Abstract
Study Design:
Systematic review.
Objective:
To conduct a systematic review and synthesis of the literature to assess the comparative effectiveness, safety, and cost-effectiveness of early (≤24 hours) versus late decompression (>24 hours) in adults with acute spinal cord injury (SCI).
Methods:
A systematic search was conducted of Medline, EMBASE, the Cochrane Collaboration Library, and Google Scholar to identify studies published through November 6, 2014. Studies published in any language, in humans, and with an abstract were considered for inclusion. Included studies were critically appraised and the overall strength of evidence was determined using methods proposed by the Grading of Recommendation Assessment, Development and Evaluation working group.
Results:
The search yielded 449 potentially relevant citations. Sixteen additional primary studies were identified through other sources. Six studies met inclusion criteria. All but 2 studies were considered to have moderately high risk of bias. Across studies and injury levels, the impact of early surgical decompression (≤24 hours) on clinically important improvement in neurological status was variable. Isolated studies reported statistically significant and clinically important improvements at 6 months (cervical injury, low strength of evidence) and following discharge from inpatient rehabilitation (all levels, very low strength of evidence) but not at other time points; another study observed a statistically significant 6 point improvement in ASIA Impairment Scale (AIS) among patients with AIS B, C, or D, but not for those with AIS A (very low strength of evidence). In one study of acute central cord syndrome without instability, a clinically and statistically meaningful improvement in total motor scores was reported at 6 and 12 months in patients treated early (versus late). There were, however, no significant differences in AIS improvement between early and late surgical groups at 6- or 12-months (very low strength of evidence). One of 3 studies found a shorter length of hospital stay associated with early surgical decompression. Of 3 studies reporting on safety, no significant differences in rates of complications (including mortality, neurologic deterioration, pneumonia or pressure ulcers) were noted between early and late decompression groups.
Conclusions:
Results surrounding the efficacy of early versus late decompressive surgery, as well as the quality of evidence available, were variable depending on the level of SCI, timing of follow-up, and specific outcome considered. Existing evidence supports improved neurological recovery among cervical SCI patients undergoing early surgery; however, evidence regarding remaining SCI populations and clinical outcomes was inconsistent.
Keywords: timing of surgery, spinal cord injury, systematic review
Introduction
Historically, the cornerstones of therapy for the acute management of spinal cord injury (SCI) rested on principles of closed reduction, with prolonged courses of external immobilization, with little priority given to acute surgical care. However, over the past few decades, clinicians within the field have prioritized the early surgical management of SCI patients.1–3 From a biological perspective, preclinical evidence suggests that persistent compression of the spinal cord after the primary injury represents a reversible form of secondary injury, which, if ameliorated in an expeditious fashion, may lead to reduced neural tissue injury and improved outcomes.4–6 A 2013 meta-analysis, collating results from 21 animal studies, found that surgical decompression of the spinal cord improves neurobehavioral outcomes by 35% and that early intervention is one of the key predictors of improvement.7 From a surgical perspective, advancements in rigid segmental fixation techniques have allowed decompression of the spinal cord to be paired with restoration of the structural integrity of the vertebral column. Such stabilization not only prevents ongoing trauma to the spinal cord but also allows for early patient mobilization and institution of physical therapy and rehabilitation.
In spite of the justification provided above, judgment about the true suitability of early surgical decompression rests on its demonstrated efficacy and safety in clinical studies. Accordingly, a number of articles investigating the impact of early surgery on clinical outcomes have emerged in recent years. Across these studies, several different time thresholds have been used to define “early” versus “late” surgical decompression. While studies investigating 48- and 72-hour thresholds have been less frequently considered, the earlier 24-hour threshold has served as the basis for the majority of recent studies, and hence is the time window most suitable for the focus of an in-depth review.8 Furthermore, previous groups, including the Spinal Trauma Study Group, identified the first 24 hours as the most promising time window during which decompression may afford neuroprotection.9 At present, no surgical guidelines exist that rigorously explore the merits of early versus late surgical decompression for SCI, relative to the 24-hour threshold.
Given this background, the objective of this systematic review is to critically appraise and summarize evidence on the comparative effectiveness, safety, and cost-effectiveness of early (≤24 hours) versus late decompression (>24 hours) in adults with acute traumatic SCI. This systematic review was undertaken to support the development of a clinical practice guideline on care of individuals with acute SCI. With respect to this topic, we sought to answer the following key questions for adult patients with acute complete or incomplete traumatic SCI:
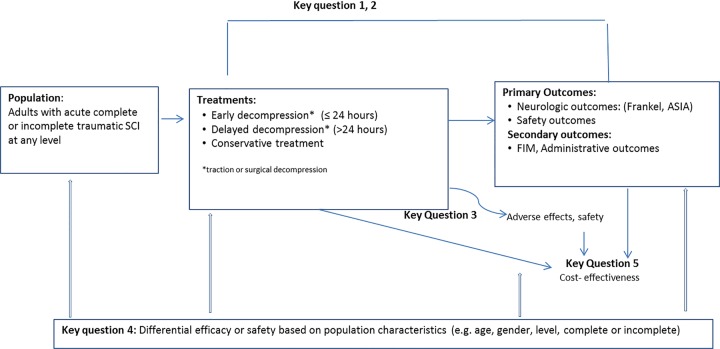
Key Question 1: What is the efficacy and effectiveness of early decompression (≤24 hours) compared with late decompression (>24 hours) or conservative therapy based on clinically important change in neurological status?
Key Question 2: Does timing of decompression influence other functional outcomes or administrative outcomes?
Key Question 3: What is the safety profile of early decompression (≤24 hours) compared with late decompression (>24 hours) or conservative therapy?
Key Question 4: What is the evidence that early decompression (≤24 hours) has differential efficacy or safety in subpopulations?
Key Question 5: What is the cost-effectiveness of the treatment options evaluated in Key Questions 1 to 4?
Figure 1 outlines the relationships between the key questions based on the primary time frame of interest.
Figure 1.
Analytic framework.
Materials and Methods
Electronic Literature Search
A systematic search of the literature was performed to identify potential studies published through November 6, 2014. Studies published in any language, on humans, and with an abstract were considered for inclusion. The PubMed interface was used to search the MEDLINE database for primary studies and systematic reviews. The Cochrane Collaboration Library, EMBASE, and the bibliographies of relevant articles were also searched. The focus of this review was to identify studies explicitly designed to evaluate the effects of early decompression (defined for the purpose of this review as ≤24 hours) compared with late decompression (>24 hours) or conservative therapy in adults with complete or incomplete traumatic SCI.
We searched for studies that used either surgical or nonsurgical methods to relieve compression of the spinal cord and that reported on outcomes of efficacy or effectiveness (neurological outcomes such as Frankel grade and American Spinal Injury Association [ASIA] Impairment Scale [AIS]), functional and patient-reported outcomes (eg, Functional Independence Measure [FIM]), administrative outcomes (eg, length of stay [LOS] in intensive care unit [ICU] and/or hospital), and safety (eg, complications and adverse events). There is currently no validated threshold to define a clinically meaningful difference for neurological outcomes; however, consultation with clinical authors suggested that a 2-grade change in outcomes such as Frankel grade and AIS, and a 5 point change in AIS Motor Score are clinically meaningful. These thresholds have been used in previous trials that examined the efficacy of several pharmacological agents on neurological recovery post–acute SCI.10,11 We excluded studies that did not adjust for injury severity at baseline. However, studies were included if injury severity at baseline was similar between groups.
The search strategy is described in the supplemental material (available in the online version of the article) and included use of controlled vocabulary (MeSH terms) as well as keywords. Terms specific to decompression included microdecompression OR microdiscectomy OR open decompression OR laminectomy OR traction OR mechanical traction OR inversion therapy. These terms were combined with terms specifying SCI and timing of intervention.
We sought randomized controlled trials (RCTs) and prospective cohort studies comparing early versus late decompression. The search was expanded to include retrospective cohort studies because only 1 RCT and 3 nonrandomized prospective cohort studies satisfied the inclusion criteria.
Study Selection and Data Abstraction
One reviewer (HH) screened the electronic search results and excluded nonrelevant studies. Two or more reviewers (HH, AS, EB, KP, EAF) then screened the remaining titles and abstracts, retrieved the full texts of potentially relevant studies, and independently evaluated the studies against inclusion and exclusion criteria. In each stage of the screening process, disagreement concerning inclusion of studies was resolved by discussion or, if necessary, by consulting a third reviewer. If an article published in a foreign language appeared to meet inclusion criteria based on the English abstract, Google Translate12 was used to translate the article into English. If any part of the translation was unclear, human translation assistance was sought. Trained research assistants (KM, EB, KP, EAF) abstracted the following data: age, sex, completeness and level of SCI, whether or not baseline assessment was conducted, if any adjunct medical therapy was administered (eg, methylprednisolone), timing of decompression, and results related to neurological, functional, and safety outcomes.
Individual Study Quality
A minimum of 2 independent systematic review methodologists (HH, EB, ACS) critically appraised each included study for risk of bias based on criteria set by The Journal of Bone & Joint Surgery, American Volume 13 for therapeutic studies, and modified to delineate criteria associated with methodological quality14 (see the supplemental material). Disagreements in ratings were resolved through discussion.
Overall Strength of Body of Literature
After rating each individual article, the strength of the overall body of evidence with respect to each outcome was determined based on precepts outlined by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) Working Group15,16 and recommendations made by the Agency for Healthcare Research and Quality (AHRQ).17 Additional qualitative analysis was performed according to AHRQ-required (risk of bias, consistency, directness, precision) and additional domains (dose-response, strength of association, publication bias).18 In general, risk of bias was determined when evaluating each individual article as described above (see the supplemental material).
The initial strength of the overall body of evidence was considered High for RCTs and Low for observational studies. The body of evidence for a given outcome may be downgraded 1 or 2 levels based on the following criteria: (1) risk of bias (study limitations), (2) inconsistency of results, (3) indirectness of evidence, (4) imprecision of the effect estimates (eg, wide confidence intervals), or (5) failure to provide an a priori statement of subgroup analyses. The body of evidence may be upgraded 1 or 2 levels based on the following criteria if no downgrades were made: (1) large magnitude of effect or, (2) dose-response gradient or (3) if all plausible biases would decrease the magnitude of an apparent effect.
The final overall strength of the body of literature expresses (1) our confidence that the effect size lies close to the true effect and (2) the extent to which the effect is believed to be stable based on the adequacy of, or deficiencies in the body of evidence.16 An overall strength of “High” means that we are very confident that the true effect lies close to that of the estimated effect. A “Moderate” rating means that we are moderately confident in the effect estimate; the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. An overall strength of “Low” means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate. Finally, a rating of “Very Low” means that we have very little confidence in the effect estimate; the true effect is likely to be substantially different than the estimated effect. In addition, this rating may be used if there is no evidence or it is not possible to estimate an effect.
Analysis
When available, we reported effect sizes from multivariate analysis (ie adjusted effect size estimates) and/or level of significance following adjustment for confounders. When data was available for studies that did not use multivariate analysis, we calculated unadjusted risk ratios (RRs) and 95% confidence intervals (95% CIs) to estimate the effect size and evaluate whether an association was present. Calculations were performed using Stata 9.0.19 Fisher’s exact test was used if cells had fewer than 5 observations. For continuous data, we computed either (1) the mean change or percent change from preoperative values if the data was not part of multivariate analysis or (2) differences in means to estimate effect size if standard deviations were provided. Key Question 4 evaluated whether special populations such as the elderly are differentially affected by treatments. In other words, this question aimed to answer if a factor such as age “modifies” treatment effect or safety. If data from RCTs was available, we assessed the extent to which the magnitude of estimates (eg, RRs and corresponding 95% CIs) were different in one stratum versus another in the same underlying population (direct comparison). Furthermore, the presence of effect modification was evaluated using a statistical test for interaction. Pooling of data was considered if studies were reasonably homogeneous with respect to study quality, clinical factors, and outcome measures. No clinically minimum important difference has been established for improved neurological status. However, based on consultation with clinical experts, an improvement of 2 or more grades for Frankel or AIS, or a 5-point improvement in AIS Motor Score was considered a priori to represent clinically meaningful improvements. Evaluation of overall quality (strength) of evidence focused on clinically meaningful improvements.
Results
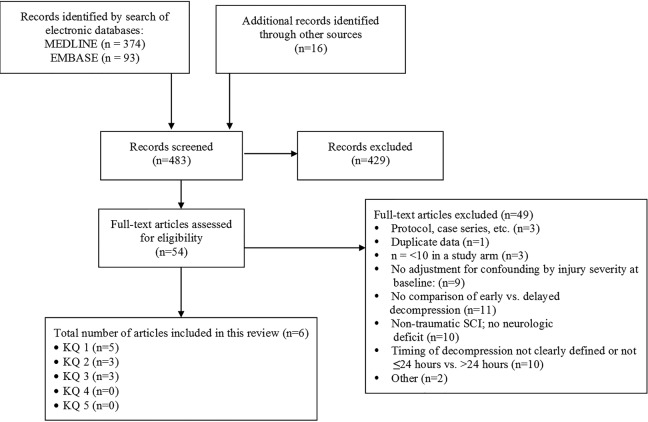
The search strategy yielded 449 citations. Sixteen additional primary studies were identified through other sources such as reference lists. Three hundred and twenty-five citations were excluded based on their title and/or abstract, while 52 were selected for full-text investigation (Figure 2). Four comparative studies (3 prospective20–22 and 1 retrospective23), 1 RCT,24 and 1 prospective observational study25 met inclusion criteria and are summarized in this systematic review. Nine additional comparative studies were identified that reported on decompression performed at ≤24 hours versus >24 hours; however, they were excluded because these studies did not adjust for injury severity at baseline. A list of excluded studies and the rationale for exclusion is provided in the supplemental material. Due to the heterogeneity of studies (injury level, measures used, and clinical characteristics), pooling of data was not done for any of the outcomes of interest (see Table 1).
Figure 2.
Study selection flow chart.
Table 1.
Patient, Intervention, Comparators, and Outcomes (PICO).
| Study Component | Inclusion | Exclusion |
|---|---|---|
| Participants | Adults with traumatic acute spinal cord injury (complete or incomplete) |
|
| ||
|
||
|
||
|
||
| Intervention |
|
|
| Comparators |
|
|
|
||
| Outcomes | Efficacy/Effectiveness | |
|
||
|
||
|
||
|
||
| Functional or Patient Reported Outcomes | ||
|
||
| Administrative Outcomes | ||
|
||
| Safety Outcomes | ||
|
||
|
||
|
||
| Study design |
|
|
|
|
|
|
||
|
||
|
||
|
||
| Publication |
|
|
|
||
|
||
|
||
|
||
|
||
|
Abbreviations: CSM, cervical spondylotic myelopathy; RCT, randomized controlled trial.
Table 2 summarizes study features and demographic information for included studies (detailed data abstraction is found in the supplemental material). The 6 included studies (1 RCT and 5 comparative cohort studies [4 prospective and 1 retrospective]) provided data on the effectiveness of surgical decompression performed within 24 hours of injury compared with after 24 hours of injury. Of these, 1 study contributed data on cervical SCI,21 1 study on thoracolumbar SCI,24 1 study on cervical and thoracolumbar SCI,23 1 study on acute central cord syndrome without instability,25 and 2 studies on all levels of SCI20,22 (detailed patient characteristics are provided in the supplemental material). No studies described timing of nonsurgical decompression.
Table 2.
Demographics for Included Studies Comparing Early (≤24 Hours) and Late (>24 Hours) Decompression.
| Author (Year), Study Design (Risk of Bias), Follow-up | Demographics | Baseline Neurological Status | Patient Characteristics | Intervention(s) and Co-intervention(s) | Inclusion/Exclusion |
|---|---|---|---|---|---|
| Cervical SCI | |||||
| Fehlings (2012) STASCIS trial (multicenter) Prospective cohort (moderately low risk of bias) Follow-up: 6 months (70.9%, n = 222/313; Early: 72.0%, n = 131/182; Late: 69.5%, n = 91/131) | Early (<24 hours) N = 182 Mean age (±SD): 45.0 ± 17.2 years Male: 76.9% Late (≥24 hours) N = 131 Mean age (±SD): 50.7 ± 15.9 years Male: 73.3% | Initial neurologic exam at presentation (within 24 hrs. of admission) ASIA grade, n (%) Early A: 65 (35.7) B: 40 (22.0) C: 32 (17.6) D: 45 (24.7) Late A: 36 (27.5) B: 14 (10.7) C: 34 (26.0) D: 47 (35.9) |
|
Interventions
|
Inclusion:
|
| Cervical and thoracolumbar SCI | |||||
| Bourassa-Moreau (2013) Retrospective cohort (moderately high risk of bias) Follow-up: Mean NR; acute hospital stay only (97.2%, n = 419/431) | Overall N = 431 Mean age (±SD): 41.8 ± 17.6 years Males: 77.7% Early (<24 hours) N = 90 Mean age (±SD): 37.0 ± 15.9 years Males: 82.2% Late I (24-72 hours) N = 231 Mean age (±SD): 40.7 ± 17.3 years Males: 78.4% Late II (>72 hours) N = 110 Mean age (±SD): 47.9 ± 18.0 years Males: 72.7% | Initial assessment time was not reported ASIA grade, n (%) Overall A: 197 (45.7) B: 71 (16.5) C: 61 (14.2) D: 102 (23.7) Early A: 55 (61.1) B: 16 (17.8) C: 8 (8.9) D: 11 (12.2) Late I A: 109 (47.2) B: 42 (18.2) C: 38 (16.5) D: 42 (18.2) Late II A: 33 (30.0) B: 13 (11.8) C: 15 (13.6) D: 49 (44.5) |
|
Intervention
|
Inclusion:
|
| Thoracolumbar SCI | |||||
| Rahimi-Movghar (2014) RCT (moderately low risk of bias) Follow-up: Early 1 month: 87.5% (n = 14/16) 3 months: 56.3% (n = 9/16) 6 months: 87.5% (14/16) 12 months: 93.8% (15/16) Late 1 month: 73.7% (14/19) 3 months: 63.2% (12/19) 6 months: 78.9% (15/19) 12 months: 94.7% (18/19) | Early (<24 hours) N = 16 Mean age (±SD): 31.7 ± 9.1 years Male: 69.0% Mean time to surgery (± SD): 18.9 ± 4.75 hours Late (24–72 hours) N = 19 Mean age (±SD): 37.8 ± 13.70 years Male: 74.0% Mean time to surgery (± SD): 45.0 ± 11.93 hours | Initial neurologic examinations performed on admission, preoperatively, immediately after surgery, and at 1, 3, 6, and 12-months follow-ups ASIA grade, n (%) Early A: 7 (44.0) B: 1 (6.0) C: 4 (25.0) D: 4 (25.0) Late A: 9 (47.0) B: 5 (26.0) C: 1 (5.0) D: 4 (21.0) |
|
Interventions
|
Inclusion:
|
| Cervical and thoracic and lumbosacral SCI | |||||
| Dvorak (2015) Prospective cohort (moderately high risk of bias) Rick Hansen Spinal Cord Injury Registry Follow-up: NR | Overall N = 888 Mean age (range): 45.7 (76) years Male: 76.5% Mean time to surgery (±SD): 60.4 ± 80 hours Early (<24 hours) N = 355 Average age: NR Male: NR Late (24–168 hours) N = 533 Average age: NR Male: NR | Initial neurologic exam performed within 72 hours of injury ASIA grade, n (%) Overall A: 292 (38.8) B: 90 (12) C: 138 (18.4) D: 232 (30.9) |
|
Interventions
|
Inclusion:
|
| Wilson (2012) Prospective cohort (moderately high risk of bias) Ontario Spinal Cord Registry Follow-up: Acute care discharge: Mean 24.8 ± 29.2 days (97.6%, n = 82/84) Inpatient rehabilitation discharge: Mean 89.6 ± 47.4 days (65.4%, n = 55/84; Early: 62.9%, n = 22/35; Late: 67.3%, n = 33/49) | Overall N = 84 Mean age: 45.3 years Males: 80.1% Early (<24 hours) N = 35 Mean age: 41.6 years Males: 83% Mean time to surgery (±SD): 12.7 ± 4.9 hours Late (≥24 hours) N = 49 Mean age: 47.9 years Males: 78% Mean time to surgery (±SD): 155.0 ± 236.7 hours | Initial assessment at acute-care admission ASIA grade, n (%) Early A: 18 (51) B: 6 (17) C: 5 (14) D: 6 (17) Late A: 15 (31) B: 3 (6) C: 6 (12) D: 25 (51) |
|
Interventions
|
Inclusion:
|
| Acute central cord injury without instability | |||||
| Lenehan (2010) Prospective observational dataset (moderately high risk of bias) Spine Trauma Study Group Follow-up: 1 year, % NR | Overall N = 73 Mean age (±SD): 58.2 ± 14.4 years Male: 81.0% Mean time from injury to admission (±SD): 25.6 ± 13.6 hours Mean time from admission to surgery (±SD): 37.7 ± 85.7 hours Early (<24 hours) N = 17 Mean age at injury (±SD): 55.0 ± 14.4 years Male: 82.3% Late (≥24 hours) N = 56 Mean age at injury (±SD): 59.1 ± 14.3 years Male: 80.3% | Timing of initial neurological examination was not reported Comorbidity (%): Overall: None: 57 (78) One: 14 (19) More: 2 (3) Early: Yes: 3 (17.65) No: 14 (82.35) Late: Yes: 13 (23.21) No: 43 (76.79) ASIA Motor Score: Overall (mean ± SD): 25.6 ± 13.6 Overall (median, range): 41.0 (1.0 to 50.0) Early (mean ± SD): 61.1 ± 29.2 Late (mean ± SD): 63.5 ± 25.1 ASIA grade (%): Overall: C: 28 (38) D: 45 (62) Early: C: 9 (52.94) D: 8 (47.06) Late: C: 19 (33.93) D: 37 (66.07) |
|
Interventions
|
Inclusion:
|
Abbreviations: AANS, American Association of Neurological Surgeons; AIDS, acquired immunodeficiency syndrome; AIS, ASIA Impairment Score; ASIA, American Spinal Injury Association; CCS, central cord syndrome; CoE, class of evidence; CT, computed tomography; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MRI, magnetic resonance imaging; MVA, motor vehicle accident; NR, not reported; RCT, randomized controlled trial; RHSCIR, Rick Hansen Spinal Cord Injury Registry; SCI, spinal cord injury; SD, standard deviation; STASCIS, Surgical Timing in Acute Spinal Cord Injury Study; TSCI, traumatic spinal cord injury.
Each article was critically appraised and assessed for risk of bias. Five studies were originally considered to have moderately high risk of bias (level of evidence [LoE] III),20–23,25 and one study, an RCT, was considered to have moderately low risk of bias (LoE II).24 Results were presented to a guideline development group who formulated recommendations for a clinical practice guideline for patients with acute SCI. Following presentation to this group, the rating for one study was revised from moderately high risk of bias to moderately low risk of bias.21 This study was initially downgraded for having a follow-up less than 80%; however, 73% of the guideline development group voted that a 70% follow-up rate was acceptable for this study. The rationale for this revision included the following: (1) there are logistical challenges associated with following patients with acute SCI; (2) the group hypothesized that patients with problems are more likely to attend their follow-up appointments despite these logistical challenges; and (3) the other components of this study were methodologically sound. This exception was only relevant for the study by Fehlings et al.21 Two studies reported independent or blind assessment of primary outcomes.21,24 Two studies indicated that co-interventions (eg, methylprednisolone, rehabilitation) were applied equally between groups.21,22 Three studies appeared to have an adequate sample size.20,21,23 All 6 studies controlled for possible confounding factors, including baseline neurologic status. One study25 did not describe patient eligibility (or number eligible), selection, attrition, or details of propensity scoring. Only 2 studies had a follow-up ≥80%23,24; a third study did not provide sufficient detail to determine attrition.20 Appraisal of individual studies and rating of overall quality (strength) of evidence are found in the supplemental material. Summaries of the overall strength of evidence for the primary outcomes are provided in Table 7.
Table 7.
Evidence Summary and Strength of Evidence: Comparison of Outcomes Between Early (≤24 Hours) Versus Late (>24 Hours) Decompression.
| Outcome | Studies (n) | Strength of Evidence | Conclusions, Effect Size |
|---|---|---|---|
| Question 1: Efficacy and effectiveness based on neurologic outcomes | |||
| Cervical SCI | 1 prospective cohort (Fehlings 2012) (N = 222) | Lowa | At 6 months: There was no significant difference in the odds of achieving a ≥1 grade improvements on AIS between patients decompressed early versus late (ORadj = 1.37, 95% CI = 0.80 to 2.57, P = 0.31) Patients decompressed early were more likely to exhibit clinically significant improvement in neurological status (a ≥2 grade improvement on AIS) than those decompressed late (ORadj = 2.83, 95% CI = 1.10 to 7.28, P = .03) |
| AIS Improvement | |||
| Cervical and thoracic and lumbosacral SCI | 1 prospective cohort (Wilson 2012); Acute care (N = 82) | Very lowb | Acute care discharge: There were no differences in mean AIS motor score improvement between early and late decompression groups (P = .18) or in frequency of ≥1 grade (RR = 1.15, 95% CI = 0.48 to 2.79, P = .7499) or ≥2 grade improvement on AIS (RR = 4.45, 95% CI = 0.48 to 41.0, P = .2974) |
| AIS Improvement | IP rehabilitation (N = 55) | Very lowb | IP Rehabilitation discharge: Patients receiving early decompression exhibited an additional 13-point improvement in AIS Motor Score compared to those treated later. A higher proportion of patients in the early decompression group exhibited a ≥2 grade improvement on AIS than in the late surgical group (RR = 8.9, 95% CI = 1.12 to 70.64, P = .0154); the stability of this estimate is questionable |
| AIS Motor Score Improvement | 1 prospective cohort (Dvorak) (N = 888) | Very lowc | Follow-up/timing not reported: AIS A: There was no difference in AIS improvement between early and late decompression groups (Beta = 0.068, 95% CI = −0.625 to 0.76, P = .848) |
| AIS B, C, D: Patients decompressed early improved by 6 more motor points than those decompressed late (Beta = 6.258, 95% CI = 0.618 to 11.897, P = .03) | |||
| Thoracolumbar SCI | 1 RCT (Rahimi-Movghar) (N = 35) | Lowb | At 12 months: There was no difference in the frequency of patients who achieved a ≥1 grade improvement on AIS between the early and late surgical groups (5 vs 7 persons; RR = 0.85, 95% CI = 0.33 to 2.16) |
| AIS Improvement | More patients in the early decompression group experienced a ≥2 grade improvement on AIS than in the late decompression group; however, this relationship did not reach statistical significance (3 vs 1; RR = 3.56, 95% CI = 0.41 to 30.99) | ||
| Definitive conclusions cannot be drawn due to the small sample size and study limitations | |||
| Acute central cord injury without instability | 1 retrospective observational study (Lenehan, 2010) (N = 73) | Very Lowb | 6 months: Early surgery, compared to late decompression, was associated with an additional 7.47 (95% CI = −0.04 to 14.91, P = .0511) point improvement in AIS Motor Score; however, this relationship was within the limits of chance. There was no significant difference in improvement on AIS between early and late surgical groups (ORadj = 3.39, 95% CI = 0.75 to 15.34, P = .1131) |
| AIS Improvement | 12 months: Early surgery, compared to late decompression, was associated with an additional 6.31 (95% CI = 0.44 to 12.18, P = .0359) point improvement in AIS Motor Score. There was no significant difference in improvement on AIS between early and late surgical groups (ORadj = 2.81, 95% CI = 0.48 to 16.6, P = .2548) | ||
| AIS Motor Score Improvement | |||
| Question 2: Functional and administrative outcomes | |||
| Functional outcomes | |||
| Acute central cord injury without instability | 1 prospective observational study (Lenehan, 2010), N = 73 | Very lowb | 12-months: Patients treated early, compared to those decompressed late, exhibited an additional 6.92 (95% CI = −0.11 to 13.96, P = .0537) point improvement on the FIM motor subscore; however, this association was within the limits of chance. |
| FIM Motor Sub-Score Improvement | 12 months: Patients treated early, compared to those decompressed late, exhibited an additional 7.79 (95% CI = 0.09 to 15.49, P = .0474) point improvement on the FIM total score. | ||
| FIM Total Score Improvement | |||
| Administrative outcomes | |||
| Cervical and thoracic and lumbosacral SCI | 1 prospective cohort (Wilson 2012) | ||
| Length of stay | Acute care (N = 82) | Very lowb | Acute care: There was no significant difference in length of stay between the early and late decompression groups (25 days for each group) |
| IP rehabilitation (N = 55) | Very lowb | IP rehabilitation: There was no significant difference in length of stay between the early and late decompression groups (102.9 vs 80.2 days, respectively) | |
| 1 prospective cohort (Dvorak) (N = 888) | Very lowc | Setting/timing not reported: In AIS A patients, there was a significantly shorter length of stay following early decompression compared to late decompression (7.5 days vs NR, respectively; P = .004). In AIS B patients, there was a significantly shorter length of stay following early decompression compared to late decompression (12.8 days vs NR, respectively; P = .003) | |
| Thoracolumbar SCI | 1 RCT (Rahimi-Movghar) (N = 35) | Lowb | There was no significant difference in length of stay between the early and late decompression groups (7 vs 9.7 days, respectively; mean difference = −2.7, 95% CI = −8.1 to 2.7) |
| Length of stay | |||
| Question 3: Safety outcomes | |||
| Cervical | 1 prospective cohort (Fehlings 2012) (N = 222) | Very lowd | There was no significant difference in rates of complications between groups; however, most were rare events and there was likely insufficient power to detect a difference (unadjusted RR, 95% CI) |
| Cardiopulmonary complications | Cardiopulmonary complications: RR = 0.68, 95% CI = 0.44 to 1.04 | ||
| Construct failure requiring surgery | Construct failure requiring surgery: RR = 2.16, 95% CI = 0.23 to 20.53 | ||
| Deep wound infection | |||
| Neurologic deterioration | Neurologic deterioration: RR = 2.88, 95% CI = 0.33 to 25.46 | ||
| Pulmonary embolism | Pulmonary embolism: RR = 0.72, 95% CI = 0.10 to 5.04 | ||
| Systemic infection | Systemic infection: RR = 0.54, 95% CI = 0.19 to 1.52 | ||
| Wound dehiscence | Wound dehiscence: RR = 0.72, 95% = 0.05 to 11.40 | ||
| Mortality; ≤30 days postinjury | Mortality; ≤30 days postinjury: RR = 0.72, 95% CI = 0.05 to 11.40 | ||
| Mortality; >30 days postinjury | |||
| Cervical and thoracolumbar | 1 retrospective cohort (Bourassa-Moreau 2013) (N =431) | Very lowc | There was no significant difference in rates of complications between groups; however, most were rare events and there was likely insufficient power to detect a difference (unadjusted RR, 95% CI). |
| Pneumonia | Pneumonia: RR = 0.62, 95% CI = 0.38 to 1.02 | ||
| Pressure ulcer | Pressure ulcer: RR = 0.66, 95% CI = 0.37 to 1.15 | ||
| Urinary tract infection | Urinary tract infection: RR = 0.82, 95% CI = 0.52 to 1.29 | ||
| Other complications | Other complications: RR = 0.76, 95% CI = 0.41 to 1.39 | ||
| Mortality | Mortality: RR = 1.26, 95% CI = 0.35 to 4.57 | ||
| Pneumonia was more common in the late surgery group (16.7% vs 26.7%, respectively) | |||
| Thoracolumbar | 1 RCT (Rahimi-Movghar) (N = 35) | Very lowb | There were no significant differences in rates of complications between groups; however, most were rare events and there was likely insufficient power to detect a difference (unadjusted RR, 95% CI) |
| Deep vein thrombosis | Deep vein thrombosis: RR = 1.2, 95% CI = 0.08 to 17.5 | ||
| Wound infection | |||
| CSF leak | |||
| Meningitis | |||
| Decubitis ulcer | |||
| Revision of surgical screws | Revision of surgical screws: RR = 0.79, 95% CI = 0.15 to 4.16 | ||
| Bilateral rod fracture | |||
| Death | Death: RR = 1.2, 95% CI = 0.08 to 17.5 | ||
Abbreviations: AIS, ASIA Impairment Score; ASIA, American Spinal Injury Association; SCI, spinal cord injury; IP, inpatient; NR, not reported.
aThere were differences in perspective among members of the guideline development group regarding the impact of and bias associated with a 30% loss to follow-up. This issue was discussed during 2 meetings and voted on. Seventy-three percent of the guideline development group agreed that a 70% follow-up rate was acceptable for this study. The rationale for this revision included the following: (1) there are logistical challenges associated with following patients with acute SCI; (2) the group hypothesized that patients with problems are more likely to attend their follow-up despite these logistical challenges; and (3) the other components of this study were methodologically sound. As a result, there was no downgrade for risk of bias and the overall strength of evidence was considered low instead of very low. This exception was not relevant for (1) the studies by Dvorak et al (2014) and Lenehan et al (2010) as attrition rate was not specified or (2) the study by Wilson et al (2012) as follow-up was less than 70%.
bDowngraded for serious risk of bias (studies did not meet 2 or more criteria of a good-quality RCT or cohort study) and lack of precision.
cDowngraded for serious risk of bias (studies did not meet 2 or more criteria of a good-quality RCT or cohort study) and lack of precision (confidence intervals are wide for estimates despite the overall study population size; this may be a function of the number of individuals in subanalyses).
dDowngraded for imprecision: these are rare outcomes and studies were not sufficiently powered to detect differences between groups; confidence intervals are large, reflecting imprecision.
eDowngraded for serious risk of bias due to high or unreported attrition rates and imprecision.
Delays in time to surgery ranged from greater than 24 hours (not further defined) to 168 hours. Inclusion and exclusion criteria varied across studies, but all patients presented with a neurologic deficit, most commonly evaluated using the AIS. The initial neurologic assessment was conducted at the time of admission in 3 studies21,22,24 and within 72 hours of injury in 1 study20; the time of evaluation was not reported in 2 studies.23,25 The most common causes of injury across studies were motor vehicle accidents and falls.21,24,25
Sample sizes ranged from 16 to 355 in the early surgical groups and from 19 to 533 in the late surgical groups. Males comprised the majority (>69%) of the study populations, and mean ages ranged from 41.8 to 58.2 years. One study had a follow-up time of 6 months,21 2 studies had a follow-up time of 12 months,24,25 and another study did not report timing of follow-up.20 Two other studies followed patients only during acute hospital stay22,23 or until discharge from inpatient rehabilitation.22 Follow-up rates ranged from 65.4% to 97.6%. Four studies reported the administration of methylprednisolone in their patients; steroid administration was at the discretion of the surgeon in 2 studies,21,22 and prescribed according to the NASCIS protocol in one.24 In a fourth study, further details were not provided about steroid use.23 Steroid injections and other co-interventions were poorly reported across studies and their impact on outcomes could not be assessed. Tables 3 to 6 summarize neurological, functional, administrative, and safety outcomes, respectively. Detailed study characteristics are provided in the supplemental material.
Table 3.
Summary Table of Neurological Outcomes Between Early (≤24 hours) and Late (>24 hours) Decompression.
| Author, Year, Study Design | Measure | Early, ≤24 Hours | Late, >24 Hours | Effect Size |
|---|---|---|---|---|
| Cervical SCI | ||||
| Fehlings, 2012 | AIS Improvement at 6 months | n = 131 | n = 91 | ORadj a: |
| Prospective cohort study | ≥1 grade improvement | 74 (56.5) | 45 (49.5) | 1.37 (95% CI = 0.80 to 2.57), P = .31 |
| ≥2 grade improvement | 26 (19.8) | 8 (8.8) | 2.83 (95% CI = 1.10 to 7.28), P = .03 | |
| Cervical and thoracic and lumbosacral SCI | ||||
| Dvorak, 2015 | AIS Improvement | Adjusted estimatesb | ||
| Prospective cohort study | “Improved score” in AIS A patients | n = NR | n = NR | Beta: 0.068 (95% CI = −0.625 to 0.76); P = .848 |
| IRR: 1.07 (95% CI = 0.54 to 2.14) | ||||
| “Improved score” in AIS B, C, and D patients | n = NR | n = NR | Beta: 6.258 (95% CI = 0.618 to 11.897); P = .03 | |
| IRR: 522.17 (95% CI = 1.855 to 146825.5) | ||||
| Wilson, 2012 | AIS Improvement (preoperative to acute-care discharge (mean 24.8 ± 29.2 days)) | n = 33 | n = 49 | Unadjusted RR: |
| Prospective cohort study | ≥1 grade improvement, n (%) | 7 (21.2) | 9 (18.4) | 1.15 (95% CI = 0.48 to 2.79), P = .7499 |
| ≥2 grade improvement, n (%) | 3 (9.1) | 1 (2.0) | 4.45 (95% CI = 0.48 to 41.0), P = .2974 | |
| AIS Improvement (preoperative to inpatient rehabilitation discharge (mean 89.6 ± 47.4 days)) | n = 22 | n = 33 | Unadjusted RR: | |
| ≥1 grade AIS improvement, n (%) | 9 (40.9) | 10 (30.3) | 1.33 (95% CI = 0.61 to 2.93), P = .4700 | |
| ≥2 grade AIS improvement, n (%) | 6 (27.2) | 1 (3.0) | 8.9 (95% CI = 1.12 to 70.64), P = .0154 | |
| AIS Motor Score Improvement (mean) | 6.2 | 9.7 | P = .18 | |
| Multivariate analysis predicting change in AIS Motor Score at rehabilitation discharge | NR | NR | Adjusted effect estimatec = 13.0, P = .01 | |
| Thoracolumbar SCI | ||||
| Rahimi-Movghar, 2014 | ASIA Impairment Grade at 12 months | n = 16 | n = 19 | RR: |
| RCT | ≥1 grade improvement, % (n) | 5 (31.2) | 7 (44) | 0.85 (95% CI = 0.33 to 2.16) |
| ≥2 grade improvement, % (n) | 3 (18.1) | 1 (5.2) | 3.56 (95% CI = 0.41 to 30.99) | |
| Mean change (± SD) from baseline in motor score improvementd | 15 (14.34) | 14 (13.3) | Difference in means: 1 (95% CI = −8.5 to 10.5, P = .8320) | |
| Acute central cord injury without instability | ||||
| Lenehan (2010) | n = 17 | n = 56 | ORadj e: | |
| Prospective observational study | AIS Improvement at 6-monthsc | NR | NR | 3.39 (95% CI = 0.75 to 15.34), P = .1131 |
| AIS Improvement at 12-monthsc | NR | NR | 2.81 (95% CI = 0.48 to 16.60), P = .2548 | |
| Total Motor Score Improvement at 6-months | NR | NR | Group differencee: 7.47 (95% CI = −0.04 to 14.91), P = .0511 | |
| Total Motor Score Improvement at 12-months | NR | NR | Group differencee: 6.31 (95% CI = 0.44 to 12.18), P = .0359 | |
Abbreviations: AIS, ASIA Impairment Score; CI, confidence interval; NR, not reported; OR, odds ratio; IRR, incidence rate ratio; RCT, randomized controlled trial; RR, risk ratio; SCI, spinal cord injury.
aOdds ratio adjusted for preoperative neurological status and steroid administration.
bAuthor reported estimates adjusted for age, injury severity score, and injury type.
cControlling for neurological level of injury and baseline neurological status, an additional 13 points in motor recovery was seen in patients treated within 24 hours of injury compared to those who underwent late decompression.
dAuthors reported no improvement in mean AIS motor score for either early or late decompression in patient with complete SCI. In contrast, improvement was observed in both groups of patients with incomplete SCI; data is not provided for comparison between early and late.
eAuthors reported that regression with propensity scoring was done to adjust for potential selection bias; however, details were not provided.
Table 4.
Summary Table of Administrative Outcomes Between Early (≤24 Hours) and Late (>24 hours) Decompression.
| Author, Year, Study Design | Measure | Early, ≤24 Hours | Late, >24 Hours | Effect Size |
|---|---|---|---|---|
| Cervical and thoracic and lumbosacral SCI | ||||
| Dvorak, 2015 | Length of stay | AIS A patients | AIS A patients | Adjusted estimatesa: |
| Prospective cohort study | Setting undefined | n = NR | n = NR | Beta: −0.358 (95% CI = −0.590 to −0.126), P = .003 |
| 7.5 days | Days: NR | IRR: 0.699 (95% CI = 0.554 to 0.881) | ||
| AIS B patients | AIS B patients | Beta: −0.181 (95% CI = −0.303 to −0.059), P = .004 | ||
| n = NR | n = NR | IRR: 0.834 (95% CI = 0.738 to 0.942) | ||
| 12.8 days | Days: NR | |||
| Wilson, 2012 | Length of stay | |||
| Prospective cohort study | Acute care | n = 33 | n = 49 | P = .97 |
| 24.9 days | 24.7 days | |||
| Rehabilitation | n = 22 | n = 33 | Mean difference: 22.7; P = .10 | |
| 102.9 days | 80.2 days | |||
| Thoracolumbar SCI | ||||
| Rahimi-Movghar, 2014 | Length of stay | n = 16 | n = 19 | Mean difference: −2.7 (95% CI = −8.1 to 2.7), P = .3137 |
| RCT | Setting undefined | 7.0 ± 7.13 days | 9.7 ± 8.28 days | |
Abbreviations: AIS, ASIA Impairment Score; CI, confidence interval; IRR, incidence rate ratio; NR, not reported; RCT, randomized controlled trial; SCI, spinal cord injury.
aAuthors reported estimates adjusted for age, sex, neurologic level, injury severity score score, and vertebral injury.
Table 5.
Summary Table of Functional Outcomes Between Early (≤24 Hours) and Late (>24 Hours) Decompression.
| Author, Year, Study Design | Measure | Early, ≤24 Hours | Late, >24 Hours | Effect Size |
|---|---|---|---|---|
| Acute central cord injury without instability | ||||
| Lenehan (2010) | n = 17 | n = 56 | Group differencea: | |
| Prospective observational study | FIM total score improvement from discharge to 12-months | NR | NR | 6.92 (95% CI = −0.11 to 13.96), P = .0537 |
| FIM motor sub-score improvement from discharge to 12-months | NR | NR | 7.79 (95% CI = 0.09 to 15.49), P = .0474 | |
Abbreviations: CI, confidence interval; FIM, Functional Independence Measure; NR, not reported.
aAuthors reported that regression with propensity scoring was done to adjust for potential selection bias; however, details were not provided.
Table 6.
Summary Table of Safety Outcomes Between Early (≤24 Hours) and Late (>24 Hours) Decompression.
| Author, Year | Measure | Early, ≤24 Hours | Late, >24 Hours | Effect Size |
|---|---|---|---|---|
| Cervical SCI | ||||
| Fehlings, 2012 | Inpatient postoperative complications, n (%) | na = 182 | na = 131 | Unadjusted RR (95% CI) |
| Prospective cohort study | Cardiopulmonary | 32 (17.6) | 34 (26.0) | 0.68 (0.44 to 1.04) |
| Construct failure requiring surgery | 3 (1.6) | 1 (0.8) | 2.16 (0.23 to 20.53) | |
| Deep wound infection | 0 (0) | 2 (1.5) | Incalculable | |
| Neurologic deterioration | 4 (2.2) | 1 (0.8) | 2.88 (0.33 to 25.46) | |
| Pulmonary embolism | 2 (1.1) | 2 (1.5) | 0.72 (0.10 to 5.04) | |
| Systemic infection | 6 (3.3) | 8 (6.1) | 0.54 (0.19 to 1.52) | |
| Wound dehiscence | 1 (0.5) | 1 (0.8) | 0.72 (0.05 to 11.40) | |
| Mortality; ≤30 days postinjury | 1 (0.5) | 1 (0.8) | 0.72 (0.05 to 11.40) | |
| Mortality; >30 days postinjury | 3 (1.6) | 0 (0) | Incalculable | |
| Cervical and thoracolumbar SCI | ||||
| Bourassa-Moreau, 2013 | Acute stay postoperative complications, n (%) | n = 90 | n = 341 | Unadjusted RR (95% CI)b |
| Retrospective cohort study | Pneumonia | 15 (16.7) | 91 (26.7) | 0.62 (0.38 to 1.02) |
| Pressure ulcer | 12 (13.3) | 73 (21.4) | 0.66 (0.37 to 1.15) | |
| Urinary tract infection | 18 (20.0) | 83 (24.3) | 0.82 (0.52 to 1.29) | |
| Other complications | 11 (12.2) | 55 (16.1) | 0.76 (0.41 to 1.39) | |
| Mortality | 3 (3.3) | 9 (2.6) | 1.26 (0.35 to 4.57) | |
| Thoracolumbar SCI | ||||
| Rahimi-Movghar, 2014 | Postoperative complications, n (%) | n = 16 | n = 19 | Unadjusted RR (95% CI) |
| RCT | Deep vein thrombosis | 1 (6.2) | 1 (5.2) | 1.2 (0.08 to 17.5) |
| Wound infection | NR | 1 (5.2) | Incalculable | |
| CSF leak | NR | 1 (5.2) | Incalculable | |
| Meningitis | NR | 1 (5.2) | Incalculable | |
| Decubitis ulcer | NR | 1 (5.2) | Incalculable | |
| Revision of surgical screws | 2 (12.5) | 3 (15.7) | 0.79 (0.15 to 4.16) | |
| Bilateral rod fracture | NR | 1 (5.2) | Incalculable | |
| Death | 1 (6.2) | 1 (5.2) | 1.2 (0.08 to 17.5) | |
Abbreviations: CSF, cerebrospinal fluid; NR, not reported; RCT, randomized controlled trial; RR, risk ratio; SCI, spinal cord injury.
aDenominator is total number of subjects enrolled because information on timing of complications and number of patients is not provided.
bRRs were calculated by combining 24-72 hour and >72 hour surgery groups into a single late surgery group of >24 hours.
Key Question 1. What Is the Efficacy and Effectiveness of Early Decompression (≤24 Hours) Compared With Late Decompression or Conservative Therapy Based on Clinically Important Change in Neurological Status?
Data on relative efficacy was obtained from 2 prospective cohort studies, 1 retrospective cohort study, 1 prospective observational dataset, and 1 RCT. One cohort study included patients with cervical SCI only,21 3 cohort studies20,22,23 included patients with SCI at multiple levels, and 1 prospective observational dataset25 included patients with acute central cord syndrome without instability. One small RCT provided limited information on the relative efficacy of early versus late decompression in patients with thoracolumbar SCI.24 The timing of initial evaluation was not well reported across studies. No studies evaluated conservative treatment or nonsurgical decompression. No studies evaluated the impact of early versus delayed surgical decompression on patient (or caregiver) related outcomes, such as quality of life.
Cervical SCI
A single prospective cohort study21 (N = 313) with moderately low risk of bias reported change in AIS at 6 months in patients with cervical SCI. Following adjustment for preoperative neurological status and steroid administration, patients decompressed early were more likely to exhibit a ≥2 grade improvement at 6 months in AIS than those decompressed late (odds ratio [OR] = 2.83, 95% CI = 1.10 to 7.28; P = .03). There was no significant difference in the odds of achieving a ≥1 grade improvement between treatment groups (OR = 1.37, 95% CI = 0.80 to 2.57; P = .31; Table 3).
Cervical, Thoracic, Lumbosacral SCI
Two prospective cohort studies20,22 examined neurological outcomes in patients with cervical, thoracic, or lumbosacral SCI (Table 3). Both studies had moderately high risk of bias.
In the largest study20 (N = 888), there was no significant difference in AIS Motor Score improvement between the early and late surgery groups in AIS A patients (mean improvements not reported for either arm; P = .848). Patients with AIS B, C, or D injuries treated early improved, on average, by 6 additional motor points than those decompressed late. The confidence interval for the regression coefficient, however, was large, indicating substantial variability. The timeframe for improvement or the timing of follow-up were also not reported.
In the second study22 (N = 84), there was no difference in mean AIS Motor Score improvement between early and late decompression groups (P = .18) at the time of discharge from acute care (mean 24.8 days). At the time of discharge from rehabilitation (mean 89.6 days), however, patients receiving early decompression exhibited an additional 13-point improvement in AIS Motor Score compared with those treated later, after adjusting for completeness and level of injury (mean improvements not reported for either arm; P = .01). Similarly, a greater percentage of patients in the early surgery group experienced a ≥2 grade AIS improvement (27.2%) than in the late surgery group (3%; unadjusted RR = 8.9, 95% CI = 1.12 to 70.64, P = 0.0154); however, the confidence interval of this risk ratio is wide and the effect estimate is unstable.
Thoracolumbar SCI
A single small RCT24 (N = 35) with moderately low risk of bias evaluated differences in AIS Motor Score improvements between early and late decompression groups in patients with complete or incomplete thoracolumbar SCI. In patients with incomplete SCI, AIS Motor Scores improved in both groups, with no differences observed between patients decompressed early versus late. No changes in Motor Scores were achieved in patients with complete injury, regardless of timing of intervention. More patients in the early decompression group experienced an improvement of ≥2 grades in AIS (3 vs 1 patient, RR = 3.56, 95% CI = 0.41 to 30.99); however, this relationship did not reach statistical significance. Furthermore, the confidence interval for the risk ratio is wide, suggesting estimate instability (Table 3).
Acute Central Cord Syndrome Without Instability
One prospective observational dataset25 (N = 73) with moderately high risk of bias compared outcomes following early and late decompression in patients with acute central cord syndrome. Early surgery was associated with an additional 7.47 (95% CI = −0.04 to 14.91, P = .0511) point improvement in total AIS Motor Score at 6 months and 6.31 (95% CI = 0.44 to 12.18, P = .0359) point improvement at 12 months after propensity score stratification. There were no significant differences in improvement in AIS between early and late surgical groups at 6 months (OR = 3.39, 95% CI = 0.75 to 15.34, P = .1131) or 12 months (OR = 2.81, 95% CI = 0.48 to 16.6, P = .2548; Table 3).
Key Question 2. How Does Timing of Decompression Influence Other Functional Outcomes or Administrative Outcomes?
One study evaluated functional outcomes and 3 studies reported on administrative outcomes.
Cervical, Thoracic, Lumbosacral SCI
Two prospective studies20,22 at moderately high risk of bias evaluated LOS between patients decompressed early versus late. A large registry study (N = 888) reported a significant difference in LOS (setting undefined) between early versus late surgical groups in patients with AIS A (7.5 vs NR days, respectively; P = .003) or B injury severity (12.8 vs NR days, respectively; P = .004).20 In a second study, there was no statistically significant difference between groups with respect to LOS in either an acute care (early: 24.9 days, late: 24.7 days; P = .97, N = 82) or rehabilitation setting (early: 102.9 days, late: 80.2 days; P = .10, N = 55)22 (Table 4).
Thoracolumbar SCI
One RCT24 with moderately low risk of bias reported no difference in LOS between early and late surgical groups (P = .3137; Table 4).
Acute Central Cord Injury Without Instability
One prospective observational dataset25 (N = 73) with moderately high risk of bias evaluated functional outcomes in patients with acute central cord syndrome by comparing FIM total and motor subscore improvements between early versus late decompression groups. Patients treated early exhibited an additional 6.92-point (95% CI = −0.11 to 13.96, P = .0537) improvement in motor subscore and 7.79-point (95% CI = 0.09 to 15.49, P = .0474) improvement in total score at 12 months (Table 5).
Key Question 3. What Is the Safety Profile of Early Decompression (≤24 Hours) Compared With Late Decompression?
Data on complications was available from one small RCT,24 one prospective cohort study,21 and one retrospective cohort study.23 There were no significant differences in rates of complications between patients treated early versus late; however, study sample sizes may have been insufficient to evaluate differences between groups, especially for rare events (Table 6).
Cervical SCI
One prospective study21 (N = 313) with moderately low risk of bias reported on rates of total and specific complications in patients with cervical SCI.
Cardiopulmonary complications occurred the most frequently; however, there were no statistical differences in rates between the early and late surgery groups (17.6% vs 26%; RR = 0.68, 95% CI = 0.44 to 1.04; Table 6). Neurologic deterioration was reported in 2.2% of patients treated early versus 0.8% of those decompressed late. Within 30 days of injury, there was 1 death in each group, whereas after 30 days of injury, there were 3 deaths in the early group and zero in the late group. Deep wound infection was more common in patients receiving early decompression, whereas construct failure and pulmonary embolism were more frequent in the late surgery group; however, these relationships did not reach statistical significance. Rates of systemic infection and wound dehiscence were similar between groups.
Overall, there was no statistical difference between early and late decompression for any safety outcome; however, for some outcomes, there may have not been sufficient statistical power to detect a difference between groups.
Cervical and Thoracolumbar SCI
One retrospective cohort study23 with moderately high risk of bias reported on safety outcomes in patients with cervical and thoracolumbar SCI. Risk of complications were not significantly different between the early and late decompression groups, with the exception of pneumonia, which was more common in the late surgery group (early: 17.7%, late: 26.7%; RR = 0.62, 95% CI = 0.38 to 1.02; P = .0496). In addition, fewer patients in the early group experienced a pressure ulcer compared to the late group (13.3% vs 21.4%, respectively; RR = 0.66, 95% CI = 0.37 to 1.15; P = .1295); however, this association did not reach statistical significance (Table 6).
Thoracolumbar SCI
In one small RCT (N = 35)24 with moderately low risk of bias, there were no differences in rates of deep vein thrombosis, revision of surgical screws, or death between patients treated early versus late (Table 6). This study was likely underpowered to detect differences between groups.
Key Question 4. What Is the Evidence That Early Decompression Has Differential Efficacy or Safety in Subpopulations?
No formal analyses evaluated the differential effectiveness or safety of early versus late decompression in subpopulations with different patient characteristics, such as level of SCI. A single study at moderately high risk of bias20 suggested that outcomes may differ in AIS A versus AIS B, C, or D patients.
Key Question 5: What Is the Evidence of Cost-Effectiveness Comparing the Treatment Options Evaluated in Key Questions 1 to 4?
No full economic studies were identified that met the inclusion criteria. A costing study was identified26 and is described in the discussion. This study provided no information on the impact of the timing of decompression surgery on cost of care.
Evidence Summary and Strength (Quality) of Evidence
Key Question 1
The overall strength of evidence was “Low to Very Low” that early (≤24 hours) decompression results in clinically meaningful improvements in neurological status (≥2 grade improvement in AIS or Frankel grade or ≥5 point improvement in AIS Motor Score) at any follow-up period. This means that there is limited to little confidence that the effect estimates reflect the true effect; the true effect is likely to be substantially different than the estimated effect. In general, across studies, timeframes, and outcome measures, a greater number of patients receiving early decompression experienced improvements in neurologic status compared with those who had late decompression; however, some results were within the limits of chance (Table 7 and supplemental material).
Based on 2 studies, patients treated early were more likely to exhibit a ≥2 grade improvement in AIS at 6-month follow-up (cervical SCI only, strength of evidence “Low”) and at discharge from inpatient rehabilitation (all levels, strength of evidence “Very Low”) than those decompressed late. In another study, AIS B, C, or D patients treated early improved, on average, by 6 additional motor points than those decompressed late. There was no significant difference in AIS Motor Score improvement between the early and late surgery groups in AIS A patients (all levels, strength of evidence “Very Low”). Patients decompressed early for acute central cord syndrome had greater total motor score improvements at 6 and 12 months than those decompressed late; however, at the same time points there were no significant differences between the groups with respect to rates of AIS grade conversion (strength of evidence “Very Low”). Finally, in thoracolumbar SCI (strength of evidence “Low”), early decompression did not significantly affect neurological recovery (defined by Motor Score or AIS grade improvement) at any follow-up point. No differences in outcomes between early and late surgery were reported at time of acute care discharge across levels (strength of evidence “Very Low”).
For most outcomes, the rating of evidence was “Very Low” due to imprecision, high or unreported attrition rates, and/or methodological concerns. We did not downgrade for inconsistency in single studies; however, the consistency of findings from single studies is unknown (Table 7 and Appendix Tables).
Key Question 2
The overall strength of evidence was “Very Low” that timing of surgery (early vs late) is associated with functional outcomes. A single study reported that patients treated early for central cord syndrome have greater FIM total score improvements from discharge to 12-month follow-up compared to those receiving late decompression; in contrast, the difference between the 2 groups at the 6-month time point was within the limits of chance. The authors of this study reported that propensity scoring was done to decrease selection bias; however, no methodology details were provided. Furthermore, wide confidence intervals and a lack of precision resulted in downgrading the level of evidence to “Very Low.”
The overall strength of evidence was “Very Low” that timing of surgical decompression (early vs late) affects administrative outcomes, including LOS. Evidence across 3 studies was inconsistent, and variability in methodology, including timing of follow-up, prevents strong conclusions. A single study reported a significantly shorter LOS in patients treated early for SCI at cervical, thoracic, or lumbosacral levels.
Key Question 3
The overall strength of evidence was “Very Low” that timing of surgical decompression (early vs late) influences safety outcomes. There were no significant differences in rates of total or specific complications between early versus late decompression groups; this may be due to a lack of statistical power to detect differences between groups, especially for rare outcomes. There was a tendency for a higher rate of pneumonia in patients treated late for cervical and thoracolumbar SCI. In general, mortality was rare, ranging from 0.5% to 3.3% in the early decompression group and from 0% to 2.9% in the late decompression group in studies that included at least 100 patients.
Key Question 4
No studies formally assessed the differential effectiveness or safety of early versus late surgical decompression in subpopulations (“Insufficient” evidence).
Key Question 5
No full economic studies were identified; there is “Insufficient” evidence regarding the economic impact of early versus late surgery.
Across Key Questions
For all outcomes, there were not enough studies or publically available data to formally evaluate the possibility of publication bias; although none of the outcomes were downgraded for publication or reporting bias, the possibility of publication bias and/or selective outcomes reporting cannot be ruled out.
Discussion
Across studies, the impact of early surgical decompression, defined as ≤24 hours after injury, on clinically important improvement in neurological status was variable depending on the neurological level of injury. The strength of evidence was low that early decompression, compared to late surgery, may lead to clinically important improvements in neurologic status in patients with cervical injury; however, this is based on a single study and confirmatory evidence in other populations is needed. Evidence was very low across 2 studies in populations with mixed injury levels (cervical, thoracic, and lumbar) that early, compared to late, surgical decompression was statistically associated with clinically important neurological improvement at the time of discharge from inpatient rehabilitation (one study) and in patients with incomplete SCI (one study). Wide confidence intervals suggest that estimates may be unstable. Statistical significance was not achieved in other studies or for different time frames, likely due in part to small sample sizes. In the only study in patients with acute central cord injury without instability, very low evidence suggests that patients treated early have greater total motor score improvements at 6 and 12 months than those decompressed late. Again, wide confidence intervals are noted. The generalizability of findings for specific injury levels from these studies is unclear.
Safety and harms were reported in 3 studies; no clinically significant differences in complication rates were observed between surgical groups.
Previous Systematic Reviews
Five previous systematic reviews provide additional context for the primary findings in this review. Three of these discussed the impact of surgical timing on neurological outcomes,8,27,28 no studies reported on functional outcomes, 4 discussed administrative outcomes,6,8,28,29 and 4 addressed safety outcomes6,8,28,29 (Table 8).
Table 8.
Summary Table of Findings From Previous Systematic Reviews.
| Assessment (Year) | Evidence Available | Primary Conclusions | AMSTAR Score |
|---|---|---|---|
| van Middendorp (2013) | 2 RCTs (including quasi RCT) 20 non-RCTs (N = 2363) | Definition of early versus late: Early decompression was defined as ≤24 hours after injury in 10 studies and as ≤72 hours after injury in 6 studies. The maximum delay for late decompression ranged from 1 week to 12 months. Fifteen of the included studies failed to report maximum surgery delay. Effectiveness: Studies included in this analysis suggest that early treatment is associated with better neurological improvement (6 studies) and higher Total Motor Scores (7 studies) than late decompression. However, the authors state that the evidence is not robust enough to make definitive conclusions and show results indicating presence of publication bias. Functional outcomes: This SR did not formally evaluate functional outcomes. Administrative outcomes: Patients in the early decompression group had a shorter length of stay than those in the late decompression group (6 studies). Safety: Patients in the late decompression group may be at higher risk of pulmonary complications. There was no significant difference in risk of mortality between the early and late decompression groups. | 9/11; High quality |
| Carreon (2011) | 2 RCTs (including quasi-RCT) 2 non-RCTs 9 reviews (N = 6416) | Definition of early versus late: Early decompression was defined as ≤24 hours after injury in 3 studies and as ≤72 hours after injury in 12 studies. Late decompression was defined as any time period after the early period. Effectiveness: This SR did not formally evaluate neurologic outcomes. Functional outcomes: This SR did not formally evaluate functional outcomes. Administrative outcomes: Patients in the early decompression group had shorter hospital (11 studies) and ICU stays (7 studies) than those in the late decompression group. This is increasingly evident in more severely injured patients. Safety: Early decompression may be associated with a lower risk of pulmonary complications (8 studies). This is more evident in more severely injured patients. Incidences of mortality (7 studies) and pneumonia (4 studies) were also evaluated but definitive conclusions could not be made. | 2/11; Low quality |
| Furlan (2011) | 22 studies—type NR (N = 4182) | Definition of early versus late: Early decompression ranged from 8 hours to 4 days after injury and late decompression from 8 hours to 5 days after injury. Effectiveness: The evidence included in this SR was inconsistent; some studies reported that early decompression results in superior neurologic outcomes, whereas others did not. Functional outcomes: This SR did not formally evaluate functional outcomes. Administrative outcomes: Patients in the early decompression group had a shorter length of hospital and ICU stay (1 study). Furthermore, early treatment requires a smaller volume of fresh frozen plasma for operations (1 study). Safety: Early surgical decompression does not increase the risk of treatment-related harm. | 6/11; Medium quality |
| Fehlings (2006) | 1 RCT 16 non-RCTs (N = NR) | Definition of early versus late: Included studies had variable definitions of early and late decompression. Early decompression ranged from <8 hours to <2 weeks postinjury, whereas late decompression ranged from >8 hours to >2 weeks postinjury. Effectiveness: Four studies concluded that early decompression is associated with higher “neurological improvement rates” whereas 4 studies reported that early decompression does not increase “neurological improvement rates.” Limited data was provided. Functional outcomes: This SR did not formally evaluate functional outcomes. Administrative outcomes: Three studies reported no difference in length of stay within the rehabilitation setting between patients treated early versus late. However, a fourth study concluded that early decompression may be associated with a shorter length of stay. Safety: Patients undergoing early decompression may experience fewer complications (12 studies). | 5/11; Medium quality |
| La Rosa (2004) | 33 studies—type NR (N = 800) | Definition of early versus late: Early decompression was defined as <24 hours after injury, and late decompression was defined as >24 hours after injury. Effectiveness: Both complete and incomplete SCI patients who underwent early (complete: 42% [95% CI = 33.1 to 50.8%]; incomplete: 89.7% [95% CI = 83.9 to 95.5%]) decompression presented with greater distal cord functional improvement than patients treated late (complete: 8.3% [95% CI = 4.8 to 11.8%]; incomplete: 58.5% [95% CI = 53.1 to 63.9%]). Patients with incomplete impairment who underwent early decompression exhibited significantly higher neurological improvement regardless of baseline Frankel Grade (numerical data NR). Functional outcomes: This SR did not formally evaluate functional outcomes. Administrative outcomes: This SR did no formally evaluate administrative outcomes. Safety: This SR did not formally evaluate safety outcomes. | 6/11; Medium quality |
Abbreviations: AMSTAR, A Measurement Tool to Assess Systematic Reviews; CI, confidence interval; ICU, intensive care unit; NR, not reported; RCT, randomized controlled trial; SR, systematic review; SCI, spinal cord injury.
The most recent systematic review with meta-analysis by van Middendorp et al was rated as high quality; however, there was substantial heterogeneity with respect to how “early” and “late” decompression were defined across included studies and most did not adjust for baseline clinical status.28 The authors also did not examine outcomes by injury level. Overall, the findings of our review are consistent with those described by van Middendorp et al in that early surgical decompression may be associated with improved neurological status. They also report that early surgery was associated with shorter LOS in the hospital. It is important to note that their analysis reveals substantial publication bias, with a large number of small studies favoring early surgery; this casts some doubt on the validity of the estimates in their review. Only 2 of the 22 studies included in the review by van Middendorp et al met the inclusion criteria for this report, both of which had relatively small sample sizes.21
Across reviews that reported on neurological outcomes, conclusions were somewhat inconsistent; one reported that neurological outcomes were more favorable in patients who received early decompression,27 whereas 2 other studies stated that the evidence is not significant enough to support early over late decompression.8,28 Patients who underwent early surgical decompression for acute SCI had reduced LOS in both hospital and ICU settings in 3 reviews.8,28,29 In 2 reviews,28,29 it was noted that early decompression may lead to fewer pulmonary complications than late decompression, particularly in severely injured patients. Based on a single systematic review, early surgical decompression was associated with fewer complications compared to late decompression6; however, 2 other reviews concluded that safety outcomes and risk of complications do not vary between treatment groups.8,28 Although there was overlap in the studies included across the systematic reviews, the earlier reviews did not include more recent studies. Furthermore, the quality of systematic reviews varied substantially based on the AMSTAR score (A Measurement Tool to Assess Systematic Reviews). None of the reviews formally assessed overall quality of evidence and only one evaluated the possibility of publication bias. Finally, this review focused on studies that adjusted for injury severity at baseline as this is one of the most important predictors of outcome.
Economic Impact
One retrospective cohort study26 (N = 477) from Québec compared cost of care for acute SCI patients undergoing early (within 24 hours; 19.4%, n = 93) versus late surgery (after 24 hours; 80.5%, n = 384) and included costs for all resources utilized during hospitalization, with the exception of physician fees. After taking into account patient clinical condition, risk of mortality, costs associated with teaching at the associated university-affiliated hospital, and patient diagnostic and therapeutic resource use, costs in Canadian dollars were calculated either using a continuous or dichotomized (≥24 hours vs <24 hours) variable for timing of surgery. This partial economic study indicated that early surgery leads to reduced costs (in Canadian dollars): (1) in the continuous model, delay of surgery led to an increase of $15.00 per hour (95% CI = $5.20 to $24.70) and (2) in the dichotomized model, surgery conducted within 24 hours of injury was estimated to be $5305.80 (95% CI = −$8809.40 to −$1802.10) less expensive than surgery conducted after 24 hours of injury. However, it should be noted that the early surgery group was significantly younger (P = .004) than the late surgery group and that the cost-analysis model does not include physician fees, as calculating those would be very complex. These differences are noted to be systematic and the researchers felt that they would not have an impact on the effect measures identified between early and late surgery patient groups. Given the differences in health care systems and reimbursement practices in other countries, however, these results may not be applicable in other settings.
Strengths
This review focused on studies that controlled for baseline factors (ie, baseline neurologic status) and had a specific definition of early decompression. Exclusion of studies that did not account for baseline severity of neurological deficit reduces the potential for confounded effect estimates.
Limitations and Gaps in Evidence
A number of limitations of the evidence base are noted, including high loss to follow-up and failure to report patient selection criteria and attrition. Small sample sizes resulted in large confidence intervals and unstable estimates. There is not an established minimum clinically important difference for any of the outcomes measures reported. Furthermore, only 3 studies reported results in terms of what we defined as a clinically important difference for the purpose of this review (eg, ≥2 grade improvement in ASIA score).21,24 Variation in measurement and definitions for some outcomes across studies prevented comparison and pooling of data. The consistency of findings is unknown for all levels given the paucity of studies and given that only single studies for most injury levels were identified. It is therefore unclear if results from included studies are generalizable across injury levels.
A number of limitations to this review should be noted. First, the a priori decision to define early decompression as ≤24 hours limited the number of available studies and prevented reporting of different timing thresholds. However, we were able to summarize studies with similar definitions of early and late surgery. There was substantial clinical variability with regard to injury levels as well as how outcomes were reported. Statistical pooling of data was not possible given the heterogeneity across studies. Publication and reporting bias could also not be rigorously assessed.
Conclusions
Results surrounding the efficacy of early (<24 hours) versus late decompressive surgery, as well as the quality of evidence available, were variable depending on the level of SCI, the timing of follow-up, and the specific outcome assessed. In general, the existing evidence supported improved neurological recovery amongst cervical SCI patients undergoing surgery ≤24 hours postinjury; evidence regarding remaining SCI populations and clinical outcomes was inconsistent. While no statistically or clinically significant differences in complication or mortality rates were noted between groups, firm conclusions regarding the safety of early surgery are difficult given small sample sizes and rare events. Finally, given the paucity of studies available, no conclusions can be made regarding cost-effectiveness of early surgery for SCI.
Supplementary Material
Acknowledgements
We thank Kathryn Mihalovich, BS, Krystle Pagarigan, BS, and Erin Anthony-Fick, BS, for assistance with data abstraction and summarization of included studies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by AOSpine and the AANS/CNS Section on Neurotrauma and Critical Care. Dr Fehlings wishes to acknowledge support from the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration and the DeZwirek Family Foundation. Dr Tetreault acknowledges support from a Krembil Postdoctoral Fellowship Award. Methodologic and analytic support for this work was provided by Spectrum Research, Inc, with funding from AOSpine North America.
Supplemental Material: The supplemental materials are available in the online version of the article.
References
- 1. Wilson J, Fehlings M. Emerging approaches to the surgical management of acute traumatic spinal cord injury. Neurotherapeutics. 2011;8:197–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rowland J, Hawryluk G, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. [DOI] [PubMed] [Google Scholar]
- 3. Hawryluk G, Roland J, Kwon B, Fehlings M. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. [DOI] [PubMed] [Google Scholar]
- 4. Guha A, Tator C, Endrenyi L. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324–339. [DOI] [PubMed] [Google Scholar]
- 5. Dimar J, Glassman S, Raque G, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24:1623–1633. [DOI] [PubMed] [Google Scholar]
- 6. Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine (Phila Pa 1976). 2006;31(11 suppl):S28–S35. [DOI] [PubMed] [Google Scholar]
- 7. Batchelor PE, Wills TE, Skeers P, et al. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. 2013;8:e72659 doi:10.1371/journal.pone.0072659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furlan J, Noonan V, Cadotte D, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–1399. doi:10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fehlings M, Wilson J. Timing of surgical intervention in spinal trauma: what does the evidence indicate? Spine (Phila Pa 1976). 2010;35(21 suppl):S159–S160. [DOI] [PubMed] [Google Scholar]
- 10. Geisler F, Coleman W, Grieco G, Poonian D; Sygen Study Group. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976). 2001;26:87–98. [DOI] [PubMed] [Google Scholar]
- 11. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi:10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 12. Google. Google Translate. https://translate.google.com/. Accessed April 3, 2017.
- 13. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85:1–3. [PubMed] [Google Scholar]
- 14. Skelly AC, Hashimoto RE, Norvell DC, et al. Cervical spondylotic myelopathy: methodological approaches to evaluate the literature and establish best evidence. Spine (Phila Pa). 2013;38(22 suppl 1):S9–S18. doi:10.1097/BRS.1090b1013e3182a1097ebbf. [DOI] [PubMed] [Google Scholar]
- 15. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi:10.1016/j.jclinepi.2010.1007.1015. [DOI] [PubMed] [Google Scholar]
- 17. West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ). 2002:1–11. [PMC free article] [PubMed] [Google Scholar]
- 18. Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions: Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010;63:513–523. doi:10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 19. StataCorp. Stata Statistical Software: Release 9. College Station, TX: StataCorp; 2005. [Google Scholar]
- 20. Dvorak MF, Noonan VK, Fallah N, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J Neurotrauma. 2015;32:645–654. doi:10.1089/neu.2014.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012;7:e32037 doi:10.31371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson JR, Singh A, Craven C, et al. Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord. 2012;50:840–843. doi:10.1038/sc.2012.59. [DOI] [PubMed] [Google Scholar]
- 23. Bourassa-Moreau E, Mac-Thiong JM, Ehrmann Feldman D, Thompson C, Parent S. Complications in acute phase hospitalization of traumatic spinal cord injury: does surgical timing matter? J Trauma Acute Care Surg. 2013;74:849–854. doi:10.1097/TA.1090b1013e31827e31381. [DOI] [PubMed] [Google Scholar]
- 24. Rahimi-Movaghar V, Niakan A, Haghnegahdar A, Shahlaee A, Saadat S, Barzideh E. Early versus late surgical decompression for traumatic thoracic/thoracolumbar (T1-L1) spinal cord injured patients. Primary results of a randomized controlled trial at one year follow-up. Neurosciences (Riyadh). 2014;19:183–191. [PMC free article] [PubMed] [Google Scholar]
- 25. Lenehan B, Fisher CG, Vaccaro A, Fehlings M, Aarabi B, Dvorak MF. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976). 2010;35(21 suppl): S180–S186. doi:10.1097/BRS.1090b1013e3181f1032a1044. [DOI] [PubMed] [Google Scholar]
- 26. Mac-Thiong JM, Feldman DE, Thompson C, Bourassa-Moreau E, Parent S. Does timing of surgery affect hospitalization costs and length of stay for acute care following a traumatic spinal cord injury? J Neurotrauma. 2012;29:2816–2822. doi:10.1089/neu.2012.2503. [DOI] [PubMed] [Google Scholar]
- 27. La Rosa G, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–512. [DOI] [PubMed] [Google Scholar]
- 28. van Middendorp JJ, Hosman AJ, Doi SA. The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2013;30:1781–1794. doi:10.1089/neu.2013.2932. [DOI] [PubMed] [Google Scholar]
- 29. Carreon LY, Dimar JR. Early versus late stabilization of spine injuries: a systematic review. Spine (Phila Pa 1976). 2011;36:E727–E733. doi:10.1097/BRS.1090b1013e3181fab1002f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.