Abstract
Objectives:
The objective of this study was to conduct a systematic review of the literature to address the following clinical questions: In adult patients with acute and subacute complete or incomplete traumatic SCI, (1) does the time interval between injury and commencing rehabilitation affect outcome?; (2) what is the comparative effectiveness of different rehabilitation strategies, including different intensities and durations of treatment?; (3) are there patient or injury characteristics that affect the efficacy of rehabilitation?; and (4) what is the cost-effectiveness of various rehabilitation strategies?
Methods:
A systematic search was conducted for literature published through March 31, 2015 that evaluated rehabilitation strategies in adults with acute or subacute traumatic SCI at any level. Studies were critically appraised individually and the overall strength of evidence was evaluated using methods proposed by the GRADE (Grades of Recommendation Assessment, Development and Evaluation) working group.
Results:
The search strategy yielded 384 articles, 19 of which met our inclusion criteria. Based on our results, there was no difference between body weight–supported treadmill training and conventional rehabilitation with respect to improvements in Functional Independence Measure (FIM) Locomotor score, Lower Extremity Motor Scores, the distance walked in 6 minutes or gait velocity over 15.2 m. Functional electrical therapy resulted in slightly better FIM Motor, FIM Self-Care, and Spinal Cord Independence Measure Self-Care subscores compared with conventional occupational therapy. Comparisons using the Toronto Rehabilitation Institute Hand Function Test demonstrated no differences between groups in 7 of 9 domains. There were no clinically important differences in Maximal Lean Test, Maximal Sidewards Reach Test, T-shirt Test, or the Canadian Occupational Performance Measure between unsupported sitting training and standard in-patient rehabilitation.
Conclusion:
The current evidence base for rehabilitation following acute and subacute spinal cord injury is limited. Methodological challenges have contributed to this and further research is still needed.
Keywords: spinal cord injuries, rehabilitation
Introduction
Spinal cord injury (SCI) often leads to profound motor, sensory and autonomic impairments, which are associated with functional limitations, reduced participation in daily activities, and altered quality of life (QoL). For individuals with a severe SCI and limited potential for neurorecovery, rehabilitation approaches focus on utilizing compensatory techniques to optimize function. As our understanding of SCI and associated mechanisms of recovery (eg, principles of motor control, activity-dependent neuroplasticity) continue to grow, there is an increasing emphasis on the restoration of function through the remediation of underlying impairments. The selection of restorative and/or compensatory techniques is affected by the severity of SCI.
The rehabilitation of individuals with SCI can be divided into 3 phases: acute, subacute, and chronic. While there has been variability in the definition and temporal demarcation of these phases, the acute and subacute periods, when combined, generally correspond with the natural history of neurorecovery (12-18 months postinjury), while the chronic phase is the period when neurorecovery has plateaued.1,2 Rehabilitation during the acute and subacute phases focuses on preventing secondary complications, promoting and enhancing neurorecovery, maximizing function, and establishing optimal conditions for long-term maintenance of health and function. In the chronic phase, compensatory or assistive approaches are often used, whereas in the acute and subacute phases, there is a greater emphasis on techniques that address underlying impairments and promote neurorecovery.
Given our enhanced insight into the mechanisms of recovery and the benefits of rehabilitation,3 it is important to understand and synthesize existing evidence on specific rehabilitation approaches. A systematic appraisal of the evidence base will inform clinical decision making and highlight current knowledge gaps that require additional investigation. The objectives of this systematic review are to address the following key questions: In adult patients with acute or subacute traumatic SCI,
does the time interval between injury and commencing rehabilitation affect outcome?
what is the comparative effectiveness of different rehabilitation strategies, including different intensities and durations of treatment?
are there patient or injury characteristics that affect the efficacy of rehabilitation?
what is the cost-effectiveness of various rehabilitation strategies?
Materials and Methods
Electronic Literature Search
A systematic search was conducted in MEDLINE, the Cochrane Collaboration Library, and Embase for literature published through March 31, 2015. The search results were limited to human studies that included an abstract. Reference lists of key articles were also systematically checked to identify additional eligible articles. Studies were included if they were on adult patients who underwent rehabilitation therapy for acute or subacute traumatic SCI at any level or severity (American Spinal Injury Association Impairment Scale [AIS] Grade A-D).4 For questions 1 and 2, we sought comparative studies with concurrent controls. Severity of impairment in patients with SCI is a well-known factor that is associated with outcome.5–8 Impairment severity following SCI may also influence clinical decision making about the timing, duration, intensity, and type of rehabilitation. Therefore, we included randomized controlled trials (RCTs) or nonrandomized observational studies that controlled for SCI severity as measured by baseline motor status or complete versus incomplete impairment. Timing, duration, and intensity can be reported as parameters that quantify the dose of rehabilitation therapy or as factors affected by injury severity. We searched for studies that evaluated the effect of rehabilitation timing, durations, and intensities on outcomes. Studies were excluded if they addressed these questions through retrospective correlation of these rehabilitation parameters with outcomes.
For question 3, studies were included if they evaluated important predictors of outcome(s) following rehabilitation, while also controlling for baseline severity. Factors of interest included patient (eg, age, sex, marital status, and education level) and injury-related factors (eg, AIS grade, neurological level, cause, and severity of injury). Relevant outcomes included neurological outcomes, performance in activities of daily living (ADLs), ambulation outcomes, QoL, and rates of mortality, rehospitalization, and secondary complications (eg, pressure ulcers).
Exclusion criteria were pediatric or pregnant patients, studies with a follow-up rate <70%, populations with >40% of patients presenting with nontraumatic SCI, and patients with cord compression due to tumor, hematoma, or degenerative disease. Other exclusions were studies with less than 10 patients per treatment group (questions 1 and 2) or less than 20 patients in total (question 3); animal, biomechanical, or cadaveric studies; and editorials, reviews, and case reports. In addition, studies were excluded if they involved neural prosthetics or explored single therapies such as speech/language, pharmacological, or respiration/breathing training that were not used in conjunction with a physical rehabilitation strategy. To address question 4 on cost-effectiveness, we searched for economic studies that evaluated and synthesized costs and consequences of SCI rehabilitation strategies (ie, cost-minimization, cost-benefit, cost-effectiveness or cost-utility) (Table 1).
Table 1.
Inclusion/Exclusion Criteria.
| Inclusion | Exclusion | |
|---|---|---|
| Patients |
|
|
| Intervention (KQ1 and 2) |
|
KQ2: neural prosthetics, cell therapy, spinal cord stimulators, speech/language therapy only, pharmacological therapy only, respiration/breathing therapy only |
| Comparison |
|
|
| Predictive Factors (KQ3) |
|
|
| Outcomes |
Efficacy/effectiveness
Safety
Cost data
|
|
| Study design |
|
|
| Publication |
|
|
Abbreviations: ASIA, American Spinal Injury Association; KQ, key question.
Data Extraction
The following data was extracted from articles included in questions 1 and 2: study design, patient demographics, follow-up duration and percent follow-up, baseline neurological or trauma severity, level and type of injury, surgical characteristics, rehabilitation strategy and/or timing, details of rehabilitation, and outcomes, including neurological status, functional impairment, and safety (Table 2). The following data was extracted from articles included in question 3: study design, funding, purpose, inclusion and exclusion criteria, patient demographics, follow-up duration, percent follow-up, and potential predictors of patient outcomes. Outcomes of interest included neurological impairment, as measured by Frankel or AIS grades; functional status, evaluated by Functional Independence Measure (FIM) or ambulatory capacity (eg, walking speed, distance, etc); patient safety and well-being, assessed by frequency of complications and QoL; and various other factors such as rehospitalization.
Table 2.
Characteristics of Studies Reporting Different Rehabilitation Strategies After SCI.
| Study (Year) Study Design | Demographics | Inclusion/Exclusion Criteria | Treatment Details | Baseline Patient Characteristics | Follow-up (% Followed) | Outcome Measures |
|---|---|---|---|---|---|---|
| Dobkin (2006, 2007) Multicenter RCT Note: 2006 report includes primary outcomes at 6 months, 2007 report includes secondary outcomes at 3 months | N = 146 Mean age: NR Males: NR |
Inclusion:
|
|
ASIA grade: At entry into study:
|
3 months after initiation of rehabilitation (80.1%) 6 months after initiation of rehabilitation (70.5%) |
|
| Harvey (2011) Multicenter RCT | N = 32 Median age: 27 (IQR: 24-31) years Males: 93.8% (30/32) |
Inclusion:
|
Intervention: Training unsupported sitting:
|
AIS:
|
6-7 weeks (84.4%) |
|
| Kohlmeyer (1996) RCT | N = 60 n = 44 Mean age: 38.8 years Males: 90.9% (40/44) |
Inclusion:
|
Intervention:
|
Severity of injury:
|
6 weeks (73.3%) |
|
| Lucareli (2011) RCT | N = 30 n = 24 Mean age: 31.5 years Male: 58.3% (14/24) |
Inclusion:
|
Therapy for 2 times/week, 30 minutes/session, for 4 months Intervention: BWSTT:
|
ASIA grade
e
:
|
4 months (80%) |
|
| Popovic (2011) RCT | N = 24 n = 21 Mean age: 43.3 (18-66) years Males: 76.2% (16/21) |
Inclusion:
|
Therapy for 10 hours/week, for 8 weeks Intervention: FES:
|
Severity of injury:
|
8 weeks (87.5%) |
|
Abbreviations: AD, autonomic dysreflexia; ADL, activities of daily living; BWSTT, body weight–supported treadmill training; CI, confidence interval; CoE, class of evidence; COPD, chronic obstructive pulmonary disease; COPM, Canadian Occupational Performance Measure; COT, conventional occupational therapy; EMG, electromyography; ES, electrical stimulation; FES, functional electrical stimulation; FIM, Functional Independence Measure; FIM-L, Functional Independence Measure Locomotor Score; IQR, interquartile range; LEMS, lower extremity motor score; LMN, lower motor neuron; MMSE, Mini-Mental State Examination; MVA, motor vehicle accident; NR, not reported; PROM, passive range of motion; RCT, randomized controlled trial; SCI, spinal cord injury; SCILT, Spinal Cord Injury Locomotor Trial; SCIM, spinal cord independence measure; TBWS, treadmill with body weight support; TRI-HFT, Toronto Rehabilitation Institute–Hand Function Test; UMN, upper motor neuron; WISCI, Walking Index for Spinal Cord Injury.
ASIA grade (Dobkin 2006, 2007; Lucareli 2011): categorizes motor and sensory impairment in patients with SCI; (A) complete—no sensory or motor function is preserved in sacral segments S4-S5; (B) incomplete—sensory, but not motor, function is preserved below the neurologic level and extends through sacral segments S4-S5; (C) incomplete—motor function is preserved below the neurologic level, and most key muscles below the neurologic level have muscle grades less than 3; (D) incomplete—motor function is preserved below the neurologic level, and most key muscles below the neurologic level have muscle grades greater than or equal to 3; (E) normal—sensory and motor functions are normal.
FIM-L scale (Dobkin 2006, 2007): (1) = dependent, total physical assistance; (2) = maximum assistance, one helper to walk 50 feet and patient performs <50% of task; (3) = moderate assistance, one helper to walk 50 feet and patient performs 50%-75% of task; (4) = minimal assistance, one helper to walk at least 50 feet and patient performs >75% of task; (5) = supervision, no contact by helper to walk at least 50 feet; (6) = independent with equipment to walk at least 150 feet in a reasonable time; (7) = independent to walk >150 feet without assistive devices in a reasonable time.
Function score (Kohlmeyer 1996): A score of (0) = unable to perform, (1) = able to perform but not functional, and (2) = able to perform functionally is assigned for each of the following tasks: (1) feeds self without use of wrist support (may use utensil cuff); (2) picks up light finger foods (popcorn, chips); (3) picks up moderate weight finger foods (cookie, half sandwich): (4) picks up and drinks from a 12-oz soda can.
Manual muscle tests (Kohlmeyer 1996): Muscles were graded based on the following scoring system: (0) = no palpation or movement; (1) = palpable (must palpate at least 3 times); (2) = moves less than ½ available range when gravity eliminated; (3) = moves more than ½ available range when gravity eliminated; (4) = moves to neutral against gravity; (5) = moves full range against gravity but takes no resistance; (6) = moves full range against gravity and takes slight resistance; (7) = moves full range against gravity and takes good resistance (can still break); (8) = moves full range against gravity and you cannot break hold; NOT TESTED = used when an extremity was not tested.
SCIM Self-Care Score (Popovic 2011): Includes feeding, bathing, dressing, and grooming, and is scored from 0 to 20.
TRI-HFT (Popovic 2011): Evaluates gross motor function of unilateral grasp, power grasp, lateral pitch, and precision grip, as well as strength of power and lateral grasps. Scored on a scale of 0 to 7: (0) = no movement elicited; that is, the subject is unable to reach for the object; (1) = the subject is able to reach for the object but unable to grasp the object; (2) = the subject is able to reach and grasp (using passive grasp) but unable to lift the object successfully off the supporting surface; (3) = the subject is able to reach and grasp (using active grasp) but unable to lift the object successfully off the supporting surface; (4) = the subject is able to reach, grasp, and lift the object (using passive grasp) but unable to manipulate the object; (5) = the subject is able to reach, grasp, and lift the object (using active grasp) but unable to manipulate the object; (6) = the subject is able to reach, grasp, lift, and manipulate the object (using passive grasp) appropriately; (7) = the subject is able to reach, grasp, lift, and manipulate the object (using active grasp) appropriately.
a Definition of unsupported sitting (Harvey 2011): 5/7 or less on the unsupported sitting item of the Clinical Outcomes Variable Scale, cited in Campbell et al (2003).
b Unsupported sitting therapy consisted of 84 potential exercises involving movement of the upper body over and outside the base of support (with 3 grades of difficulty = 252 exercises). Developed by Boswell-Ruys et al (2010), written on cards and chosen arbitrarily by the patient during each session.
c Standard physiotherapy and occupational therapy which included training for transfers, wheelchair skills, dressing, and showering.
d Conventional physiotherapy consisted of the following: (1) passive stretching for 30 seconds for all muscle groups of the lower limbs, taking around 8 minutes in total, (2) passive mobilization of the hip, knee and ankle joints for 5 minutes, and (3) overground gait training conducted and supervised by a physiotherapist (verbal commands and manual contact for correction of movements). When necessary, the parallel bars were used to ensure the safety of the patient. All of the patient’s weight was placed on the floor, and the upper limbs were used as supports on the parallel bars when necessary.
e There were discrepancies in the initial ASIA scores summarized in the authors' demographic table (Lucareli, 2011).
The risk of bias for each article was evaluated using criteria adopted by The Journal of Bone and Joint Surgery 9 for therapeutic studies and modified to delineate criteria associated with methodological quality and risk of bias based on recommendations made by the Agency for Healthcare Research and Quality (see supplemental material for risk of bias evaluation, available in the online version of the article).10
The overall body of evidence with respect to each primary outcome was determined based on precepts outlined by the Grades of Recommendation Assessment, Development and Evaluation (GRADE) Working Group.11,12 The initial strength of evidence was considered HIGH if the majority of the studies were RCTs and LOW if the majority of the studies were observational studies. The body of evidence could be downgraded 1 or 2 levels based on the following criteria: (1) serious risk of bias, (2) inconsistency of results, (3) indirectness of evidence, (4) imprecision of the effect estimates (eg, wide confidence intervals), or (5) non–a priori statement of subgroup analyses. Alternatively, the body of evidence could be upgraded 1 or 2 levels based on the following factors: large magnitude of effect or dose-response gradient. The final overall strength of the body of literature expresses (1) our confidence that the effect size lies close to the true effect and (2) the extent to which it is believed to be stable based on the adequacy of or deficiencies in the body of evidence. An overall strength of “HIGH” means that we are very confident that the true effect lies close to that of the estimated effect. A “MODERATE” rating means that we are moderately confident in the effect estimate; the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. An overall strength of “LOW” means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate. Finally, a rating of “VERY LOW” means that we have very little confidence in the effect estimate; the true effect is likely to be substantially different than the estimated effect.
Analysis and Synthesis
Between-group comparisons were conducted for questions 1 and 2 and stratified by baseline severity when available. For continuous outcomes, the mean or median change in scores from the initiation of rehabilitation to follow-up were presented if available. For outcomes where no data was available at the initiation of rehabilitation, only follow-up mean or median scores were compared. Percent improvement from baseline for dichotomous outcomes is also summarized. For question 3, we attempted to collect effect sizes (relative risks, hazard ratios, or odds ratios) in order to assess the magnitude of the association between various predictors and outcomes; however, reporting of these was rare. Instead, most authors reported a correlation or regression coefficient or a P value. Therefore, we simply indicated whether a prognostic factor was associated with a specific outcome (no association, association with a negative outcome or association with a positive outcome). One large multicenter collaborative investigation (SCIRehab study) included 6 inpatient rehabilitation facilities in the United States and reported different associations between various factors and outcomes, in part due to different modeling strategies. When this occurred, we displayed both results (ie, that the predictive factor was associated in one report but not another).
Results
Study Selection and Quality
The search strategy yielded 384 potential articles (Figure 1). After reviewing the titles and abstracts, we excluded 313 articles and retrieved 71 articles for full text review. A total of 52 studies were excluded after full text investigation, leaving 19 publications to be included in this systematic review. See supplemental material for a list of excluded articles. For question 1, no comparative studies were identified that evaluated the association between the timing of implementation and the effectiveness of a rehabilitation treatment. For the second question, 5 studies compared the efficacy of different rehabilitation strategies. For question 3, we included 14 publications (10 studies) that evaluated important predictors of outcomes following rehabilitation. For question 4, no studies assessed the cost-effectiveness of different rehabilitation strategies.
Figure 1.
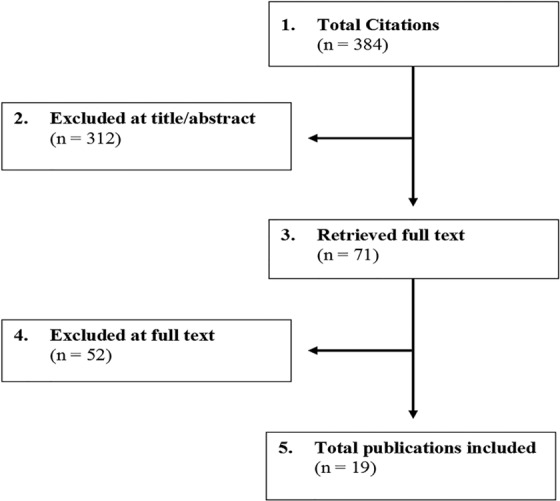
Results of literature search
Question 1: Timing of Implementation of Rehabilitation
No studies were identified that directly compared the impact of timing on the effectiveness of rehabilitation.
Question 2: Rehabilitation Strategies
Five studies compared the effectiveness of various rehabilitation strategies and were summarized in this review (Table 2).
Two RCTs compared body weight–supported treadmill training (BWSTT) with conventional overground gait training.13,14 Dobkin et al13 evaluated whether AIS B, C or D patients treated with BWSTT would have superior outcomes compared to a control group receiving defined overground mobility training of similar intensity (Table 2). Patients were enrolled within 8 weeks of injury and within 1 week of rehabilitation admission and received 12 weeks of treatment. The BWSTT group had a slightly higher proportion of males, a higher proportion of cervical injuries and poorer lower extremity motor scores (LEMS) at baseline than the control group.13 Outcomes were assessed at 6 weeks, and at 3-, 6-, and 12-months following rehabilitation. There were no significant differences between the groups with regard to FIM Locomotor scores (FIM-L), LEMS, walking velocity or walking distance at any follow-up (Table 3).13 This study had a moderately high risk of bias (see supplemental material). Random sequencing and allocation concealment were judged to be appropriate. The authors claimed intent to treat analysis; however, they randomized 146 patients and subsequently excluded 29 from the analysis because the patients did not receive the allocated intervention or were judged to be ineligible after treatment was initiated. Follow-up rate was 71% at 6 months.
Table 3.
Outcomes Comparing BWSTT With Conventional Rehabilitation.
| Authors (Year) | Outcome | ASIA B | ASIA C/D | Authors’ Conclusions | ||
|---|---|---|---|---|---|---|
| BWSTT | Conventional | BWSTT | Conventional | |||
| Dobkin et al (2007) | FIM-L a | |||||
| 6 weeks (mean ± SD) | 1.07 ± 0.27 | 1.06 ± 0.24 | 3.0 ± 2.1 | 3.9 ± 2.1 | No statistical difference | |
| 3 months (mean ± SD) | 1.31 ± 1.11 | 1.94 ± 1.73 | 4.7 ± 2.1 | 5.5 ± 1.4 | No statistical difference | |
| 6 months (mean ± SE) | 2.0 ± 0.6 | 2.5 ± 0.5 | 5.3 ± 0.3 | 5.6 ± 0.27 | No statistical difference | |
| 12 months (mean ± SE) | 2.7 ± 0.7 | 2.1 ± 0.6 | 5.8 ± 0.3 | 5.6 ± 0.32 | No statistical difference | |
| LEMS b | ||||||
| 6 weeks (mean ± SD) | 4.1 ± 5.5 | 4.6 ± 6.5 | 29.1 ± 14.2 | 29.5 ± 11.5 | No statistical difference | |
| 3 months (mean ± SD) | 6.1 ± 8.6 | 7.3 ± 10.3 | 34.7 ± 13.3 | 35.7 ± 11.3 | No statistical difference | |
| Distance walked in 6 min (m) | ||||||
| 3 months (mean ± SD) | 10.7 ± 32 | 16.4 ± 36.3 | 247.7 ± 187.6 | 251.3 ± 203.7 | No statistical difference | |
| Timed walk (m/s for 15.2 m) | ||||||
| 6 weeks (mean ± SD) | 0.11 ± NR | 0.16 ± 0.08 | 0.69 ± 0.40 | 0.51 ± 0.42 | No statistical difference | |
| 3 months (mean ± SD) | 0.41 ± NR | 0.27 ± 0.13 | 0.85 ± 0.55 | 0.84 ± 0.54 | No statistical difference | |
| 6 months (mean ± SE) | 0.22 ± 0.07 | 0.24 ± 0.09 | 0.98 ± 0.10 | 1.09 ± 0.10 | No statistical difference | |
| 12 months (mean ± SE) | 0.25 ± 0.08 | 0.72 ± 0.08 | 1.21 ± 0.11 | 1.09 ± 0.10 | No statistical difference | |
| Authors (Year) | Angular kinematic parameters | Mean Differenceb (95% CI) | ||||
| Lucareli et al (2011) | DF stance (deg) | 0.0 (−0.4, 0.4) | 0.7 (0.2, 1.1) | No statistical difference | ||
| PF preswing (deg) | 0.0 (−1.8, 1.9) | −9.7 (−11.6, −7.8) | BWSTT more effective than control | |||
| Knee extension stance (deg) | −1.4 (−4.9, 2.1) | −1.1 (−4.6, 2.4) | No statistical difference | |||
| Knee flexion swing (deg) | −0.4 (−4.4, 3.5) | 1.5 (−2.4, 5.5) | No statistical difference | |||
| Hip flexion walking (deg) | 0.7 (−2.7, 4.1) | 1.1 (−2.3, 4.5) | No statistical difference | |||
| Hip extension stance (deg) | −0.2 (−1.4, 1.08) | −7.8 (−9.1, −6.6) | BWSTT more effective than control | |||
| Spatial-temporal parameters | ||||||
| Gait velocity (m/s) | 0.4 (0.09, 0.71) | 0.02 (−0.51, 0.55) | BWSTT more effective than control | |||
| Time of gait cycle (s) | 0.85 (0.24, 1.46) | −0.1 (−0.74, 0.54) | BWSTT more effective than control | |||
| Stance/duration of supportd (% of cycle) | −4.04 (−5.45, −2.63) | −0.1 (−2.05, 1.85) | BWSTT more effective than control | |||
| Swing/balance duratione (% of cycle) | 3.91 (2.47, 5.35) | −0.7 (−2.61, 1.21) | BWSTT more effective than control | |||
| Step length (cm) | 10.25 (8.34, 12.16) | 0.5 (−1.68, 2.68) | BWSTT more effective than control | |||
| Distance (m) | 10.75 (3.19, 18.31) | 1.8 (−4.14, 7.74) | BWSTT more effective than control | |||
| Cadence (steps/min) | 15.0 (7.94, 22.06) | 4.19 (−2.94, 11.32) | BWSTT more effective than control | |||
Abbreviations: ASIA, American Spinal Injury Association; BWSTT, body weight–supported treadmill training; DF, dorsiflexion; FIM-L, Functional Independence Measure–locomotor score; LEMS, lower extremity motor score; NR, not reported; PF, plantarflexion; SD, standard deviation; SE, standard error.
a FIM-L: assesses the amount of assistance a patient requires to walk 50 feet (levels 2 to 5) or 150 feet (levels 6 and 7): level 1 (patient requires total physical assistance), level 2 (maximum assistance of one person), level 3 (moderate assistance), level 4 (minimal assistance, hands-on contact), level 5 (patient requires supervision, no physical help), level 6 (independent with equipment), level 7 (no need for assistive devices).
b LEMS: measures the strength of the lower extremities, range 0 to 50, with lower scores indicating greater disability.
c Mean difference between preintervention and postintervention for the treatment group; a negative mean was assumed as a loss of motion compared with baseline.
d Control treatment group reported spatial temporal “stance” outcome; BWSTT treatment group reported spatial-temporal “duration of support” outcome.
e Control treatment group reported spatial temporal “swing” outcome; BWSTT treatment group reported spatial-temporal “balance duration” outcome.
A second RCT by Lucareli et al14 included patients with traumatic incomplete SCI that occurred within 12 months of study enrollment. The aim of this study was to compare kinematic gait parameters (range of motion and spatial-temporal variables) between patients treated with BWSTT and those receiving conventional gait training. Both groups underwent a total of 30 half-hour training sessions twice a week. The mean age of the sample was 31.5 years, 58% were males and patients were classified as either AIS C or D (n = 11, n = 13). Groups were similar with regard to baseline characteristics.14 Compared with the control group, patients receiving BWSTT achieved superior improvements in maximum hip extension during stance (mean difference from baseline: BWSTT: −0.2°, Conventional: −7.8°; P < .001) and maximum plantarflexion during preswing (mean difference from baseline: BWSTT: 0.0°, Conventional: −9.7°; P < .001) (Table 3).14 There were no differences between groups with regard to other range of motion variables, including dorsiflexion stance, knee extension stance, knee flexion swing and hip flexion while walking (Table 3). BWSTT was more effective at improving spatial-temporal gait parameters (gait velocity, time of gait cycle, stance time/duration of support, swing time/balance duration, step length, distance, and cadence) than the control group. This study had moderately low risk of bias (see supplemental material). Concealed allocation was documented, but the method of random sequence generation was not reported. Though the authors claimed intent to treat analysis, they excluded 3 patients from each group for failure to attend at least 85% of the training sessions. Follow-up was 80% at 12 weeks.
Two RCTs compared outcomes following functional electrical stimulation (FES) versus conventional therapy. In a study by Popovic et al,15 patients with incomplete cervical SCI were recruited within 6 months of injury and either received FES in addition to conventional occupational therapy or only occupational therapy. Injury etiology was different between the FES intervention and the control group: In the FES group, 58% were injured in a motor vehicle accident compared with only 22% in the control group. Patients received treatment for 8 weeks and 87.5% had complete follow-up (Table 2). The FES group had significantly greater improvements on the FIM Motor subscore (15.0 vs 4.1 points), FIM Self-Care subscore (20.1 vs 10 points) and Spinal Cord Independence Measure (SCIM) Self-Care subscore (10.2 vs 3.1 points) than the control group (Table 4).15 FES was also significantly more effective than the control therapy at improving 2 of the 9 components of the Toronto Rehabilitation Institute Hand Function Test: (1) the ability to hold an instrumented cylinder and (2) the ability to hold a credit card.15 This study had moderately high risk of bias (see supplemental material). The authors did not complete an intent-to-treat analysis. The authors reported that there were no clinically significant differences between the 2 groups with respect to baseline patient characteristics; however, variations in injury etiology (more motor vehicle accidents in the FES group) may reflect differences in injury severity. For example, the baseline FIM self-care, motor, and sphincter subscores were all lower in the control group, suggesting that patients in the control group had more severe injuries.
Table 4.
Percent Improvement From Baseline Between Patients Treated With FES and Controls.
| Authors (Year) | Outcome | Baseline | Discharge | Authors’ Conclusions | ||
|---|---|---|---|---|---|---|
| FES | Control | FES | Control | |||
| Popovic et al (2011) | FIM Motor subscore | 7.2 | 6.8 | 22.2 | 10.9 | FES more effective than controla |
| FIM Self-Care subscore | 8.1 | 7.8 | 28.2 | 17.8 | FES more effective than control | |
| SCIM Self-Care subscore | 1.9 | 3.3 | 12.1 | 6.4 | FES more effective than control | |
| TRI HFT | ||||||
| 10 objects | 37.1 | 27.2 | 53.8 | 38.5 | No statistical difference | |
| 9 rectangular blocks | 49.7 | 29.3 | 49.7 | 38.4 | No statistical difference | |
| Cylinder (able to hold) | 1.0 | 1.90 | 1.7 | 1.33 | FES more effective than control | |
| Cylinder (torque, N·m) | 0.26 | 0.26 | 1.13 | 2.59 | No statistical difference | |
| Credit card (able to hold) | 1.0 | 1.33 | 1.7 | 1.41 | FES more effective than control | |
| Credit card (force, nm) | 4.42 | 2.67 | 12.5 | 8.76 | No statistical difference | |
| Wooden bar (able to hold) | 0.8 | 0.63 | 1.5 | 0.96 | No statistical difference | |
| Wooden bar (thumb direction, length values, cm) | 1.67 | 2.88 | 10.94 | 10.5 | No statistical difference | |
| Wooden bar (little finger direction, length values, cm) | 5.56 | 3.17 | 12.78 | 11.85 | No statistical difference | |
Abbreviations: FES: functional electrical stimulation; FIM, functional independence measure; SCIM, Spinal Cord Independence Measure; TRI-HFT, Toronto Rehabilitation Institute–Hand Function Test.
a Calculated based on data provided in the article, but not analyzed by Popovic et al (2011).
The other RCT compared the effectiveness of FES, biofeedback and a combination of these treatments versus conventional strengthening therapy for recovering tenodesis grasp.16 All patients suffered a traumatic SCI and began rehabilitation, on average, 2.5 to 3.2 weeks after injury. The sample size was small for each group and, as a result, there were differences in patient characteristics at baseline (Table 2). Injury severity was less severe in the biofeedback group compared with the other groups. Patients in the FES group were younger and started rehabilitation later than the other groups. With regard to outcomes, there were no significant differences between treatment groups in terms of tenodesis grasp. The sample size, however, was small and no summary data was presented.16 This study had high risk of bias (see supplemental material). The authors did not report details for intent-to-treat analysis or about concealed allocation. Sixty subjects were initially randomized, but 16 were excluded because they were unsuitable for treatment or did not complete the treatment sessions. Follow-up rate was 73.3% at 6 weeks (rehabilitation discharge).
One RCT conducted at 2 centers compared outcomes between patients who received additional training time devoted to unsupported sitting exercises and those treated with standard inpatient therapy.17 The purpose of this study was to determine if additional motor retraining improves a patient’s ability to sit unsupported. The median time from injury was 11 weeks and patients received 18 training sessions over 6 weeks. Patients randomized to the experimental group had higher motor levels of injury and more severe injuries as evaluated by AIS than the control group (Table 2). The predetermined “minimally worthwhile treatment effect” was not achieved on any of the outcome measures, including the SCI Falls Concern Scale, Maximal Lean Test, and the Canadian Occupational Performance Measure (Table 5).17 Therefore, there were no added benefits of unsupported sitting training.17 The study had moderately low risk of bias (see supplemental material). Control patients at 1 of the 2 centers received a short duration of unsupported sitting training in addition to standard rehabilitation. If this extra training conferred benefit to these control patients, this could attenuate the between-group differences and increase the possibility of a type II error. Follow-up rate was 84.4% at 6 weeks (rehabilitation discharge).
Table 5.
Outcomes Comparing Training With Unsupported Sitting With Control.
| Authors | Outcome | Between-Group Differences | Minimally Worthwhile Treatment Effect | Authors’ Conclusions |
|---|---|---|---|---|
| Harvey et al (2011) | Maximal Lean Test (mm), mean (95% CI) | −20 (−64, 24) | 24 | No statistical difference |
| Maximal Sideward Reach Test (% arm length), mean (95% CI) | 5 (−3, 13) | 20 | No statistical difference | |
| T-Shirt Test (s), mean (95% CI) | 8 (−5, 20) | 14 | No statistical difference | |
| SCI Falls Concern Scale (points/64), mean (95% CI) | −2 (−8, 3) | NA | No statistical difference | |
| COPM (points/10), median (IQR) | 0.5 (−0.5, 1.5) | 2 | No statistical difference | |
| COPM Satisfaction (points/10), median (IQR) | −1.0 (−2.5, 0.0) | 2 | No statistical difference | |
| Participants’ impression of change (points/7), median (IQR) | 1.0 (0.0, 2.0) | 1 | No statistical difference | |
| Clinicians’ impression of change (points/7), median (IQR) | 0.0 (−1.0, 1.0) | 1 | No statistical difference |
Abbreviations: CI, confidence interval; COPM, Canadian Occupational Performance Measure; IQR, interquartile range; NA, not applicable; SCI, spinal cord injury.
Question 3: Factors That Affect Outcomes Following Rehabilitation
Ten studies (3 prospective, 7 retrospective) reported in 14 publications evaluated important predictors of outcomes following rehabilitation (Table 6). The most commonly assessed factors were age, sex, AIS grade at admission, and FIM at admission. The average age of the patients enrolled in these studies ranged from 31.8 to 42.1 years and 76.9% to 89.8% were males. The mean follow-up across studies was 2 to 12 months. Surgical treatment details were not reported in most studies.
Table 6.
Characteristics of Studies Reporting Patient or Injury Factors Affecting Outcome Following Rehabilitation.
| Investigator (Year) | Study Design, Funding | Demographics | Predictive Factors Assessed | Outcome Measures |
|---|---|---|---|---|
| Abdul-Sattar (2014) | Single center prospective cohort study Funding NR | N = 90 Mean age: 38.1 ± 18.3 years Males: 82.2% (74/90) Follow-up: 123 ± 45 days (% NR) | Patient factors Age Gender Marital status Education level Presence of family caregiver during inpatient rehabilitation SCI factors Etiology of injury Time from injury to admission Length of stay Level of SCI injury Severity of injury/ASIA score at admission Motor FIM score at admission Depression Anxiety Urinary tract infection Spasticity Pressure ulcer Pain Destination at discharge |
|
| Cifu (1999) | Multicenter (n = 16) retrospective cohort study (SCI Model Systems Project) Study was supported in part by a grant from NIDRR. No funds were provided by a commercial party with direct financial interest in the results of this study | N = 300 Mean age: NR Males: 79.7% (239/300) Follow-up: At discharge (80.0%) | Patient factors Age |
|
| Coleman (2004) | Multicenter (n = 28) retrospective cohort study (GM-1 ganglioside drug study) Nothing of value received from a commercial entity related to this research | N = 760 Mean age: NR Males: NR Follow-up: 26 weeks postinjury (94.2%) | SCI factors Anatomical region of injury |
|
| Daverat (1990) | Single center prospective study Funding NR | N = 99 Mean age: 41 (12-84) years Males: 78% (n/N NR) Follow-up: 18 months (94.9%) | Patient factors Age SCI factors Initial conscious level Level of lesion Yale Scale score |
|
| DeJong (2013) | Multicenter (n = 6) retrospective study (used SCIRehab dataset) No commercial party with direct financial interest in the results of this research has or will confer a benefit on the authors or on any organization with which the authors are associated Likely overlap in patient population with other SCIRehab studies; extent of overlap unknown | N = 1032 Mean age: 37.9 ±16.5 years Males: 81.3% (836/951) Follow-up: 12 months postinjury (79.1%) | Patient factors Age BMI Education Employment status (admission) Marital status Primary language Primary payer Race Sex SCI factors CMG-TW CSI (admission) FIM Cognitive score (admission)b FIM Motor score (admission)b |
|
| Furlan (2009) | Multicenter retrospective study (used NASCIS-3 database) Authors disclose no conflicting financial interests | N = 499 Mean age: NR Males: 89.8% (448/499) Follow-up: 12 months postinjury (79.3%) | Patient factors Age BAC Ethnicity Sex SCI factors Cause of SCI Complete/incomplete injury (severity of SCI) Level of injury Glasgow Coma Scale (admission) |
|
| Horn (2013) CoE: II | Multicenter (n = 6) retrospective cohort (used SCIRehab dataset) No commercial party with direct financial interest in the results of this research has or will confer a benefit on the authors or on any organization with which the authors are associated Likely overlap in patient population with other SCIRehab studies; extent of overlap unknown | N = 1031 Mean age: 37.7 ± 16.7 years Males: 81.2% Follow-up: 12 months postinjury (83.3%) | SCI factors CCI CMG-TW CSI (admission, maximum) | Motor FIM scoreb Hospitalization by 1 year postinjury |
| Iseli (1999) Abstraction of traumatic only patients | Prospective cohort study Study was supported by the Swiss National Science Foundation and the International Research Institute for Paraplegia | N = 39 Mean age: 40±16.8 years Males: 76.9% (30/39) Follow-up: 6 months (100%) | Patient factors Age SCI factors Complete/incomplete injury (severity of SCI) Initial ASIA motor score Initial ASIA sensory score SSEP recordings (tibial, pudendal) |
|
| Kay (2007) CoE: II | Retrospective cohort study No funds were provided by a commercial party with direct financial interest in the results of this study | N = 343 Mean age: 42.1 ± 18.8 years Males: 79.9% Follow-up: 60.4 ± 32.5 days (96%) | Patient factors Age SCI factors AIS Central cord syndrome Completeness of neurologic impairment |
|
| Putzke (2003) | Multicenter (n = 18) retrospective cohort study (used NSCISC data of patients admitted to Department of Education–funded MSCIS centers) Resources for the production of this manuscript were provided by the University of Alabama at Birmingham Model Spinal Cord Injury System of Care Grant from the NIDRR, OSERS, and Department of Education | N = 6128 Mean age: 39.0±13.3 years Males: 80% (4913/6128) Follow-up: 12 months (79.4%) | Patient factors Age |
|
| Sipski (2004) | Multicenter (n = 20) retrospective cohort study (used data of patients admitted to MSCIS centers) No funds were provided by a commercial party with direct financial interest in the results of this study | N = 14 433 Mean age: 31.8 ± 15.0 years Males: 81.5% Follow-up: 12 months postinjury (83.7%) | Patient factors Age Sex |
|
| Teeter (2012) | Multicenter (n = 6) retrospective cohort study (used SCIRehab dataset and NSCISC database) No commercial party with direct financial interest in the results of this research has or will confer a benefit on the authors or on any organization with which the authors are associated Likely overlap in patient population with other SCIRehab studies; extent of overlap unknown | N = 1032 Mean age: 37.7 ± 16.7 years Males: 81.2% Follow-up: 12 months postinjury (80.4%) | Patient factors Age BMI Education Employment status (admission) Marital status Primary language Primary payer Race/ethnic group Sex SCI factors AIS Cause of SCI CSI (admission) FIM cognitive score (admission)b FIM motor score (admission)b Time from injury to admission |
|
| Tian (2013) | Multicenter (n = 6) retrospective cohort study (used SCIRehab dataset and NSCISC database) No commercial party with direct financial interest in the results of this research has or will confer a benefit on the authors or on any organization with which the authors are associated Likely overlap in patient population with other SCIRehab studies; extent of overlap unknown | N = 1032 Mean age: 37.7 ± 16.7 years Males: 80.9% Follow-up: 12 months postinjury (85.2%) | Patient factors BMI |
|
| Whiteneck (2012) | Multicenter (n = 6) retrospective cohort study (used SCIRehab dataset and NSCISC database) The contents of this article were developed under grants provided to the Craig Hospital, the Mount Sinai School of Medicine, and the Rehabilitation Institute of Chicago from the NIDRR, Office of Rehabilitative Services and US Department of Education Likely overlap in patient population with other SCIRehab studies, extent of overlap unknown | N = 1032 Mean age: 37.7 ± 16.7 years Males: 81% Follow-up: 12 months postinjury (83.2%) | Patient factors Age BMI Education Employment status (admission) Marital status Primary language Primary payer Race/ethnic group Sex SCI factors AIS Cause of SCI CSI (admission) FIM cognitive score (admission)b FIM motor score (admission)b Time from injury to admission |
|
Abbreviations: AIS, ASIA Impairment Scale; ASIA, American Spinal Injury Association; BAC, blood alcohol concentration; BMI, body mass index; CCI, Charlston Comorbidity Index; CMG-TW, case-mix group-tier weight; CoE, class of evidence; CSI, comprehensive severity index; FIM, Functional Independence Measure; MSCIS, Model Spinal Cord Injury Systems; NASCIS, National Acute Spinal Cord Injury Study; NIDRR, National Institute on Disability and Rehabilitation Research; NR, not reported; NSCISC, National Spinal Cord Injury Statistical Center; OSERS, Office of Special Education and Rehabilitation Services; PHQ, patient health questionnaire; SCI, spinal cord injury; SF-12, Short Form-12; SSEP, somatosensory-evoked potentials; SWLS, Satisfaction With Life Scale.
ASIA grade/AIS: categorizes motor and sensory impairment in patients with SCI: (A) complete—no sensory or motor function is preserved in sacral segments S4-S5; (B) incomplete—sensory, but not motor, function is preserved below the neurologic level and extends through sacral segments S4-S5; (C) incomplete—motor function is preserved below the neurologic level, and most key muscles below the neurologic level have muscle grades less than 3; (D) incomplete—motor function is preserved below the neurologic level, and most key muscles below the neurologic level have muscle gradess greater than or equal to 3; (E) normal—sensory and motor functions are normal.
ASIA Motor Score: sum of strength grades for all 10 key muscles bilaterally; scores range from 0 – 100, with lower scores indicating greater disability.
Barthel Scale Independence: Independent if patient can perform basic self-care activities such as feeding, dressing, washing, toileting, transferring, and moving (in wheelchair or walking) without the presence of a helping individual, but perhaps with assistive devices. In this sense, independence corresponds to a Barthel index of more than 60.
FIM score: a clinician-reported measure for the level of a patient’s disability; indicates how much assistance is required for the individual to carry out activities of daily living; scores range from 18 to 126, with lower scores indicating greater disability. In Cifu et al (1999), motor scores range from 13 to 91 and cognitive scores range from 5 to 35 (higher scores denoting greater levels of independence).
Level of preserved neurological function: Improvement was defined as a dichotomous (0 to 1) variable for left side, right side, either side, and both sides. For left-side improvement, a score of 1 was assigned to patients whose left level of preserved neurologic function was 1 or more levels greater at 1-year than at MSCIS admission; a score of 0 was assigned in all other cases. Right-side improvement was likewise defined. Patients with improvement on either side were given a score of 1 for “either side improvement” and 0 otherwise. Bilateral improvement was defined as improvement in both the left and the right side.
NASCIS motor score: Motor scores included assessment of 14 muscle segments from each body side. Each segment was scored 0 (no contraction), 1 (reduced contraction), 2 (active movement without gravity), 3 (active movement with resistance), 4 (function reduced but active movement against resistance), or 5 (normal function). Unilateral expanded motor scores varied from 0 (no contraction in any muscle) to 70 (normal motor function in 14 muscles).
PHQ-9: Contains nine questions about the frequency of depression symptoms. A higher score indicates greater symptomatology; proxy responses were not allowed.
SWLS: Life satisfaction based on responses to 5 questions addressing global life satisfaction: 5 statements on a 7-point Likert-type scale (1: completely disagree, 7: completely agree). Scores range from 7 to 35, with higher scores indicating greater life satisfaction.
a Coleman’s “marked recovery” was defined as improvement of at least 2 grades on the Modified Benzel Scale at week 26.
b FIM motor and transfer scores were Rasch-transformed.
Patient Factors
Older age was an inconsistent predictor of most outcomes. Two studies reported that older age was associated with decreased QoL (Table 7).18,19 Medicaid recipients consistently had worse performance of ADLs and QoL, and a higher risk of rehospitalization.19–21 Patients with Workers’ Compensation as the primary payer also had worse QoL outcomes; however, associations with other reported outcomes were inconsistent or absent.19,20 One study found that a higher blood alcohol concentration at acute care admission predicted worse performance in ADLs, but not other outcomes.22 Four of the SCIRehab publications19,20,23,24 analyzed the predictive value of body mass index (BMI) and reported a consistent association between a high BMI (≥30 kg/m2 ) and better QoL outcomes.19,20 A higher educational background was also related with a positive QoL outcome.19,20
Table 7.
Association Between Patient and SCI Factors and Outcomes Following Rehabilitationa
| Prognostic Factors | Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Neurologicalb | ADLs25,c | Ambulation | QoLd | Mortality | Rehospitalization | Pressure Ulcers | |
| Patient factors | |||||||
| Older age | (–),38 (O)22 ,39 | (–)19,20 ,40 (O)22,25 ,38 | (O)6,26 | (–)18,19 | (O)22 | (–/O)19,20,21 | (O)19,20 |
| Higher BAC (per 1% increase) | (O)22 | (–)22 | (O)22 | ||||
| Body mass index (≥30 kg/m2) | (–/O)19,20,24 | (+)19,20 | (–/O)19–21 | (O)19,20 | |||
| Higher education level | (+/O),19,20 (O)25 | (+)19,20 | (O)19–21 | (O)19,20 | |||
| Employment status at injury (working as reference) | |||||||
| Retired | (O)19,20 | (O)19–21 | (O)19,20 | ||||
| Student | (O)19,20 | (+/O)19–21 | (O)19,20 | ||||
| Unemployed | (O)19,20 | (–)19,20 | (O)19–21 | (O)19,20 | |||
| Presence of family caregiver during inpatient rehabilitation | (O)25 | ||||||
| Marital status at discharge | (O)19,25 | (O)19–21 | (O)19,20 | ||||
| Primary language (English) | (O)19,20 | (O)19–21 | (O)19,20 | ||||
| Primary payer (private insurance as a reference) | |||||||
| Medicaid | (–)19,20 | (–)19,20 | (–)19–21 | (O)19,20 | |||
| Medicare | (O)19,20 | (O)19 | (O)19–21 | (O)19,20 | |||
| Worker’s compensation | (–/O)19,20 | (–)19,20 | (O)19–21 | (O)19,20 | |||
| Race/Ethnicity | (O)22 | (O)19,20,22 | (O)22 | (O)19–21 | (O)19,20 | ||
| Sex (female) | (O)22 ,39 | (O)19,20,22,25 ,39 | (–)20 | (O)22 | (–/O)19–21 | (O)19,20 | |
| SCI factors | |||||||
| Cause of injury (vehicular as a reference) | (O)22 | (O)22,25 | (O)22 | ||||
| Fall or falling object | (O)19,20,25 | (O)19,20 | |||||
| Sports | (O)19,20,25 | (O)19,20 | |||||
| Violence | (O)19,20 | (O)19,20 | |||||
| Work related | (O)19,20 | (O)19,20 | |||||
| Other | (O)19,20,25 | (–)19,20 | (O)19,20 | ||||
| Higher CCI | (–)23 | (O)23 | |||||
| Central cord syndrome | (O)6 | ||||||
| Higher CMG-TW | (–)21,23 | ||||||
| Completeness of neurologic impairment | (O)6 | ||||||
| Initial conscious level | (O)40 | ||||||
| Higher CSI (admission) | (O)19,20,23 | (–/O)19–21,23 | (–/O)19,20 | ||||
| Higher CSI (maximum) | (–)23 | (O)15 | |||||
| Level of injury (cervical) | (–),22 (O)5 | (O),22 ,40 (–)25 | (O)22 | ||||
| Lower ASIA grade/scale (admission) | (–)19,20,25 | (–)6 | (–/O)19,20 | (–/O)19,20 | |||
| Lower ASIA motor score (admission) | (–)26 | ||||||
| Lower ASIA sensory score (admission) | (–)26 | ||||||
| Lower FIM cognitive score (admission) | (+)19,20 | (–/O)19–21 | (O)19,20 | ||||
| Lower FIM motor score (admission) | (–)19,20,25 | (–/O)19–21 | (–)19,20 | ||||
| Lower Glasgow Coma Scale (admission) | (O)22 | (O)22 | (O)22 | ||||
| Lower Yale Scale Score (admission) | (–)40 | ||||||
| More days from injury to rehabilitation | (–)19,20,25 | (–)19,20 | (–/O)19,20 | (–/O)19,20 | |||
| Shorter length of stay | (–)25 | ||||||
| Severity of injury (complete injury) | (–)22 | (O)22 | (–)26 | (–)22 | |||
| Stronger SSEP recordings (pudendal nerves) | (+)26 | ||||||
| Stronger SSEP recordings (tibial nerves) | (+)26 | ||||||
| Depression/Anxiety | (–)25 | ||||||
| Urinary tract infection | (O)25 | ||||||
| Spasticity | (O)25 | ||||||
| Pressure ulcer | (O)25 | ||||||
| Pain | (O)25 | ||||||
| Destination/Discharged to home | (O)25 | ||||||
Abbreviations: (+) = association with a positive outcome; (–) = association with a negative outcome; (O) = no association; (+/O) = publications from same dataset; association with a positive outcome in one publication, no association in a second publication; (–/O) = publications from same dataset; association with a negative outcome in one publication, no association in a second publication. AIS, ASIA Impairment Scale; ASIA, American Spinal Injury Association; BAC, blood alcohol concentration; BMI, body mass index; CCI, Charlston Comorbidity Index; CMG-TW, case-mix group-tier weight; CoE, class of evidence; CSI, comprehensive severity index; MSCIS, Model Spinal Cord Injury Systems; NASCIS, National Acute Spinal Cord Injury Study; PHQ, patient health questionnaire; SCI, spinal cord injury; SSEP, somatosensory-evoked potentials; SWLS, Satisfaction With Life Scale.
a References 19, 20, 23 and 24 are publications from the same study dataset (SCIRehab) but with different modeling strategies.
b ASIA Grade/Scale, change in level of preserved neurological function, marked recovery, NASCIS Motor score.
c Barthel Scale (independence/dependence), FIM Motor score.
d PHQ-9, SF-12 Physical Health, SWLS.
Injury Factors
A lower FIM Motor Score was consistently associated with diminished independence with ADLs and a higher risk of pressure ulcers (Table 7).19,20,25 Complete injuries were related to poorer neurologic and ambulation outcomes and higher rates of mortality.22,26 Stronger somatosensory-evoked potential (SSEP) recordings (pudendal and tibial nerves) were predictive of better ambulation outcomes.26 Increased time from injury to rehabilitation was associated with worse ADL and QoL outcomes, but not with increased risk of rehospitalization or pressure ulcers.19,20,25 Increased injury severity at admission, as reflected by components of the International Standards for the Neurological Classification of SCI (including AIS impairment grade), was an important predictor of worse performance of ADLs and ambulation6,19,20,26; however, associations with risk of rehospitalization or development of pressure ulcers were inconsistent.19,20
Evidence Summary
There was no difference between BWSTT and conventional rehabilitation with regard to the FIM-L Score, LEMS Score, the distance walked in 6 minutes or gait velocity over 15.2 m in patients with acute SCI. The strength of evidence for these findings is low. There is low evidence that FES may result in slightly better FIM Motor, FIM Self-Care and SCIM Self-Care subscores compared with conventional occupational therapy in patients with acute cervical SCI (Table 8). Comparisons using the Toronto Rehabilitation Institute Hand Function Test demonstrated no differences between groups in 7 of 9 domains. There were no clinically important differences in Maximal Lean Test, Maximal Sideward Reach Test, T-shirt Test, or the Canadian Occupational Performance Measure between unsupported sitting training and standard inpatient rehabilitation. The evidence for this conclusion is low.
Table 8.
Strength of Evidence Summary Table.
| No. of Studies/N/Follow-up | Strength of Evidence | Conclusions, Effect Size | |
|---|---|---|---|
| Body weight–supported treadmill training (BWSTT) vs conventional rehabilitation | |||
| FIM-L Score (± SD) | 1 RCT/146/12 months | Lowa | There were no differences between BWSTT and conventional rehabilitation for ASIA B (mean difference, 0.6 ± 1.2) or ASIA C and D patients (mean difference, 0. 2 ± 0.94) |
| LEMS Score (± SD) | 1 RCT/146/12 months | Lowa | There were no differences between BWSTT and conventional rehabilitation for ASIA B (mean difference, −1.2 ± 3.5) or ASIA C and D patients (mean difference, −1.0 ± 2.7) |
| Distance walked in 6 min (m) (±SD) | 1 RCT/146/12 months | Lowa | There were no differences between BWSTT and conventional rehabilitation for ASIA B (mean difference, −5.7 ± 14.9 m) or ASIA C and D patients (mean difference, −3.6 ± 46.7 m) |
| Difference in gait velocity over 15.2 m (m/s) (±SD) | 1 RCT/146/12 months | Lowa | There were no differences between BWSTT and conventional rehabilitation for ASIA B (mean difference, −0.47 ± 0.14 m/s) or ASIA C and D patients (mean difference, 0.12 ± 0.34 m/s) |
| Functional electrical stimulation vs control/conventional occupational therapy | |||
| FIM Motor subscore | 1 RCT/24/8 weeks | Lowa | FES treatment resulted in a significant 11.3 point increase in FIM Motor subscore compared with COT |
| FIM Self-Care Subscore | 1 RCT/24/8 weeks | Lowa | FES treatment resulted in a significant 10.4 point increase in FIM Self-Care subscore compared with COT |
| SCIM Self-Care subscore | 1 RCT/24/8 weeks | Lowa | FES treatment resulted in a significant 5.7 point increase in SCIM Self-Care subscore compared with COT |
| TRI HFT | 1 RCT/24/8 weeks | Lowa | Of the 9 subdomains evaluated, only ability to hold a cylinder and credit card showed that FES was slightly more effective than COT |
| Training unsupported sitting (TUS) vs control/standard inpatient therapy (control) | |||
| Maximal Lean Test (mm) | 1 RCT/32/6-7 weeks | Lowa | There were no differences between TUS and standard inpatient therapy (mean difference, −20 mm (95% CI −64, 24)) |
| Maximal Sideward Reach Test (% arm length) | 1 RCT/32/6-7 weeks | Lowa | There were no differences between TUS and standard inpatient therapy (difference in mean % arm length, 5% (95% CI −3%, 13%)) |
| T-Shirt Test (seconds) | 1 RCT/32/6-7 weeks | Lowa | There were no differences between TUS and standard inpatient therapy (difference in mean duration, 8 seconds (95% CI −5, 20)) |
| COPM Performance (points/10) | 1 RCT/32/6-7 weeks | Lowa | There were no differences between TUS and standard inpatient therapy (median difference, 0.5 (IQR −0.05, 1.5)) |
| COPM Satisfaction (points/10) | 1 RCT/32/6-7 weeks | Lowa | There were no differences between TUS and standard inpatient therapy (median difference, −1.0 (IQR −1.0, 1.0)) |
Abbreviations: BWSTT: Body Weight–Supported Treadmill Training; CI, confidence interval; COPM, Canadian Occupational Performance Measure; COT, conventional occupational therapy; FES, functional electrical stimulation; FIM, Functional Independence measure; FIM-L, Functional Independence Measure–locomotor score; IQR, interquartile range; NR, not reported; SCIM, Spinal Cord Independence Measure; TUS, Training Unsupported Sitting; TRI-HFT, Toronto Rehabilitation Institute Hand Function Test.
a Downgraded for serious risk of bias and imprecision
Discussion
Rehabilitation improves functional status and clinical outcomes following acute SCI.27,28 The contribution of individual treatment interventions, however, has been exceedingly difficult to tease out due to the nature of and challenges associated with rehabilitation research. Identified issues include the lack of standardization of interventions, therapeutic doses, and outcome measures, as well as heterogeneous populations, superimposed spontaneous recovery, and problems with group assignment and active contrast for control groups.29 Compared with interventions such as drug trials or surgical procedures, contemporary interdisciplinary rehabilitation typically involves the simultaneous application of multiple treatments by multiple team members, with individual team members having considerable professional discretion as to the nature of delivered services. This complex milieu of multiple concurrent and interacting treatments makes it extremely difficult to identify the contribution of single interventions. Consequently rehabilitation following SCI has been likened to the proverbial “Black Box” or even a “Russian Doll.”30
In response to the above challenges, there have been significant efforts in recent years to develop a classification system or taxonomy for rehabilitation treatments.31 Using this paradigm, rehabilitation treatments are organized by their known or hypothesized “active ingredients” (mechanisms of action).30 The SCIRehab project has recently employed this taxonomy to systematically document and quantify interventions provided by 7 rehabilitation disciplines at 6 US inpatient SCI rehabilitation facilities.32 Practice-based research (comparative effectiveness) of this nature will help identify specific interventions or combinations of interventions that are associated with positive outcomes following SCI rehabilitation.
The nature and content of rehabilitation are also subject to considerable variance depending on local context and jurisdiction. Timing, type, intensity, and duration of therapies can be driven by available funding and health care policy, as much as by medical evidence. Similar to other types of clinical SCI research, the heterogeneity and low incidence of SCI pose additional challenges to accruing the required number of subjects to adequately power trials.2
Acknowledging these limitations, we discuss and summarize the evidence specific to our questions.
1. Does the timing of implementing a rehabilitation strategy affect outcome?
Currently there are no targeted human trials that directly assess the impact of timing on the efficacy of rehabilitation. Observational and retrospective studies have reported an association between increased time from injury to rehabilitation and poorer QoL and performance of ADLs.19–21,33,34 This suggests that a delay in the initiation of specialized rehabilitation could be detrimental.
Looking to the future, it is anticipated that there will continue to be significant barriers to performing comparative studies, as there are strong drivers for initiating rehabilitation as early as possible. When confronted with a life-altering event such as a SCI, patients and individuals are understandably eager to initiate rehabilitation and begin working toward recovery as soon as possible. From the perspective of health care delivery systems, there is typically great pressure to transition patients from acute care and initiate rehabilitation as soon as feasible, assuming there is medical stability. This is driven by the ongoing need to both minimize costs associated with acute care and maintain patient flow and resource availability (eg, acute beds) for newly injured individuals. For the above reasons, a comparative trial of early versus delayed rehabilitation would be untenable, and the timing of rehabilitation will likely continue to be driven by medical stability and the availability of rehabilitation resources.
Future investigations could study and compare outcomes between jurisdictions where variations in treatment patterns and timing of rehabilitation already exist.35 The challenge with such an approach, however, is the need to identify and adequately control for all potential confounders. This is problematic due to the sheer number of potential confounders (eg, patient, injury, clinician, center, and health scheme characteristics) in a complex system and the fact that some confounders are likely to remain unknown.
2. What is the comparative effectiveness of different rehabilitation strategies, including the intensity and duration of rehabilitation?
For individuals with incomplete SCI, the RCT by Dobkin et al13 suggests that BWSTT is as effective as overground mobility training of similar duration and intensity for improving ambulation outcomes. It is not known whether there are specific contexts and subpopulations that favor one approach over the other. A small RCT by Lucareli et al14 suggests that, compared with conventional gait training, BWSTT might have advantages for improving very specific gait parameters. It was not shown, however, that these differences affect the degree of required assistance and independence, nor function outside an experimental context. Further research is needed to identify the appropriate dose of BWSTT and potential patient subpopulations who may benefit from aspects of progressive limb loading, sensory input, control of posture, correction of gait kinematics or speed, in sequence or in combination with conventional therapy. A disadvantage to BWSTT is that it is a labor-intensive approach that can require 2 to 3 experienced therapists to manually assist the patient through the gait cycle.
In regard to upper extremity function, there is evidence suggesting that FES is better than conventional rehabilitation in individuals with incomplete cervical SCI; however, this evidence is only from 1 center.15 This strategy warrants further investigation to confirm its generalizability and feasibility in diverse clinical settings. Hand function is one of the most important priorities for individuals with cervical SCI and so the desirable consequences of FES probably outweigh the undesirable consequences in most settings.
Outside the above studies, there is no compelling evidence in support of other specific rehabilitation interventions for acute or subacute SCI. It is important to state that this does not mean that current conventional approaches are ineffective. Since it is universally considered unethical to withhold conventional rehabilitation, studies have compared new therapeutic approaches to conventional, established approaches. In this context, we can only say that alternative approaches have generally failed to demonstrate superiority when compared with conventional therapy in the context of acute or subacute SCI. A possible exception is FES for upper extremity function in individuals with incomplete cervical SCI.
There are additional studies that have investigated the efficacy of rehabilitation interventions for individuals with chronic SCI; however, this patient population fell outside the defined scope of this systematic review. For example, Yang and colleagues36 found that either repetitive mass practice or precision practice improves walking performance in individuals with chronic SCI, and that mass practice leads to comparatively better endurance. In another study, Field-Fote and Roach37 demonstrated that walking speed improved with both overground training and treadmill-based training combined with FES to assist dorsiflexion; however, walking distance improved to a greater extent with overground training. It can be postulated that interventions that have efficacy in chronic SCI could also be applicable in acute SCI populations.
In the future, clinical effectiveness research designs and pilot studies could be used to better define the essential elements of task-specific interventions and their mechanisms of action, determine dose-response and timing, establish a meaningful contrasting control treatment, select subpopulations most likely to respond to rehabilitation strategies and estimate numbers needed for multicenter studies.29
3. Are there patient or injury characteristics that impact the efficacy of rehabilitation?
Prior studies have identified the following patient characteristics as important predictors of outcomes following rehabilitation for individuals with SCI: older age,18,19 Medicaid enrollment (United States),19–21 work-related injury with associated compensation,19,20 and increased serum alcohol concentration at the time of injury. Several of these variables hint at the importance and impact of social and societal factors on the outcomes of rehabilitation. Medicaid enrollment is associated with lower income in the United States, as it is a public program designed for individuals whose annual income is below specific thresholds. In regard to Workers’ Compensation, the continued payment of benefits is often contingent on the persistence of functional impairments and associated disability. Finally, serum alcohol levels at hospitalization reflect preinjury behaviors and decision-making that could contribute to an individual’s postinjury function, adaptability, and general well-being.
Some patient characteristics are protective and enhance rehabilitation outcomes. Educational background has been associated with enhanced QoL postrehabilitation.19,20 Potential explanations for this include differences in employment and income, social supports and marital status, adaptability, and coping strategies. Interestingly, increased BMI has also been associated with increased QoL.19,20
Not surprisingly, parameters that reflect injury severity have been associated with poorer outcomes following rehabilitation. These include lower FIM motor scores,19,20,25 lesion completeness,22,26 SSEP characteristics,26 time from injury to rehabilitation,19,20,25 and increased severity as measured by International Standards for the Neurological Classification of SCI.6,19,20,26
Patient and injury characteristics have important implications for rehabilitation and influence the selection of patients most likely to respond to a specific treatment strategy. Response to treatment strategies may vary depending on factors such as age, comorbidities, pattern of impairment/spared function, time postinjury, natural history of change over time, and compensation and accommodation of impairments or disabilities.
4. What is the evidence that one rehabilitation strategy is more cost-effective compared with a different strategy?
Despite the intensive resources and associated costs of SCI rehabilitation, there have been no studies comparing the cost-effectiveness of different strategies and approaches. In addition, even when one sets aside costs, there is still a relative dearth of studies that objectively assess the efficiency of different rehabilitation methodologies for SCI.27 It will be important to assess cost-effectiveness in future trials, as this will inevitably affect feasibility and widespread adoption.
Conclusion
Rehabilitation plays a central role in maximizing function and facilitating community reintegration following SCI. The results of this rigorous, systematic review reveal, however, that the evidence base is limited for many fundamental questions related to rehabilitation following acute and subacute SCI. These knowledge gaps include the timing of rehabilitation, nature of rehabilitation (specific interventions), therapeutic dose (intensity, frequency, duration), role and impact of patient and injury characteristics, cost-effectiveness and efficiency of alternative interventions. As discussed, this is largely attributable to the challenges and barriers associated with assessing efficacy of concurrent treatments administered by multiple interdisciplinary team members. Recent studies have begun to address identified methodological challenges. Future studies will need to build on these efforts in order to establish and expand the evidence base for rehabilitation after SCI.
Supplementary Material
Acknowledgments
The authors thank Krystle T. Pagarigan.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by AOSpine and the AANS/CNS Section on Neurotrauma and Critical Care. Dr Fehlings wishes to acknowledge support from the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration and the DeZwirek Family Foundation. Dr Tetreault acknowledges support from a Krembil Postdoctoral Fellowship Award. Analytic support for this work was provided by Spectrum Research, Inc., with funding from the AOSpine North America.
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Burns AS, Marino RJ, Flanders AE, Flett H. Clinical diagnosis and prognosis following spinal cord injury. Handb Clin Neurol. 2012;109:47–62. doi:10.1016/B978-0-444-52137-8.00003-6. [DOI] [PubMed] [Google Scholar]
- 2. Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi:10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 3. Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil. 2012;93:1487–1497. doi:10.1016/j.apmr.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 4. Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–546. doi:10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coleman WP, Geisler FH. Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. 2004;4:373–378. doi:10.1016/j.spinee.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 6. Kay ED, Deutsch A, Wuermser LA. Predicting walking at discharge from inpatient rehabilitation after a traumatic spinal cord injury. Arch Phys Med Rehabil. 2007;88:745–750. doi:10.1016/j.apmr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 7. Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr Neurologic recovery after traumatic spinal cord injury: data from the model spinal cord injury systems. Arch Phys Med Rehabil. 1999;80:1391–1396. [DOI] [PubMed] [Google Scholar]
- 8. Tee JW, Chan CH, Gruen RL, et al. Early predictors of health-related quality of life outcomes in polytrauma patients with spine injuries: a level 1 trauma center study. Global Spine J. 2014;4:21–32. doi:10.1055/s-0033-1358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A:1–3. [PubMed] [Google Scholar]
- 10. West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ). 2002;47:1–11. [PMC free article] [PubMed] [Google Scholar]
- 11. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490 doi:10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi:10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 13. Dobkin B, Barbeau H, Deforge D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21:25–35. doi:10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucareli PR, Lima MO, Lima FP, de Almeida JG, Brech GC, D’Andréa Greve JM. Gait analysis following treadmill training with body weight support versus conventional physical therapy: a prospective randomized controlled single blind study. Spinal Cord. 2011;49:1001–1007. [DOI] [PubMed] [Google Scholar]
- 15. Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–442. doi:10.1177/1545968310392924. [DOI] [PubMed] [Google Scholar]
- 16. Kohlmeyer KM, Hill JP, Yarkony GM, Jaeger RJ. Electrical stimulation and biofeedback effect on recovery of tenodesis grasp: a controlled study. Arch Phys Med Rehabil. 1996;77:702–706. [DOI] [PubMed] [Google Scholar]
- 17. Harvey LA, Ristev D, Hossain MS, et al. Training unsupported sitting does not improve ability to sit in people with recently acquired paraplegia: a randomised trial. J Physiother. 2011;57:83–90. [DOI] [PubMed] [Google Scholar]
- 18. Putzke JD, Barrett JJ, Richards JS, DeVivo MJ. Age and spinal cord injury: an emphasis on outcomes among the elderly. J Spinal Cord Med. 2003;26:37–44. [DOI] [PubMed] [Google Scholar]
- 19. Teeter L, Gassaway J, Taylor S, et al. Relationship of physical therapy inpatient rehabilitation interventions and patient characteristics to outcomes following spinal cord injury: the SCIRehab project. J Spinal Cord Med. 2012;35:503–526. doi:10.1179/2045772312y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whiteneck G, Gassaway J, Dijkers MP, Heinemann AW, Kreider SE. Relationship of patient characteristics and rehabilitation services to outcomes following spinal cord injury: the SCIRehab project. J Spinal Cord Med. 2012;35:484–502. doi:10.1179/2045772312y.0000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeJong G, Tian W, Hsieh CH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil. 2013;94(4 suppl):S87–S97. doi:10.1016/j.apmr.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 22. Furlan JC, Kattail D, Fehlings MG. The impact of co-morbidities on age-related differences in mortality after acute traumatic spinal cord injury. J Neurotrauma. 2009;26:1361–1367. doi:10.1089/neu.2008-0764. [DOI] [PubMed] [Google Scholar]
- 23. Horn SD, Smout RJ, DeJong G, et al. Association of various comorbidity measures with spinal cord injury rehabilitation outcomes. Arch Phys Med Rehabil. 2013;94:S75–S86. [DOI] [PubMed] [Google Scholar]
- 24. Tian W, Hsieh CH, DeJong G, Backus D, Groah S, Ballard PH. Role of body weight in therapy participation and rehabilitation outcomes among individuals with traumatic spinal cord injury. Arch Phys Med Rehabil. 2013;94(4 suppl):S125–S136. doi:10.1016/j.apmr.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 25. Abdul-Sattar AB. Predictors of functional outcome in patients with traumatic spinal cord injury after inpatient rehabilitation: in Saudi Arabia. NeuroRehabilitation. 2014;35:341–347. [DOI] [PubMed] [Google Scholar]
- 26. Iseli E, Cavigelli A, Dietz V, Curtz A. Prognosis and recovery in ischaemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. J Neurol Neurosurg Psychiatry. 1999;67:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burns AS, Yee J, Flett HM, Cournoyea N. Impact of benchmarking and clinical decision making tools on rehabilitation length of stay following spinal cord injury. Spinal Cord. 2013;51:165–169. doi:10.1038/sc.2012.91. [DOI] [PubMed] [Google Scholar]
- 28. Yarkony GM, Roth EJ, Heinemann AW, Wu YC, Katz RT, Lovell L. Benefits of rehabilitation for traumatic spinal cord injury. Multivariate analysis in 711 patients. Arch Neurol. 1987;44:93–96. [DOI] [PubMed] [Google Scholar]
- 29. Dobkin BH. Confounders in rehabilitation trials of task-oriented training: lessons from the designs of the EXCITE and SCILT multicenter trials. Neurorehabil Neural Repair. 2007;21:3–13. doi:10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whyte J, Hart T. It’s more than a black box; it’s a Russian doll: defining rehabilitation treatments. Am J Phys Med Rehabil. 2003;82:639–652. doi:10.1097/01.PHM.0000078200.61840.2D. [DOI] [PubMed] [Google Scholar]
- 31. Whiteneck G, Gassaway J, Dijkers M, Jha A. New approach to study the contents and outcomes of spinal cord injury rehabilitation: the SCIRehab project. J Spinal Cord Med. 2009;32:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gassaway J, Whiteneck G, Dijkers M. Clinical taxonomy development and application in spinal cord injury research: the SCIRehab project. J Spinal Cord Med. 2009;32:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scivoletto G, Morganti B, Molinari M. Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study. Arch Phys Med Rehabil. 2005;86:512–516. doi:10.1016/j.apmr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 34. Sumida M, Fujimoto M, Tokuhiro A, Tominaga T, Magara A, Uchida R. Early rehabilitation effect for traumatic spinal cord injury. Arch Phys Med Rehabil. 2001;82:391–395. doi:10.1053/apmr.2001.19780. [DOI] [PubMed] [Google Scholar]
- 35. Whiteneck G, Gassaway J, Dijkers M, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Inpatient treatment time across disciplines in spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34:133–148. doi:10.1179/107902611X12971826988011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang JF, Musselman KE, Livingstone D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair. 2014;28:314–324. doi:10.1177/1545968313508473. [DOI] [PubMed] [Google Scholar]
- 37. Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91:48–60. doi:10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


