Abstract
Background:
Mycobacterium can develop drug resistance (DR) by mutation of its existing gene. However, the existence of DR without mutation shows the need to look for an alternative mechanism such as the role of efflux pumps. In this study, we examined the effect of efflux pump inhibitors on isoniazid (INH) susceptibility in clinical isolates of Mycobacterium tuberculosis (Mtb).
Materials and Methods:
Resazurin microtiter assay was used to examine the effect of efflux pump inhibitors on minimum inhibitory concentration (MIC) levels of INH in eighteen Mtb clinical isolates.
Results:
The observed reduction in INH-MIC was 2–16-fold in INH-resistant isolates with katG and inhA gene mutations, 2–8-fold in INH-resistant isolates without mutation and 2–4-fold in INH-sensitive isolates. The MIC reduction by verapamil (VER) was observed in 83% isolates, by carbonyl cyanide m-chlorophenylhydrazone (CCCP) 61% isolates, by chloropromazine (CPZ) 61% isolates, by reserpine (RES) in 61% isolates and by 2,4-dinitro phenol (DNP) in 55% isolates.
Interpretation and Conclusions:
The results obtained in this study confirm that MIC of INH decreased in the presence of efflux pump inhibitors (VER, CCCP, CPZ, DNP, RES) in clinical isolates of Mtb and that the inhibition of efflux pumps by the efflux pump inhibitors can enhance the clinical effect of a drug. The results showed that these efflux pump inhibitors are active against both drug susceptible and drug resistant isolates, indicating that the effect of efflux pump inhibitors is not dependent on the mutational profile of the isolate. We observed in this study that VER was the most effective efflux pump inhibitor.
KEY WORDS: Efflux pump inhibitors, mechanism, minimum inhibitory concentration, Mycobacterium tuberculosis, resazurin microtitre assay
INTRODUCTION
The steady increase in tuberculosis (TB) and drug resistance TB (DR-TB), is one of the most common causes of morbidity and mortality in developing countries.[1] In India, the trend in DR is increasing and its magnitude is also found to be very high among retreatment patients.[2,3,4] Mechanism of DR in bacteria characteristically involves drug inactivation or modification, target alteration or decrease in drug accumulation.[5,6,7] In Mycobacterium tuberculosis (Mtb) sequential accumulation of spontaneous mutations in target genes has been considered as the single cause of DR.[8] However, such mutations are not found in low-level drug resistant isolates.[9] The decrease in drug accumulation by efflux pump is another probable mechanism which may be responsible for the existence of low-level DR in nonmutated isolates.[10,11] The main function of efflux pump is to extrude out antimicrobial substances. Efflux pump allows a better tolerance of drugs and thus may potentiate a higher level of DR when it co-exists with mutations.[12]
Isoniazid (INH) and rifampicin are the two most effective drugs being used in TB therapy. Activation of prodrug “INH” requires the enzyme catalase-peroxidase (katG) and the latter targets the NADH-dependent enoyl carrier protein reductase (InhA).[13,14] Mutations in the most common genes, namely katG (primary) and inhA (secondary) are responsible for INH resistance. Studies have shown that approximately 75% of the INH DR is due to mutations in these two genes, while 20%–30% of clinical isolates do not have a mutation in any of the gene associated with INH resistance.[15,16,17] The genome analysis of mycobacteria showed the existence of efflux pumps in various species of Mycobacterium, and also showed the association with low level of INH resistance due to increased efflux activity, in contrast to the high-level resistance caused by mutations in genes encoding for the primary targets of INH.[18]
MATERIALS AND METHODS
Materials
Five efflux pump inhibitors: Verapamil (VER), Carbonyl cyanide m-chlorophenylhydrazone (CCCP), Chloropromazine (CPZ), Reserpine (RES) and 2,4-Dinitro phenol (DNP), and INH drug were purchased from Sigma-Aldrich (St. Louis, MO, USA). VER and INH were dissolved in distilled water and CCCP, CPZ, DNP, and RES were dissolved in dimethyl sulfoxide. Middlebrook 7H9 broth and oleic albumin dextrose catalase were purchased from Becton Dickinson (BD Biosciences, USA). Microtiter 96-well plates were purchased from (Nunc, Denmark). The bacterial suspension was prepared in 7H9-S broth of No. 1 McFarland standard. A stock solution of 0.02% resazurin was prepared in distilled water, filtered through a syringe with a membrane sterilized filter of pore size 0.22 μm and kept at 4°C.
Mycobacterium tuberculosis isolates
Seventy (50 INH resistant, 20 INH-susceptible) clinical isolates were included in the study after drug susceptibility test for the first-line anti-tubercular drug: streptomycin (S), INH, rifampicin (R), and ethambutol (E).[19] These isolates were obtained from sputum samples of previously treated pulmonary TB cases referred to the TB laboratory, Department of Microbiology, King George's Medical University, Lucknow. The level of INH resistance was confirmed by absolute concentration method.[20] A rapid DNA extraction procedure was performed for Mtb isolates. Amplification of katG and inhA genes was done in a thermal cycler and the products, so obtained were used for DNA sequencing.
Sequencing of katG and inhA gene
Polymerase chain reaction (PCR) products of katG and inhA genes were purified using exonuclease I and shrimp alkaline phosphatase. The purified PCR products were sequenced by using forward and reverse primers on an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, USA) using Big Dye Terminator chemistry (version 3.1). Nucleotide and amino acid sequences of the amplified products were analyzed by using BLAST with a reference strain of Mtb (H37Rv).
Analysis of sequencing data
The sequencing data so obtained were analyzed by sequencing analysis software v5.2 (Applied Biosystems, CA, 94404, USA) and freely available web-based software National Centre for Biotechnology Information (NCBI) BLAST (accessible at: http://www.blast.ncbi.nlm.nih.gov). The sequencing data of mutations have been deposited in the NCBI under GenBank accession number KC844268 to KC844289, KC800647 to KC800661, and KF704009 to KF704041 for katG gene, KJ652027 to KJ652085 and KJ545536 for inhA gene.
After sequencing analysis, we randomly selected six INH-resistant isolates with no mutation in katG and inhA genes, six INH-resistant isolates (five isolates with mutation in katG gene and only one isolate with mutation in both katG and inhA) and six INH-susceptible isolates.
All 18 isolates were subjected to INH-MIC on a 96-well plate. INH-MICs were determined in the presence of each efflux pump inhibitor using resazurin microtier assay (REMA) in all the isolates. Broth microdilution was based on the NCCLS guideline.[21,22] The lowest concentration of each efflux pump inhibitors to which could sustain the growth of Mtb H37 Rv strain was optimized and selected for the final experiment.
Determination of isoniazid minimum inhibitory concentration in presence and absence of efflux pump inhibitors by resazurin microtitre assay plate method
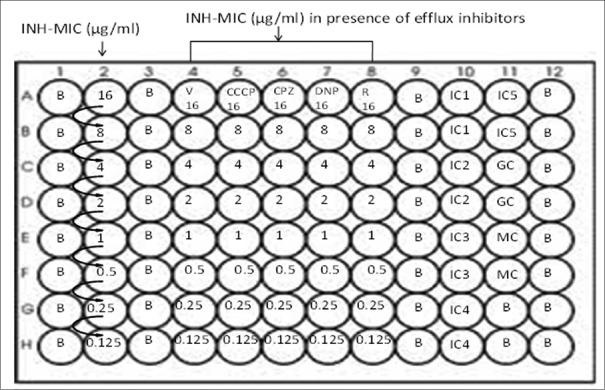
The protocol described by Martin and Palomino was used to determine the MIC of INH in the presence and absence of efflux pump inhibitors for each isolate.[23] A 96-well flat bottom plate was used for the experiment. Hundred μl 7H9-S broth was suspended in each of the test well, and 200 μl distilled water was added in the blank wells to prevent the evaporation during incubation [Figure 1].
Figure 1.
Minimum inhibitory concentration of INH in the presence and absence of efflux pump inhibitors by resazurin microtitre assay. B: Blank, INH: Isoniazid, MIC: Minimal inhibitory concentration, MC: Medium control, GC: Growth control, IC: Inhibitory control, VER: Verapamil, CCCP: Carbonyl cyanide m-chlorophenyl hydrazone, CPZ: Chloropromazine, DNP: 2,4 di-nitro phenol, RES: Reserpine
*Drug concentration of INH in each well is mentioned in the figure
Column 1 A-H = Blank (B); Column 2 = 7H9 broth + INH + Inoculum; Column 4 = 7H9 broth + INH + Inoculum + V (4μg/ml); Column 5 = 7H9 broth + INH + Inoculum + CCCP (1.5μg/ml); Column 6 = 7H9 broth + INH + Inoculum + CPZ (3.75μg/ml); Column 7 = 7H9 broth + INH + Inoculum + DNP (25μg/ml); Column 8 = 7H9 broth + INH + Inoculum + R (20μg/ml); Column 10 A-B (IC1) = 7H9 broth + Inoculum + V (4μg/ml); C-D (IC2) = 7H9 broth + Inoculum + CCCP (1.5μg/ml); E-F (IC3) = 7H9 broth + Inoculum + CPZ (3.75μg/ml); G-H (IC4) =7H9 broth + Inoculum + DNP (25μg/ml); Column 11 A-B (IC5) =7H9 broth + Inoculum + R (20μg/ml); C-D (GC) =7H9 broth + Inoculum; E-F (MC) =7H9 broth
For each isolate 100 μl INH (64 μg/ml) was added in the wells of the first row and 2-fold serial dilution was obtained vertically. Hundred μl of diluted (1:100) bacterial suspensions were added to each well except in the control and blank wells. The optimized concentration of V (4 μg/ml), CCCP (1.5 μg/ml), CPZ (3.75 μg/ml), DNP (25 μg/ml) and R (20 μg/ml) was added in their respective wells [Figure 1]. The plate was sealed and incubated at 37°C for 7 days. After incubation, 30 μl of freshly prepared resazurin solution was added to each well, and the sealed plate was re-incubated overnight. MIC for INH in the presence and absence of efflux pump inhibitors were determined by observing the change in color (blue to pink). H37Rv strain was used as control (susceptible at 0.2 μg/ml levels of INH). The INH resistance was defined as MIC >0.25 μg/ml and INH susceptibility as MIC of <0.25 μg/ml. The efflux activity was determined by observing a reduction of 2-fold or more in MIC of INH. The REMA assay was repeated thrice and results were validated for each isolate.
RESULTS
Selection of isolates
Among seventy Mtb isolates, 18 isolates were categorized in three groups, Group A; INH-resistant without mutations in katG and inhA genes (six isolates), Group B; INH-resistant with mutation Ser315Thr, Ser315Asn in katG and Prol17Gln in inhA genes (six isolates), and Group C; INH-susceptible (six isolates). The details are shown in Figure 2.
Figure 2.
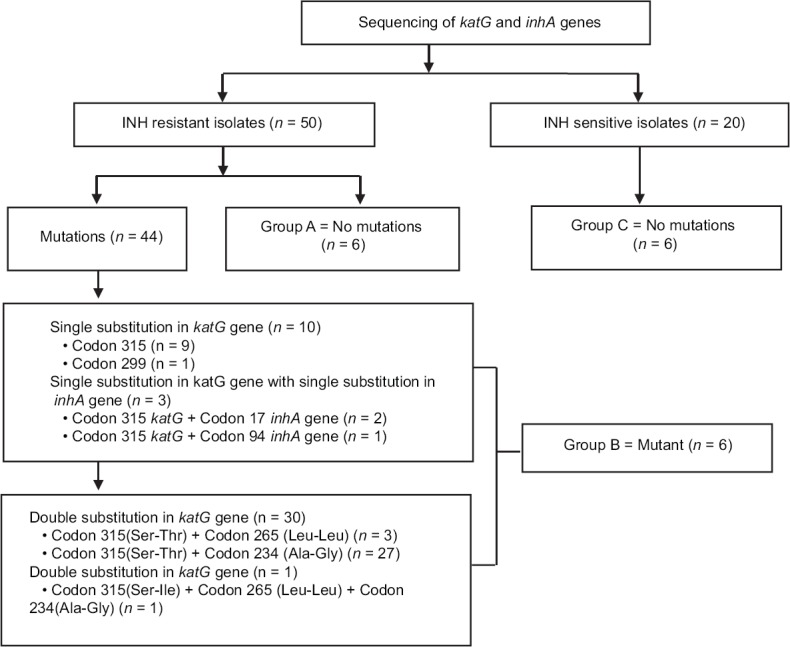
Selection of M. tuberculosis isolates (Group A, B and C) to check the effect of efflux pump inhibitors
Isoniazid minimum inhibitory concentration in the presence of efflux pump inhibitors in Group A (isoniazid-resistant isolates with no mutation in katG and inhA genes)
The MICs of INH and their fold change in INH-MIC in the presence of efflux pump inhibitors (VER, CCCP, CPZ, DNP, RES) among six isolates are shown in Table 1.
Table 1.
Effect of efflux pump inhibitors on isoniazid resistant isolates with no mutations in katG and inhA genes
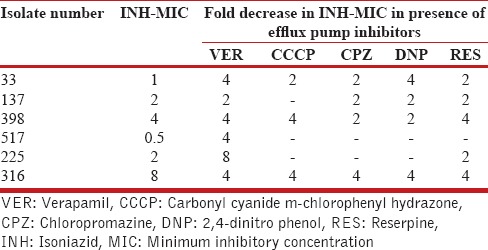
In Group A, out of six isolates, 2-fold decrease (1/6 isolates: VER; 1/6 isolates: CCCP; 3/6 isolate: CPZ; 2/6 isolates: DNP; 3/6 isolates: RES), 4-fold decrease (4/6 isolates: VER; 2/6 isolates: CCCP; 1/6 isolate: CPZ; 2/6 isolate: DNP; 2/6 isolate: RES), and 8-fold decrease (1/6 isolate: VER) in MICs of INH was observed in presence of efflux pump inhibitors [Table 2].
Table 2.
Fold change in isoniazid minimum inhibitory concentration of Mycobacterium tuberculosis isolates in presence of efflux inhibitor in Group A, B and C

Isoniazid minimum inhibitory concentration in the presence of efflux pump inhibitors in Group B (isoniazid-resistant five isolates with mutation in katG gene and only one isolate with mutation in both, katG and inhA genes)
Among six isolates, five isolates showed decrease in INH MIC (2–16-fold) with all five efflux pump inhibitors (VER, CCCP, CPZ, DNP, RES) and in one isolate no inhibitor could decrease the MIC of INH.
There was a gradual decrease in the MICs of INH in the presence of efflux pump inhibitors (VER, CCCP, CPZ, DNP, RES) among six isolates and detail of mutation pattern are shown in Table 3.
Table 3.
Effect of efflux pump inhibitors on isoniazid esistant isolates with mutations in katG and inhA genes
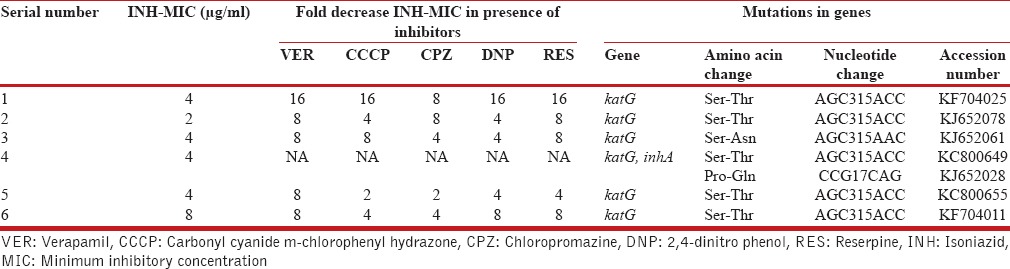
In Group B, out of six isolates 2-fold decrease (1/6 isolate: CCCP; 1/6 isolate: CPZ), 4-fold decrease (2/6 isolates: CCCP; 2/6 isolates: CPZ; 3/6 isolates: DNP; 1/6 isolates: RES), 8-fold decrease (4/6 isolates: VER; 1/6 isolates: CCCP; 2/6 isolates: CPZ; 1/6 isolates: DNP; 3/6 isolates: RES), and 16-fold decrease (1/6 isolate: VER; 1/6 isolate: CCCP; 1/6 isolate: DNP; 1/6 isolate: RES) in MIC of INH was observed in presence of efflux pump inhibitors [Table 2].
Isoniazid minimum inhibitory concentration in the presence of efflux pump inhibitors in Group C (isoniazid susceptible isolates)
All the six isolates had MIC <0.25 μg/ml. Among the six isolates, one isolate showed decrease in MIC level of INH in presence of all the five efflux pump inhibitors (VER, CCCP, CPZ, DNP, RES), 3 isolates showed decrease in MIC of INH in presence of more than one inhibitor and two isolates did not show any change in MIC level of INH.
In Group C out of six isolates, 2-fold decrease (1/6 isolate: CCCP; 1/6 isolate: CPZ), 4-fold decrease (3/6 isolates: VER; 2/6 isolates: CCCP; 1/6 isolate: CPZ; 1/6 isolate: DNP; 1/6 isolate: RES) in MIC of INH was observed in presence of efflux pump inhibitors [Table 2].
The effect of five efflux pump inhibitors viz. VER, CCCP, CPZ, DNP, and RES on MIC of INH in 18 isolates was observed. All the five efflux pump inhibitors caused MIC reduction in nine isolates (50%), however in four isolates (22%) the reduction was achieved by more than one efflux pump inhibitor. VER caused MIC reduction in two isolate (11%), and no reduction in MIC of INH was observed in three isolates (16%) [Table 4].
Table 4.
Effect of more than one efflux pump inhibitors on resistance level of INH-MIC in Mycobacterium tuberculosis
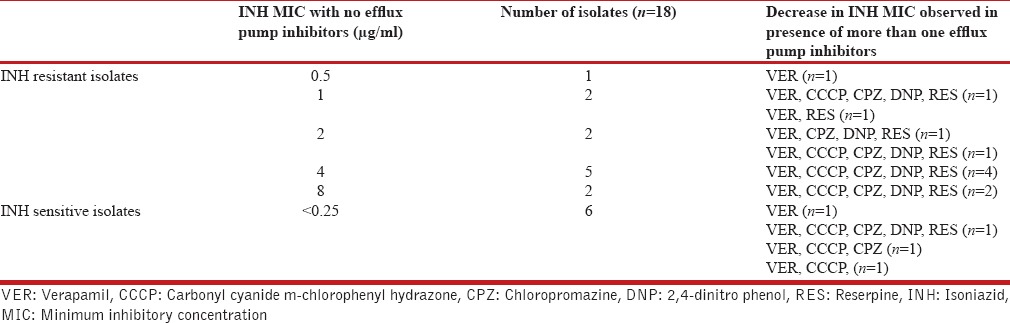
The MIC reduction was observed in 15 isolates (83%) by VER, 11 isolates (61%) by CCCP, 11 isolates (61%) by CPZ, ten isolates (55%) by DNP and 11 isolates (61%) by RES. It was observed that VER was the most effective efflux pump inhibitor for INH-resistant isolates [Table 4].
DISCUSSION
The lengthy treatment required with current anti-tubercular drugs is a major challenge in TB management. The consequences of poor adherence to the drug are serious both for the individual patient and for the community leading to DR, treatment failure, and further TB transmission.[24] During the first 2 days of treatment with INH, more than 99% of the initial sputum bacillary are killed, after which the rate of killing drops off noticeably. The residual bacteria become phenotypically resistant, while INH minimum inhibitory concentrations are unchanged.[25]
Earlier studies reported that mutations in drug target genes and drug efflux pump are responsible for the DR in Mtb.[11,26,27] Efflux pumps have multi drug extrusion ability, which confer clinically significant levels of DR.[10] The aim of the present study was to determine the effect of different efflux pump inhibitors on the mechanism of INH DR in isolates of Mtb.
In the present study, we found that 16.66% isolates changed their MIC phenotypically from resistant to susceptible in the presence of VER. Similarly, Singh et al.[28] also reported that 20% isolates phenotypically changed their MIC from resistant to susceptible. Earlier studies have shown a decrease in the minimum inhibitory concentrations of INH, rifampicin, streptomycin, ciprofloxacin, ofloxacin and linezolid in the presence of efflux pump inhibitors (CCCP and VER).[29,30] Although in vivo data are limited, a recent study found that VER restored the activity of INH, rifampicin, and pyrazinamide against MDR-TB in mice.[26]
Of the 18 isolates, 83% isolates showed decrease in MIC of INH with VER, 61% isolates with CCCP, CPZ, and RES, while the lowest number of isolates (55%) showed decrease in MIC of INH with DNP. An Indian study reported that in 66% ofloxacin resistant isolates, the active efflux pump was blocked by efflux pump inhibitors resulting in decreased ofloxacin MIC levels in these isolates.[28] Banerjee et al.[31] reported that the CCCP, VER, DNP, RES and CPZ increases the accumulation of a drug due to inhibition of active efflux.
A 2–16-fold MIC reduction in INH-resistant isolates with katG mutations is in agreement with the results of other studies also.[12,32,33] We found 2–8-fold decrease in INH MIC in resistant isolates with no mutation, While the decrease was 2–4-fold in susceptible isolates. These observations confirm the effect of efflux pump inhibitors on INH-resistant isolates (with and without mutations in katG and inhA) and INH-susceptible isolates.[34] These observations also show that these efflux pump inhibitors are active against both drug susceptible and drug resistant isolates indicating that the effect of these compounds is not dependent on the mutational profile of the isolates.[12]
A study found that the combination of VER and first line anti-TB drugs significantly reduced pulmonary bacilli burden in mice.[26] Another study showed that MIC of INH and ethambutol decreased when efflux pump inhibitors such as CCCP and VER were used.[35]
The role of efflux pumps in promoting drug tolerance opens up a potentially powerful approach for shortening the duration of TB treatment. The use of efflux pump inhibitors would target not only bacteria but also drug tolerance. In the laboratory, macrophage-induced tolerance is inhibited by VER, a calcium channel antagonist in clinical use for years, which has also been shown to inhibit multiple bacterial efflux pumps in vitro.[36,37,38] VER also reduces intracellular mycobacterial growth in the absence of drugs.[36,39]
The clinical implications of efflux pump are quite serious. In areas where facilities for mycobacterial cultures are limited, the standard TB regimens prescribed to unrecognized DR-TB patients show minimum efficacy in such patients, thus limiting the treatment options. Efflux pump inhibitors not only help in overcoming drug tolerance but also reduce the emergence of genetically drug resistant Mtb.
CONCLUSION
The present study was planned to observe the effect of efflux pump inhibitors verapamil (VER), carbonyl cyanide m-chlorophenyl hydrazone (CCCP), chloropromazine (CPZ), 2,4-dinitro phenol (DNP) and reserpine (RES) on susceptibility of Mtb to INH. We observed that VER is the most effective efflux pump inhibitor.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jin J, Zhang JY, Guo N, Sheng H, Li L, Liang JC, et al. Farnesol, a potential efflux pump inhibitor in Mycobacterium smegmatis. Molecules. 2010;15:7750–62. doi: 10.3390/molecules15117750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawat J, Sindhwani G, Juyal R, Dua R. Five-year trend of acquired antitubercular drug resistance in patients attending a tertiary care hospital at Dehradun (Uttarakhand) Lung India. 2009;26:106–8. doi: 10.4103/0970-2113.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahiri S, Mukherjee A, Hazra S, Jana P, Roy S, Saha BK. First-line anti-tubercular drug resistance of mycobacterial strains from re-treatment cases that were smear-positive at 4th month onwards under the revised national tuberculosis control program. Lung India. 2015;32:127–31. doi: 10.4103/0970-2113.152619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rai SP, Bhattacharyya D, Kashyap M. Pattern of initial drug resistance and its impact on short course chemotherapy of pulmonary tuberculosis. Lung India. 2007;24:51–3. [Google Scholar]
- 5.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: An update. Drugs. 2009;69:1555–623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen L, Thompson CJ. Foundations of antibiotic resistance in bacterial physiology: The mycobacterial paradigm. Trends Microbiol. 2006;14:304–12. doi: 10.1016/j.tim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Nikaido H. Preventing drug access to targets: Cell surface permeability barriers and active efflux in bacteria. Semin Cell Dev Biol. 2001;12:215–23. doi: 10.1006/scdb.2000.0247. [DOI] [PubMed] [Google Scholar]
- 8.Somoskovi A, Parsons LM, Salfinger M. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res. 2001;2:164–8. doi: 10.1186/rr54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues L, Villellas C, Bailo R, Viveiros M, Aínsa JA. Role of the Mmr efflux pump in drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2013;57:751–7. doi: 10.1128/AAC.01482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado D, Couto I, Perdigão J, Rodrigues L, Portugal I, Baptista P, et al. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS One. 2012;7:e34538. doi: 10.1371/journal.pone.0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho T, Machado D, Couto I, Maschmann R, Ramos D, von Groll A, et al. Enhancement of antibiotic activity by efflux inhibitors against multidrug resistant Mycobacterium tuberculosis clinical isolates from Brazil. Front Microbiol. 2015;6:330. doi: 10.3389/fmicb.2015.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–3. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 14.Dubnau E, Chan J, Mohan VP, Smith I. responses of Mycobacterium tuberculosis to growth in the mouse lung. Infect Immun. 2005;73:3754–7. doi: 10.1128/IAI.73.6.3754-3757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Seet Q, Denkin S, Parsons L, Zhang Y. Molecular characterization of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis from the USA. J Med Microbiol. 2006;55(Pt 11):1527–31. doi: 10.1099/jmm.0.46718-0. [DOI] [PubMed] [Google Scholar]
- 16.Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. A balancing act: Efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother. 2009;53:3181–9. doi: 10.1128/AAC.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramaswamy SV, Reich R, Dou SJ, Jasperse L, Pan X, Wanger A, et al. Single nucleotide polymorphisms in genes associated with isoniazid resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2003;47:1241–50. doi: 10.1128/AAC.47.4.1241-1250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rossi E, Arrigo P, Bellinzoni M, Silva PA, Martín C, Aínsa JA, et al. The multidrug transporters belonging to major facilitator superfamily in Mycobacterium tuberculosis. Mol Med. 2002;8:714–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Sen MR, Mohapatra TM, Anupurba S. Evaluation of the performance of nitrate reductase assay for rapid drug-susceptibility testing of Mycobacterium tuberculosis in North India. J Health Popul Nutr. 2011;29:20–5. doi: 10.3329/jhpn.v29i1.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandjean L, Moore DA. Tuberculosis in the developing world: Recent advances in diagnosis with special consideration of extensively drug-resistant tuberculosis. Curr Opin Infect Dis. 2008;21:454–61. doi: 10.1097/QCO.0b013e32830ce783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues L, Wagner D, Viveiros M, Sampaio D, Couto I, Vavra M, et al. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J Antimicrob Chemother. 2008;61:1076–82. doi: 10.1093/jac/dkn070. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes: Approved Standard M24-a. Wayne, PA USA: NCCLS; 2003. [PubMed] [Google Scholar]
- 23.Martin A, Palomino JC. Procedure Mannual; Resazurin Microtiter Assay (REMA) colorimetric Redox Indicator (CRI), Drug Susceptibility Testing for Mycobacterium tuberculosis. 2009. [Last accessed on 2012 Aug 15]. pp. 1–17. Available from: http://tbevidence.org/documents/rescentre/sop/Procedure%20manual%20CRI%2006-2012.pdf .
- 24.Johnson JL, Hadad DJ, Dietze R, Maciel EL, Sewali B, Gitta P, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180:558–63. doi: 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchison D, Davies G. The chemotherapy of tuberculosis: Past, present and future. Int J Tuberc Lung Dis. 2012;16:724–32. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Hernandez-Pando R, et al. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med. 2011;184:269–76. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida Da Silva PE, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: Classical and new drugs. J Antimicrob Chemother. 2011;66:1417–30. doi: 10.1093/jac/dkr173. [DOI] [PubMed] [Google Scholar]
- 28.Singh M, Jadaun GPS, Ramdas K. Efflux pump inhibitors on drug susceptibility of ofloxacin resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2011;133:535–40. [PMC free article] [PubMed] [Google Scholar]
- 29.Escribano I, Rodríguez JC, Llorca B, García-Pachon E, Ruiz M, Royo G. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to fluoroquinolones and linezolid. Chemotherapy. 2007;53:397–401. doi: 10.1159/000109769. [DOI] [PubMed] [Google Scholar]
- 30.Gupta AK, Chauhan DS, Srivastava K, Das R, Batra S, Mittal M, et al. Estimation of efflux mediated multi-drug resistance and its correlation with expression levels of two major efflux pumps in mycobacteria. J Commun Dis. 2006;38:246–54. [PubMed] [Google Scholar]
- 31.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis.Science-AAAS-Weekly Paper Edition-including Guide to Scientific Information. 1994;263:227–9. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 32.Böttger EC. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol Infect. 2011;17:1128–34. doi: 10.1111/j.1469-0691.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 33.Cambau E, Viveiros M, Machado D, Raskine L, Ritter C, Tortoli E, et al. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother. 2015;70:686–96. doi: 10.1093/jac/dku438. [DOI] [PubMed] [Google Scholar]
- 34.Ramón-García S, Martín C, Aínsa JA, De Rossi E. Characterization of tetracycline resistance mediated by the efflux pump Tap from Mycobacterium fortuitum. J Antimicrob Chemother. 2006;57:252–9. doi: 10.1093/jac/dki436. [DOI] [PubMed] [Google Scholar]
- 35.Gupta AK, Reddy VP, Lavania M, Chauhan DS, Venkatesan K, Sharma VD, et al. jefA (Rv2459), a drug efflux gene in Mycobacterium tuberculosis confers resistance to isoniazid and ethambutol. Indian J Med Res. 2010;132:176–88. [PubMed] [Google Scholar]
- 36.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87:1137–47. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues L, Aínsa JA, Amaral L, Viveiros M. Inhibition of drug efflux in mycobacteria with phenothiazines and other putative efflux inhibitors. Recent Pat Antiinfect Drug Discov. 2011;6:118–27. doi: 10.2174/157489111796064579. [DOI] [PubMed] [Google Scholar]
- 39.Martins M, Viveiros M, Amaral L. Inhibitors of Ca2+ and K+ transport enhance intracellular killing of M. tuberculosis by non-killing macrophages. In Vivo. 2008;22:69–75. [PubMed] [Google Scholar]