Abstract
The HIV reservoir forming at the earliest stages of infection is likely composed of CCR5+ cells, because these cells are the targets of transmissible virus. Restriction of the CCR5+ reservoir, particularly in the gut, may be needed for subsequent cure attempts. Strategies for killing or depleting CCR5+ cells have been described, but none have been tested in vivo in nonhuman primates, and the extent of achievable depletion from tissues is not known. In this study we investigate the efficacy of two novel cytotoxic treatments for targeting and eliminating CCR5+ cells in young rhesus macaques. The first, an immunotoxin consisting of the endogenous CCR5 ligand RANTES fused with Pseudomonas exotoxin (RANTES-PE38), killed CCR5+ lamina propria lymphocytes (LPLs) ex vivo, but had no detectable effect on CCR5+ LPLs in vivo. The second, a primatized bispecific antibody for CCR5 and CD3, depleted all CCR5+ cells from blood and the vast majority of such cells from the colonic mucosa (up to 96% of CD4+CCR5+). Absence of CCR5-expressing cells from blood endured for at least 1 week, while CCR5+ cells in colon were substantially replenished over the same time span. These data open an avenue to investigation of combined early ART treatment and CCR5+ reservoir depletion for cure of HIV-infected infants.
Keywords: : HIV, SIV, CCR5, bispecific antibodies, immunotoxins, RANTES-PE38
Introduction
The HIV reservoir formed during early infection is likely composed of CCR5+ cells, which are critical targets of infectious, transmissible HIV virus.1,2 In early infection, patients heterozygous for the CCR5 delta 32 allele exhibit significantly lower cell-associated HIV DNA loads than those homozygous for the normal allele, suggesting delayed establishment of the virus in reservoirs.3 Furthermore, most HIV-infected but untreated people maintain a predominantly CCR5-tropic viral population for extended periods (∼8 years), with potent antiretroviral therapy creating the conditions only in some patients for emergence of X4 variants in cellular reservoirs after 30–60 months of treatment.4 Thus, a large fraction of the HIV reservoir found in chronic infection is also contained in CCR5+ cells.4,5 We propose that while heterogeneity in latently infected cell populations may prove a major barrier to virus eradication later in infection, the earliest reservoir is concentrated in CCR5+ cells and therefore vulnerable to targeted elimination.
To deplete CCR5+ cells, we tested two cytotoxic strategies: immunotoxin and bispecific antibodies. Immunotoxins are fusion proteins consisting of a ligand or antibody fused with a toxin capable of driving cell death.6 Often the latter component is derived from Pseudomonas exotoxin, which causes apoptosis by ADP ribosylating and inactivating translation elongation factor 2.7 Domain I, which consists of the first 38 N-terminal amino acids of the protein and is responsible for binding of target cells,8 is replaced with the targeting ligand or antibody. Bispecific antibodies are another form of cytotoxic treatment that have shown some success in cancer therapy9,10 and are emerging as a potential therapy for HIV. In particular, bispecific T cell engagers (BiTEs) comprising an anti-CD3 antibody and an anti-target antibody cause cell death by linking CD3 on cytotoxic T lymphocytes (CTLs) to target cell surface antigens. The cross-linking mediated by the bispecific antibody leads to degranulation of the T cell and subsequently to target cell death.11,12
We created (i) an immunotoxin consisting of truncated PE (PE38) and an endogenous ligand for CCR5, RANTES13,14 and (ii) a bispecific antibody consisting of Fab arms reactive to CD3 and CCR5.13 We then tested each agent for its ability to deplete CCR5+ cells from blood and tissue of rhesus macaques. We find that CD3/CCR5 bispecific antibody rapidly depletes CCR5+ cells from blood and tissues and is thus a promising candidate for use in future cure therapies.
Materials and Methods
Cell cultures
CHO-K1 cells were acquired from ATTC and maintained in F-12K medium with 50 U/ml penicillin and streptomycin, 200 mM l-glutamine, and 10% fetal bovine serum. The CCR5 coding sequence was amplified by real-time polymerase chain reaction, using primers 5′-GTTATGGATTATCAAGTGTCAAGTCCAAC-3′ and 5′-TCACAAGCCCACAGATGTTTCC-3′, from reverse-transcribed RNA isolated from rhesus lamina propria lymphocyte (LPL) cells. The resulting amplicon was cloned into the mammalian expression vector pEF6/V5-His TOPO® TA (Invitrogen) by enzymatic ligation. The resulting vector was transfected into CHO-K1 cells using Lipofectamine 2000 (Invitrogen) and the cells selected in 5 μg/ml blasticidin (Thermo Fisher). Cells were stained with Brilliant Violet-labeled anti-CCR5 antibody and sorted for CCR5 expression on a BD FACSAria™ (BD Biosciences) and subsequently maintained in 5 μg/ml blasticidin.
Synthesis of RANTES-PE38 immunotoxin
The RANTES-PE38 immunotoxin was synthesized by the Resource for NHP Immune Reagents at the New Iberia Research Center, University of Louisiana at Lafayette. Mature RANTES proteins of rhesus macaques and humans have identical amino acid sequences.15 Expression of RANTES-PE38 was performed using an expression vector generously provided by Mack and colleagues13 that encodes the periplasmic signal peptide OmpA, which is cleaved off cleanly from the N-terminus of mature human RANTES, followed by PE38 with a C-terminal 6x histidine tag. The plasmid was transformed into BL21 (DE3) strain of Escherichia Coli, and protein production was induced with 1 mM isopropyl-B-d-thiogalactopyranoside for 3 h. RANTES-PE38 was prepared from E. coli inclusion bodies purified using Ni-NTA agarose (QIAGEN) and refolded by stepwise equilibration in decreasing concentrations of urea. Endotoxin was removed using three rounds of Triton X-114 phase separation,16 and the protein was dialyzed against bicarbonate buffer and lyophilized. RANTES-PE38 was tested for sterility, absence of endotoxin using the limulus amebocyte lysate end point clot test (Charles River Laboratories), and protein content was determined by the bicinchoninic acid assay (Pierce). Protein purity was evaluated by running samples on 4%–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Lonza) gels stained with Bio-Safe Coomassie G-250 (Bio-Rad) and the fusion partners verified by Western blot with anti-RANTES clone VL1 (BioLegend) or mouse anti-Histidine tag (GE Healthcare Life Sciences) (Fig. 1A).
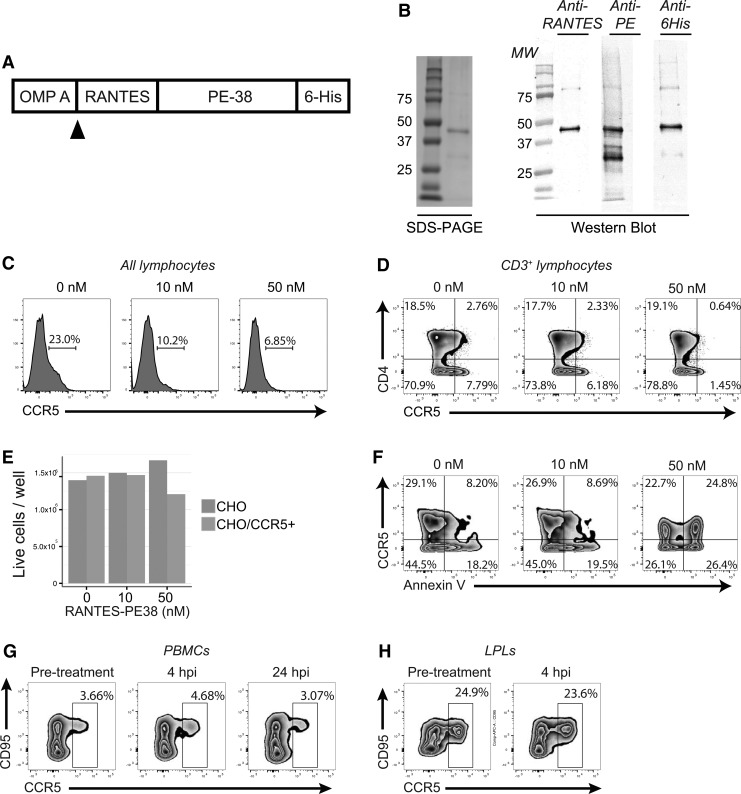
FIG. 1.
RANTES-PE38 depletes CCR5+ cells in vitro but not in vivo. (A, B) Outline of the macaque RANTES fusion protein construct, with RANTES and the Histidine tag flanking PE38 (A), and evaluation of protein purification through Coomassie staining and Western blot (B). (C, D) All LPLs (C) or CD3+ lymphocytes (D) after incubation for 40 h with 0, 10, or 50 nM of toxin. (E) Live cell counts after incubation of CHO-K1 cells or CHO-K1 cells stably transfected with CCR5 expression vector with RANTES-PE38 for 48 h. (F) Annexin V staining of a mixture of CCR5-expressing and unmodified CHO-K1 cells after a 48-h incubation with RANTES-PE38. (G, H) CCR5 expression on CD3+CD4+ PBMCs (G) or LPLs (H) of 1.5-year-old rhesus macaques treated with 1.25 mg/kg of RANTES-PE38. LPLs, lamina propria lymphocytes; PBMCs, peripheral blood mononuclear cells.
Generation and characterization of rhesus CD3-CCR5 bispecific antibody
An antibody capable of engaging both CD3 and CCR5 was engineered using the controlled Fab arm exchange method.17 The hybridoma expressing anti-CCR5 antibody 5C718 that had been shown to cross-react with macaque CCR519 was obtained from ATCC. The hybridoma expressing the broadly primate species cross-reactive antibody SP3420,21 was a gift from Dr. Cox Terhorst (Beth Israel Deaconess Medical Center). The heavy and light chain variable regions of both antibodies were sequenced and CDRs grafted into rhesus macaque frameworks. The SP34 light chain was engineered with a rhesus macaque lambda constant region and the 5C7 light chain engineered with a rhesus macaque kappa constant region. Both antibody heavy chains were constructed with rhesus macaque IgG1 constant regions containing the following mutations in CH3 to enable Fab arm exchange: 5C7, Y404F, and F405L; SP34, Y404F, K409R.
Both parental antibodies' engineered heavy and light chain genes were inserted into an expression vector and used for large-scale transient transfection of CHO cells through the ExpiCHO expression system (Life Technologies). Antibodies expressed after culture in serum-free medium were purified using protein A affinity chromatography and adjusted to 3.5 mg/ml in PBS pH 7.0. Equimolar quantities of both antibodies were mixed and incubated at 37°C for 90 min in the presence of the mild reducing agent, 2-mercaptoethylamine. After this incubation step, the reductant was removed by extensive dialysis against PBS pH 7.0.
Efficiency of Fab arm exchange was confirmed by hydrophobic interaction chromatography (HIC) and capillary isoelectric focusing (cIEF)—analytic methods which could resolve the parental and bispecific antibodies. Briefly, for HIC, samples of individual parental antibody, a mixture of two parental antibodies, or bispecific product were loaded onto a 7.8 × 75 mm ProPac HIC-10 column (Thermo Fisher Scientific) by a predilution to 1.0 mg/ml in HIC equilibration buffer (100 mM phosphate containing 1.2 M ammonium sulfate, pH 7.0). The high ionic strength in the loading buffer facilitates the interaction between the hydrophobic chromatographic medium and the hydrophobic patches present on protein molecules. In the later separation, the salt concentration is gradually decreased in order of increasing hydrophobicity, and the antibodies are eluted off of the HIC column. cIEF is a method that separates native protein species by their isoelectric points (pI). The antibodies were characterized under their condition using a PA 800 Protein Characterization System (Beckman Coulter, Inc., Fullerton, CA) equipped with UV detector. A 30.2 cm long, 50 μm I.D. neutral capillary, maintained at 20°C, was used for separation. Test samples were injected after dilution to 0.2 mg/ml in a diluent containing four synthetic peptide pI standards, Pharmalyte 3–10 carrier ampholytes, and a gel matrix. By subsequently changing the pH of the mobile phase from basic to acidic, species with different charges are focused with different migration times. The pI of each peak are determined by a linear calibration curve calibrated by the internal pI standards.
To confirm that the final product possessed bispecific binding properties for both CCR5 and CD3, CHO cells were transduced with the rhesus macaque CCR5 gene for CCR5 surface expression. Parental or bispecific antibodies were incubated with CCR5-expressing CHO cells, washed, and then incubated with biotinylated rhesus CD3ɛ protein. Cells were washed and incubated with phycoerythrin-streptavidin and analyzed by flow cytometry to determine if the bispecific antibody could bridge binding between cell surface-expressed CCR5 and soluble CD3 protein.
In vitro depletion assays
LPLs were isolated from the colon tissue of donor rhesus macaques by collagenase digestion. Frozen LPLs were thawed and allowed to rest at 4°C overnight before plating. Cells were plated in 24-well plates and incubated for 40 h with 0, 10, or 50 nM of RANTES-PE38 at 37°C. The extent of CCR5 depletion was assessed by flow cytometry.
To evaluate apoptosis, 1 million each of CHO-K1 and CCR5+ CHO-K1 cells were plated into 96-well plates and incubated for 4 h at 37°C in various concentrations of RANTES-PE38 immunotoxin diluted in F-12K complete medium: 0, 10, 20, or 50 nM. Cells were then stained for flow cytometry as described below, including fluorescently labeled anti-CCR5 and Annexin V reagents.
In vivo animal studies
This study was performed under strict compliance with the NIH Guide for the Care and Use of Laboratory Animals. Established policies of the Institutional Animal Care and Use Committee of the University of California, Davis were followed for sample collections, housing, and medical care. The study was performed at the California National Primate Research Center, which is one of eight centers supported by the National Institutes of Health, Office of the Director, and is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Four 1.5-year-old Rhesus macaques were initially assigned to this study. Blood samples and colon and lymph node biopsies were collected immediately before treatment. Two animals received 1.25 mg/ml of RANTES-PE38 and two received 1 mg/ml of CCR5xCD3 bispecific antibody. After 4 weeks, three animals received 3 mg/ml of CCR5xCD3 bispecific antibody, two that previously were administered RANTES-PE38 and one that received bispecific antibody previously. Colon and lymph node biopsies were taken from all animals at 4 h and again at 4 weeks plus 4 h (i.e., 4 h after the second treatment). All animals were necropsied 8 weeks after first drug administration. Two additional 8-month-old rhesus macaques received 3 mg/ml of CCR5xCD3 bispecific antibody. Colon and lymph node biopsies were taken before treatment and 4 h after treatment. Both animals were necropsied 1 week after treatment. Colon and lymph node samples were processed immediately for flow cytometry analysis. Blood was collected weekly for the duration of each trial. Whole blood was assessed by flow cytometry.
Flow cytometry
CHO-K1 cells or rhesus macaque peripheral blood mononuclear cells (PBMCs), LPLs, or lymph node cells were stained for flow cytometry using predetermined optimal concentrations of the following antibodies: anti-CD3-Alexa 700 (clone SP34-2), anti-CD95-APC or anti-CD95-PacBlue (clone DX2), anti-CD8-PE-Cy5.5 (clone 3B5), anti-CD4-BV650 (clone L200), anti-CCR5-BV421 (clone 3A9), anti-CD28-APC-H7 (clone CD28.2), and anti-HLA-DR-ECD. A stain reagent for dead cells (Invitrogen Aqua LIVE/DEAD Fixable Dead Cell Stain) was included in the panel. Cells were washed, fixed in phosphate-buffered saline containing 1% paraformaldehyde, and acquired on a BD LSRFortessa cytometer. Analysis was performed using FlowJo.
Results
RANTES-PE38 depletes CCR5+ cells in vitro but not in vivo
RANTES-PE38 was created by fusing the DNA sequence for rhesus macaque RANTES with a truncated version of Pseudomonas exotoxin (PE38) based on the vector previously reported by Mack and colleagues (Fig. 1A).13 Successful fusion was verified by sequencing, protein production was achieved in E. coli, the quality of the recombinant protein was checked (Fig. 1B), and the absence of endotoxin was verified as described in the Materials and Methods section.
Since the overall goal of the experiment is to deplete CCR5+ cells from HIV target tissues, including intestine, we first tested efficacy of RANTES-PE38 for depleting CCR5+ LPLs. Incubation of rhesus macaque LPLs in 50 nM fusion protein resulted in a decreased frequency of CCR5+ lymphocytes, as well as CCR5-expressing CD4+ T cells (Fig. 1C, D). To test if this decreased expression was a result of cell death and not downregulation of the receptor, we created CHO-K1 cells stably expressing macaque CCR5 and incubated these cells for 40 h in the presence of varying concentrations of RANTES-PE38. Low-dose RANTES-PE38 (10 nM) had no effect on the number of live cells, but a higher concentration (50 nM) led to a modest reduction in the number of living cells after 40 h (Fig. 1E). At this higher dose, however, both CCR5+ and CCR5− cells demonstrated increased Annexin V staining, suggesting that RANTES-PE38 is only poorly specific (Fig. 1F).
Finally, we administered 1.25 mg/kg of RANTES-PE38 to two 1.5-year-old rhesus macaques and took colon biopsies immediately before treatment and 4 h after treatment. Administration of RANTES-PE38 in vivo had no detectable effect on the frequency of CCR5+ expressing cells in either PBMCs or colon LPLs (Fig. 1G, H).
CD3-CCR5 bispecific primatized antibody is efficiently produced by Fab arm exchange
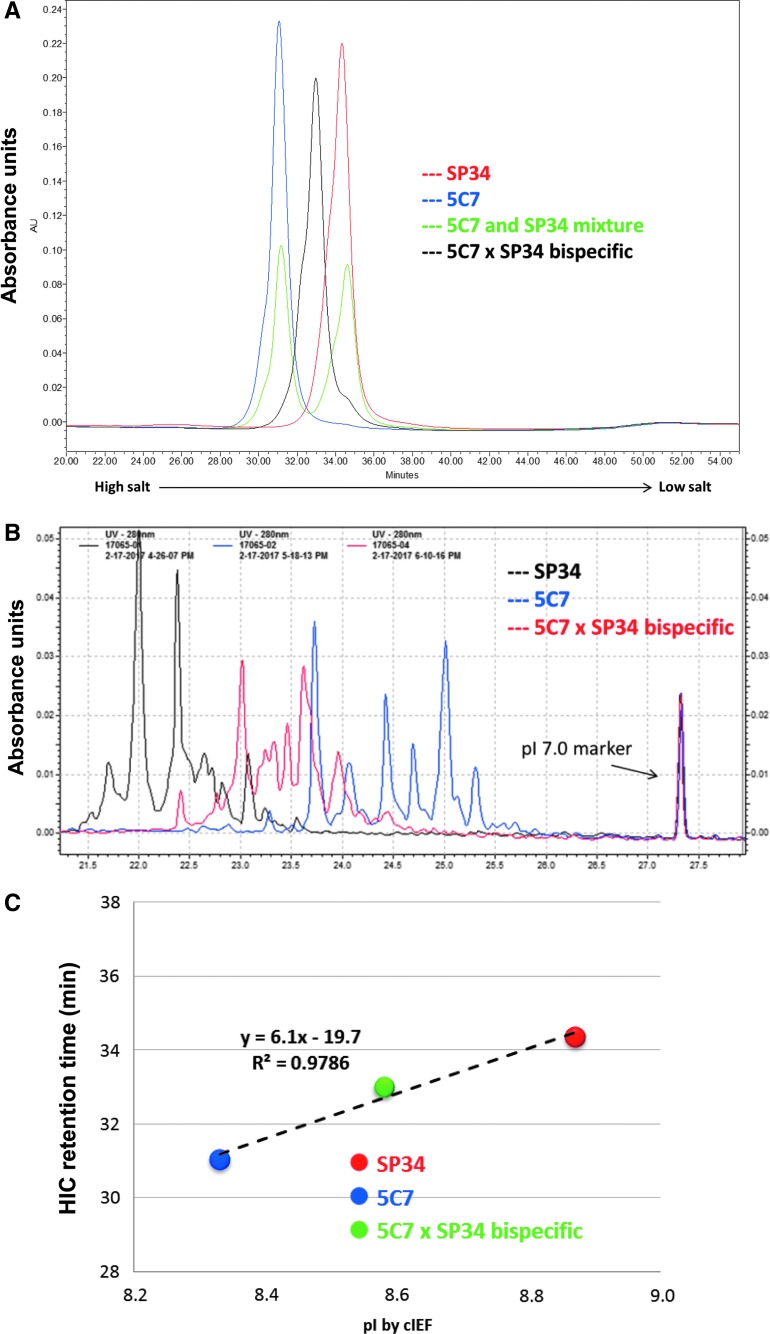
We next pursued an alternative strategy for CCR5+ cell depletion. We hypothesized that a bispecific antibody targeting both CD3 and CCR5 might function by cross-linking CTLs to CCR5-expressing cells and simultaneously activating the CTLs. An antibody with these characteristics was generated from primatized forms of anti-CD3 and anti-CCR5 by controlled Fab arm exchange. The efficiency of Fab arm exchange was examined by comparing characteristics of the parental antibodies with the final bispecific product by HIC and cIEF. HIC chromatographic analysis was able to clearly distinguish the parental antibodies when tested separately or as a mixture (Fig. 2A). 5C7 and SP34 were eluted at retention times of 31.0 and 34.4 min, respectively. Under the same chromatographic conditions, the bispecific product was eluted at a retention time of 33.0 min, midway between the two parental antibodies. No parental antibody was detectable in the final product. The same comparisons were made using cIEF, in which the bispecific product was shown to migrate midway between the parental antibodies (Fig. 2B). We also plotted the HIC retention time against the measured pI of the strongest cIEF peak of the main species, demonstrating that the results of HIC and cIEF are in good agreement (Fig. 2C). The HIC analysis also confirmed the purity of the bispecific antibody product and the high efficiency of Fab arm exchange. In addition, a flow cytometry-based assay confirmed that the bispecific antibody retained specificity for both CCR5 and CD3 (data not shown).
FIG. 2.
Creation of a CCR5xCD3 bispecific antibody. (A) Overlay of HIC peaks for parental antibodies, loaded alone or as a mixture, and for bispecific product following controlled Fab arm exchange. (B) cIEF results for parental antibodies and bispecific product. (C) Plot of HIC retention times versus pI calculated from cIEF (highest peak), confirming that bispecific product had expected characteristics relative to the parental antibodies. cIEF, capillary isoelectric focusing; HIC, hydrophobic interaction chromatography; pI, isoelectric points.
CCR5xCD3 bispecific antibody causes CD3+ lymphopenia and CCR5+ cell depletion from blood in vivo
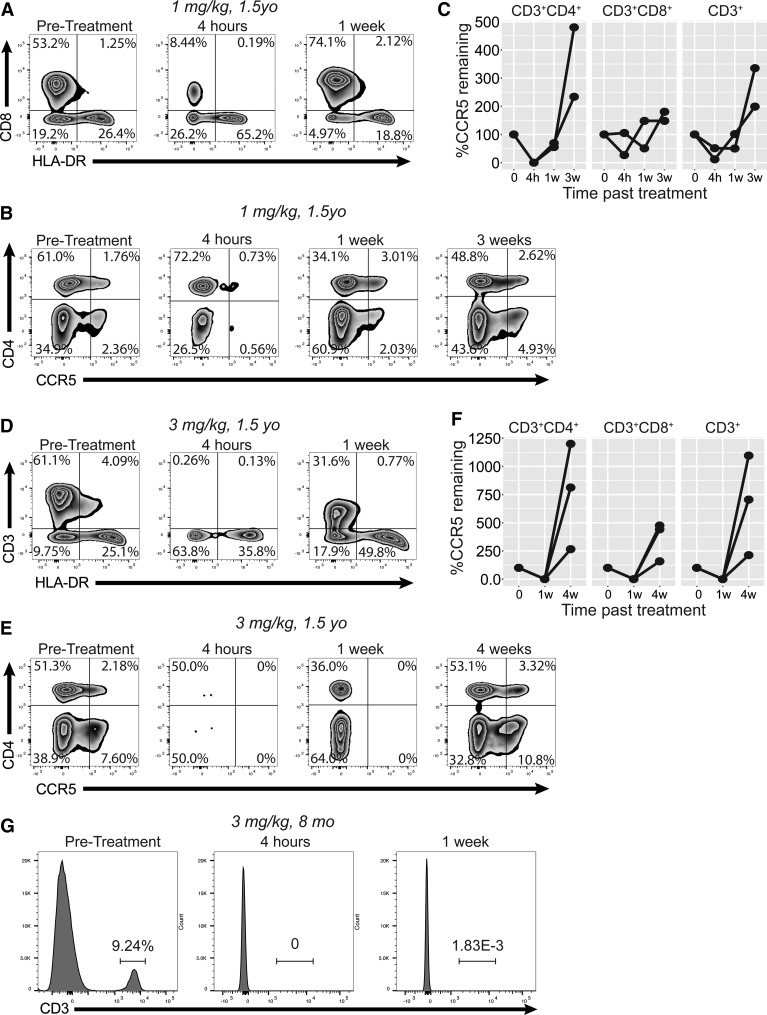
To assess the ability of the bispecific antibody to deplete CCR5+ cells in vivo, we initially administered 1 mg/kg antibody to two 1.5-year-old rhesus macaques (Fig. 3A–C). Unexpectedly, CD3+ cells were rapidly depleted from peripheral blood within 4 h (Fig. 3A, second cytogram). By 1 week after treatment, these cells had been reconstituted. In addition, at 4 h after treatment, a substantial reduction in the frequency of blood CCR5+ cells was identified in both CD8+ and CD4+ T cell subsets (Fig. 3B, C). By 3 weeks after treatment the CCR5+ cells had returned to their pretreatment frequency (Fig. 3C). We next tested the antibody at a higher dose, 3 mg/kg, in three 1.5-year-old macaques. At this dose the CD3+ lymphopenia was nearly complete by 4 h after treatment (Fig. 1D). HLA-DR+CD3− cells were also depleted at this time point, although to a lesser degree and with a concentrated effect on CCR5-expressing cells. More significantly, however, we observed complete depletion of CCR5+ cells from both CD4+ and CD8+ T cell subsets by 1 week after treatment, in all animals treated (Fig. 3E, F). Animals tolerated therapy well and continued to gain weight and maintain healthy blood counts up until necropsy (Table 1).
FIG. 3.
Lymphopenia and reduction of CCR5+ populations in blood after CCR5xCD3 bispecific antibody treatment. (A) Flow cytometry of whole blood from 1.5-year-old rhesus macaques treated with 1 mg/kg bispecific antibody, gated on forward/side scatter, single cells, and live/dead only. Blood was taken before treatment, 4 h after treatment, and 1 week after treatment. (B, C) Representative cytograms (B) and longitudinal plots (C) of CD3+ lymphocytes from rhesus macaques receiving 1 mg/kg at pretreatment, 4 h, 1 week, and 3 weeks from drug administration. (D) Flow cytometry of whole blood from 1.5-year-old rhesus macaques treated with 3 mg/kg bispecific antibody. (E, F) Representative cytograms (E) and longitudinal plots (F) of CD3+ lymphocytes from rhesus macaques receiving 3 mg/kg. (G) Whole blood staining of 8-month-old rhesus macaques treated with 3 mg/kg of bispecific antibody, demonstrating loss of CD3+ cells from blood.
Table 1.
Complete Blood Counts for 1.5-Year-Old Macaques Receiving bsAb
| Animal | Timepoint | Weight (KG) | WBC ( × 103/μl) | RBC ( × 106/μl) | Seg. neutrophils (%) | Seg. neutrophils (/μl) | Lymphocytes (%) | Lymphocytes (/μl) |
|---|---|---|---|---|---|---|---|---|
| 44845 | Pre | 2.35 | 9.4 | 5.56 | 61 | 5,734 | 36 | 3,384 |
| 44845 | 4 h | 2.52 | 4.5 | 6.06 | 75 | 3,375 | 17 | 765 |
| 44845 | Necropsy | 2.57 | 21.8 | 5.54 | 69 | 15,042 | 27 | 5,886 |
| 44866 | Pre | 1.93 | 8.4 | 5.89 | 51 | 4,284 | 47 | 3,948 |
| 44866 | 4 h | 3.29 | 7.7 | 5.87 | 41 | 3,157 | 57 | 4,389 |
| 44866 | Necropsy | 3.39 | 16.1 | 6.32 | 15 | 2,415 | 81 | 13,041 |
| 45361 | Pre | 2.3 | 8.6 | 4.85 | 45 | 3,870 | 52 | 4,472 |
| 45361 | 4 h | 2.63 | 8.6 | 5.35 | 77 | 6,622 | 18 | 1,548 |
| 45361 | Necropsy | 2.74 | 5.7 | 4.92 | 46 | 2,622 | 52 | 2,964 |
bsAb, bispecific antibody.
Finally, we treated two 8-month-old macaques with 3 mg/kg of bispecific antibody. Interestingly, complete CD3+ lymphopenia persisted for a full week after treatment, unlike our observation in the older macaques (Fig. 3G). These younger animals were necropsied 1 week after treatment to allow assessment of tissue depletion.
The CCR5xCD3 bispecific antibody leads to nearly complete depletion of CCR5+ colonic and lymph node T cells in vivo
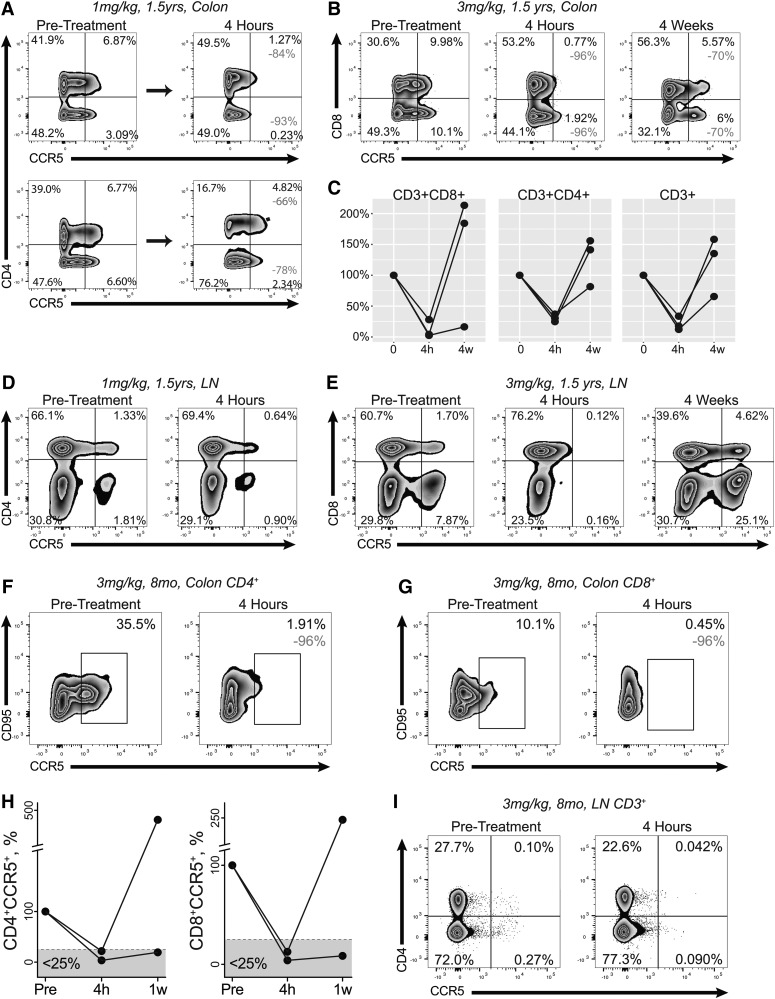
One of the greater challenges in drug delivery is to achieve therapeutic effect in tissues that may have poor drug penetration. For the CCR5xCD3 bispecific antibody, successful depletion of CCR5+ cells from intestine is of special importance because HIV undergoes robust replication in this anatomic site.22 We assessed the frequency of CCR5+ cells among LPLs isolated from colon biopsies taken before treatment and at 4 h after treatment in all treatment groups. Each of the two 1.5-year-old macaques receiving 1 mg/kg bispecific antibody showed substantial depletion of CCR5-expressing CD8+ T cells (78%; 93%). Only one animal treated with this low dose achieved substantial depletion of CD4+CCR5+ T cells, however (Fig. 4A, top row). In contrast, all three 1.5-year-old macaques that received high-dose antibody (3 mg/kg) manifested ∼75% depletion of CD3+CCR5+ cells within 4 h of treatment (Fig. 4B, C). These animals were necropsied 4 weeks after treatment, at which time point one animal retained a depressed fraction of CCR5-expressing CD8+ T cells in gut, but the others saw the frequency of CCR5+ cells rebound to a level exceeding pretreatment levels (Fig. 4C). Depletion of CCR5+ cells from lymph nodes followed a similar pattern, with some loss of such cells in animals receiving a low dose but complete depletion in those receiving a high dose of antibody (Fig. 4D, E).
FIG. 4.
Depletion of CCR5+ lymphocytes from colon and lymph node after CCR5xCD3 bispecific antibody administration. (A) Flow cytograms of colonic CD3+ LPLs in two 1.5-year-old rhesus macaques receiving 1 mg/kg bispecific antibody. The top row demonstrates nearly complete depletion in one of these two animals (84% and 93% of CD4+ and CD4− cells, respectively). (B, C) Essentially complete depletion of colonic CCR5+ cells among CD3+ LPLs from 1.5-year-old rhesus macaques receiving 3 mg/kg antibody. Representative flow cytograms (B) show 96% depletion of both CD8+ and CD8− T cells. (D, E) Depletion of CCR5+ cells from among lymph node CD3+ lymphocytes of 1.5-year-old rhesus macaques receiving 1 mg/kg (D) or 3 mg/kg (E) antibody. (F, I) Depletion of CCR5+ cells from among colon CD3+CD4+ (F), colon CD3+CD8+ (G), or lymph node CD3+ (I) cells of 8-month-old rhesus macaques receiving 3 mg/kg bispecific antibody. A graph of the extent of depletion in colon is shown in (H).
The two 8-month-old macaques treated with 3 mg/kg bispecific antibody experienced more uniform and profound depletion of CCR5+ T cells from colon tissue. We observed greater than 75% depletion of CCR5+ cells from both CD4+ and CD8+ T cell subsets in both animals (Fig. 4F, H). We necropsied these animals 1 week after administering the antibody. Interestingly, one animal retained a substantial deficit of CCR5+ cells at 1 week, while the other demonstrated a significant rebound (Fig. 4H). In lymph nodes, these younger animals possessed a very low percentage of CCR5+ cells before treatment, but still saw substantial depletion of CCR5+ cells 4 h after drug administration (Fig. 4I).
Discussion
Immunotoxins target specific cells for destruction by combining a cell type-specific targeting moiety with a toxic moiety that causes cell death. Immunotoxins have been shown to be effective treatment for some hematologic malignancies, and many such treatments have proceeded to clinical trials, depending on specific targeting for their relatively lower toxicity.23–25 With the development of antiretroviral therapy for HIV that is capable of fully suppressing plasma viremia, but which fails to clear infection, there has been increasing urgency to identify cells that make up the latent viral reservoir. Initial groundbreaking studies identified resting CD4+ memory cells as important constituents,26–28 while more recent studies have identified potentially specific surface markers.29 As the reservoir is identified with increasing specificity, it makes sense to consider immunotoxins as a means of reservoir elimination.
In this study we demonstrated that the immunotoxin RANTES-PE38 eliminates CCR5-expressing LPLs in vitro, but was unable to achieve the same effect in vivo. RANTES-PE38 has previously been used in murine studies to reduce pulmonary inflammation from Aspergillus fumigatus,14,30 but to our knowledge this study represents the first attempt to utilize RANTES-PE38 in rhesus macaques. Failure of RANTES-PE38 to deplete CCR5+ cells in vivo may be related to insufficient dosing, poor biodistribution, poor internalization of the toxic moiety, poor affinity of the fusion protein for the CCR5 receptor, or other factors that differ between in vitro experiments and the complexity of in vivo efficacy. Interestingly, the immunotoxin we created had relatively poor activity against CCR5-expressing CHO cells, especially compared to previously reported results with a similar immunotoxin.13 This difference may be due to differences between our constructed immunotoxin and the previously discussed RANTES-PE38 or due to differences in the expressed receptor (mouse vs. human) that cause different toxin binding or internalization.
As an alternative approach, we demonstrated the efficacy of linking cytotoxic T cells with target cells through an antibody that binds both CD3 and a target epitope. Some authors have termed these drugs bispecific T cell engagers, or BiTEs.31 CTLs have previously been recruited to cancer targets using bispecific antibodies, including a CD3 arm.12,32,33 These studies demonstrated that both CD4+ and CD8+ effector cells are engaged and that both upregulate cytotoxic effector molecules such as granzyme B,32 resulting sometimes in remarkable single-agent efficacy.11,34,35 Although bispecific antibodies have also been synthesized that simultaneously target specific HIV epitopes and CD3,36 targeting of HIV proteins may be inefficient at clearing latently infected cells because latent virus produces very few peptide products on the surface of infected cells,37 limiting vulnerability of the cells to cross-linking and apoptosis and allowing viral escape from treatment. For this reason we instead pursued targeting of a coreceptor for viral entry, reasoning that cells expressing such coreceptors are a superset of the latently infected pool. In early infection, furthermore, we believe that infected cells are most likely to express CCR5 because CCR5-tropic viruses are most frequently transmitted.1,2,38
We were initially uncertain if CCR5-expressing cells are stably committed to CCR5 expression or if many T cells might be capable of such expression under the right conditions in various tissue niches. Indeed, our experience is that flow cytometric analysis of CCR5 expression requires either rapid staining of minimally manipulated cells or incubation of cells at 37°C to allow re-expression of the receptor—experience suggesting that expression of this receptor might be somewhat plastic and variable. In contrast, however, our in vivo results suggest strongly that a pool of stable CCR5+ cells exists, which can be depleted and remain deficient for weeks afterward. Such stable depletion was seen in blood, for example, 1 week after drug administration and was seen for up to 4 weeks in colon tissue.
Elimination of the HIV reservoir from tissue will be necessary to achieve persistent cure. Penetration of pharmaceuticals from blood into target tissues is a common obstacle to effective therapy,39 but this bispecific antibody proved effective at eliminating CCR5+ cells in colon tissues at a modest dose. We will test in subsequent work if repeat dosing of bispecific antibody can lead to enhanced and perhaps complete depletion of CCR5+ cells from all intestinal tissue.
The development of CD3+ lymphopenia was a surprising development although such lymphopenia has sometimes been observed after administration of the parental anti-CD3 antibody, suggesting the possibility that lymphopenia can result from widespread T cell activation.40 Most common forms of drug-induced lymphopenia favor depletion of CD4+ lymphocytes,41 but our treatment demonstrated a total depletion of CD3+ cells from blood. Treatment with single-specificity monoclonal antibodies has led to lymphopenia in some patients.42,43 Lymphopenia has also been identified as a common adverse side effect of bispecific antibodies such as Blinatumomab, a bispecific antibody that engages CD3 and CD19 for treating B cell lymphomas.34 Other bispecific antibody therapies similarly show transient lymphopenia with few other side effects.9,10 In this study lymphopenia was transitory in older animals, but persisted in younger animals for at least a week. As part of the development of our bispecific antibody, the antibodies were primatized to reduce the likelihood of deleterious drug-associated effects, but additional strategies might be useful in seeking to eliminate the observed depletion of CD3+ T cells. For example, a slight excess of parental anti-CCR5 antibody might be used to ensure that no monospecific anti-CD3 antibody remains in the drug preparation.
Pharmacologic depletion of the HIV reservoir is an underexplored but potentially effective component of HIV cure strategies. Other investigators have proposed temporary depletion of certain cells as a means to clear the reservoir,44 using targeting molecules ranging from the very specific (CD32a29) to broad (all CD4+ T cells). The results reported in this study demonstrate the feasibility of this approach in an NHP model and constitute a critical first step toward systematic evaluation of this strategy.
Acknowledgments
The authors thank Matthias Mack for his support of these studies. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number R21 AI116230 to D.J.H.O.C., by the National Center for Research Resources (P51RR000169) and subsequently the Office of Research Infrastructure Programs/OD (P51OD011107) through their funding of the California National Primate Research Center, by the California HIV/AIDS Research Program under award ID13-D-564, and by the Floyd and Mary Schwall Fellowship in Medical Research. Development of the bispecific antibody was supported by grants from the NIH (R24 OD10976 and U24 AI126683).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Keele BF, et al. : Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Shaw GM, McMichael AJ, Haynes BF: Acute HIV-1 Infection. N Engl J Med 2011;364:1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzenstein TL, et al. : Cell-associated HIV DNA measured early during infection has prognostic value independent of serum HIV RNA measured concomitantly. Scand J Infect Dis 2002;34:529–533 [DOI] [PubMed] [Google Scholar]
- 4.Delobel P, et al. : R5 to X4 switch of the predominant HIV-1 population in cellular reservoirs during effective highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2005;38:382–392 [DOI] [PubMed] [Google Scholar]
- 5.Soulié C, et al. : HIV-1 X4/R5 co-receptor in viral reservoir during suppressive HAART. AIDS 2007;21:2243–2245 [DOI] [PubMed] [Google Scholar]
- 6.Madhumathi J, Verma RS: Therapeutic targets and recent advances in protein immunotoxins. Curr Opin Microbiol 2012;15:300–309 [DOI] [PubMed] [Google Scholar]
- 7.Pastan I, FitzGerald D: Recombinant toxins for cancer treatment. Science 1991;254:1173–1177 [DOI] [PubMed] [Google Scholar]
- 8.Hwang J, Fitzgerald DJ, Adhya S, Pastan I: Functional domains of pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell 1987;48:129–136 [DOI] [PubMed] [Google Scholar]
- 9.Valone FH, et al. : Clinical trials of bispecific antibody MDX-210 in women with advanced breast or ovarian cancer that overexpresses HER-2/neu. J Hematother 1995;4:471–475 [DOI] [PubMed] [Google Scholar]
- 10.Frankel SR, Baeuerle PA: Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol 2013;17:385–392 [DOI] [PubMed] [Google Scholar]
- 11.Goebeler ME, et al. : Bispecific T-cell engager (BiTE) antibody construct Blinatumomab for the treatment of Patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase I study. J Clin Oncol 2016;34:1104–1111 [DOI] [PubMed] [Google Scholar]
- 12.Nagorsen D, et al. : Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk Lymphoma 2009;50:886–891 [DOI] [PubMed] [Google Scholar]
- 13.Bruhl H, et al. : Depletion of CCR5-expressing cells with bispecific antibodies and chemokine toxins: A new strategy in the treatment of chronic inflammatory diseases and HIV. J Immunol 2001;166:2420–2426 [DOI] [PubMed] [Google Scholar]
- 14.Schuh JM, Blease K, Brühl H, Mack M, Hogaboam CM: Intrapulmonary targeting of RANTES/CCL5-responsive cells prevents chronic fungal asthma. Eur J Immunol 2003;33:3080–3090 [DOI] [PubMed] [Google Scholar]
- 15.Bostik P, et al. : Expression and in vitro evaluation of rhesus macaque wild type (wt) and modified CC chemokines. J Med Primatol 1998;27:113–120 [DOI] [PubMed] [Google Scholar]
- 16.Aida Y, Pabst MJ: Removal of endotoxin from protein solutions by phase separation using triton X-114. J Immunol Methods 1990;132:191–195 [DOI] [PubMed] [Google Scholar]
- 17.Labrijn AF, et al. : Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A 2013;110:5145–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, et al. : CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med 1997;185:1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottman JB, et al. : Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol 1997;151:1341–1351 [PMC free article] [PubMed] [Google Scholar]
- 20.Conrad ML, Davis WC, Koop BF: TCR and CD3 antibody cross-reactivity in 44 species. Cytom Part A 2007;71:925–933 [DOI] [PubMed] [Google Scholar]
- 21.Pessano S, Oettgen H, Bhan AK, Terhorst C: The T3/T cell receptor complex: Antigenic distinction between the two 20-kd T3 (T3-delta and T3-epsilon) subunits. EMBO J 1985;4:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenchley JM, et al. : CD4 + T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazor R, Onda M, Pastan I: Immunogenicity of therapeutic recombinant immunotoxins. Immunol Rev 2016;270:152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreitman RJ: Immunotoxins in cancer therapy. Curr Opin Immunol 1999;11:570–578 [DOI] [PubMed] [Google Scholar]
- 25.Ashorn P, Moss B, Berger EA: Activity of CD4-Pseudomonas exotoxin against cells expressing diverse forms of the HIV and SIV envelope glycoproteins. J Acquir 1992;5:70–77 [PubMed] [Google Scholar]
- 26.Chun T-W, et al. : Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997;94:13193–13197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finzi D, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 28.Wong JK: Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278:1291–1295 [DOI] [PubMed] [Google Scholar]
- 29.Descours B, et al. : CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 2017;543:564–567 [DOI] [PubMed] [Google Scholar]
- 30.Hogaboam CM, et al. : The therapeutic potential in targeting CCR5 and CXCR4 receptors in infectious and allergic pulmonary disease. Pharmacol Ther 2005;107:314–328 [DOI] [PubMed] [Google Scholar]
- 31.Schlereth B, et al. : Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res 2005;2882–2889. DOI: 10.1158/0008-5472.CAN-04-2637 [DOI] [PubMed] [Google Scholar]
- 32.Haas C, et al. : Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology 2009;214:441–453 [DOI] [PubMed] [Google Scholar]
- 33.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA: Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 2006;43:763–771 [DOI] [PubMed] [Google Scholar]
- 34.Topp MS, et al. : Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29:2493–2498 [DOI] [PubMed] [Google Scholar]
- 35.Bargou R, et al. : Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science (80-.) 2008;321:974–977 [DOI] [PubMed] [Google Scholar]
- 36.Petrovas C, et al. : Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med 2017;9:eaag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siliciano RF, Greene WC: HIV latency. Cold Spring Harb Perspect Med 2011;1:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw GM, Hunter E: HIV transmission. Cold Spring Harb Perspect Med 2012;2:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergeron MG: Tissue penetration of antibiotics. Clin Biochem 1986;19:90–100 [DOI] [PubMed] [Google Scholar]
- 40.Herold KC, et al. : Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gergely P: Drug-induced lymphopenia. Focus on CD4+ and CD8+ cells. Drug Saf 1999;21:91–100 [DOI] [PubMed] [Google Scholar]
- 42.Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B: Sirukumab, a human anti-interleukin-6 monoclonal antibody: A randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2014;73:1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suntharalingam G, et al. : Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006;355:1018–1028 [DOI] [PubMed] [Google Scholar]
- 44.Zanin MKB, Duvall MR: Back-burning to cure HIV: Temporary depletion of all CD4+ cells and elimination of the extracellular reservoir with HIV Immunotoxin Therapy. Med Hypotheses 2009;72:592–595 [DOI] [PubMed] [Google Scholar]