Abstract
Older patients with acute myeloid leukemia (AML) have worse rates of complete remission and shorter overall survival than younger patients. The epigenetic modifier CC-486 is an oral formulation of azacitidine with promising clinical activity in patients with AML in Phase I studies. The Phase III, randomized, double-blind, placebo-controlled QUAZAR AML Maintenance trial (CC-486-AML-001) examines CC-486 maintenance therapy (300 mg/day for 14 days of 28-day treatment cycles) for patients aged ≥55 years with AML in first complete remission. The primary end point is overall survival. Secondary end points include relapse-free survival, safety, health-related quality of life and healthcare resource utilization. This trial will investigate whether CC-486 maintenance can prolong remission and improve survival for older patients with AML.
KEYWORDS : acute myeloid leukemia, CC-486, de novo, elderly, maintenance therapy, oral azacitidine, Phase III, secondary
Acute myeloid leukemia (AML) is characterized by malignant clones of myeloid lineage found in the bone marrow and peripheral blood, and disseminated into tissues [1]. Most patients with AML are older adults, with a median age at diagnosis of 67 years in the USA [2]. While improvements in survival have been observed over the past three decades for all but elderly patients, overall survival (OS) still remains dismal [3–5]. Currently, the standard treatment paradigm for AML consists of induction chemotherapy with the aim of inducing complete remission (CR), followed by variable postremission consolidation strategies [6–9]. Older patients with AML exhibit a markedly different disease course than their younger counterparts. Older age is associated with adverse cytogenetics, more aggressive and resistant disease, and disease more commonly evolved from an antecedent hematologic disorder [10]. Compared with younger patients, older patients undergoing intensive chemotherapy have lower CR rates, shorter relapse-free survival (RFS) and OS, and higher rates of treatment-related mortality [10,11]. There is a desperate need for new treatment options to improve outcomes in older patients with AML [11,12].
Maintenance therapy is not currently part of standard treatment for patients with most forms of AML [6,13–14]. However, it has become part of the standard treatment in acute lymphoblastic leukemia (ALL) [13,15–17]. Maintenance chemotherapy based on a backbone of daily mercaptopurine and weekly methotrexate is an established part of treatment for most subtypes of ALL. In addition, targeted agents have successfully been incorporated into maintenance regimens for specific ALL subtypes, including tyrosine kinase inhibitors such as imatinib for Philadelphia chromosome-positive ALL and anti-CD20 monoclonal antibodies such as rituximab for CD20-expressing B-cell ALL [13,15–17].
For patients with AML, maintenance chemotherapy has failed to demonstrate improved survival outcomes [18–23]. However, in acute promyelocytic leukemia (APL), similar strategies have been used to reduce toxicity and relapse rates, suggesting that some leukemia lineages would also be amenable to maintenance therapy. The introduction of novel treatment options with antileukemic activity, including demethylating agents, immunotherapies and targeted agents, makes it possible to re-explore maintenance therapy in AML [13,14]. Single-agent maintenance chemotherapy with IFN or IL-2 has been examined, but so far neither has led to slowing of disease progression or improved OS [24–26]. Combination therapy with IL-2 and the immune modulator histamine dihydrochloride has demonstrated significantly longer leukemia-free survival compared with observation or IL-2 monotherapy in patients with AML [27,28]. This has led to the approval of this combination as maintenance therapy for patients with AML in first remission in some European countries; however, efficacy has not been demonstrated in older patients aged >60 years [29], and this treatment does necessitate repeated injections. The use of demethylating agents in maintenance therapy is currently under active investigation and is explored in the trial described here.
In studies of older patients with AML, treatment with subcutaneous azacitidine (75 mg/m2/day for 7 days of 28-day cycles) resulted in clinically meaningful improvements in OS compared with conventional care regimens [30,31]. In patients with relapse post-stem cell transplant, low-dose azacitidine (16–40 mg/m2 for 5 days of 28-day cycles) has been shown to induce durable remissions [32]. Also, additional cycles of low-dose azacitidine maintenance therapy (8–40 mg/m2 given for 5 days of 30-day cycles) post-stem cell transplant prior to relapse has been associated with prolonged OS [33].
Recently, the feasibility of maintenance therapy with hypomethylating agents following achievement of CR with induction chemotherapy has been investigated [34,35]. Patients with myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) or AML secondary to MDS received maintenance therapy with subcutaneous azacitidine (60–75 mg/m2 for 5 days of 28-day cycles) within 28 days of achieving CR [34]. The median duration of CR was 13.5 months in patients who received maintenance therapy. Median OS was 20 months in patients who received maintenance therapy versus 8.2 months for the entire population. Azacitidine maintenance therapy was well tolerated at a dose of 60 mg/m2, although higher starting doses were associated with higher rates of grade 3/4 neutropenia. In another study, patients with AML were randomized to receive maintenance therapy with decitabine or conventional care regimens including low-dose cytarabine, prolonged intensive chemotherapy or observation [35]. Although the study was terminated early due to a higher incidence of relapse at 1 year with decitabine treatment, with longer follow-up (44.9 months) fewer patients in the decitabine arm relapsed versus the conventional care arm (50 vs 60%), although this difference was not significant. Decitabine maintenance therapy was well tolerated, with neutropenia and thrombocytopenia as the most common adverse events (AEs).
The epigenetic modifier CC-486 (Celgene Corporation, NJ, USA) is a bioavailable oral formulation of azacitidine [36]. Compared with the injectable formulation, oral administration eliminates injection-site reactions and may maximize convenience. The biological and clinical activity of CC-486 in patients with MDS, CMML or AML was demonstrated in a recent Phase I trial [37–39]. In part 1 of this trial, patients received one cycle of subcutaneous azacitidine at 75 mg/m2 daily for the first 7 of 28 days, followed by CC-486 at 120–600 mg daily for 7 days of repeating 28-day treatment cycles [37]. Part 2 of this trial investigated extended dosing regimens of CC-486 of 300 mg daily or 200 mg twice a day for 14 or 21 days of 28-day cycles [38]. In part 1, three of eight patients with AML (38%) achieved an overall response that included one hematologic improvement, one red blood cell transfusion independence (RBC-TI), two morphologic CRs and one morphologic partial response [38]. In part 2, seven of 15 patients with AML (47%) achieved an overall response that included four hematologic improvements, four RBC-TIs, one platelet transfusion independence and three morphologic partial responses [38]. No patients achieved complete or partial response. These data demonstrated preliminary clinical activity of CC-486 in patients with AML, even though this population included patients with complex cytogenetics and those in whom prior therapy had failed. Extended dosing schedules of CC-486 also prolonged methylation reversal through treatment cycle end [40]. Combined results for part 1 and part 2 of the Phase I trial showed that CC-486 was reasonably tolerated in patients with AML and that AEs of any severity were most commonly gastrointestinal in nature [38]. The most common grade 3/4 AEs in patients with AML with CC-486 treatment were febrile neutropenia (35%), pneumonia (17%), syncope (17%) and nausea (13%) [38]. While CC-486 and injectable azacitidine has shown promising activity in patients with AML in these early-phase studies and the maintenance studies described above, the present trial was designed to confirm these observations in a larger population and to determine the degree to which CC-486 as maintenance therapy can prolong remissions and improve survival in older patients.
The QUAZAR AML Maintenance trial
The Phase III, randomized, double-blind, placebo-controlled QUAZAR AML Maintenance trial (CC-486-AML-001) was initiated to assess the safety and efficacy of maintenance therapy with CC-486 for older patients (aged ≥55 years) with AML who are in first CR or CR with incomplete blood count recovery (CRi) following induction therapy with or without consolidation chemotherapy. The study is registered on ClinicalTrials.gov (NCT01757535) and is sponsored by Celgene Corporation. Positive results from this trial are expected to facilitate global regulatory approvals and expand treatment options for older patients with AML.
Design
• Objectives
The primary objective of this study is to demonstrate whether maintenance therapy with CC-486 improves OS compared with placebo in patients with AML, aged ≥55 years, who are in first CR/CRi after induction with intensive chemotherapy with or without consolidation chemotherapy. Secondary objectives include RFS, safety and tolerability, health-related quality of life (HRQOL) and healthcare resource utilization.
• Eligibility criteria
Eligible patients must be aged ≥55 years and have newly diagnosed, histologically confirmed de novo AML or AML secondary to prior MDS by WHO 2008 classification [41]. Patients with therapy-related AML are excluded. Patients must have had induction therapy with intensive chemotherapy with or without consolidation therapy and have achieved first CR/CRi within 90 days prior to randomization. Patients must also have an Eastern Cooperative Oncology Group performance status of 0–3. They must have adequate bone marrow function as well as adequate organ function. Patients may not have suspected or proven APL based on morphology, immunophenotype, molecular assay or karyotype, or have secondary AML unless preceded by MDS or CMML. Patients with AML associated with inv(16), t(8;21), t(16;16), t(15;17) or t(9;22) karyotypes or molecular evidence of these translocations are not eligible. Patients should not have received prior bone marrow or stem cell transplant and should not be a candidate for a planned allogeneic transplant at screening. Patients who achieved CR/CRi following therapy with hypomethylating agents or prior therapy with hypomethylating agents for MDS within 4 months of developing AML are also excluded.
• Study design & treatment
The QUAZAR AML Maintenance trial is an international, multicenter, randomized, double-blind, placebo-controlled, Phase III study of maintenance therapy with CC-486 with or without best supportive care versus placebo with or without best supportive care for patients with AML who are in first CR/CRi. This is a global study, with study sites in 21 countries in North America (USA, Canada and Mexico), South America (Brazil), Europe (Austria, Belgium, Czech Republic, Finland, Germany, Ireland, Israel, Italy, Poland, Portugal, Spain and UK), Asia (Russia and South Korea) and Australia.
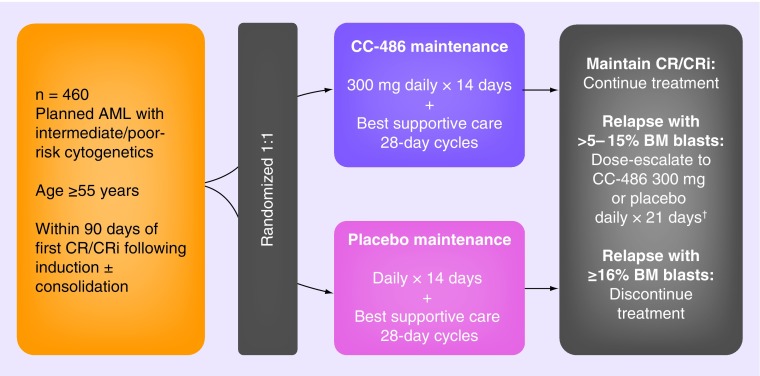
Patients will be randomized 1:1 to receive CC-486 300 mg or placebo once daily for the first 14 days of each 28-day treatment cycle (Figure 1). Patients will be stratified on the basis of age (55–64 years or ≥65 years), prior history of MDS, cytogenetic risk category and receipt of consolidation therapy following induction, and must be enrolled within 90 days of first CR/CRi. Patients will be evaluated for CR/CRi status at cycle 3. Those who maintain a CR/CRi continue on study treatment and will be assessed for disease status at the end of every third cycle. Patients who experience disease relapse with >5–15% blasts in the bone marrow or peripheral blood may escalate the dose schedule at the investigators’ discretion to CC-486 300 mg once daily for 21 days of each 28-day cycle, a total dose increment of 50%. Patients with disease progression with ≥16% blasts in the bone marrow or peripheral blood will discontinue study treatment. Patients may also discontinue study treatment because of AEs or if they become eligible for an allogeneic stem cell transplant.
Figure 1. . Study schema of the CC-486-AML-001 trial.
†May also discontinue treatment based on investigator's decision.
AML: Acute myeloid leukemia; BM: Bone marrow; CR: Complete remission; CRi: Complete remission with incomplete blood count recovery.
This study is being conducted in accordance with Good Clinical Practice E6 Guidelines set forth by the International Conference on Harmonization and as outlined in the Declaration of Helsinki. The CC-486-AML-001 study protocol must receive approval from the institutional review board/ethics committee for each institution prior to commencement.
• Planned study period
The study was initiated in April 2013, and the estimated completion date is August 2018.
• Study procedures
Efficacy assessments
The primary efficacy end point of OS is defined as the time from randomization to death from any cause. All patients who discontinue, regardless of reason for discontinuation, will be followed for survival until death, loss to follow-up, withdrawal of consent from further follow-up or study closure. Patients will be assessed for CR/CRi status maintenance or disease relapse every third cycle of treatment. Similar to the International Working Group 2003 criteria [42], CR is defined as <5% bone marrow blasts, absence of blasts with Auer rods, absence of extramedullary disease, blood transfusion independence unless attributed to recent chemotherapy, neutrophil counts >1.0 × 109/l and platelet counts ≥100 × 109/l. CRi is defined as <5% bone marrow blasts, absence of blasts with Auer rods, absence of extramedullary disease, blood transfusion independence, neutrophil counts <1.0 × 109/l or platelet counts <100 × 109/l.
A bone marrow aspirate and biopsy will be required at baseline. Bone marrow aspirate samples (or biopsy if adequate aspirate is not attainable) will be collected on day 1 of every third cycle and additionally as clinically indicated. Cytogenetic testing will be performed whenever a bone marrow aspirate of biopsy is obtained for efficacy assessment.
Safety assessments
Safety analyses will be performed in all randomized patients who received at least 1 dose of any study treatment (CC-486 or placebo). AEs, serious AEs and second primary malignancies will be evaluated. It is of interest to evaluate whether CC-486 may lead to reduction in the occurrence of second primary malignancies. The severity or intensity of AEs will be graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Health-related quality of life assessments
HRQOL with CC-486 versus placebo will be evaluated using the symptom-specific Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale and the EQ-5D, as well as with exploratory HRQOL questions. The FACIT-F is a subscale of the general questionnaire, the FACIT-G, that assesses fatigue associated with anemia through a 13-item questionnaire [43–45], and the EQ-5D is a generalized measure of health status that is not disease-specific [46].
Healthcare resource utilization
Medical resource utilization and cost–effectiveness/cost utility analyses will be conducted to determine the effect of CC-486 compared with placebo on healthcare resource utilization. Information collected on each hospitalization includes the reason for hospitalization (e.g., disease relapse, AML-related illness, treatment-related AE) and days of hospitalization by treatment setting (e.g., inpatient, special care unit). Information will also be collected on all medications and resources used related to treatment for subsequent AML.
Correlative assessments
Exploratory correlative analyses will be performed to assess the relationship between CC-486 concentration data and pharmacodynamics and biomarkers. Exploratory assessments of the bone marrow and peripheral blood include evaluation of cytogenetic abnormalities, gene variants (gene sequencing, single nucleotide polymorphism array), immunophenotyping by flow cytometry, measurements of DNA methylation, mRNA expression, miRNA expression and other cellular protein measurements relevant to disease biology or drug mechanism. Minimal residual disease (MRD) will be evaluated at baseline and during maintenance treatment.
• Statistical analyses
Analyses methods
The primary efficacy analyses will compare OS distributions with CC-486 versus placebo and will be conducted in the intent-to-treat population. The null hypothesis is that the OS distributions for the two treatment groups are equivalent. OS curves and median OS will be estimated using Kaplan–Meier methods. A stratified log-rank test, stratifying by age at time of induction therapy, prior history of MDS, whether consolidation therapy was administered and cytogenetic risk category at time of induction therapy, at a two-sided alpha level of 0.05, will be used to compare the OS distributions. The p-value from the stratified log-rank test will be the confirmatory p-value. A stratified Cox proportional hazards model will be used to estimate the corresponding hazard ratio and 95% CI for CC-486 relative to placebo.
The key secondary efficacy analysis of RFS is defined as the interval from the date of randomization to the date of documented relapse after CR, CRi or death from any cause, whichever occurs first. The analysis of RFS will be performed using the intent-to-treat population and will be analyzed using the same methods as those used for the primary efficacy analysis of OS.
Sample size
An estimated 460 patients will be enrolled in this study. Power and sample size were determined based on an assumption of a median OS of 16 months in the placebo-treated group [47,48], a median OS of 22.9 months in the CC-486-treated group (43% improvement) and a study duration of 60 months with a dropout rate of 5% from both treatment groups over the duration of the study. This design requires 330 deaths and approximately 460 randomized patients (230 per treatment arm) to achieve at least 90% power to detect a constant hazard ratio of 0.70 and demonstrate a statistically significant difference in OS. Sample size calculations are based on a one-sided alpha of 0.025 with one interim analysis for futility after 30% of the events have occurred.
Conclusion
AML occurs most frequently in the elderly. These patients have poor prognoses, including those eligible to receive intensive chemotherapy [10–11,49–50], which is the population being examined in this study. Long-term maintenance therapy with CC-486 is anticipated to improve survival outcomes for older patients with AML in first CR/CRi following induction chemotherapy and to prolong duration of remissions. This study is powered to detect a significant improvement in OS with CC-486 maintenance therapy versus placebo as the primary end point. Placebo is used as the comparator because there is no globally recognized maintenance standard-of-care therapy for patients with AML.
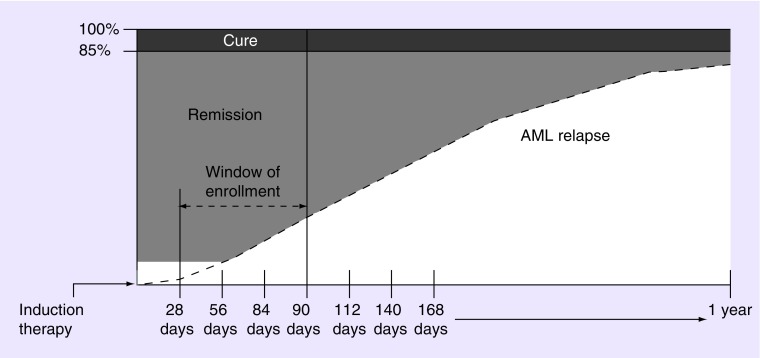
To avoid enriching the study population with patients with better prognoses, participants must be randomized within 90 days of achieving first CR/CRi. Longer durations of remissions have been associated with better outcomes in patients with AML (Figure 2) [51]. Additionally, patients with AML who are in remission after treatment may relapse rapidly, and older patients often exhibit shorter remission durations than their younger counterparts [10–11,49]. Therefore, timely initiation of maintenance therapy may provide the greatest benefit for extending remissions in this patient population.
Figure 2. . A 90-day randomization window.
Only a small proportion of patients with AML are cured (10–15%, dark gray) and relapse (indicated by the dashed line) begins within weeks of achieving complete remission (CR). To avoid enriching for patients with better prognoses who remain in remission longer (shaded portion of the figure), study participants must be enrolled within 90 days of achieving first CR/CR with incomplete blood count recovery. A later enrollment cutoff, for example, beyond 140 days, would include a greater proportion of patients who are likely to be cured long term. This would falsely elevate the number of patients who appear to benefit from the study. The 90-day randomization window provides a more realistic picture of the entire AML population.
AML: Acute myeloid leukemia.
Patients will be assessed every third cycle for CR/CRi status to determine maintenance of remission. Because AML occurring in the elderly frequently has clinical and laboratory features which suggest that it may have transformed from underlying undiagnosed MDS, in this study when AML relapses, the progression may be slow, akin to the progression seen in MDS transforming through refractory anemia with excess blasts-1/2 toward AML. For patients who meet criteria for relapse but progress slowly, with >5–15% blasts in peripheral blood or bone marrow, treatment with CC-486 may still provide clinical benefit. Therefore, this study allows these patients to escalate dosing by 50% from a 14 to 21 out of 28-day dosing schedule at the investigators’ discretion. Patients who progress rapidly with ≥16% blasts in bone marrow or peripheral blood, will be discontinued from treatment.
HRQOL is impaired in patients with AML at diagnosis [52], and HRQOL of older patients is negatively affected by intensive chemotherapy [53,54]. Following treatment, HRQOL gradually improves over time and is especially associated with achievement of CR [53] as well as discharge from the hospital [54]. Prolonging CR with maintenance therapy may have positive effects on HRQOL. Therefore, the impact of long-term maintenance therapy with CC-486 on HRQOL is assessed as a secondary end point in this study. Because CC-486 is an oral formulation of azacitidine, frequent clinic visits for drug administration are not required during the treatment cycle, which may also contribute to improved HRQOL.
The management of patients with AML is associated with substantial resource utilization largely due to the high costs of inpatient hospitalizations [55–58]. In patients with AML with 20–30% blasts, azacitidine treatment demonstrated significantly lower rates of fever requiring intravenous antimicrobials, hospitalization admissions and days in hospital compared with conventional care regimens [30]. The impact of CC-486 treatment on medical resource utilization in the maintenance setting will be examined as a secondary end point.
Exploratory molecular and cellular assessments will also be performed. Gene sequencing will be used to identify recurrent mutations in genes that have prognostic impact in AML [7,59–60]. Somatic mutations occur in almost all patients with AML and may refine risk stratification for a large number of them [61]. This study offers the unique opportunity to evaluate the mutational heterogeneity and clonal evolution that occurs over time in patients with AML. The placebo arm allows for analysis of the natural history of the disease, whereas the CC-486 arm allows for investigation of the impact of treatment on the molecular course of the disease. These findings may improve understanding of the relevance of using genetic aberrations in predicting disease relapse, maintenance of stable disease and remission status, and to discern the instances in which CC-486 treatment is most efficacious.
Following CR, small numbers of residual leukemia cells (≥0.5% leukemic blasts) can often be detected by multidimensional flow cytometry employing a standardized panel of monoclonal antibodies [62]. Their presence often portends clinical relapse of the AML. In this study, remission durations of MRD-positive and MRD-negative patients can be compared within both the placebo and CC-486 treatment arms to help understand the effects of the presence of MRD on clinical relapse and long-term outcome. Comparison between arms can reveal effects of treatment on patients with MRD positivity, and this study uniquely should be able to compare outcomes when detecting MRD by molecular methods versus flow cytometry, as the sensitivity of these methods is likely to vary. Furthermore, the presence of certain molecular mutations may not necessarily dictate that disease progression is likely. It will be important to determine the clinical implications resultant from these specific abnormalities.
To the best of our knowledge, the QUAZAR AML Maintenance trial represents the first double-blind, placebo-controlled, Phase III study of maintenance therapy in patients with newly diagnosed AML (non-APL). Unlike many other ongoing Phase III trials in this treatment setting [63], this trial is focused on maintenance only and does not follow any one specific induction and/or consolidation regimen as part of the trial. Patients who received any intensive chemotherapy with or without consolidation therapy, excluding hypomethylating agents, are eligible for inclusion. As such, the results of this study will be broadly applicable for this population of patients with limited treatment options.
CC-486 is also being investigated in a Phase III trial in lower-risk MDS. The QUAZAR Lower-Risk MDS trial (AZA-MDS-003) is investigating CC-486 for the treatment of patients with International Prognostic Scoring System low/intermediate-1 risk MDS with red blood cell transfusion-dependent anemia and thrombocytopenia.
The QUAZAR AML Maintenance trial is a registration trial and is currently ongoing and actively recruiting eligible patients. CC-486-AML-001 enrolled and randomized the first patient on 10 May 2013. The target enrollment is 460 patients. Results from this trial may expand therapy options for patients with AML by supporting global regulatory approvals. For additional information, please contact QUAZARTrialsInfo@celgene.com or visit the NCT website [64].
EXECUTIVE SUMMARY.
CC-486
Extended dosing regimens of the epigenetic modifier CC-486, an oral formulation of azacitidine, has demonstrated clinical and biological activity in patients with acute myeloid leukemia (AML) in Phase I studies.
The QUAZAR AML Maintenance trial
This Phase III, randomized, placebo-controlled, international QUAZAR AML Maintenance trial (CC-486-AML-001) was created to evaluate the efficacy and safety of maintenance therapy with CC-486 in patients aged ≥55 years with AML who are in first complete remission (CR) or CR with incomplete blood count recovery (CRi) following induction therapy with or without consolidation chemotherapy.
Approximately 460 patients will be randomized 1:1 to either placebo or CC-486 300 mg daily for the first 14 days of 28-day cycles.
Patients must be enrolled within 90 days of the first CR/CRi and will be stratified based on age (55–64 years or ≥65 years), cytogenetic risk category, prior history of myelodysplastic syndromes and receipt of consolidation therapy following induction.
Objectives
The primary objective of this study is to demonstrate whether maintenance therapy with CC-486 improves overall survival compared with placebo in older patients with AML who are in first CR/CRi after induction therapy.
Secondary objectives include relapse-free survival, safety and tolerability, health-related quality of life, and healthcare resource utilization.
Exploratory analyses will assess biomarkers of prognostic impact such as cytogenetic abnormalities, gene variants and immunophenotyping, as well as biomarkers of response including measures of DNA methylation, RNA expression and protein measurements related to drug mechanism.
Footnotes
Trial registration
Financial & competing interests disclosure
The CC-486-AML-001 trial is sponsored by Celgene Corporation. GJ Roboz’ institution received a grant from Celgene Corporation. PM Fernandes’ institution received a grant from Celgene Corporation. A Wei received a grant from Celgene Corporation. MT Voso received support for travel to the meeting for the study or other purposes from Celgene Corporation. F Ravandi-Kashani's institution received a grant from Celgene Corporation. B Skikne is employed by Celgene Corporation in which he has an equity interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by J Leslie of MediTech Media and was funded by Celgene Corporation.
Open access
This work is licensed under the Creative Commons Attribution-NonCommercial 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Liesveld JL, Lichtman MA. Acute myelogenous leukemia. In: Lichtman MA, editor. Williams Hematology. McGraw-Hill; NY, USA: 2005. pp. 1183–1236. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. 2014. http://seer.cancer.gov/csr/1975_2011/
- 3.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119(15):2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derolf AR, Kristinsson SY, Andersson TM, Landgren O, Dickman PW, Bjorkholm M. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113(16):3666–3672. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, Andersson TM, Rachet B, Bjorkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971–2006: a population-based study. Br. J. Haematol. 2013;162(4):509–516. doi: 10.1111/bjh.12425. [DOI] [PubMed] [Google Scholar]
- 6.Milligan DW, Grimwade D, Cullis JO, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br. J. Haematol. 2006;135(4):450–474. doi: 10.1111/j.1365-2141.2006.06314.x. [DOI] [PubMed] [Google Scholar]
- 7.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia. 2015. www.nccn.org/professionals/physician_gls/f_guidelines.asp Version 1.
- 9.Roboz GJ. Current treatment of acute myeloid leukemia. Curr. Opin. Oncol. 2012;24(6):711–719. doi: 10.1097/CCO.0b013e328358f62d. [DOI] [PubMed] [Google Scholar]
- 10.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br. J. Haematol. 2011;152(5):524–542. doi: 10.1111/j.1365-2141.2010.08470.x. [DOI] [PubMed] [Google Scholar]
- 11.Luger SM. Treating the elderly patient with acute myelogenous leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2010;2010:62–69. doi: 10.1182/asheducation-2010.1.62. [DOI] [PubMed] [Google Scholar]
- 12.Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2011;2011:43–50. doi: 10.1182/asheducation-2011.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Baer MR. Is there a role for maintenance therapy in acute myeloid leukaemia? Best Pract. Res. Clin. Haematol. 2009;22(4):517–521. doi: 10.1016/j.beha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Krug U, Lubbert M, Buchner T. Maintenance therapy in acute myeloid leukemia revisited: will new agents rekindle an old interest? Curr. Opin. Hematol. 2010;17(2):85–90. doi: 10.1097/MOH.0b013e3283366bf4. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia. 2015. www.nccn.org/professionals/physician_gls/f_guidelines.asp Version 1.
- 16.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J. Clin. Oncol. 2011;29(5):532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 17.Gokbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2006;2006(1):133–141. doi: 10.1182/asheducation-2006.1.133. [DOI] [PubMed] [Google Scholar]
- 18.Buchner T, Urbanitz D, Hiddemann W, et al. Intensified induction and consolidation with or without maintenance chemotherapy for acute myeloid leukemia (AML): two multicenter studies of the German AML Cooperative Group. J. Clin. Oncol. 1985;3(12):1583–1589. doi: 10.1200/JCO.1985.3.12.1583. [DOI] [PubMed] [Google Scholar]
- 19.Löwenberg B, Suciu S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy – the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J. Clin. Oncol. 1998;16(3):872–881. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 20.Buchner T, Hiddemann W, Berdel WE, et al. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): a randomized trial of the German AML Cooperative Group. J. Clin. Oncol. 2003;21(24):4496–4504. doi: 10.1200/JCO.2003.02.133. [DOI] [PubMed] [Google Scholar]
- 21.Cassileth PA, Harrington DP, Hines JD, et al. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J. Clin. Oncol. 1988;6(4):583–587. doi: 10.1200/JCO.1988.6.4.583. [DOI] [PubMed] [Google Scholar]
- 22.Sauter C, Berchtold W, Fopp M, et al. Acute myelogenous leukaemia: maintenance chemotherapy after early consolidation treatment does not prolong survival. Lancet. 1984;1(8373):379–382. doi: 10.1016/s0140-6736(84)90424-0. [DOI] [PubMed] [Google Scholar]
- 23.Preisler H, Davis RB, Kirshner J, et al. Comparison of three remission induction regimens and two postinduction strategies for the treatment of acute nonlymphocytic leukemia: a Cancer and Leukemia Group B study. Blood. 1987;69(5):1441–1449. [PubMed] [Google Scholar]
- 24.Goldstone AH, Burnett AK, Wheatley K, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 25.Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J. Clin. Oncol. 2010;28(5):808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 26.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J. Clin. Oncol. 2008;26(30):4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized Phase 3 trial. Blood. 2006;108(1):88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 28.Berry SM, Broglio KR, Berry DA. Addressing the incremental benefit of histamine dihydrochloride when added to interleukin-2 in treating acute myeloid leukemia: a Bayesian meta-analysis. Cancer Invest. 2011;29(4):293–299. doi: 10.3109/07357907.2011.568563. [DOI] [PubMed] [Google Scholar]
- 29.Ceplene® (histamine dihydrochloride), prescribing information. Immune Pharmaceuticals, New York, NY, USA. 2013.
- 30.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]; • Subanalysis of the AZA-001 Phase III trial in patients showing azacitidine treatment significantly improves survival of patients with acute myeloid leukemia (AML) with 20–30% blasts compared with conventional care regimens.
- 31.Dombret H, Seymour JF, Butrym A, et al. International Phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]; • AZA-AML-001 Phase III trial demonstrating clinically meaningful improvements in survival of patients with AML with >30% blasts with azacitidine compared with conventional care regimens.
- 32.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115(9):1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116(23):5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grovdal M, Karimi M, Khan R, et al. Maintenance treatment with azacytidine for patients with high-risk myelodysplastic syndromes (MDS) or acute myeloid leukaemia following MDS in complete remission after induction chemotherapy. Br. J. Haematol. 2010;150(3):293–302. doi: 10.1111/j.1365-2141.2010.08235.x. [DOI] [PubMed] [Google Scholar]
- 35.Boumber Y, Kantarjian H, Jorgensen J, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012;26(11):2428–2431. doi: 10.1038/leu.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22(9):1680–1684. doi: 10.1038/leu.2008.145. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J. Clin. Oncol. 2011;29(18):2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phase I study demonstrating the tolerability and biological and clinical activity of extended dosing regimens of CC-486 in patients with myelodysplastic syndromes (MDS) and AML.
- 38.Gore SD, Cogle CR, Skikne B, et al. Oral azacitidine (AZA) activity in patients with acute myelogenous leukemia (AML) Blood. 2011;118:1546. [Google Scholar]; •• Subanalysis of a Phase I study of CC-486 in patients with AML showing activity including in patients with high-risk features such as complex cytogenetics and failed prior therapy.
- 39.Garcia-Manero G, Gore SD, Kambhampati S, et al. Safety and efficacy of oral azacitidine (CC-486) administered in extended treatment schedules to patients with lower-risk myelodysplastic syndromes. Blood. 2012;120(21):424. [Google Scholar]; • Subanalysis of a Phase I study of CC-486 in patients with International Prognostic Scoring System low- or intermediate-1 risk MDS. Demonstrated activity of CC-486 in extended dosing regimens of 14 or 21 days of 28-day treatment cycles.
- 40.Laille E, Shi T, Garcia-Manero G, et al. Extended dosing of oral azacitidine (CC-486) for 14 and 21 days provides more effective methylation reversal than a 7-day schedule. Blood. 2012;120(21):1337. [Google Scholar]; •• Pharmacodynamics of CC-486 in 7-day and extended 14- and 21-day dosing regimens in a Phase I study in patients with MDS and AML. Showed significant reductions in DNA methylation from baseline with extended dosing regimens of CC-486.
- 41.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 42.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 43.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur. J. Cancer. 2006;42(7):846–863. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 45.Functional Assessment of Chronic Illness Therapy. www.facit.org/FACITOrg
- 46.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann. Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 47.Baer MR, George SL, Sanford BL, et al. Treatment of older patients with de novo acute myeloid leukemia (AML) with one or more postremission chemotherapy courses: analysis of four CALGB studies. J. Clin. Oncol. 2010;28(Suppl.):6531. [Google Scholar]
- 48.Baer MR, George SL, Sanford BL, et al. Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B Study 9720. Leukemia. 2011;25(5):800–807. doi: 10.1038/leu.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J. Clin. Oncol. 2009;27(1):61–69. doi: 10.1200/JCO.2007.15.4245. [DOI] [PubMed] [Google Scholar]
- 50.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 2005;23(9):1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Oliva EN, Nobile F, Alimena G, et al. Quality of life in elderly patients with acute myeloid leukemia: patients may be more accurate than physicians. Haematologica. 2011;96(5):696–702. doi: 10.3324/haematol.2010.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alibhai SM, Leach M, Gupta V, et al. Quality of life beyond 6 months after diagnosis in older adults with acute myeloid leukemia. Crit. Rev. Oncol. Hematol. 2009;69(2):168–174. doi: 10.1016/j.critrevonc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809–816. doi: 10.1038/sj.leu.2403289. [DOI] [PubMed] [Google Scholar]
- 55.Nerich V, Lioure B, Rave M, et al. Induction-related cost of patients with acute myeloid leukaemia in France. Int. J. Clin. Pharm. 2011;33(2):191–199. doi: 10.1007/s11096-010-9462-1. [DOI] [PubMed] [Google Scholar]
- 56.Redaelli A, Botteman MF, Stephens JM, Brandt S, Pashos CL. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat. Rev. 2004;30(3):237–247. doi: 10.1016/j.ctrv.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl. Health. Econ. Health. Policy. 2013;11(3):275–286. doi: 10.1007/s40258-013-0032-2. [DOI] [PubMed] [Google Scholar]
- 58.Leunis A, Blommestein HM, Huijgens PC, Blijlevens NM, Jongen-Lavrencic M, Uyl-de Groot CA. The costs of initial treatment for patients with acute myeloid leukemia in The Netherlands. Leuk. Res. 2013;37(3):245–250. doi: 10.1016/j.leukres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J. Clin. Oncol. 2011;29(5):475–486. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro AF, Pratcorona M, Erpelinck-Verschueren C, et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood. 2012;119(24):5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- 61.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012;366(12):1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sievers EL, Lange BJ, Alonzo TA, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. 2003;101(9):3398–3406. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- 63.Clinical trials database. https://clinicaltrials.gov/
- 64.http://clinicaltrials.gov/show/NCT01757535 Efficacy of Oral Azacitidine Plus Best Supportive Care as Maintenance Therapy in Subjects With Acute Myeloid Leukemia in Complete Remission (QUAZAR AML-001)