Abstract
Substance misusers, including adolescent smokers, often have reduced reward system activity during processing of non-drug rewards. Using a psychophysiological interaction approach, we examined functional connectivity with the ventral striatum during reward anticipation in a large (n=206) sample of adolescent smokers. Increased smoking frequency was associated with 1) increased connectivity with regions involved in saliency and valuation, including the orbitofrontal cortex; 2) reduced connectivity between the ventral striatum and regions associated with inhibition and risk aversion, including the right inferior frontal gyrus. These results demonstrate that functional connectivity during reward processing is relevant to adolescent addiction.
Introduction
Adolescence is a period of substantial behavioral and brain changes and of heightened propensity for risk-taking. Adolescence is also a time of increased risk for impulse-control disorders, including addiction (Chambers, Taylor & Potenza, 2014; Paus, Keshavan & Giedd, 2008). The most common addiction in adolescence is nicotine (Young Corley, Stallings, Rhee Crowley & Hewitt, 2002). Smoking is the leading cause of preventable deaths in the U.S., and nearly one in five adults is a smoker (U.S. Department of Health and Human Services, 2014). Next to alcohol, cigarettes are one of the most widely available addictive substances, meaning that it is much easier for adolescents to try cigarettes than other drugs. Adolescent smoking differs widely in its frequency and regularity, but can broadly be categorized into four smoking trajectories: 1) Adolescents who start smoking at an early age and go on to become regular smokers, 2) individuals who follow the same path but initiate smoking at a later age, 3) adolescents who experiment with smoking but don’t become addicted or stop smoking, and 4) non-smokers (Audrain-McGovern, Rodriguez, D., Tercyak, Cuevas, Rodgers, & Patterson 2004; Chassin, Presson, Pitts & Sherman, 2000; Mayhew, Flay & Mott, 2000).
While the behavioral and personality differences between adolescents in different smoking trajectories are subtle and difficult to pinpoint, the differences between adolescent smokers and non-smokers are well established: Adolescent smokers show increased novelty seeking, reduced harm avoidance, and increased choice impulsivity (Audrain-McGovern et al., 2004a, 2004b; Wills, Windle & Cleary, 1998). However, these traits are not only characteristic of adolescent smokers compared with non-smokers, but also of adolescents compared with adults (Brändström, Sigvardsson, Snylander & Richter, 2008; Steinberg, Graham, O’Brien, Woolard, Cauffman & Banich, 2009). A number of neurobiological models have attributed these characteristics of the adolescent developmental period to a difference in the balance between different brain systems in adolescence. The dual-system model (e.g. Steinberg, Albert, Cauffman, Banich, Graham & Woolard, 2008), the triadic model (Ernst, Pine & Hardin, 2006) and the imbalance model (Casey, Jones & Hare, 2008) all distinguish between the reward system and the cognitive control systems. Among the structures involved in cognitive control are the dorsolateral prefrontal cortex (dlPFC) which is one of the most important executive control regions (Alvarez & Emory, 2006), the orbitofrontal cortex (OFC) which has been attributed a role in saliency and value attribution (O’Doherty, 2004), the anterior cingulate cortex (ACC) which has been implicated in selective attention (Alvarez & Emory, 2006), and the right inferior frontal gyrus (IFG) which has been established as a central region in behavioral inhibition (Chikazoe, Konishi, Asari, Jimura & Miyashita, 2007; Jacobson et al., 2003).
There are many interacting regions involved in reward processing (see Haber & Knutson, 2010). Among these regions, the ventral striatum (VS) is particularly important. The VS receives dopaminergic input from the ventral tegmental area and is connected to frontal areas such as the orbitofrontal and ventromedial cortices. The VS is not only central to processing reward-related stimuli, but also plays a key role in integrating affective and cognitive information, and in action selection and motivation (Floresco, 2015). Along with decreases in impulsive choice from adolescence to adulthood, activation in the VS during reward-related decision making decreases, and activations in prefrontal cognitive control regions have been shown to increase with age (Christakou, Brammer & Rubia, 2011). The functional connectivity between the VS and prefrontal cortex (PFC) during reward outcomes also increases over the course of adolescence (Van den Bos, Cohen, Kahnt & Crone, 2012). Furthermore, ventral striatal dopamine D2 receptor availability was associated with alcohol cue-induced activation in the ACC and medial prefrontal cortex, confirming a role for dopamine in VS-medial prefrontal interactions (Heinz et al., 2004).
In adult smokers, lifetime tobacco use is associated with structural brain alterations in both the reward and cognitive control systems (Gallinat et al., 2006; Zhang, Salmeron, Ross, Geng, Yang, & Stein, 2011). Furthermore, adult smokers show reduced connectivity between the striatum and anterior cingulate cortex (ACC), associated with the severity of nicotine dependence (Hong et al., 2009). While these findings suggest a role of long-term chronic cigarette smoking in brain deficits in these systems, there is robust evidence linking the VS to adolescent impulsivity and smoking. VS hypoactivity during reward anticipation can be observed in adolescents with ADHD compared to control subjects (Scheres, Milkam, Knutson & Castellanos, 2007), and is associated with risk-taking bias in typically developing adolescents (Schneider et al., 2012). It appears that VS activity is negatively associated with impulsivity, independent of age (Ripke et al., 2012). VS hypoactivity can be seen in dependent adult smokers compared to occasional smokers (Bühler et al. 2010), and is associated with level of nicotine use in adults (Rose, Ross, Salmeron & Lee, 2012). Importantly, a reduction in VS activation during reward anticipation has also been observed in adolescents prenatally exposed to nicotine (Müller et al., 2013) and in adolescent smokers (Peters et al., 2011). Furthermore, Peters et al. reported that ventral striatal activity during reward anticipation was negatively correlated with smoking frequency in adolescents. These findings point toward a possible deficit in the processing of rewarding stimuli in individuals who are at risk for developing nicotine dependence.
Whereas the majority of studies to date have used measures of regional changes in Blood Oxygen Level Dependent (i.e., BOLD) activation to examine differences between substance using groups and non-users, a number of recent studies have used BOLD to evaluate differences in brain connectivity between these groups. However, the majority of these studies have focused on resting-state connectivity (Fedota & Stein, 2015). Compared with resting state measures of functional connectivity, examining differences in connectivity in relation to specific conditions, such as different reward cue types, has the potential to be more informative with regard to differences in reward processing. For instance, a study examining reward cue reactivity in smokers found greater functional connectivity between the left insula and a widespread network including the OFC, ACC, and dorsal striatum during smoking compared to food cues (Claus, Blaine, Filbey, Mayer & Hutchison, 2013). While examining smokers’ reactivity to smoking cues is a valuable tool for understanding the mechanisms of craving and relapse in addicted smokers, the way in which non-smoking rewards are processed has the potential to offer more insight into factors associated with smoking initiation and smoking trajectories in adolescents.
A task which has widely been used to examine generalized reward processing in the context of functional magnetic resonance imaging (fMRI) is the Monetary Incentive Delay (MID) task (Knutson, Westdorp, Kaiser & Hommer, 2000). The paradigm has the distinct advantage of temporally separating anticipation and receipt of positive or negative outcomes, making it possible to examine the activation patterns associated with each separately. VS activity is observed during the anticipation of rewards in the MID (Adcock, Thangavel, Whitfield-Gabrieli, Knutson & Gabrieli, 2006; Knutson, Fong, Bennett, Adams & Hommer, 2003). Other regions associated with reward anticipation in this task include the dorsal striatum, cuneus, thalamus, ACC, ventromedial PFC, OFC, insula, and midbrain (Haber & Knutson, 2010; Van Leijenhorst, Zanolie, Van Meel, Westenberg, Rombouts & Crone, 2010).
Here, we examine the association between adolescent smoking frequency and functional connectivity in the VS during anticipation of large rewards compared to no reward in the MID task, using Psychophysiological Interaction (PPI) analysis (Friston et al., 1997). We employed a powerful machine learning procedure to examine the connectivity patterns associated with smoking. Such approaches have previously been used to investigate adolescent binge-drinking (Whelan et al., 2014) and intelligence (Jollans et al., 2015). This approach has the potential to detect relatively subtle differences, while guarding against spurious findings, using both cross-validation and random-label permutation. We included 206 adolescents from a large multisite study, with a wide spectrum of nicotine use. As our aim was to identify effects associated with smoking frequency, rather than with smoking initiation, we included only adolescents who had smoked on three or more occasions in their lifetime at the point of data collection. In line with a recent review examining resting state functional connectivity in nicotine addiction (Fedota & Stein, 2015), which concluded that disruptions in nicotine addiction appear to be focused on the salience network as well as frontal cognitive control systems, we hypothesized that frequency of smoking would be associated with reduced VS connectivity to fronto-parietal cognitive control regions (Garavan & Weierstall, 2012) and increased connectivity to regions associated with salience or valuation of stimuli, such as the anterior cingulate and orbitofrontal and insular cortices (Seeley et al., 2007).
Method
Characteristics of the IMAGEN Study
A large sample of 14-year olds was recruited at eight recruitment sites. Adolescents completed an extensive battery of psychiatric and neuropsychological assessments, including fMRI. Details of the full study protocol and data acquisition are provided elsewhere (Schumann et al., 2010).
Participants
Participants were a subset of 206 adolescents from the multisite study (110 female). Further information on the distribution of smoking frequency is provided in Table 1, and other details about the sample are provided in Table 2.
Table 1.
Distribution of smoking frequency across the sample.
| Lifetime smoking occasions
|
n | |
|---|---|---|
| ESPAD score | ESPAD range | |
| 2 | 3 to 5 | 57 |
| 3 | 6 to 9 | 37 |
| 4 | 10 to 19 | 32 |
| 5 | 20 to 39 | 20 |
| 6 | 40+ | 60 |
Table 2.
Characteristics of the sample
| Mean | SD | Correlation with nicotine use
|
||
|---|---|---|---|---|
| r | p | |||
| Age | 14.58 | 0.46 | 0.11 | 0.13 |
| Socioeconomic Status | 17.50 | 4.36 | −0.16 | 0.025 |
| Pubertal Development Status | 3.66 | 0.70 | 0.13 | 0.065 |
| WISC-IV Perceptual Reasoning | 103.66 | 12.97 | −0.01 | 0.92 |
| WISC-IV Verbal Comprehension | 107.80 | 13.79 | −0.10 | 0.13 |
| ESPAD Lifetime Alcohol use | 3.21 | 1.63 | 0.26 | 0.0002* |
| ESPAD Lifetime Cannabis use | 0.64 | 1.45 | 0.21 | 0.0029* |
| SURPS Anxiety Sensitivity | 2.24 | 0.49 | −0.14 | 0.045 |
| SURPS Impulsivity | 2.60 | 0.42 | −0.05 | 0.44 |
| SURPS Hopelessness | 1.93 | 0.40 | 0.02 | 0.77 |
| SURPS Sensation Seeking | 2.80 | 0.54 | −0.08 | 0.22 |
| TCI-R Disorderliness | 23.71 | 4.33 | 0.07 | 0.26 |
| TCI-R Exploratory Excitability | 33.44 | 4.74 | 0.03 | 0.70 |
| TCI-R Extravagance | 30.79 | 6.02 | 0.04 | 0.52 |
| TCI-R Impulsivity | 27.82 | 5.01 | −0.06 | 0.41 |
| TCI-R Novelty Seeking | 115.77 | 14.43 | 0.05 | 0.47 |
p<0.003125, p value corrected for multiple comparisons
Substance use questionnaire
Lifetime smoking, alcohol, and cannabis use were measured using the European School Survey Project on Alcohol and Other Drugs questionnaire (ESPAD, Hibell et al., 1997), which was administered using the computerized assessment platform Psytools. Psytools presented questionnaire items and response alternatives on a computer screen. The reliability of individual data was checked in a two-stage procedure: Before every task, adolescents were asked to report on the current testing context including questions about their attentional focus and the confidentiality of the setting. Potentially problematic testing situations were followed-up by research assistants face-to-face in a confidential setting. Exclusion criteria for substance use measures included an indication that the participant was in a hurry, somebody was watching, or an indication to have known or taken the sham drug Relevin. Scores on the ESPAD are ranked as follows: 0: no lifetime use, 1: 1 to 2 uses, 2: 3 to 5 uses, 3: 6 to 9 uses; 4: 10 to 19 uses, 5: 20 to 39 uses, 6:40 or more uses. Participants were included if they had a score of 2 or higher on the ESPAD item measuring lifetime smoking. ESPAD scores for lifetime smoking are reported in Table 1.
Wechsler Intelligence Scale for Children
Participants completed a version of the Wechsler Intelligence Scale for Children WISC-IV (Wechsler, 2003), of which we included the following subscales: Perceptual Reasoning, consisting of Block Design (arranging bi-colored blocks to duplicate a printed image) and Matrix Reasoning (a series of colored matrices are presented and the child is asked to select the consistent pattern from a range of options); and Verbal Comprehension, consisting of Similarities (two similar but different objects or concepts are presented and the child is asked to explain how they are alike or different) and Vocabulary (a picture is presented or a word is spoken aloud by the experimenter and the child is asked to provide the name of the depicted object or to define the word).
Substance Use Risk Profile Scale
The Substance Use Risk Profile Scale (SURPS; Woicik, Stewart, Pihl, & Conrod, 2009) assesses personality traits that confer risk for substance misuse and psychopathology. This scale measures four distinct and independent personality dimensions; anxiety sensitivity, hopelessness, sensation seeking, and impulsivity. The anxiety sensitivity dimension is characterized by the fear of symptoms of physical arousal. The hopelessness dimension is identified as a risk factor for the development of depression and characterized by dismal feelings. The sensation seeking dimension is characterized by the desire for intense and novel experiences. The impulsivity dimension involves difficulties in the regulation (controlling) of behavioral responses.
Temperament and Character Inventory
The novelty seeking scale of the Temperament and Character Inventory – Revised (TCI-R; Cloninger, 1999) was administered. The Novelty seeking scale is composed of four sub-scales. Exploratory Excitability contrasts with ‘stoic rigidity’ and reflects sensation-seeking and novelty-seeking behaviors. Impulsiveness describes behavior on a dimension from impulsivity to reflection and captures elements of emotional reactivity, and unreflective, careless behavior. The Extravagance subscale assesses overspending behavior and poor planning and is believed to reflect a tendency to approach reward cues. Disorderliness reflects disorganized, uncontrolled, and antinormative behavior.
Puberty Development Scale
The Puberty Development Scale (PDS; Petersen, Crockett & Richards, 1988) was used to assess the pubertal status of our adolescent sample. This scale provides an eight-item self-report measure of physical development based on the Tanner stages with separate forms for males and females. For this scale, there are five categories of pubertal status: (1) prepubertal, (2) beginning pubertal, (3) midpubertal, (4) advanced pubertal, (5) postpubertal. Participants answered questions about their growth in stature and pubic hair, as well as menarche in females and voice changes in males.
Monetary incentive Delay Task
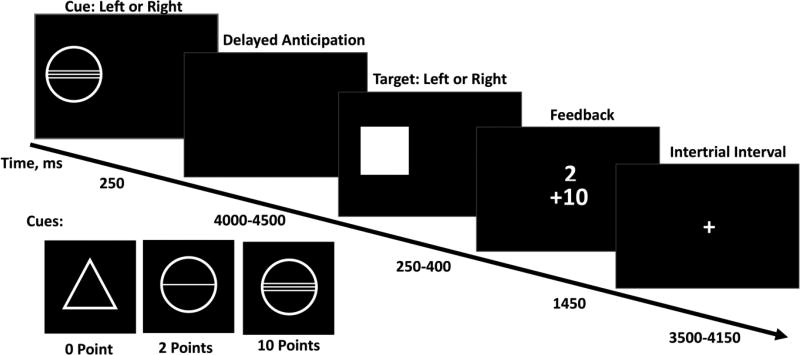
Participants completed a modified version of the MID task, involving small and large possible gains. On each trial, the amount of points that could be won on that trial was signaled by a cue, displayed for 4–4.5 s. Participants could win a reward by responding as quickly as possible to a target stimulus presented after a random time interval, by means of a button press, after which feedback was presented. The response and feedback phase lasted a total of 2 s. The response interval was dynamically adjusted so that subjects won on 66% of all trials. Trials were separated by a 3.5–4.15 s inter-trial interval, during which a fixation cross was presented. The cue stimuli were a circle with two lines signaling a large reward (10 points), a circle with one line signaling a small reward (2 points), and a triangle signaling that no reward could be gained. 22 trials per condition were completed, resulting in 66 total trials. Task stimuli and timings are presented in Figure 1.
Figure 1. Stimuli and timings in the MID Task.
Cues signaling the task condition (no reward, small reward, large reward) were displayed for 4-4.5 s. The response and feedback phase lasted a total of 2 s. Trials were separated by a 3.5 – 4.15 s inter-trial interval.
fMRI Data Acquisition
Full details of the magnetic resonance imaging (MRI) acquisition protocols and quality checks have been described previously, including an extensive period of standardization across MRI scanners (Schumann et al., 2010). MRI Acquisition Scanning was performed at the eight assessment sites with a 3T whole body MRI system made by several manufacturers (Siemens: 4 sites, Philips: 2 sites, General Electric: 1 site, and Bruker: 1 site). To ensure a comparison of MRI data acquired on these different scanners, we implemented image-acquisition techniques using a set of parameters compatible with all scanners that were held constant across sites, for example, those directly affecting image contrast or fMRI preprocessing. Standardized hardware for visual and auditory stimulus presentation (NordicNeurolabs, Bergen Norway, http://www.nordicneurolab.com) was used at all sites. BOLD functional images were acquired with a gradient-echo echoplanar imaging (EPI) sequence using a relatively short echo-time to optimize imaging of subcortical areas. For the MID, 300 volumes consisting of 40 slices were acquired for each subject. Scanning time for this task was a total of 11 minutes.
fMRI preprocessing and analysis
Briefly, the functional imaging processing was as follows: Time series data were first corrected for slice-timing, then corrected for movement, non-linearly warped onto MNI space using a custom EPI template, and gaussian-smoothed at 5mm-full width half maximum. Nuisance variables were also added to the design matrix: estimated movement was added in the form of 6 additional regressors (3 translations, 3 rotations). These analysis steps were carried out in SPM8. All subsequent analyses were conducted in SPM12.
Three conditions (No-win, Small-win, Big-win) in addition to individual movement parameters were specified in a general linear model. An F contrast for effects of interest was conducted after model estimation. Subsequently, BOLD signals from 3-mm radius spherical ROIs in the left ventral striatum (MNI coordinates: [−12, 10, −10]) and right ventral striatum (MNI coordinates: [12, 10, −10]) were adjusted by effects of interest and extracted. These extracted signal time series were used as the physiological regressors, and the effect of condition (Big-win versus No-win) was used as the psychological regressor. The Psychophysiological Interaction (PPI) term was computed using the PPI toolbox in SPM12. For further details on the PPI analysis, see Supplementary materials.
Functional connectivity during reward anticipation
A one-sample t-test to identify clusters in which functional connectivity for reward anticipation differed significantly from zero was conducted in SPM12. Data acquisition site, sex, and PDS were also entered into the analysis as nuisance covariates. The family-wise error (p<.05) was corrected for by using an uncorrected p-value of 0.001 in combination with a minimum cluster extent of 14 contiguous voxels, calculated using SPM.
Functional connectivity associated with smoking frequency
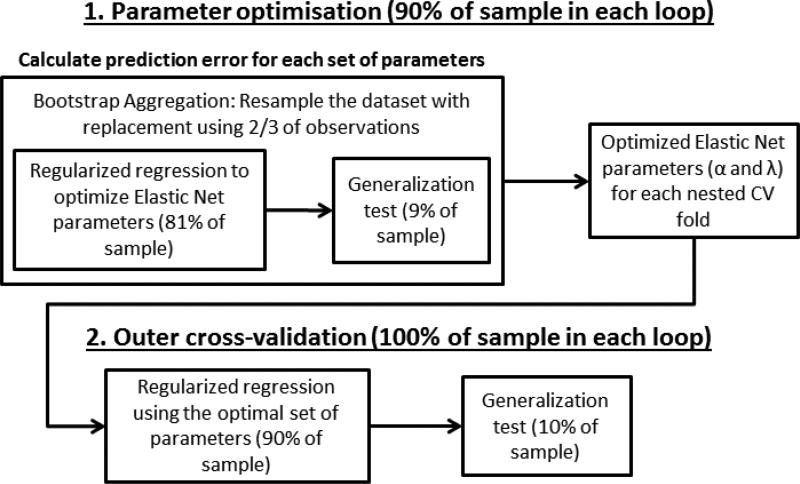
Data from 92 ROIs based on the AAL atlas (Tzourio-Mazoyer et al., 2002) and two masks for the subthalamic nuclei (x=-12, y=-10, z=-5; x=12, y=-13, z=-5), as well as lifetime alcohol and cannabis use, data acquisition site, sex, and pubertal development status were entered into the analysis. Data were z-scored. The analysis procedure is shown in Figure 2. A similar approach has previously been used by Whelan et al. (2014) and Jollans et al. (2015). To assess the effect of lifetime smoking on VS connectivity, two ROI regularized multiple regression analyses for the left and right VS seed were carried out in Matlab R2014a, via the Elastic Net (Zou & Hastie, 2005). Regression with Elastic Net regularization is an example of a sparse regression method, which imposes a hybrid of both L1- and L2-norm penalties (i.e., penalties on the absolute (L1 norm) and squared values of the β weights (L2 norm)). This allows relevant but correlated coefficients to coexist in a sparse model fit, by doing automatic variable selection and continuous shrinkage simultaneously, and selects or rejects groups of correlated variables. Least absolute shrinkage and selection operator (LASSO, Tibshirani, 1996) and ridge regression (Hoerl & Kennard, 1970) are special cases of the Elastic Net.
Figure 2. Machine Learning analysis procedure.
The machine learning analysis was carried out in two stages: (1) The optimal Elastic Net parameters for each main cross-validation (CV) fold were identified using nested CV within each main CV fold. Bootstrap aggregation was used in this step. (2) The optimal Elastic Net parameters for each main CV fold were applied to the full training set (90% of the data) to generate beta weights for all input variables. These beta weights were then used to generate outcome predictions for the remaining, untouched 10% of the dataset in each main CV fold. The goodness-of-fit was estimated using the outcome predictions for the entire dataset.
We used 10-fold nested cross-validation, in which 10 separate regression models were generated, with the beta weights for all parameters being generated on 90% of the dataset (the training set), and tested on 10% of the dataset (the test set). Within the test set, additional 10-fold cross-validation was used to identify the optimal Elastic Net parameters α and λ. Alpha represents the weight of lasso vs. ridge regularization which the Elastic Net uses, and λ is the regularization coefficient.
We additionally applied 50-fold bootstrap aggregation to introduce an additional level of stability (Breiman, 1996). That is, parameter optimization was repeated 50 times, using sampling with replacement (i.e., on average two thirds of the data in each iteration). The results from all iterations within each training fold were then averaged. In addition to bootstrap aggregation this entire analysis procedure was repeated 50 times, and the results (correlation coefficients and beta weights) were averaged across all 50 iterations of the analysis procedure. Overall, this yielded 500 sets of beta weights, from 10 cross-validation folds across 50 analysis iterations. Beta weights were averaged for each variable.
Two null models were also computed using the same method. For these, the same analysis procedure was carried out using random label permutations with the same dataset (i.e., subjects were randomly assigned to ESPAD scores). These null models yielded average beta weights of 0.018 and 0.016, and average correlation coefficients of r=-0.006 and r=-0.01. Based on the null models, the threshold for reporting ROIs was set at a minimum absolute beta weight of 0.048 this was the 95th percentile of the distribution of beta weights in the null models). The reporting thresholds for the minimum frequency with which ROIs should be included in the regression models across iterations was set at 84% (left) and 81% (right, this was the 95th percentile of the distribution of occurrence frequency across iterations in the null models).
Results
A series of Spearman’s rank correlations were conducted (see Table 2). Using Bonferroni correction for multiple comparisons, lifetime smoking was significantly positively correlated with alcohol and cannabis use.
VS connectivity during reward anticipation
A number of cortical and subcortical clusters showed altered functional connectivity with the VS during anticipation of a large reward vs. no reward. Clusters with significantly increased or decreased functional connectivity are reported in Table 3.
Table 3.
Clusters which showed significant changes in functional connectivity with the VS during anticipation of a large reward vs. no reward
| x | y | z | k | max t | |
|---|---|---|---|---|---|
| Clusters with increased functional connectivity | |||||
| Left VS | |||||
| −6 | −1 | 64 | 27 | 4.27 | Supplemental Motor Area (L) |
| 12 | 20 | 37 | 15 | 4.15 | Middle Cingulum (R) |
| 6 | 11 | 61 | 22 | 4.15 | Supplemental Motor Area (R) |
| Right VS | |||||
| 24 | −70 | −11 | 16 | 4.12 | Fusiform Gyrus (R) |
|
| |||||
| Clusters with decreased functional connectivity | |||||
| Left VS | |||||
| −30 | −91 | −11 | 138 | 7.30 | Inferior Occipital Gyrus (L) |
| 27 | −94 | 1 | 105 | 6.24 | Middle Occipital Gyrus (R) |
| −42 | 26 | 25 | 16 | 3.80 | IFG, triangular part (L) |
| Right VS | |||||
| −27 | −91 | −11 | 68 | 6.00 | Inferior Occipital Gyrus (L) |
| 33 | −88 | −11 | 59 | 4.98 | Inferior Occipital Gyrus (R) |
R: right; L: left; k; cluster extent; IFG: Inferior Frontal Gyrus
Changes in VS connectivity associated with lifetime smoking
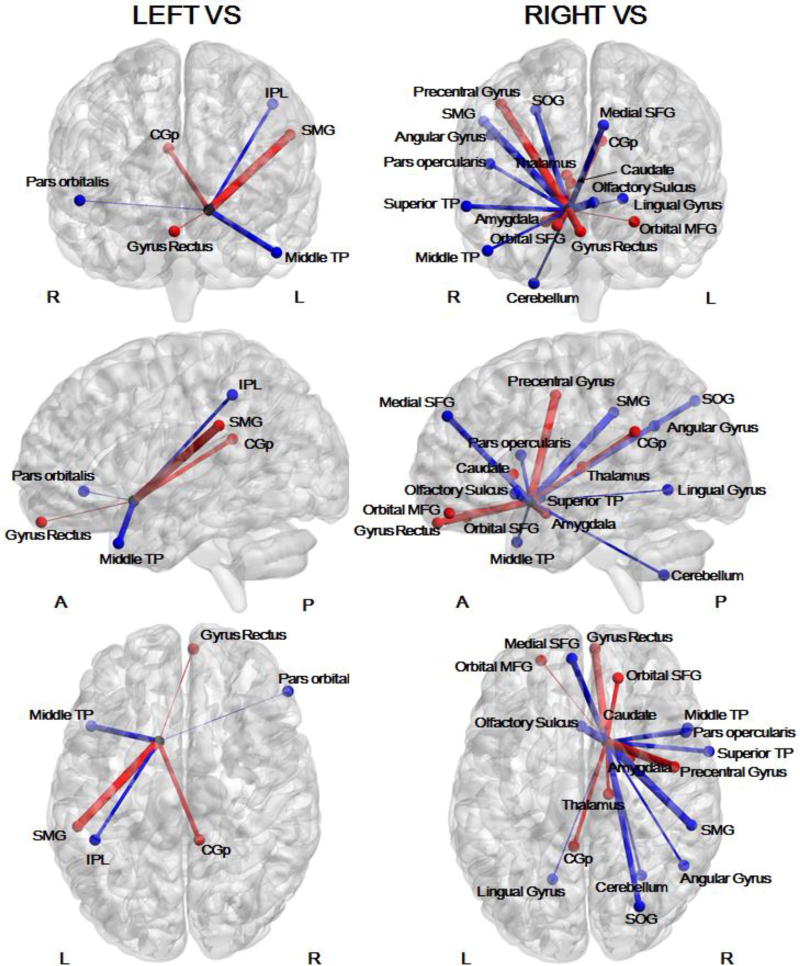
There was a significant association between lifetime smoking and both right (mean r=.27) and left (mean r=.21) VS functional connectivity. ROIs which passed the thresholds for absolute beta weights and frequency of occurrence across cross-validation folds determined using the null models are reported (see Table 5 and Figure 3 for ROIs associated with lifetime smoking).
Figure 3. ROIs for which functional connectivity with the VS during anticipation of a large reward vs. no reward was associated with lifetime smoking.
L: Left; R: Right; A: Anterior; P: Posterior; PCC: Posterior Cingulate; IPL: Inferior Parietal Lobule; TP: Temporal Pole; SMG: Supramarginal Gyrus; SOG: Superior Occipital Gyrus, SFG: Superior Frontal Gyrus; MFG: Middle Frontal Gyrus. Functional connectivity between the VS and nodes drawn in red was positively associated with smoking frequency. Functional connectivity between the VS and nodes drawn in blue was negatively associated with smoking frequency. This figure was generated using BrainNet Viewer (Xia, Wang, & He, 2013).
Discussion
A Psychophysiological Interaction (PPI) analysis of a large (n=206) sample of adolescent smokers has produced two key findings with respect to adolescent smoking frequency and functional connectivity with the VS during anticipation of rewards: (1) a positive association within the reward system; specifically, between the VS and OFC and amygdala, (2) a negative correlation between the reward system and inhibitory control and attention networks; specifically, between VS and the right IFG, inferior parietal cortex, and medial PFC. We also found that smoking frequency was not significantly associated with measures of impulsivity or novelty seeking, which is in line with previous studies that were not able to distinguish between adolescent smokers in different smoking trajectories on the basis of novelty-seeking or choice impulsivity (Audrain-McGovern et al., 2004a; 2009).
Smoking frequency was associated with an increase in connectivity between the OFC and VS. The VS can indirectly modulate frontal cortical activity, by means of the thalamus. However, the ACC, mPFC and OFC also provide direct input to the VS (Cohen et al., 2012; Haber & Knutson, 2010). The OFC has previously been implicated in a study comparing occasional and dependent smokers (Bühler et al., 2010). This study found that dependent smokers exhibited significantly less orbitofrontal activation during anticipation of monetary rewards than occasional smokers, supporting our finding of altered function of this region associated with frequency of smoking. Interestingly, the same study also reported increased activity during reward anticipation in the right medial OFC and gyrus rectus in short-term abstinent compared to non-abstinent smokers, for monetary and cigarette rewards (Bühler et al., 2010). In line with the proposed role of the OFC in attribution of saliency and valuation (O’Doherty, 2004), our finding of increased striatal connectivity with these same medial orbitofrontal regions associated with smoking frequency suggest that adolescent smoking is associated with generalized increased reward valuation; similar to the pattern demonstrated during nicotine withdrawal by Bühler and colleagues.
Thalamus-VS connectivity was also positively associated with smoking frequency. The thalamus has been highlighted as an important region in incentive processing in adolescents and adults, along with the insula (Cho et al., 2013). Cho et al. (2013) suggest that interoceptive information from the insula, and alerting signals about opportunities for incentive processing from the thalamus converge in the nucleus accumbens (NAc), which forms part of the VS. Considering findings of increased activation in the thalamus during reward anticipation in alcoholics (Wrase et al., 2007), our finding of increased connectivity between the VS and thalamus points toward a heightened sensitivity toward salient external stimuli. We also observed increased functional connectivity between the bilateral VS and the contralateral posterior cingulate cortex (PPC), associated with smoking frequency. A general role for the PPC in directing the focus of attention internally or externally, and in determining the width or breadth of the attentional focus has been proposed (Leech & Sharp, 2014), which is consistent with its role as a central node of the default-mode network (DMN, Buckner et al., 2008). In monkeys PPC activity was also found to be mediated by actual and expected reward value (McCoy et al., 2003), and in humans the PPC has been shown to play a role in integrating motivational information and spatial attention (Mohanty et al., 2008). Along with the OFC, the PPC showed heightened activation during motivationally salient cues in humans (Mohanty et al., 2008), which suggests that the heightened functional connectivity between the VS and PPC may reflect a similar effect of heightened attention to highly valued and motivationally salient events as the heightened connectivity with the OFC.
In line with previous research which found that smokers show less IFG activity than non-smokers to negative emotional images (Froeliger et al., 2013), we found that functional connectivity between the VS and right IFG was negatively associated with smoking frequency. The right IFG is a central region for response inhibition (Chikazoe, Konishi, Asari, Jimura & Miyashita, 2007; Jacobson et al., 2003) and attentional control (Hampshire, Chamberlain, Monti, Duncan & Owen, 2010). The right IFG can also be considered part of a ventral frontoparietal attention network, which further includes the inferior parietal cortex and supramarginal gyri (Corbetta et al., 2008). This network plays a role in attentional shifting and filtering sensory input according to behavioral relevance. We also observed a strong negative association between smoking frequency and VS connectivity to regions in the medial PFC (mPFC). Studies of patients with lesions to the mPFC have shown that this region is involved in decision-making under risk, biasing healthy individuals toward more conservative choices (Clark et al., 2008). Taken together with the finding of increased connectivity between the VS and OFC, the deficit in right IFG, inferior parietal (and superior occipital) cortex, and mPFC connectivity is consistent with the imbalance model’s account of an over-active motivational system, receiving heightened input from regions central in the valuation of stimuli, and not being reigned in sufficiently by an underactive inhibitory control system and a deficit in directing attention toward behaviorally relevant stimuli.
In addition to the above mentioned ROIs, we also observed a significant association between smoking frequency and functional connectivity between the VS and the amygdala. Connectivity between the right VS and the right amygdala has been found to be associated with the relevance of stimuli (Ousdal, Reckless, Server, Andreassen & Jensen, 2012). This is consistent with our findings of higher VS connectivity to regions associated with salience and valuation of stimuli. VS connectivity to the adjacent bilateral temporal poles on the other hand showed a strong negative association with smoking frequency. A previous study found that adult smokers’ level of nicotine dependence was positively associated with activation in the temporal pole and insula during presentation of smoking compared to food cues (Claus et al., 2013). While the majority of studies examining temporal pole function have focused on social cognition and emotion processing, there is some evidence that the temporal pole could serve as a hub integrating emotional and sensory cues (Fan et al., 2014; Pehr et al., 2015; Olson et al., 2007). Furthermore, reduced grey matter volume in the temporal pole has been reported in cocaine users (Albein-Urios et al., 2013), making this region a promising target for further investigation in substance use.
While PPI analysis is a valuable tool for identifying functional differences in connectivity, it is not able to identify anatomical or structural alterations in connectivity. Conducting PPI in conjunction with tractography (e.g., Cohen, Elger & Weber, 2008) would allow the identification of structural differences associated with functional connectivity alterations in smokers. Furthermore, PPI analyses often suffer from a lack of power, particularly when event-related tasks are used (O’Reilly, Woolrich, Behrens, Smith & Johansen-Berg, 2012). However, low power is a chronic problem in neuroimaging research (Button et al., 2013). In this study we addressed this issue by using a large sample, and a very rigorous analysis protocol. Cross-validation and bootstrapping are valuable tools for guarding against false positives (Whelan & Garavan, 2014) and identifying true, but small, effects. In addition, the random-label permutation (null model) approach which we adopted is an effective means of quantifying the validity of our results.
In conclusion, the use of a PPI analysis in conjunction with a robust machine learning approach identified differences in VS connectivity during reward anticipation associated with adolescent smoking frequency. The increased functional connectivity between the VS and OFC and PPC with increased cigarette use suggests that adolescent smoking may be associated with increased attribution of salience to reward-related stimuli. Furthermore, the finding of reduced functional connectivity between the VS and the right IFG, mPFC, and inferior parietal cortex with increased smoking indicates a deficit in inhibitory control and attentional orienting. Taken together, these findings paint a picture of increased valuation of rewards, alongside difficulties inhibiting behavior, and possibly a deficit in the integration of sensory and motivational cues in adolescent smokers. Notably, our findings extend the literature showing differences in the neural networks underpinning reward processing between adolescent smokers and non-smokers, showing that reward processing also differs between different adolescent smoking trajectories. While it is not possible to deduce whether these differences in VS connectivity preceded smoking initiation, the link between reward-related activity in the VS and adolescent impulsivity supports the conclusion that differences in VS connectivity may pose a risk for adolescent smoking. Future longitudinal studies should evaluate whether VS connectivity can be established as a predictive biomarker of substance use risk in adolescence.
Supplementary Material
Table 4.
ROIs for which functional connectivity with the VS during anticipation of a large reward vs. no reward was associated with lifetime nicotine use
| Left VS | Right VS | ||||
|---|---|---|---|---|---|
|
|
|||||
| Beta weight | % of CV folds | Beta weight | % of CV folds | ||
| Gyrus Rectus (R) | 0.105 | 93.2 | 0.305 | 100 | |
| SFG, orbital part (R) | 0.191 | 93.6 | |||
| MFG, orbital part (L) | 0.077 | 84.6 | |||
| SFG, medial part (L) | −0.251 | 86.6 | |||
| Olfactory gyrus (L) | −0.325 | 93.4 | |||
| IFG, opercular part (R) | −0.176 | 92.4 | |||
| IFG, orbital part (R) | −0.099 | 91.8 | |||
| Amygdala (R) | 0.323 | 90.4 | |||
| Thalamus (R) | 0.150 | 89.6 | |||
| Caudate (R) | 0.076 | 81.2 | |||
| Posterior Cingulate (L) | 0.184 | 88.0 | |||
| Posterior Cingulate (R) | 0.238 | 88.6 | |||
| Precentral gyrus (R) | 0.337 | 93.6 | |||
| Supramarginal Gyrus (L) | 0.381 | 84.8 | |||
| Supramarginal Gyrus (R) | −0.311 | 95.4 | |||
| Angular Gyrus (R) | −0.138 | 89.6 | |||
| Inferior parietal lobule (L) | −0.201 | 84.0 | |||
| Superior occipital gyrus (R) | −0.245 | 83.0 | |||
| Lingual gyrus (L) | −0.100 | 82.6 | |||
| Middle Temporal Pole (L) | −0.281 | 85.0 | |||
| Middle Temporal Pole (R) | −0.146 | 84.4 | |||
| Superior Temporal Pole (R) | −0.204 | 96.0 | |||
| Cerebellum (R) | −0.144 | 92.8 | |||
CV: Cross-validation; R: right, L: left; SFG: Superior frontal gyrus; MFG: Middle frontal gyrus; IFG: Inferior Frontal Gyrus
Acknowledgments
Funding
Irish Research Council: GOIPG/2014/418
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Albein-Urios N, Martinez-Gonzalez JM, Lozano Ó, Moreno-López L, Soriano-Mas C, Verdejo-Garcia A. Negative urgency, disinhibition and reduced temporal pole gray matter characterize the comorbidity of cocaine dependence and personality disorders. Drug and alcohol dependence. 2013;132(1):231–237. doi: 10.1016/j.drugalcdep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiology Biomarkers & Prevention. 2004a;13(12):2023–2034. [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychology of Addictive Behaviors. 2004b;18(1):64. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändström S, Sigvardsson S, Nylander PO, Richter J. The Swedish Version of the Temperament and Character Inventory (TCI) European Journal of Psychological Assessment. 2008;24(1):14–21. [Google Scholar]
- Breiman L. Bagging predictors. Machine learning. 1996;24(2):123–140. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry. 2010;67(8):745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Münte TF. Functional connectivity of reward processing in the brain. Frontiers in Human Neuroscience. 2009;2(19) doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychology. 2000;19(3):223. [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of Cognitive Neuroscience. 2007;19(1):69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2013;66:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38(12):2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. The temperament and character inventory–revised. St Louis, MO: Center for Psychobiology of Personality, Washington University; 1999. [Google Scholar]
- Cohen MX, Bour L, Mantione M, Figee M, Vink M, Tijssen MA, Denys D. Top-down-directed synchrony from medial frontal cortex to nucleus accumbens during reward anticipation. Human Brain Mapping. 2012;33(1):246–252. doi: 10.1002/hbm.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 2008;39(3):1396–1407. doi: 10.1016/j.neuroimage.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(03):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Wang J, Zhang Y, Han W, Yu C, Jiang T. Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cerebral cortex. 2014;24(12):3365–3378. doi: 10.1093/cercor/bht196. [DOI] [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Annals of the New York Academy of Sciences. 2015;1349(1):64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annual Review of Psychology. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, Garland EL, Addicott MA, McClernon FJ. Frontoparietal attentional network activation differs between smokers and nonsmokers during affective cognition. Psychiatry Research: Neuroimaging. 2013;211(1):57–63. doi: 10.1016/j.pscychresns.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. European Journal of Neuroscience. 2006;24(6):1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Weierstall K. The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Preventive medicine. 2012;55:S17–S23. doi: 10.1016/j.ypmed.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Bartenstein P. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hibell B, Andersson B, Bjarnason T, Kokkevi A, Morgan M, Narusk A, Ahlström S. The 1995 ESPAD report. Alcohol and other drug use among students in 26 European countries. Stockholm: Swedish Council for Information on Alcohol and Other Drugs; 1997. [Google Scholar]
- Hoerl AE, Kennard RW. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics. 1970;12(1):55–67. [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Archives of General Psychiatry. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M, Sharpe V, Angell M, Ashford N, Blum A, Chary L, … Wing S. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Jollans L, Watts R, Duffy D, Spechler P, Garavan H, Whelan R The IMAGEN Consortium. A Method for the Optimisation of Feature Selection with Imaging Data; Poster presented at the Organisation of Human Brain Mapping Annual Meeting; Honolulu, HI. 2015. Jun, [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Mayhew KP, Flay BR, Mott JA. Stages in the development of adolescent smoking. Drug and Alcohol Dependence. 2000;59:61–81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40(5):1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex. 2008;18(11):2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KU, Mennigen E, Ripke S, Banaschewski T, Barker GJ, Büchel C, Smolka MN. Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking. JAMA Psychiatry. 2013;70(8):847–856. doi: 10.1001/jamapsychiatry.2013.44. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Reckless GE, Server A, Andreassen OA, Jensen J. Effect of relevance on amygdala activation and association with the ventral striatum. Neuroimage. 2012;62(1):95–101. doi: 10.1016/j.neuroimage.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd J. Why do many psychiatric disorders emerge during adolescence? Nature reviews. Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrs C, Zaki J, Schlochtermeier LH, Jacobs AM, Kuchinke L, Koelsch S. The Temporal Pole Top-Down Modulates the Ventral Visual Stream During Social Cognition. Cerebral Cortex. 2015:bhv226. doi: 10.1093/cercor/bhv226. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Büchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. American Journal of Psychiatry. 2011;168(5):540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Ripke S, Hübner T, Mennigen E, Müller KU, Rodehacke S, Schmidt D, Smolka MN. Reward processing and intertemporal decision making in adults and adolescents: the role of impulsivity and decision consistency. Brain Research. 2012;1478:36–47. doi: 10.1016/j.brainres.2012.08.034. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis MA, Stein EA. Acute nicotine differentially impacts anticipatory valence-and magnitude-related striatal activity. Biological Psychiatry. 2013;73(3):280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF IMAGEN consortium. Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T IMAGEN Consortium. Risk taking and the adolescent reward system: a potential common link to substance abuse. American Journal of Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, Struve M. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society. Series B (Methodological) 1996:267–288. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. [Accessed 7th November 2015];The Health Consequences of Smoking – 50 Years of Progress. A Report of the Surgeon General. 2014 http://www.surgeongeneral.gov/library/reports/50-years-of-progress/50-years-of-progress-by-section.html.
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. The Journal of Neuroscience. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-WISC-IV. Psychological Corporation 2003 [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T IMAGEN Consortium. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biological Psychiatry. 2014;75(9):746–748. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Wills TA, Windle M, Cleary SD. Temperament and novelty seeking in adolescent substance use: convergence of dimensions of temperament with constructs from Cloninger’s theory. Journal of Personality and Social Psychology. 1998;74(2):387. doi: 10.1037//0022-3514.74.2.387. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Stewart SH, Pihl RO, Conrod PJ. The substance use risk profile scale: A scale measuring traits linked to reinforcement-specific substance use profiles. Addictive Behaviors. 2009;34(12):1042–1055. doi: 10.1016/j.addbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS one. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug and Alcohol Dependence. 2002;68(3):309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54(1):42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2005;67(2):301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.