Abstract
Objective:
The dramatic increase in the population with dementia expected in the next decades is accompanied by the establishment of novel and innovated methods that will offer accurate and efficient detection of the disease in its early stages. While Alzheimer’s disease is the most common cause of dementia, by the time it is typically diagnosed, substantial neuronal loss and neuropathological lesions can damage many brain regions. The aim of this study is to investigate the main risk factors that affect and increase Alzheimer’s disease progression over time even in cases with no significant memory impairment present. Several potential markers are discussed such as oxidative stress, metal ions, vascular disorders, protein dysfunctions and alterations in the mitochondrial populations.
Conclusion:
A multiparametric model of Alzheimer’s biomarkers is presented according to the latest classification of the disease.
Keywords: Alzheimer’s disease biomarkers, oxidative stress, metal ions, vascular disorders, protein dysfunctions, mitochondrial dynamics, mild cognitive impairment
1. INTRODUCTION
Alzheimer’s disease (AD) is referred as one of the most common causes of dementia and frailty [1]. Typically, the symptoms of the disease begin with mild memory difficulties and evolve towards cognitive impairement, dysfunctions in complex daily activities, and several other aspects of cognition [1]. By the time that AD is clinically diagnosed, neuronal loss and neuropathologic lesions occur in many brain regions [2]. Crucial role for the suspension of the potential damages is the timely drug delivery of neuroprotective medications before AD turns into mildly symptomatic [2].
To approach this goal, our capability to identify individuals with very mild symptoms prior to dementia needs to be improved [3]. A few diagnostic criteria concerning imaging techniques and cerebrospinal fluid biomarkers have been already published in order to establish a multivariate classification for AD [4].
With the pessimistic projection of AD population and its corresponding social cost in the years between 2030 and 2050, the scientific and clinical research in the area of AD is nowadays directed to the early diagnosis of the transitional phase between normal aging, mild cognitive impairment (MCI) and dementia [4]. Lately, the concept of MCI has been expanded to address observed clinical heterogeneity. Two subtypes are recognized, amnesic and nonamnesic, with the later including deficits in executive functioning such as attention, planning, problem-solving, multitasking, monitoring and behavioral control, impaired mental flexibility, increased distractibility and difficulty in learning novel tasks. While amnesic syndromes are the most common symptoms of AD early onset, researchers are particularly focused on the analysis of the medial temporal lobe memory system [5]. When patients are diagnosed with AD dementia, memory impairments appear to be significantly correlated with medial temporal lobe atrophy and hypoactivation [5]. Mitochondrial electrophysiology or electrodermal activity skin conductance analysis may be particularly useful for detecting alterations in brain function that may be present very early in the progression of AD, possibly a long time before the development of clinical symptoms and even significant neuropathology [6-7].
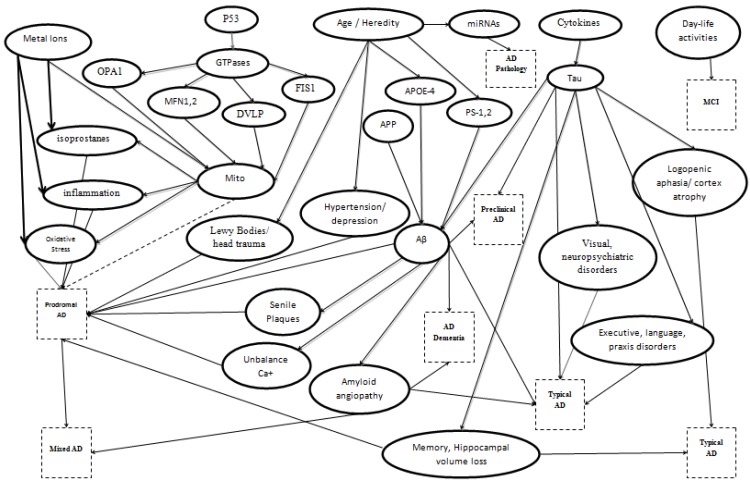
According to the latest National Institute on Aging and Alzheimer’s Association workgroup, an accurate diagnosis can be based on the general clinical and pathophysiological conditions and the assessment of several in vivo biomarkers and memory tests (Fig. 1). Albert et al. proposed a classification of 8 categories for AD: Prodromal AD, AD dementia, Typical AD, Atypical AD, Mixed AD, Preclinical States of AD, Alzheimer’s Pathology and MCI [8]. The term Prodromal AD or Predementia Stage of AD is used for early symptomatic, predementia stage of AD where clinical symptoms such as episodic memory loss of the hippocampal type are visible, but do not affect the daily life activities and do not support dementia diagnosis. Also, in this stage the biomarkers existence from Cerebrospinal fluid (CSF) or imaging can proof AD pathology. In the case of AD dementia, several serious cognitive symptoms are present among with social functioning and instrumental activities of daily living consequences. The last state would be considered as a threshold between the episodic memory modifications and in another at least cognitive domain. Also, meaningful dementia threshold would be clinical trials or social/economic evaluations. The third category is called Typical AD and includes the most common clinical phenotype of AD. This phenotype is characterized by early and progressive episodic memory deficit that dominates in the following stages of the disease and coexists with other cognitive disorders (executive dysfunction, language, praxis, and complex visual processing impairments). An incident integrates into this category if there is one or more in-vivo positive biomarker of AD pathology. The case of Atypical AD characterizes certain clinical phenotype of Alzheimer’s pathology. Such incidents include primary progressive non-fluent aphasia, logopenic aphasia, frontal variant of AD, and posterior cortical atrophy. Also, strong in vivo evidence of amyloidosis in the brain or in the CSF and one of the above clinical stages, the diagnosis of AD is certain. The fifth category is called Mixed AD and refers to patients who fulfill the diagnostic criteria for Typical AD and present clinical and brain imaging/biological evidence with other diseases which have a similar pattern with AD, such as cerebrovascular or Lewy Bodies diseases. The Preclinical States of AD are divided into two subcategories. This case consists of an asymptomatic period between the early pathogenic events such as brain lesions of AD and the very first appearance of specific cognitive modifications. The first subcategory is characterized as Asymptomatic at-risk state for AD, where brain amyloidosis or amyloidosis in the CSF is the primary evidence. The second subcategory is called Presymptomatic AD, where patients are going to be evolved into AD. It referred that this state mainly appears in families that are carriers of a rare autosomal dominant monogenic AD mutation. Alzheimer’s Pathology covers the very first pathogenic events in the brain such as synaptic loss, and vascular amyloid deposits. This term is used regardless of the clinical view. Finally, the last category contains incidents with measurable MCI. This state describes the case where there is no evidence for disease. It is a term of exclusion for individuals who have memory symptoms that do not match with AD pattern or have negative biomarkers of AD pathology [8].
Fig. (1).
Alzheimer's disease classification based on potential biomarkers.
This review study aims to highlight the significance of several potential biomarkers of AD and their correlation with the attempt of an accurate diagnosis or even more an early prognosis. The proposed diagnostic model covers a broad range of AD biomarkers such as genetic mutations, hereditary risk factors, MCI and other comorbidities, referring both to the cases of sporadic and familial AD.
2. biomarkers and risk factors
When a patient presents visuospatial deficit and significant atrophy in the parietooccipital region on Magnetic Resonance Imaging (MRI), we can easily conclude to neurodegeneration, leading to posterior cortical atrophy or optical dysfunction of AD. Typically, symptoms can be mentioned such as logopenia, aphasia, frontal form of AD, language, praxis and complicated visual process and neuropsychiatric changes in everyday activities [9-10]. Furthermore, patients with presenile dementia and hemiparkinsonism have similar characteristics with AD pathology. However, the coexistence of these two diseases is rare while the one condition will prevail against the other [11, 12].
At the same time, biomarkers that reveal high probability of AD due to MCI could be more accurate if Amyloid-β (Aβ) and neuronal injury biomarkers were also positively tested. Exclusively the Aβ protein assessment can give us only an intermediary probability for developing AD due to MCI. In the case where only one biomarker of neurologic damage exists and Aβ cannot be measured, then these patients will be assumed with a lower probability to develop AD.
In another study related to age and the way that age influent the aggravation of AD, the density of the pathological lesions are mentioned about the age of the subject [13, 14]. Moreover, the age marker exists as a primate factor in several studies [8, 15, 16]. In conjunction with the above, AD can also be divided into early onset familial AD where the disease is mainly developed before the age of 60 years. In this case, the appearance of AD reveals a hereditary disease and is inherited in an autosomal dominant manner [9, 17, 18].
Alzheimer’s disease is mainly characterized by the Aβ protein pathology which is found in amyloid precursor protein gene (APP, 21q21), of the long arm of chromosome 21 [18]. Aβ deposits lead to plaques creation, the amyloid fibrils accumulated in the cell’s outer space and grouped into globe shape. Amyloid-β can also be deposited in media and adventitia of small and mid-sized arteries, in which case we refer to Cerebral Αmyloid Αngiopathy [19, 20]. Besides, Aβ can be detected and quantified in CSF and plasma with Positron Emission Tomography scanning method, detecting fibrillar Aβ, while both techniques can detect neurological injury [21]. Few individuals with DS mutation due to trisomy 21, show high levels of Aβ and present the classic pathology by the age of 50 [18]. Another biomarker of neuronal injury is tau/phosphorylated tau protein. When the two biomarkers Aβ and tau/phosphorylated tau proteins are positively measured, the probability of AD development increases [9, 22]. Both Aβ and phosphorylated tau are conventional biomarkers for other disorders as well and can be detected in vivo or in vitro. In vitro Scanning Tunneling Microscopy detects Ab (1-42) and two Photon Rayleigh Scattering Assay technique can be also used for tau detection. In vivo with mMRI and Optical (Fluorescent) Imaging, we can detect Ab plaques [23].
Moreover, biomarkers of neurological injury are considered the hippocampal volume or medial temporal lobe atrophy in MRI, the temporoparietal/precuneus hypometa-bolism or the hypoperfusion on Positron Emission Tomography scanning method or single-photon emission computerized tomography [9]. In a recent study, increased levels of Aβ and abnormal tau were detected in neocortical regions [24, 25], and the left precuneus, the superior temporal gyrus, and the fusiform gyrus have been also observed with a decreased volume on MRI studies [26]. Family and population studies prove that individuals have increased the probability to develop AD with the fourth form of Apolipoprotein E gene of chromosome 14, while types 2 and 3 of this gene do not affect their carriers. Ages between 65 and 75 are also at high risk to develop AD [11, 27, 28]. In many recent studies, the CSF a-synuclein has been identified in samples of patients with AD or Parkinson’s disease and is possibly correlated to other biochemical biomarkers [12, 29].
Lately, scientists are also focused on mitochondrial function. The mitochondrion is a subcellular organelle that is responsible for ATP production and since neurons require high energy, low ATP levels signify cell’s death. Mitochondrial fusion and fission occur continuously but in chaotic distributions and mutations in proteins that mediate their processes can cause irreparable loss. There are a few proteins that are involved in mitochondrial dynamics like the Optic Atrophy-1, the Dynamin-Related Protein-1 (DLP-1), the Mitochondrial Fission 1, the Mitofusin-1 and Mitofusin-2 [30]. Optic Atrophy-1 is found in membrane’s inner-space and mediate in fusion process of the inner mitochondrial membrane, while DLP-1 is found in mitochondrial membrane’s interface to mediate during fission process. Dynamin Related Protein-1 is believed to concentrate long oligomers which use Guanosine Triphosphate hydrolysis to constrict mitochondrial tubules during fission process. Mitochondrial Fission 1 is an outer-membrane protein function with DLP-1 during the fission process. Mitofusin-1 and Mitofusin-2 belong to GTPases family, which can be found in the outer membrane space and mediate during the fusion process. Mitofusin-1 and Mitofusin-2 are also responsible for mitochondrial lashing [23, 30]. Furthermore, mutations in Presenilin-1 and Presenilin-2 proteins lead to AD expression [31]. These two proteins encode amyloid precursor protein, and in the presence of Presenilin-1,2 mutations individuals have high probability to develop AD. Presenilin-1,2 mutations affect γ-secratase activity, which is responsible for disruption of amyloid precursor protein and Aβ cytotoxic accumulation [19, 28, 32]. Moreover, mitochondrial phenotype present fragment, cristae structures are devastated, the number of mitochondria in dendrites is decreased, the mobility of mitochondria is decreased, and the KGDH-PDH-COX complexes present dysfunctions due to these proteins dysfunction. Additionally, Aβ concentration interacts with DLP-1, Cyclin-Dependent Kinase 1 activity increases and Kinesin protein interacts with mitochondria in the cerebral cortex [16-19]. Individuals who inherit Presenilin-1,2 mutations present AD characteristics earlier than the age of 40-45. Families with these mutations present AD heredity which attends the autosomal dominant pattern with 50% probability for each generation to develop AD [11, 33, 34]. These mutations lead to plaque creation, tangles, cell loss and dementia. However, the percentage of AD patients due to genetic mutations are less than 2% of the total AD population [11]. Education is referred as a controversial marker while individuals with high educational level have fewer probabilities to develop AD; the reason could be a network of highly stable neuronal synapses in their brain. Important AD risk factors are the metal ions, which can affect negatively the AD development. In any case that proteins and lipid membranes with toxic effect are affected, the main result is reactive oxygen species production or even more the presence of metalloprotein Aβ amyloid peptide. Zinc and Copper are released from brain’s cortical neurons and cause Aβ accumulation and Aβ deposits, through histidine amino acid interactions. Additionally, Fe2+ and Cu2+ interactions with Aβ lead to H2O2 production, H2O2 is partially responsible for the oxidative action. Also, Zn2+ and Cu2++ enhance Αβ interactions with cell’s membranes, increasing Aβ toxicity. Furthermore, Fe3+ and Cu2+ interact with Aβ protein leading to oxidative stress, Aβ oligomerization lead to Calcium channels creation; these channels affect calcium homeostasis, causing oxidative stress [23, 35, 36].
The p53 protein contributes to disease development, and specifically the unfolded p53 conformation leads to cell’s death. Aβ peptides interact with HIPK-2 protein degradation affecting p53 conformation. Proteins p53 and tau are also related and found in patients with AD. p53 induces phosphorylation of human 2N4R tau causing neuronal death and other tauopathies [37-39]. In a similar study, BRCA1 and p53 accumulations have been detected in neurons at early AD onset [40]. Even if it has been reported in a few studies, gender seems to have been abandoned as a potential AD marker, due to women longer life expectancy. Also nationality does not appear to be a significant factor, however, certain people of the Asiatic origin appear to be differentiated [13]. Additionaly, a new protein is under consideration, the YKL-40, which initially is characterized as brain cell injury biomarker, while it is increasingly detected in AD individuals between 50’s and 70’s years old [14]. D-serine levels have been identified and measured in higher levels in the hippocampus and parietal cortex of AD patients and incriminated as a potential risk factor [41]. Four miRNAs, the miR-31, miR-93, miR-143, and miR-146a are observed to be decreased in AD patient’s serum, therefore, are recently characterized as novel biomarkers of AD pathology and vascular dementia [42]. Blood pressure has been formulated as precursor marker for disease manifestation. Decades before the appearance of disease, high blood pressure can be observed when senile plaques, neurofibrillary tangles, and hippocampal atrophy are already present [43]. Also, it has been declared that the age and the blood pressure are related in AD development. As mentioned above high blood pressure revealed decades before AD diagnosis, however in later life of an AD patient low levels of blood pressure occur. Unfortunately, the way that blood pressure affects AD is still unknown, even though high blood pressure seems to be a lower risk factor for AD patients [44-46].
CONCLUSION
Since a definitive and accurate diagnosis for AD and other related disorders can be made only at autopsy, neuroimaging techniques face challenges related to clinicopathologic heterogeneity. Although all patients with AD progress through some form of an MCI phase before dementia, the converse is not true. That is some patients who fulfill MCI criteria may have non-AD disease states [47]. Furthermore, the rate at which individuals with MCI will develop dementia may also vary considerably. Thus, although prodromal AD may be clinically identifiable as MCI [48], it is important to recognize the heterogeneity within this clinical construct. Cerebrospinal fluid and plasma biomarkers, as well as amyloid imaging markers, can offer information about neuropathological symptoms of AD, when no evidence markers for hippocampal volume loss can be accurately exported from MRI scanning [49]. Especially structrural MRI biomarkers conclude to major variations among young and elderly populations, associating different neuropathological underpinnings of cognitive impairment in the very old populations [50].
Latest studies reveal also the significance of the nerve growth factor precursor protein (proNGF) with cognitive impairement, underlying the effectiveness of this diagnostic biomarker [51].
In AD patients, who have been tested in structural imaging biomarker’s detection, left and right hippocampal gradings, cortical thicknesses of the left precuneus, left superior temporal sulcus and right anterior part of the parahippocampal gyrus, offer 72% accuracy in AD diagnosis [52]. Additionally, genetic mutations affect less than 2% of total AD patients and age seems to play a dominant role in the aggravation of disease even though it has been observed that the first lesions begin at least 20 years earlier from the first symptoms. The educational level shows some resistance in the disease, but it should not be considered as a valid marker. Mitochondrial dynamics is a latest crucial element in the puzzle of AD etiology and development, concerning metal ions concentrations in the brain and metal ions interactions with Aβ protein, increasing cells and brain toxicity [53-55].
It is obvious that early diagnosis of AD could be a cost effective approach to prevent its irreversible and uncontrollable consequences. In every case, a correct diagnosis needs to be performed before the underlying pathology has become severe enough to present itself clinically [56-58].
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The publication of this study has been supported by the AFnP Engineering, Chemicals and Consumables GmbH. Alexiou A. received grant from AFnP Engineering, Chemicals and Consumables GmbH; Mantzavinos V., Greig NH. and Kamal MA. have nothing to disclose.
REFERENCES
- 1.Kukull W.A., Bowen J.D. Dementia epidemiology. Med. Clin. North Am. 2002;86:573–590. doi: 10.1016/s0025-7125(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 2.DeKosky S.T., Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson B.C., Salat D.H., Greve D.N., Chua E.F., Rand-Giovannetti E., Rentz D.M., et al. Sperling RA increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., et al. Scheltens P research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson B.C., Sperling R.A. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia. 2008;46(6):1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexiou A., Rekkas J., Vlamos P. Modeling the mitochondrial dysfunction in neurogenerative diseases due to high H+ concentration. Bioinformation. 2011;6(5):173–175. doi: 10.6026/97320630006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexiou A., Rekkas J. In (Eds: Vlamos P, Alexiou A). From Advances in Experimental Medicine and Biology pp 822. GeNeDis 2014 (2014). 2014. Superconductivity in Human Body; Myth or Necessity. Springer International Publishing Switzerland 2015. [DOI] [PubMed] [Google Scholar]
- 8.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., et al. Mild cognitive impairment (MCI) due to Alzheimers Disease Workgroup. Curr. Med. Chem. Immunol. Endocr. Metab. Agents. 2010;3:371–383. [Google Scholar]
- 10.Ringman J.M., Liang L.J., Zhou Y., Vangala S., Teng E., Kremen S., et al. Early behavioural changes in familial Alzheimer’s disease in the dominantly inherited alzheimer network. Brain. 2015;138(4):1036–1045. doi: 10.1093/brain/awv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi E.J., Bang H., Im J.H., Chung S.J. Lee JH. A case of biopsy-proven early-onset Alzheimer’s disease with hemiparkinsonism. J. Clin. Neurol. 2005;1(1):97–100. doi: 10.3988/jcn.2005.1.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biomarkers across neurodegenerative diseases. Available from http://www.alz.org/research/alzheimers_grants/biomarkers-across.asp
- 13.Mohs R.C., Haroutunian V. Alzheimer disease: from earliest symptoms to end stage. In: Davis K.L., Charney D., Coyle J.T., Nemeroff C., editors. Alzheimer disease: from earliest symptoms to end stage. Neuropsychopharmacology: The Fifth Generation of Progress. Am Coll Neuropsychopharmacol 82: 1189-97. 2002. [Google Scholar]
- 14.Sutphen C.L., Jasielec M.S., Shah A.R., Macy E.M., Xiong C., Vlassenko A.G., et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72(9):1029–1042. doi: 10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis K.L., Mohs R.C., Marin D., Purohit D.P., Perl D.P., Lantz M., et al. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281(15):1401–1406. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- 16.Arendash G.W., Gordon M.N., Diamond D.M., Austin L.A., Hatcher J.M., Jantzen P., et al. Behavioral assessment of Alzheimer’s transgenic mice following long-term ab vaccination: task specificity and correlations between ab deposition and spatial memory. DNA Cell Biol. 2001;20:737–744. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- 17.Howlett D.R. Protein misfolding in disease: cause or response? Curr. Med. Chem. Immunol. Endocr. Metab. Agents. 2003;3(4):371–383. [Google Scholar]
- 18.Moncaster J.A., Pineda R., Moir R.D., Lu S., Burton M.A., Ghosh J.G., et al. Alzheimer’s disease amyloid-b links lens and brain pathology in down syndrome. PLoS One. 2010;5(5):e10659. doi: 10.1371/journal.pone.0010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mentenopoulos G., Mpouras K., editors. The disease of Alzheimer. University Studio Press; 2002. [Google Scholar]
- 20.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R., et al. Amyloid Biomarker Study Group Prevalence of cerebral amyloid pathology in persons without dementia. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, Wiesje M, van der Flier Bart NM, et al. the Amyloid PET Study Group. Prevalence of amyloid pet positivity in dementia syndromes a meta-analysis. . JAMA . 2015;313(19):1939–50. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buée L., Bussière T., Buée-Scherrer V., Delacourte A. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000;33(1):95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 23.Nazem A., Mansoori G.A. Nanotechnology for Alzheimer’s disease detection and treatment. Insciences J. 2011;1(4):169–193. [Google Scholar]
- 24.Chan D.C. Mitochondrial dynamics in disease. N. Engl. J. Med. 2007;356(17):1707–1709. doi: 10.1056/NEJMp078040. [DOI] [PubMed] [Google Scholar]
- 25.Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mézenge F., Egret S., et al. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J. Neurosci. 2015;35(29):10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thordardottir S., Ståhlbom A.K., Ferreira D., Almkvist O., Westman E., Zetterberg H., et al. Preclinical cerebrospinal fluid and volumetric magnetic resonance imaging biomarkers in Swedish familial Alzheimer’s disease. J. Alzheimers Dis. 2015;43(4):1393–02. doi: 10.3233/JAD-140339. [DOI] [PubMed] [Google Scholar]
- 27.Risacher S.L., Kim S., Nho K., Foroud T., Shen L., Petersen R.C., et al. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11(12):1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauwenberghe C.V., Broeckhoven C.V. Sleegers DSc, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel G. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Su B., Lee H., Li X., Perry G., Smith M.A., et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabezas-Opazo F.A., Vergara-Pulgar K., Pérez M.J., Jara C., Osorio-Fuentealba C., Quintanilla R.A. Mitochondrial dysfunction contributes to the pathogenesis of alzheimer’s disease. Oxid. Med. Cell. Longev. 2015;2015:509654. doi: 10.1155/2015/509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaël N., Wallon D., Charbonnier C., Quenez O., Rousseau S., Richard A.C., et al. Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. Eur. J. Hum. Genet. 2016;24(5):710–716. doi: 10.1038/ejhg.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quiroz Y.T., Schultz A.P., Chen K., Protas H.D., Brickhouse M., Fleisher A.S., et al. Brain imaging and blood biomarker abnormalities in children with autosomal dominant alzheimer disease: a cross-sectional study. JAMA Neurol. 2015;72(8):912–919. doi: 10.1001/jamaneurol.2015.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler S.E., Fagan A.M. Autosomal dominant Alzheimer disease: a unique resource to study csf biomarker changes in preclinical ad. Front. Neurol. 2015;6:142. doi: 10.3389/fneur.2015.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duce J.A., Bush A.I., Adlard P.A. Role of Amyloid-β-metal interactions in Alzheimer’s disease. Future Neurol. 2015;6(5):641–659. [Google Scholar]
- 36.Sastre M., Ritchie C.W., Hajji N. Metal ions in Alzheimer’s disease brain. JSM Alzheimer’s Dis Related Dementia. 2015;2(1):1014. [Google Scholar]
- 37.Hooper C., Meimaridou E., Tavassoli M., Melino G., Lovestone S., Killick R. p53 is upregulated in Alzheimer’s disease and induces tau phosphorylation in HEK293a cells. Neurosci. Lett. 2007;418(1):34–37. doi: 10.1016/j.neulet.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanga S., Lanni C., Govoni S., Uberti D., D’Orazi G., Racchi M. Unfolded p53 in the pathogenesis of Alzheimer’s disease: is HIPK2 the link? Aging. 2010;2(9):545–554. doi: 10.18632/aging.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buizza L., Prandelli C., Bonini S.A., Delbarba A., Cenini G., Lanni C., et al. Conformational altered p53 affects neuronal function: relevance for the response to toxic insult and growth-associated protein 43 expression. Cell Death Dis. 2013;4:e484. doi: 10.1038/cddis.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi A., Minami A., Kitagishi Y., Ogura Y., Matsuda S. BRCA1 and p53 tumor suppressor molecules in Alzheimer’s disease. Int. J. Mol. Sci. 2015;16(2):2879–2892. doi: 10.3390/ijms16022879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madeira C., Lourenco M.V., Vargas-Lopes C., Suemoto C.K., Brandão C.O., Reis T., et al. D-serine levels in Alzheimer’s disease: implications for novel biomarker development. Transl. Psychiatry. 2015;5:e561. doi: 10.1038/tp.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong H., Li J., Huang L., Chen X., Li D., Wang T., et al. Serum microrna profiles serve as novel biomarkers for the diagnosis of Alzheimer’s disease. Dis. Markers. 2015;2015:625659. doi: 10.1155/2015/625659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoog I., Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol. Res. 2006;28(6):605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 44.Kennelly S., Collins O. Walking the cognitive “minefield” between high and low blood pressure. J. Alzheimers Dis. 2012;32(3):609–621. doi: 10.3233/JAD-2012-120748. [DOI] [PubMed] [Google Scholar]
- 45.Skoog I., Gustafson D. Hypertension, hypertension-clustering factors and Alzheimer’s disease. Neurol. Res. 2003;25(6):675–680. doi: 10.1179/016164103101201986. [DOI] [PubMed] [Google Scholar]
- 46.Østergaard S.D., Mukherjee S., Sharp S.J., Proitsi P., Lotta L.A., Day F., et al. Associations between potentially modifiable risk factors and alzheimer disease: a mendelian randomization study. PLoS Med. 2015;12(6):e100184. doi: 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen R.C., Parisi J.E., Dickson D.W., Johnson K.A., Knopman D.S., Boeve B.F., et al. Neuropathologic features of amnestic mild cognitive impairment. Arch. Neurol. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 48.Grundman M., Petersen R.C., Ferris S.H., Thomas R.G., Aisen P.S., Bennett D.A., et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch. Neurol. 2004;61(1):59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 49.Belmokhtar N., Benamrane N. Classification of Alzheimer’s disease from 3d structural mri data. Intern J Comp App. 2012;47(3):40–44. [Google Scholar]
- 50.Yang Z., Wen W., Jiang J., Crawford J.D., Reppermund S., Levitan C., et al. Structural MRI biomarkers of mild cognitive impairment from young elders to centenarians. Curr. Alzheimer Res. 2016;13(3):256–267. doi: 10.2174/1567205013666151218150534. [DOI] [PubMed] [Google Scholar]
- 51.Counts S.E., He B., Prout J.G., Michalski B., Farotti L., Fahnestock M., et al. Cerebrospinal fluid prongf: a putative biomarker for early alzheimer’s disease. Curr. Alzheimer Res. 2016;13(7):800–808. doi: 10.2174/1567205013666160129095649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eskildsen S.F., Coupé P., Fonov V.S., Pruessner J.C., Collins D.L. Structural imaging biomarkers of Alzheimer’s disease: predicting disease progression. Neurobiol. Aging. 2015;36(1):23–31. doi: 10.1016/j.neurobiolaging.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 53.Corrado M., Scorrano L., Campello S. Mitochondrial dynamics in cancer and neurodegenerative and neuroinflammatory diseases, Hindawi Publishing Corporation. Int. J. Cell Biol. 2012:2012. doi: 10.1155/2012/729290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L., Atwood C.S., et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin L.J. Mitochondrial and cell death mechanisms in neurodegenerative diseases. Pharmaceuticals. 2010;3(4):839–915. doi: 10.3390/ph3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlamos P., Alexiou A. Computational biology and bioinformatics. Springer Series: advances in experimental medicine and biology. ; World Congress on Geriatrics and Neurodegenerative Disease Research. Content level: Research (2015). ; 2015. [Google Scholar]
- 57.Vlamos P., Alexiou A. Geriatrics, Springer Series: advances in experimental medicine and biology.. In: Vlamos P, Alexiou A, editors. World Congress on Geriatrics and Neurodegenerative Disease Research, Content level: Research; 2015. [Google Scholar]
- 58.Vlamos P., Alexiou A. Computational biology and bioinformatics, Springer Series: Advances in experimental medicine and biology. ; World congress on geriatrics and neurodegenerative disease research, content level: research (2015).; 2015. [Google Scholar]