Abstract
Background:
Food allergy occurs in a significant portion of pediatric asthma. Various cells and their mediators/cytokines play a pivotal role in orchestrating the airway inflammatory response in asthma.
Objective:
To study the cutaneous hypersensitivity, Th1, Th2, and Th17 response of pediatric population with asthma and genetic predisposition to atopy, by determining total immunoglobulin E (IgE) level in response to various food allergens.
Materials and Methods:
Fifty asthmatic children with a history of worsening symptoms by various food allergens (study group) and twenty healthy children (control group) were included. Food allergy was assessed through skin prick test (SPT) of various food allergens. Total serum IgE level was measured by sandwich ELISA, and T-cell (Th1, Th2, and Th17)-dependent cytokines were measured by flow cytometry.
Results:
All 50 asthmatic children in the study group showed SPT positivity against various food allergens (rice = 17; banana, fish and groundnut = 10; wheat = 9; milk and orange = 7; egg = 6; and mango = 4). The average total IgE level in the study group was 316.8 ± 189.8 IU/mL. A significant positive correlation of total IgE with interleukin 17 (IL-17) (r = 0.796; P < 0.0001), IL-13 (r = 0.383; P = 0.01), and IL-4 (r = 0.263; P = 0.043) level was noted. A significant negative correlation of total IgE was noted with interferon gamma (r = −0.5823; P < 0.0001) and IL-10 (r = −0.4474; P < 0.001) level and the duration of breastfeeding (r = −0.31, P = 0.03).
Conclusions:
The present study found a positive correlation between total serum IgE level and Th2, Th17 cytokines in a pediatric population with asthma. A significant negative correlation was found between the duration of breastfeeding and the cytokines.
KEY WORDS: Asthma, children, cytokines, food allergen, skin prick test
INTRODUCTION
Food allergy occurs in a high proportion of children with asthma.[1] Food allergies are emerging as the second epidemic of allergen disease in the developed world as well as the developing world.[2] Asthma is an allergic airway disease having underlying chronic inflammation, the etiology of which remains largely obscured.[3] A major advance in the last few decade is the discovery of various cells and their mediators/cytokines that play a pivotal role in orchestrating the airway inflammatory response in asthma.
Although allergic sensitization during childhood period and subsequent asthma development are not entirely clear, genetic factors act probably synergistically with the environmental factors to decide the same.[4] The result is an immunological dysregulation with either the response being skewed toward Th2 response or an improper/deficient Th1 response leading to promotion of allergic sensitization. It is believed that Th2 cytokines play an important role in the development of various allergic diseases including asthma, which is marked by increased levels of various interleukins (IL-4, IL-5, IL-9, and IL-13).[5] The Th2 response is also compounded by environmental factors such as allergen exposure, infections (mainly viral), and pollutants. Th2 cells release cytokines that are essential for immunoglobulin E (IgE) synthesis, chemokine production, and mucous production in the airways and other places.[6,7] A coordinated interaction between proinflammatory/allergic cells (eosinophils, mast cells, T- and B-cells) and anti-IgE antibodies result in chronic airway inflammation and resultant hyperresponsiveness.[8]
It is proposed that breastfeeding protects against allergy probably by limiting exposure to harmful allergens (e.g., those in formula feed or cow milk) or through a balanced act of cytokines or production of Ig having role in allergy.[9,10] Exclusive breastfeeding up to 6 months has been shown to be protective against allergy and asthma.
During the last decade, it was found that the third subset of effector T-cells, i.e., Th17 cells also play role in allergic inflammation.[11,12] In mouse asthma models, Th17 cells have been found to enhance both neutrophilic and eosinophilic airway inflammation.[13,14] In addition, Th17 cells play an interlinking role to decrease the gap between Th1 and Th2 cells by secreting certain ILs (IL-17A, and IL-17F) and contributing to the immunity against certain extracellular pathogens (bacteria, fungi, etc.).[15] Evidence shows that an increase in IL-17 level is associated with a different inflammatory disease states.[16] However, its role in food allergy in children with asthma and other allergies has rarely been investigated. We set out to study the correlation between food sensitization, total IgE level, and cytokine response of Th1, Th2, and Th17 cells in children with asthma. Secondarily we also aimed to study the correlation between duration of breastfeeding and cytokines in children with asthma.
MATERIALS AND METHODS
Subjects
This prospective observational study was conducted at the Pediatric Allergy and Asthma Clinic, PGIMER, Chandigarh, from the year 2010 to 2013. Seventy children (5–12 years of age) presenting with allergy or worsening symptoms of asthma were enrolled. A total of fifty children were included in the study group. Study group consisted of children who showed exacerbation of asthma and were found to be positive for at least one of the nine food allergens by skin prick test (SPT). Written informed consent from one of their parents was obtained before enrollment into the study. The sera of age- and sex-matched nonatopic children were taken as control. The Institute's Ethics Committee of PGIMER approved this study.
Methodology
Diagnosis of food allergy to various food allergens was done by SPTs and measurement of serum total IgE level. Five milliliter of blood collected by aseptic venipuncture was taken for evaluation of cytokines (Th1, Th2, and Th17).
Skin prick test
Standard allergen extracts of 9 food allergens (Alcit India Pvt. Ltd., Delhi, India) preserved at 4°C were used. Then, SPT was performed with the following food allergens extracts: wheat, milk, egg white, rice, fish, ground nut, mango, banana, and orange. A drop (10 μl) of each allergen extract was placed on the forearm (volar aspect) with a disposable 26-gauge hypodermic needle and scratched. The reaction was read after 20 min. Positive result was denoted by a mean wheal diameter of >3 mm above the negative control. Glycerol (20%) in phosphate buffer saline served as negative control and histamine (1 mg/mL) served as positive control.
Estimation of total immunoglobulin E
The serum total IgE levels of the study group were measured using a readily available kit (PATHOZYME IMMUNOGLOBULIN E OD 417). Manufacturer's instruction was followed: test sera applied, incubation done with zero buffer, washing done with distilled water, and IgE-HRP conjugate was added to the wells. After addition of tetramethylbenzidine hydrochloride and dilute hydrochloric acid, absorbance was measured at 450 nm.
Measurement of expressions of cytokines
The serum levels of cytokines secreted by Th1 cell (interferon gamma [IFN-γ]), Th2 cell (IL-2, IL-4, IL-6, IL-10, IL-12, IL-13), and Th17 cell (IL-17) were assessed using Becton Dickinson Cytometric Bead Array Flex Set. According to the standard procedure, the analysis was carried out by flow cytometry (FACS Canto [Becton Dickinson, Mountain View, CA, USA] with FACSDiva™ software).
Statistical analysis
Data were analyzed with SPSS statistical software (version 16.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 5.0; GraphPad Software Inc., Le Jolla, CA, USA). The mean values and their standard deviation were calculated. The spearman's r rank correlation coefficient was used to evaluate the relationship between different variables. Student's t-test was used while assessing the flow cytometry data. P < 0.05 was considered statistically significant.
RESULTS
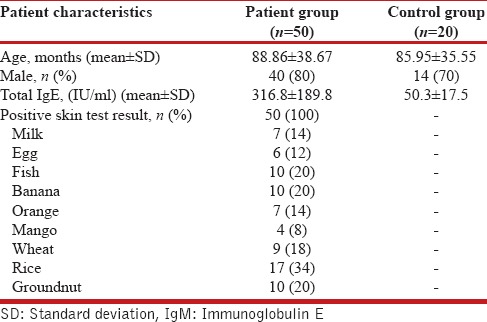
The demographic feature of included patients and results of SPT are given in Table 1. Among the study group, 17 were allergic to rice and 10 each to groundnut, fish, and banana.
Table 1.
Demographic and other parameters of study and control participants
Serum total immunoglobulin E level
The average serum total IgE level in the study group was 316.8 ± 189.8 IU/mL and in control group was 50.3 ± 17.5 IU/mL [Figure 1].
Figure 1.
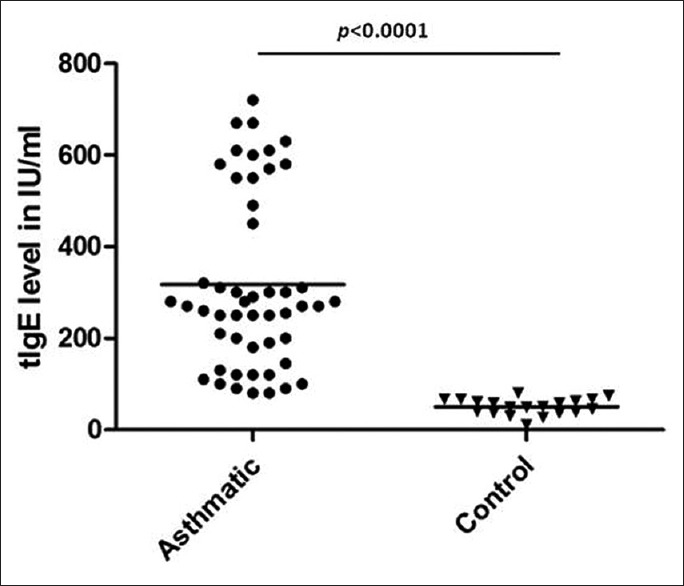
Total immunoglobulin E level in children with asthma and control group
Expression of cytokines
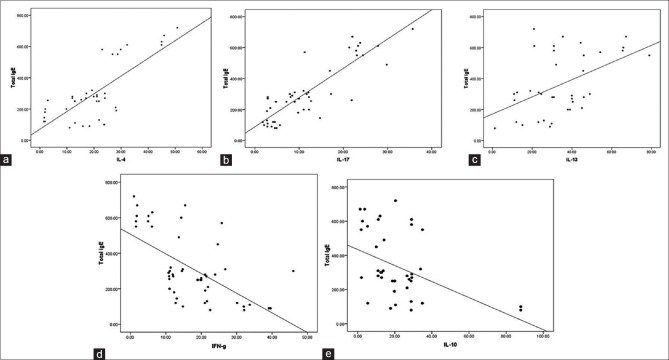
The IL-17 level in the study group was 12.09 ± 8.67 pg/mL, which was significantly higher than the control group (2.01 ± 1.27 pg/mL, P = 0.001). The IFN-γ was significantly lower in the study population (12.08 ± 8.67 pg/mL) compared to the control (21.00 ± 7.53 pg/mL, P = 0.02). No significant differences between the study group and control group were observed for IL-2 (P > 0.05); IL-4 (P > 0.05); IL-6 (P > 0.05); IL-10 (P > 0.05); IL-12 (P > 0.05); and IL-13 (P > 0.05) Table 2. A significant positive correlation of serum total IgE level with IL-17 (r = 0.796; P < 0.0001), IL-13 (r = 0.383; P = 0.01), and IL-4 (r = 0.605; P < 0.001) was found whereas, a significant negative correlation of serum total IgE level was found with IFN-γ (r = −0.5823; P < 0.0001) and IL-10 (r = −0.4474; P < 0.001) [Figure 2]. No statistically significant correlation was found between serum total IgE and other ILs (IL-2, IL-6, IL-12).
Table 2.
Cytokine expressions
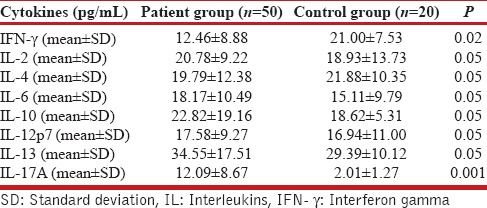
Figure 2.
(a) Correlation between total immunoglobulin E and interleukin-4; (b) correlation between total immunoglobulin E and interleukin-17; (c) correlation between total immunoglobulin E and interleukin-13; (d) correlation between total immunoglobulin E and interferon gamma; (e) correlation between total immunoglobulin E and interleukin 10
Correlation of breastfeeding with serum total immunoglobulin E level
The duration of breastfeeding was as follows: no breastfeeding at all (n = 2); 1–3 months (n = 6); 3–6 months (n = 6); up to 6 months (n = 10); and >6 months (n = 26). A significantly negative correlation between the duration of breastfeeding and serum total IgE level (r = −0.31, P = 0.03) was found [Figure 3]. Relationship of breastfeeding with other cytokines can be made indirectly through the correlation of other cytokines with serum total IgE level [Figure 2].
Figure 3.
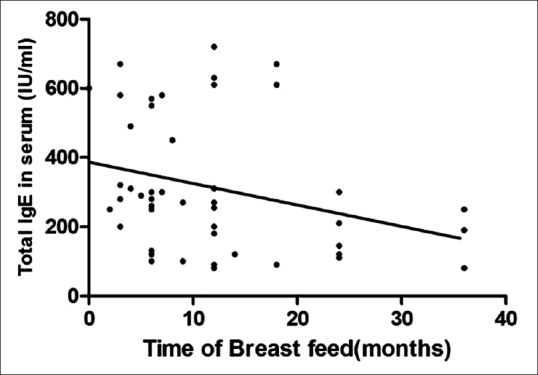
Correlation between breastfeeding time and total serum immunoglobulin E
DISCUSSION
In the present study, all fifty asthmatic children showed SPT positivity against various food allergens (most common being rice, banana, fish, and groundnut). A significant positive correlation of total IgE with IL-17, IL-13, and IL-4 level was noted. A significant negative correlation of total IgE was noted with IFN-γ and IL-10 level and the duration of breastfeeding.
Th1 type cells are involved in the cell-mediated immune response which when activated by microbial antigens result in increased IgG (not IgE) level.[17] Th1 cells typically produce cytokines such as IL-2, IFN-γ, and TNF. In contrast, Th2 type cells are involved in humoral immune response which when activated result in elevated IgE levels (not IgG) and eosinophils that modulate the development of allergic response including asthma.[18] Th2 cells typically produce cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13. Studies on soluble IL-2 receptor have demonstrated elevated levels in pediatric asthma population suggesting important role of activated T-cells in the latter.[18] Recent data support the dominating role of Th2 phenotype in pediatric asthma.[19] However, the role of Th1 cytokines (IL-2 and IFN-γ) in the promotion of allergic inflammation has been supported as well.[20]
A decreased level of IL-10 has been demonstrated in several human studies on asthma,[21] and those having severe asthma are likely to have lower production of IL-10 due to promoter polymorphisms.[22] IL-12 is produced predominantly by phagocytic and mature dendritic cells and also by B-cells and eosinophils.[23] IL-12 is essential for a Th1 dominant immune response, which leads to increased production of IFN-γ. Decreased production of IL-12 in the lung tissues of participants with asthma may play an important role in the pathogenesis of asthma by causing cytokine imbalance in the lungs.
In the present study, the mean serum total IgE level was significantly higher in the study group with food allergy than in the control group. It thus confirmed that the serum total IgE level is directly correlated with the allergic diseases such as asthma. The present study also showed significant correlation of marked change in the cytokine level with serum total IgE level: IL-4, IL-13, and IL-17 being positively correlated, and IFN-γ, IL-10 being negatively correlated in children with food allergy.[24] IL-10 has been found to inhibit both Th1 and Th2 cells, IgE synthesis, and eosinophil survival.[25] The latter has been shown by our study in that the IL-10 activity was inversely correlated with serum total IgE level.[26]
Th1, Th2, and Th17 cells orchestrate cellular infiltration that results in a state of chronic airway inflammation underlying asthmatic response.[27] The present study also showed that IL-17 was significantly higher in the study group than in the control group. No significant intergroup change was observed in the levels of following cytokines: IL-2, IL-4, IL-6, IL-10, IL-12, and IL-13. The mouse asthma model experiments have shown that IL-17 is secreted by Th17 cells, and after allergen exposure in sensitized individual, it migrates to the airways causing asthmatic symptoms.[28] In humans, the same mechanism may also happen with increased expression of IL-17 supporting the Th17-mediated pathogenesis of asthma.[29,30] The present study also demonstrated the fact that IL-17 is an important factor for the development of manifestations of asthma, and its level was upregulated in the study group compared to the control.
An interesting observation made in the present study was the presence of high prevalence of rice allergy (almost in one-third children), not previously reported in any study from India. Rice allergy is common in Japan, but much lower in the USA and Europe.[31] Rice allergy can manifest as atopic dermatitis, eczema or asthma, and in severe cases, anaphylactic reactions may occur.[31] A multigene family of 14-16 kDa protein represent the major allergens that show significant homology to the alpha-amylase/trypsin inhibitor family from barley and wheat.[32,33]
A significantly negative correlation between the duration of breastfeeding and serum total IgE level as well as other cytokines was found. Studies have shown different immunologic constituents of allergic and nonallergic mothers and breast milk contain some factors that may prevent atopy at an early age.[34,35] The present study finding was in accordance with them.
A direct interaction exists between the different types of T-cells (Th1, Th2, and Th17), but this interaction is influenced by various extraneous factors too. In the present study, a negative correlation was found between IL-10 and IL-17. IL-6 has been found to be a strong inducer of Th17 cell,[36] but the present study found a positive but nonsignificant correlation between these two. Not limited only to its pathogenetic role in asthma, IL-17 regulation may be an attractive therapeutic approach for asthma also.[37]
CONCLUSIONS
The present study found a positive correlation between total serum IgE level and Th2, Th17 cytokines in a pediatric population with asthma. A significant negative correlation was found between the duration of breastfeeding and the cytokines.
Financial support and sponsorship
This research is supported by Indian Council of Medical Research, New Delhi, India (grant no. 62/1/2006-BMS). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Woods RK, Thien F, Raven J, Walters EH, Abramson M. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann Allergy Asthma Immunol. 2002;88:183–9. doi: 10.1016/S1081-1206(10)61994-1. [DOI] [PubMed] [Google Scholar]
- 2.Varga EM, Nouri-Aria K, Till SJ, Durham SR. Immunomodulatory treatment strategies for allergic diseases. Curr Drug Targets Inflamm Allergy. 2003;2:31–46. doi: 10.2174/1568010033344507. [DOI] [PubMed] [Google Scholar]
- 3.Kumar RK, Hitchins MP, Foster PS. Epigenetic changes in childhood asthma. Dis Model Mech. 2009;2:549–53. doi: 10.1242/dmm.001719. [DOI] [PubMed] [Google Scholar]
- 4.Gern JE, Lemanske RF, Jr, Busse WW. Early life origins of asthma. J Clin Invest. 1999;104:837–43. doi: 10.1172/JCI8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ. Novel approaches and targets for treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S72–9. doi: 10.1164/ajrccm.160.supplement_1.17. [DOI] [PubMed] [Google Scholar]
- 6.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet. 2008;372:1073–87. doi: 10.1016/S0140-6736(08)61449-X. [DOI] [PubMed] [Google Scholar]
- 7.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 8.Grünig G. IL-13 and adenosine: Partners in a molecular dance? J Clin Invest. 2003;112:329–31. doi: 10.1172/JCI19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minniti F, Comberiati P, Munblit D, Piacentini GL, Antoniazzi E, Zanoni L, et al. Breast-milk characteristics protecting against allergy. Endocr Metab Immune Disord Drug Targets. 2014;14:9–15. doi: 10.2174/1871530314666140121145045. [DOI] [PubMed] [Google Scholar]
- 10.Orivuori L, Loss G, Roduit C, Dalphin JC, Depner M, Genuneit J, et al. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: The PASTURE cohort study. Clin Exp Allergy. 2014;44:102–12. doi: 10.1111/cea.12199. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. Adistinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: The Th17 lineage. Curr Opin Immunol. 2006;18:349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–32. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–85. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov S, Lindén A. Interleukin-17 as a drug target in human disease. Trends Pharmacol Sci. 2009;30:95–103. doi: 10.1016/j.tips.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Fu CL, Ye YL, Lee YL, Chiang BL. Both allergen-specific CD4 and CD8 Type 2 T cells decreased in asthmatic children with immunotherapy. Pediatr Allergy Immunol. 2003;14:284–91. doi: 10.1034/j.1399-3038.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 18.Raedler D, Ballenberger N, Klucker E, Böck A, Otto R, Prazeres da Costa O, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135:81–91. doi: 10.1016/j.jaci.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal S, Townley RG. Role of periostin, FENO, IL-13, lebrikzumab, other IL-13 antagonist and dual IL-4/IL-13 antagonist in asthma. Expert Opin Biol Ther. 2014;14:165–81. doi: 10.1517/14712598.2014.859673. [DOI] [PubMed] [Google Scholar]
- 20.Afshar R, Medoff BD, Luster AD. Allergic asthma: A tale of many T cells. Clin Exp Allergy. 2008;38:1847–57. doi: 10.1111/j.1365-2222.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomita K, Lim S, Hanazawa T, Usmani O, Stirling R, Chung KF, et al. Attenuated production of intracellular IL-10 and IL-12 in monocytes from patients with severe asthma. Clin Immunol. 2002;102:258–66. doi: 10.1006/clim.2001.5176. [DOI] [PubMed] [Google Scholar]
- 22.Woodfolk JA, Platts-Mills TA. The immune response to intrinsic and extrinsic allergens: Determinants of allergic disease. Int Arch Allergy Immunol. 2002;129:277–85. doi: 10.1159/000067595. [DOI] [PubMed] [Google Scholar]
- 23.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–23. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott-Taylor TH, Hourihane JB, Harper J, Strobel S. Patterns of food allergen-specific cytokine production by T lymphocytes of children with multiple allergies. Clin Exp Allergy. 2005;35:1473–80. doi: 10.1111/j.1365-2222.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 25.Koulis A, Robinson DS. The anti-inflammatory effects of interleukin-10 in allergic disease. Clin Exp Allergy. 2000;30:747–50. doi: 10.1046/j.1365-2222.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- 26.Chernyshov PV. B7-2/CD28 costimulatory pathway in children with atopic dermatitis and its connection with immunoglobulin E, intracellular interleukin-4 and interferon-gamma production by T cells during a 1-month follow-up. J Eur Acad Dermatol Venereol. 2009;23:656–9. doi: 10.1111/j.1468-3083.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 27.Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003;18:415–28. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 28.Lindén A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–72. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183:5113–20. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H) 17 cells. Nat Med. 2008;14:337–42. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber RW. Allergen of the month – Rice. Ann Allergy Asthma Immunol. 2012;108:A11. doi: 10.1016/j.anai.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Hirano K, Hino S, Oshima K, Nadano D, Urisu A, Takaiwa F, et al. Evaluation of allergenic potential for rice seed protein components utilizing a rice proteome database and an allergen database in combination with IgE-binding of recombinant proteins. Biosci Biotechnol Biochem. 2016;80:564–73. doi: 10.1080/09168451.2015.1116927. [DOI] [PubMed] [Google Scholar]
- 33.González-De-Olano D, Pastor-Vargas C, Pérez-Bustamante MS, Maroto AS, González-Mancebo E, Gandolfo-Cano M, et al. Occupational allergy to rice involving a-amylase inhibitor as the relevant allergen. Ann Allergy Asthma Immunol. 2012;109:71–2. doi: 10.1016/j.anai.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Böttcher MF, Jenmalm MC, Garofalo RP, Björkstén B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:157–62. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 35.Casas R, Böttcher MF, Duchén K, Björkstén B. Detection of IgA antibodies to cat, beta-lactoglobulin, and ovalbumin allergens in human milk. J Allergy Clin Immunol. 2000;105(6 Pt 1):1236–40. doi: 10.1067/mai.2000.105805. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SJ, Lee YC. Interleukin-17 regulation: An attractive therapeutic approach for asthma. Respir Res. 2010;11:78. doi: 10.1186/1465-9921-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]