzzelli L, Moss B, et al. : Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine 2011;29:3124–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, et al. : IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardeshir A, Narayan NR, Méndez-Lagares G, Lu D, Rauch M, Huang Y, et al. : Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci Transl Med 2014;6:252ra120–252ra120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. : IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967–974 [DOI] [PubMed] [Google Scholar]
- 18.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. : Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007;448:480–483 [DOI] [PubMed] [Google Scholar]
- 19.Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, et al. : Paucity of IL-21–producing CD4(+) T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood 2012;120:3925–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannello A, Boulassel M-R, Samarani S, Debbeche O, Tremblay C, Toma E, et al. : Dynamics and consequences of IL-21 production in HIV-infected individuals: A longitudinal and cross-sectional study. J Immunol 2010;184:114–126 [DOI] [PubMed] [Google Scholar]
- 21.Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL: Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol 2010;40:3085–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novy P, Huang X, Leonard WJ, Yang Y: Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol 2011;186:2729–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, et al. : HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T Cell function. J Virol 2011;85:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi JS, Ingram JT, Zajac AJ: IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol 2010;185:4835–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. : IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 2007;448:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micci L, Ryan ES, Fromentin R, Bosinger SE, Harper JL, He T, et al. : Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J Clin Invest 2015;125:4497–4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi JS, Du M, Zajac AJ: A vital role for IL-21 in the control of a chronic viral infection. Science 2009;324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney KM, Freeman GJ, McDermott DF: The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015;37:764–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, et al. : Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–e356 [DOI] [PMC free article] [PubMed] [Google Scholar]