Abstract
Study Design:
Systematic review and meta-analysis.
Objective:
The objective of this study was to conduct a systematic review to assess the comparative effectiveness and safety of high-dose methylprednisolone sodium succinate (MPSS) versus no pharmacological treatment in patients with traumatic spinal cord injury (SCI).
Methods:
A systematic search was performed in PubMed and the Cochrane Collaboration Library for literature published between January 1956 and June 17, 2015. Included studies were critically appraised, and Grades of Recommendation Assessment, Development and Evaluation methods were used to determine the overall quality of evidence for primary outcomes. Previous systematic reviews on this topic were collated and evaluated using the Assessment of Multiple Systematic Reviews scoring system.
Results:
The search yielded 723 citations, 13 of which satisfied inclusion criteria. Among these, 6 were primary research articles and 7 were previous systematic reviews. Based on the included research articles, there was moderate evidence that the 24-hour NASCIS II (National Acute Spinal Cord Injury Studies) MPSS regimen has no impact on long-term neurological recovery when all postinjury time points are considered. However, there is also moderate evidence that subjects receiving the same MPSS regimen within 8 hours of injury achieve an additional 3.2 points (95% confidence interval = 0.10 to 6.33; P = .04) of motor recovery compared with patients receiving placebo or no treatment.
Conclusion:
Although safe to administer, a 24-hour NASCIS II MPSS regimen, when all postinjury time points are considered, has no impact on indices of long-term neurological recovery. When commenced within 8 hours of injury, however, a high-dose 24-hour regimen of MPSS confers a small positive benefit on long-term motor recovery and should be considered a treatment option for patients with SCI.
Keywords: methylprednisolone sodium succinate, MPSS, spinal cord injury, systematic review, traumatic spinal cord injury
Introduction
Given its potent anti-inflammatory actions, methylprednisolone sodium succinate (MPSS) has a long history of use across a wide spectrum of disease. Within the context of traumatic spinal cord injury (SCI), preclinical animal studies have demonstrated mixed results with regard to the neuroprotective efficacy of MPSS.1–4 From the standpoint of clinical investigation, randomized trials, namely, the National Acute Spinal Cord Injury Studies (NASCIS), investigating the potential efficacy and safety of MPSS, have formed the basis for the largest therapeutic studies completed in the history of SCI research. Although interpretation of, and reaction to, the results of these studies have varied over time, their publication led to the widespread adoption of this therapy by clinicians throughout the world. As evidence of this, in a 2006 survey study polling the membership of the North American Spine Society, 86% of respondents indicated that they would choose to administer MPSS to SCI patients as per the recommendations of the NASCIS II and III studies; however, concern surrounding medicolegal reprisal for not administering MPSS was listed as the major factor motivating decision making in a large faction of these respondents.5
In spite of the extensive use of MPSS for SCI over the past several decades, the appropriateness of this treatment approach remains a contentious topic.6,7 Opponents of the routine use of MPSS for acute SCI have highlighted concerns regarding the conduct of the NASCIS trials and the reported results. These include the reliance on subgroup analysis (particularly based on timing of MPSS initiation), the small reported effect size for neurologic improvement, and the potential for harmful and serious adverse events.8 In order to quell the existing controversy, a number of attempts have been made by several different groups to review the existing evidence, with the aim of providing clinicians with specific evidence-based recommendations related to this treatment.9,10 In spite of such attempts, debate within the clinical community continues, leaving the physician caring for acute SCI patients in a precarious position where administering or not administering MPSS can be questioned and challenged.
Based on this background, the purpose of this systematic review was to address the following key questions (KQ): In adult patients with acute complete or incomplete traumatic SCI: (1) What is the efficacy and effectiveness of MPSS compared with no pharmacologic treatment? (2) What is the safety profile of MPSS compared with no pharmacologic treatment? (3) What is the evidence that MPSS has differential efficacy or safety in subpopulations?
Materials and Methods
Electronic Literature Search
We conducted a systematic search in PubMed and the Cochrane Collaboration Library for literature published between January 1956 and June 17, 2015, on patients with acute traumatic SCI treated with MPSS. The search was limited to human studies published in or translated to the English language. Reference lists of key articles were also systematically checked to identify additional eligible articles. We included studies that evaluated the efficacy and safety of MPSS compared with no treatment or placebo in patients ≥13 years with acute SCI (Table 1). With respect to study design, all randomized controlled trials were included. Severity of injury in SCI patients is a well-known factor that is associated with outcome.11–14 Severity of SCI may also influence the clinical decision of whether to administer MPSS. Therefore, we included observational studies that controlled for SCI severity, as measured by baseline motor status and/or complete versus incomplete injury. We excluded studies on patients with penetrating injuries to the spinal cord; cord compression due to tumor, hematoma, or degenerative disease; and no neurological deficit following trauma. Furthermore, we excluded animal studies, nonclinical studies, studies with a follow-up rate of <50%, small studies with n<10 per treatment group, and studies reporting nonclinical outcomes of efficacy or safety. Two investigators (JRD, JRW) reviewed the full texts of potential articles to obtain a final collection of relevant studies.
Table 1.
Inclusion and Exclusion Criteria.
| Study Component | Inclusion | Exclusion |
|---|---|---|
| Participants |
|
|
| Intervention |
|
|
| Comparators |
|
|
| Outcomes |
Efficacy/effectiveness
|
|
| Study design |
|
|
| Publication |
|
|
Abbreviations: CSM, cervical spondylotic myelopathy; MPSS, methylprednisolone sodium succinate; KQ, key question; RCT, randomized controlled trial.
Data Extraction
From the included articles, the following data was extracted: study design, patient demographics, treatment details, study inclusion/exclusion criteria, injury severity, follow-up duration, rates of follow-up for each treatment group, and outcomes assessed. We attempted to identify studies with overlapping data and only reported the data from the most complete study (largest sample size) in order to prevent double counting.
Risk of Bias and Overall Strength of Body of Literature
Risk of bias was assessed by combining epidemiologic principles with characteristics of study design. Risk of bias was determined for each article using criteria set by The Journal of Bone and Joint Surgery 15 for therapeutic studies and modified to delineate criteria associated with methodological quality and risk of bias based on recommendations made by the Agency for Healthcare Research and Quality16,17 (see Supplemental Digital Material for risk of bias evaluation).
After individual article evaluation, the strength of the overall body of evidence with respect to each primary outcome was determined based on precepts outlined by the Grades of Recommendation Assessment, Development and Evaluation (GRADE) Working Group.18,19
The initial strength of the overall body of evidence was considered “High” if the majority of the studies were randomized controlled trials and “Low” if the majority of the studies were observational studies. Criteria for downgrading published evidence 1 or 2 levels included (1) serious risk of bias, (2) inconsistency of results, (3) indirectness of evidence, (4) imprecision of the effect estimates (eg, wide confidence intervals), or (5) non–a priori statement of subgroup analyses. Alternatively, the body of evidence could be upgraded 1 or 2 levels based on the following factors: (1) large magnitude of effect or (2) dose-response gradient. The final overall strength of the body of literature expresses our confidence that the effect size lies close to the true effect and the extent to which it is believed to be stable based on the adequacy of or the deficiencies in the body of evidence. An overall strength of “High” means that we are very confident that the true effect lies close to that of the estimated effect. A “Moderate” rating means that we are moderately confident in the effect estimate; the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different. An overall strength of “Low” means that our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate. Finally, a rating of “Very Low” means that we have very little confidence in the effect estimate; the true effect is likely to be substantially different than the estimated effect. In addition, this rating may be used if there is no evidence or it is not possible to estimate an effect.
Data Analysis
Results were pooled when 2 or more studies presented the same outcomes at similar time periods. We considered the risk of bias when deciding whether to pool data between the prospective cohort studies and randomized controlled trials. Specifically, we pooled data from prospective cohort studies if they had a low risk of bias and controlled for potential confounding factors. For effectiveness outcomes, pooled data was stratified by study design to demonstrate the effect of adding nonrandomized results. To compare the estimates of procedure effectiveness across studies using continuous outcomes, weighted mean differences were computed with 95% confidence intervals (CIs). For safety outcomes, we calculated the risk difference (RD) and 95% CIs. We assumed a random-effect model using the Mantel-Haenszel method. Calculations and plots for effectiveness outcomes were implemented in RevMan,20 while the complications plot was made with R (version 3.2.1).21
To explore the possibility of differential effectiveness, we compared outcomes within subgroup stratum when data was available. We tested the difference between subgroups by calculating the I 2 statistics. We displayed the estimates visually with Forest plots to demonstrate the differential effect. When the stratum-specific effect measures and their CIs fall on opposite sides of the overall effect, this represents a differential effect.
Results
Study Selection
Our electronic and bibliography search yielded 723 citations. Of these, we excluded 693 based on information available in the title or abstract. The full texts of 30 articles were obtained and further investigated. After full text review, we excluded 17 studies for the following reasons: no control for baseline severity (n = 13), no outcome of interest (n = 1), dexamethasone was evaluated instead of MPSS (n = 1), penetrating wounds (n = 1), and population size <10 (n = 1). A list of excluded articles can be obtained in the Supplemental Material.
Among the remaining 13 studies, 7 were systematic reviews published between 2000 and 2014. The systematic reviews differed with respect to inclusion criteria, methodology, and conclusions (Table 2). The quality of the systematic reviews ranged from 2/11 (low quality) to 9/11 (high quality) as assessed by the Assessment of Multiple Systematic Reviews (AMSTAR) evaluation tool (see Supplemental Material for details). All included the NASCIS II study. Of the remaining 6 studies that met our inclusion criteria, four were randomized controlled trials and 2 were prospective cohort studies (Figure 1).
Table 2.
Overview of Previous Systematic Reviews of MPSS vs Control (Placebo or no MPSS) in the Treatment of Acute Spinal Cord Injury.
| Assessment (Year) | Literature Search Dates | Purpose | Inclusion Criteria | Evidence Base Availablea,b | Follow-up Range | Primary Conclusions | AMSTAR Scorec |
|---|---|---|---|---|---|---|---|
| Short (2000)35 | 1966 to December 1999 | To summarize the evidence evaluating the effect of high-dose MPSS on neurological improvement following acute SCI |
Inclusion:
|
|
24 hours to 4+ years | Efficacy: The evidence produced by this systematic review does not support the use of high-dose MPSS in acute SCI to improve neurological recovery. Safety: A deleterious effect on early mortality and morbidity cannot be excluded by this evidence. Economic: Not addressed | 4/11; medium quality |
| Hurlbert (2001)36 | NR | To review available literature and formulate evidence-based recommendations for the use of MPSS in acute SCI | NR |
|
2 months to 30 months | Efficacy: All studies failed to demonstrate improvement from steroid administration in any of the a priori hypotheses tested; MPSS cannot be recommended for routine use in acute nonpenetrating SCI. Safety: Prolonged administration of high-dose steroids (48 hours) may be harmful. Economic: Not addressed | 2/11; low quality |
| Hugenholtz (2002)37 | MEDLINE January 1, 1966, to April 2001 CINAHL 1982-2001 HealthSTAR 1990-2000 | To address controversy surrounding the use of MPSS infusion after acute SCI |
Inclusion:
|
|
6 weeks to 1 year | Efficacy: There is insufficient evidence to support the use of high-dose MPSS within 8 hours following an acute closed SCI as a treatment standard or as a guideline for treatment. MPSS, prescribed as a bolus IV infusion of 30 mg/kg of body weight over 15 minutes within 8 hours of closed SCI, followed 45 minutes later by an infusion of 5.4 mg/kg of body weight per hour for 23 hours, is a treatment option with weak clinical evidence (Level I to II-1). There is insufficient evidence to support extending MPSS infusion beyond 23 hours if chosen as a treatment option. Safety: In well-designed studies, there are no statistically significant complications to MPSS therapy; there are, however, trends to increased sepsis and hyperglycemia. Economic: The NASCIS II and III protocols would cost $322.02 and $579.32, respectively, per patient. Nursing time and equipment costs are not included. | 4/11; medium quality |
| Sayer (2006)38 | NR | To summarize the evidence evaluating the use of MPSS in acute SCI | NR |
|
6 weeks to 2+ years | Efficacy: There is insufficient evidence to support the use of MPSS as a standard treatment in acute SCI. Safety: MPSS use is associated with increased risk of infections. Economic: Not addressed | 2/11; low quality |
| Botelho (2009)39 | MEDLINE, LILACS, and EMBASE | To review RCTs evaluating the use of MPSS compared with placebo for SCI |
Inclusion:
|
|
6 months to 1 year | Efficacy: The results do not suggest clinical benefits of MPSS due to only modest differences between treatment strategies. Safety: The use of MPSS is associated with an increased risk of pulmonary complications and gastrointestinal bleeding in patients aged approximately 60 years. Economic: Not addressed | 6/11; medium quality |
| Bracken (2012)30 | Through August 2011 | To assess the effects of steroids in patients with acute SCI |
Inclusion: RCTs including patients with:
|
|
2 weeks to 1 year | Efficacy: MPSS enhances neurologic recovery if therapy is started within 8 hours of injury by using an initial bolus of 30 mg/kg by IV for 15 minutes, followed 45 minutes later by a continuous infusion of 5.4 mg/kg/h for 24 hours. Safety: Not addressed Economic: Not addressed | 9/11; high quality |
| Hurlbert (2013)9 | 1966-2011 | To build upon a medical evidence-based guideline on the use of MPSS and GM-1 Ganglioside previously published by the AANS and CNS |
Inclusion: NR Exclusion:
|
|
2 weeks to 1 year | Efficacy: There is no Class I or II medical evidence suggesting any beneficial effect of MPSS in an acute SCI population; however, Class III medical evidence has supported the neuroprotective effect of MPSS. Safety: Class I, II, and III evidence suggests that high-dose steroids are associated with harmful side effects including death. Economic: Not addressed | 2/11; low quality |
Abbreviations: AANS, American Association of Neurological Surgeons; CINAHL, Current Index to Nursing and Allied Health Literature; CNS, Congress of Neurological Surgeons; EMBASE, Excerpta Medical Database; HealthSTAR, Health Services Technology, Administration, and Research; LILACS, Literatura Latino Americana em Ciências da Saúde; MEDLINE, Medical Literature Analysis and Retrieval System Online; MPSS, methylprednisolone sodium succinate; N/A, not available; NASCIS, National Acute Spinal Cord Injury Study; NR, not reported; RCT, randomized controlled trial; SCI, spinal cord injury.
aThe NASCIS RCTs were published as multiple reports with different follow-up times.
bShort (2000): Included 2 studies with penetrating spinal cord injury (gunshot).
cAssessment of Multiple Systematic Reviews evaluation tool: high quality, 8 to 11; medium quality, 4 to 7; low quality, 0 to 3.
Figure 1.
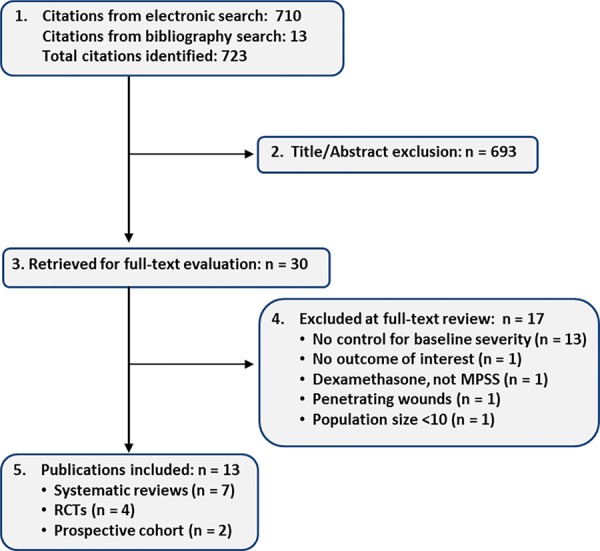
Literature search.
Characteristics of Included Studies (Table 3)
Table 3.
Study Characteristics*.
| Author (Year), Design, Risk of Bias | Sample and Characteristics | Treatment | Inclusion Criteria | Severity (on Admission) | Outcome Measures | Follow-up Time (%) | Funding |
|---|---|---|---|---|---|---|---|
| Bracken (1990/92)22,23 RCT Low | N = 487 MPSS: Male: 86.4% Age: 13-29: 55.5% 30-44: 27.2% ≥45: 17.3% Naloxone: Male: 80.5% Age: 13-29: 63.6% 30-44: 18.7% ≥45: 17.6% Placebo: Male: 84.8% Age: 13-29: 52.6% 30-44: 28.7% ≥45: 18.8% | MPSS: n = 162 IV bolus dose of 30 mg/kg body weight over a 15-minute period followed by a 45-minute pause, then infusion at 5.4 mg/kg/h for 23 hours Naloxone: n = 154 IV bolus dose of 5.4 mg/kg body weight over a 15-minute period followed by a 45-minute pause, then infusion at 4.0 mg/kg/h for 23 hours Placebo: n = 171 IV bolus dose and then an infusion given—no other info given as to the time or amount |
Inclusion:
|
|
Motor score: 0-5 (no contraction to normal function) for 14 muscles—range from 0 to 70 Response to pinprick and light touch: 1-3 (absent, dysfunction, or normal) in 29 segments—range 29 to 87 | 6 weeks: 477/487 (97.9%) 6 months: 470/487 (96.5%) 1 year: 427/487 (87.7%)a | National Institute of Neurological Disorders and Stroke (Grant NS 15078) |
| Otani (1994)b,24 RCT Moderately high | N = 117 Male: 76.1% Age: 40-49: 14.5% 50-59: 26.5% 60-69: 18.8% | MPSS: n = 70 IV bolus dose of 30 mg/kg of body weight over a 15-minute period followed by a 45-minute pause and then an infusion at 5.4 mg/kg/h for 23 hours Control: n = 47 Drug therapies without a corticosteroid and surgical treatment |
Inclusion:
|
|
Frankel Classification:
|
6 months: 117/158 (74.1%) | NR |
| Bracken (1997-8)29,40 RCT Low | N = 499 MPSS 24 hours: Male: 85.5% Age: 14-29: 42.3% 30-44: 29.5% ≥45: 28.3% TM 48 hours: Male: 86.8% Age: 14-29: 47.4% 30-44: 31.2% ≥45: 21.6% MPSS 48 hours: Male: 81.9% Age: 14-29: 45.9% 30-44: 32.5% ≥45: 21.6% | MPSS 24 hours: n = 166 IV bolus dose of 30 mg/kg body weight followed by 5.4 mg/kg/h for 24 hours, then placebo given every hour for next 24 hours TM 48 hours: n = 167 IV bolus dose of 30 mg/kg body weight followed by 2.5 mg/kg every 6 hours for 48 hours MPSS 48 hours: n = 166 IV bolus dose of 30 mg/kg body weight followed by 5.4 mg/kg/h for 48 hours |
Inclusion:
|
|
Motor score: 0-5 (no contraction to normal function) for 14 muscles—range from 0 to 70 Response to pinprick and light touch: 1-3 (absent, dysfunction or normal) in 29 segments—range 29 to 87 Functional Independence Measure (FIM): 18-126 (need for assistance in all areas to complete independence) | 6 weeks: 465/499 (93.2%) 6 months: 444/499 (89.0%) 1 year: 431/499 (86.4%)d | National Institute of Neurological Disorders and Stroke (Grant NS-15078) |
| Pointillart (2000); Petitjean (1998)e,25 RCT Low | N = 106 % Male: NR MPSS: Age: 32 (25-44) NP: Age: 32 (26-47) MPSS and NP: Age: 28 (20-39) Placebo: Age: 28 (25-42)f | MPSS: n = 27 IV bolus dose of 30 mg/kg over 1 hour, then 5.4 mg/kg/h for 23 hours NP: n = 27 NP dose of 0.15 mg/kg/h for 2 hours, then 0.03 mg/kg/h for 7 days MPSS and NP: MPSS and NP at the same doses Placebo: n = 25 Neither medication received |
Inclusion:
|
|
ASIA motor, pinprick sensation, and pain scores: Neurological examination at admission and 1 year later | 1 year: 100/106 (94.3%) 5 patients lost to death | NR |
| Matsumoto (2001)27 RCT Moderately high | N = 46 Male: 91.3% Mean age (range): 60.6 (20-84) MPSS: Male: 91.3% Mean age (range): 60.9 (41-84) Placebo: Male: 91.3% Mean age (range): 60.4 (20-84) | MPSS: n = 23 IV bolus dose of 30 mg/kg of body weight over a 15-minute period followed by a 45-minute pause and then an infusion at 5.4 mg/kg/h for 23 hours Placebo: n = 23 15-minute bolus of 30 mg/kg followed by a 45-minute pause, and then a 23-hour maintenance infusion of 5.4 mg/kg |
Inclusion:
|
Frankel grade
|
Frankel Classification:
|
2 months, 46/46 (100%) | NR |
| Wilson (2012)28 STASCIS trial, prospective cohort Moderately high | N = 411 Mean age: 44.4 ±17.0 Male: 308/411 (74.9%) | MPSS: 233/411 (56.7%) 24-hour, low-dose MPSS regimen from the NASCIS II study |
Inclusion:
|
AIS Grade:
e
|
|
NR | Christopher and Dana Reeve Foundation, Cervical Spine Research Society, AANS/CNS Section on Disorders of the Spine and Peripheral Nerves, and Rick Hansen Institute |
| Evanview (2015)26 Prospective cohort RHSCIR Moderately low | N = 88 MPSS: Male: 81.8% Mean age: 45.4 ± 16.2 No MPSS: Male: 93.2% Mean age: 45.5 ± 16.6 | MPSS: n = 44 MPSS regimen from the NASCIS II study given within 8 hours of injury and administered for 24 or 48 hours Control: n = 44 Propensity matched SCI patients with no MPSS administration |
Inclusion:
|
AIS Grade:
|
Motor score: International Standards for Neurologic Classification of Spinal Cord Injury (ISNCSCI) In-hospital complications | Median number of days: 127 (MPSS) and 117 (no MPSS) 44/46 (95.7%) | Rick Hansen Institute, Health Canada, Western Economic Diversification Canada, and the Governments of Alberta, British Columbia, Manitoba, and Ontario |
Abbreviations: AANS, American Association of Neurological Surgeons; ASIA, American Spinal Injury Association; CNS, Congress of Neurological Surgeons; IV, intravenous; MAP, mean arterial pressure; MPSS, methylprednisolone sodium succinate; NP, nimodipine; NR, not reported; RCT, randomized controlled trial; RHSCIR, Rick Hansen Spinal Cord Injury Registry; SCI, spinal cord injury; TM, tirilazad mesylate.
*Bracken studies are reported as a primary report and then a follow-up report 1 year later; 6-week and 6-month follow-up information are based on primary reports; 1-year follow-up is based on the follow-up report.
aBracken (1990/1992): Measured in 161 patients in the methylprednisolone group, 153 in the naloxone group, and 170 in the placebo group.
bEnglish translation of an article originally published in Japanese.
cBracken (1997/98): Motor function percentages are averaged from percentages of each treatment group in Table 2.
dBracken (1984/5): 330 refers to number of patients randomized, 24 patients were excluded from analysis after randomization, n = 306.
ePetitjean 1998 was published in a French journal, and Pointillart 2000 was published in an English journal.
fPetitjean 1998: Age values expressed as averages with 25th and 75th percentiles in parentheses.
Three randomized controlled trials (4 publications)22–25 and 1 prospective cohort study26 evaluated the efficacy and safety of MPSS, while 3 additional studies (2 randomized controlled trials and 1 prospective cohort study) provided further evidence on its safety.27–29 In 1990 and 1992, Bracken et al published a double-blind randomized controlled trial, also known as NASCIS II, with 6 weeks, 6 months, and 1 year of follow-up. They randomized 487 patients across 3 treatment arms: (1) MPSS bolus dose of 30 mg/kg at hospital admission, followed by an infusion at 5.4 mg/kg/h for the following 23 hours; (2) naloxone; or (3) placebo. Naloxone and its placebo were provided in 100-ampule sets of 2-mL parabens-free ampules and prepared at a concentration of 25.0 mg/mL. This was followed by a third and final NASCIS study that compared 24-hour versus 48-hour MPSS infusion using the same dose as NASCIS II, as well as to a 48-hour infusion of the putative neuroprotective drug Tirilazad. All patients were randomized to receive treatment within 8 hours of injury, with the analysis stratified according to whether treatment was initiated before or after 3 hours of injury (Table 4).29
Table 4.
Evidence Summary Table.
| KQ1. What is the efficacy and effectiveness of MPSS compared with no pharmacologic treatment? | Effect Size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. of Studies; Sample Size | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Quality of Evidence | Mean Difference (95% CI, P Value) | ||
| Motor scores | 6 months 2 RCTs (N = 414)23,24 12 months 2 RCTs (N = 335)22,25 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 6 months 1.19 (−2.34, 4.72); P = .51 12 months −1.17 (−4.80, 2.47); P = .53 | ||
| Pinprick | 6 months 1 RCTs (N = 296)23 12 months 2 RCTs (N = 334)22,25 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 6 months 3.37 (0.75, 5.99); P = .01 12 months 0.18 (−2.66, 3.02); P = .90 | ||
| Light touch | 6 months 1 RCTs (N = 294) 23 12 months 2 RCTs (N = 334) 22,25 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 6 months 2.88 (0.10, 5.66); P = .04 12 months 0.74 (−2.12, 3.61); P = .61 | ||
| KQ2. What is the safety profile of MPSS compared with no pharmacologic treatment? | MPSS % | Control % | ||||||||
| Death | 3 RCTs (N = 530)22–24,27 1 non RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 2.58% (8/310) | 4.87% (15/308) | −1.51 (−4.13, 1.12); P = .26 |
| Wound infection | 3 RCTs (N = 434)22,23,25,27 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 4.26% (11/258) | 2.27% (6/264) | 0.98 (−1.70, 3.66); P = .47 |
| GI hemorrhage | 3 RCTs (N = 434)22,23,25,27 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 6.07% (13/214) | 2.27% (5/20) | 4.51 (−1.92, 10.94); P = 17 |
| Sepsis | 3 RCTs (N = 434)22,23,25,27 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 5.81% (15/258) | 4.92% (13/264) | 0.74 (−2.88, 4.35); P = .69 |
| PE | 2 RCTs (N = 238)22,23,25 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 8.09% (19/235) | 4.592% (111/241) | 2.94 (−0.15, 6.03); P = .06 |
| Urinary infection | 3 RCTs (N = 434)22,23,25,27 1 Non-RCT (N = 88) | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 35.27% (91/258) | 34.47% (1/264) | 1.73 (−5.04, 8.49); P = .62 |
| Pneumonia | 1 RCT (N = 156) 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 25.50% (51/200) | 21.33% (45/211) | 4.69 (−3.19, 12.57); P = .24 |
| Decubitis | 2 RCTs (N = 238)22,23,27 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | 15.7% (35/223) | 14.96% (35/234) | 20.99 (−6.01, 7.98); P = .78 |
| One or more complications | 1 Non- RCT28 (N = 411) | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Very low | 33.48% (78/233) | 46.07% (82/178) | −12.59 (−22.10, −3.09); P = .009 |
| KQ3. What is the evidence that MPSS has differential efficacy or safety in subpopulations? | ||||||||||
| MPSS administered within 8 hours | ||||||||||
| Motor scores | Final follow-up (6-12 months) 3 RCTs (N = 300)22,24,25 Median time 3 months 1 Non-RCT (N = 88)26 | No serious risk of bias | No serious inconsistency | No serious indirectness | Serious risk of imprecision | Undetected | Moderate | RCTs: 3.88 (0.50, 7.27); P = .02 RCTs + Prospective cohort: 3.21 (0.10, 6.33); P = .04 | ||
Abbreviations: CI, confidence interval; GI, gastrointestinal; MPSS, methylprednisolone sodium succinate; PE, pulmonary embolism; RCT, randomized controlled trial.
Otani et al24 published an article in Japanese that randomized 117 patients to a MPSS group (n = 70) or a standard care group (n = 47). Treatment with other drugs, concomitant procedures (such as decompression of the spinal cord or reduction of a dislocation or a fracture), and rehabilitation were performed at the discretion of the attending physician. Neither the patients nor the outcome assessors were blinded to the treatment. Seventy-four percent of patients attended the 6-month follow-up visit.
Matsumoto et al27 compared the incidence of complications during the first 2 months after injury in 46 patients with cervical SCI. Patients were randomized to receive MPSS using the NASCIS II protocol or a placebo, though the methods of random generation and concealment were not clear. All patients received treatment within 8 hours. At baseline, the MPSS group presented with more severe injuries: 39% in the MPSS group versus 26% in the placebo group with Frankel grade A, and 4% in the MPSS group versus 30% in the placebo group with Frankel grade D.
Wilson et al28 assessed inpatient complications after traumatic cervical SCI from the Surgical Timing in Acute Spinal Cord Injury Study data registry. Patient information was collected on adults with cervical SCIs who were enrolled at 6 North American centers over a 7-year period. This study included patients who underwent a standardized American Spinal Injury Association neurological examination within 24 hours of injury and had follow-up information at the index hospital discharge. Decisions surrounding the administration of MPSS were made at the discretion of the spinal surgeon and the treating team.
Evaniew et al performed a prospective multicenter study using the Rick Hansen Spinal Cord Injury Registry to evaluate the impact of MPSS on motor recovery at the end of inpatient rehabilitation or discharge to the community from acute care.26 Forty-four patients received MPSS within 8 hours of acute injury following the NASCIS II regiment. Patients treated with an additional 24 hours of MPSS were also included. The control group consisted of 44 subjects who did not receive MPSS and was selected using propensity score matching. Despite this matching, those who received MPSS had a longer time from injury to first assessment of motor scores (median 72 vs 56 hours). Motor function scores (upper extremity, lower extremity, and total) were determined using the International Standards for Neurologic Classification of Spinal Cord Injury. Safety was determined by collecting rates of in-hospital mortality, urinary tract infections, pneumonia, ulcers, deep vein thrombosis or pulmonary embolism, surgical site infections, and sepsis using International Classification of Diseases, Tenth Edition, codes from the Canadian Institute for Health Information’s Discharge Abstract Database.
What Is the Efficacy of Methylprednisolone Sodium Succinate Compared With No Pharmacologic Treatment?
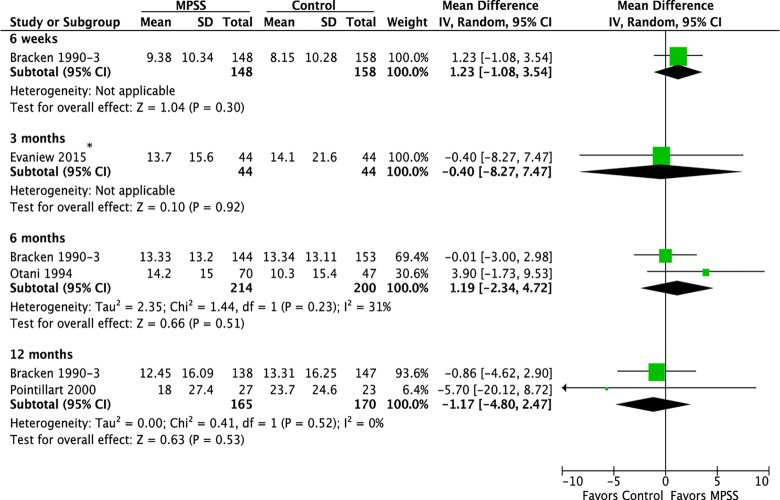
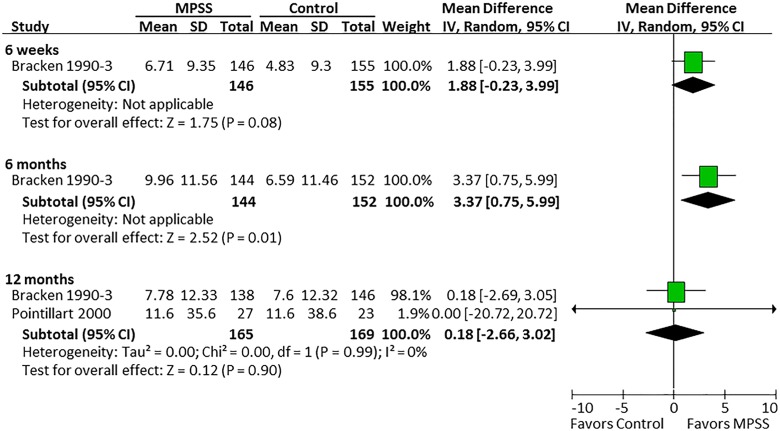
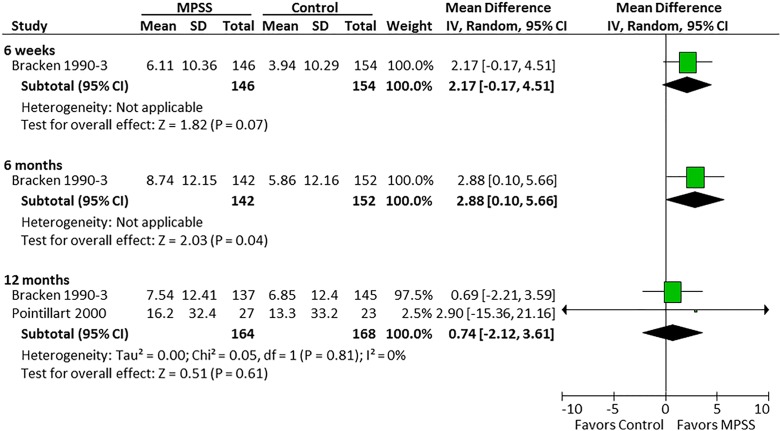
Three randomized trials22–25 and 1 prospective observational study evaluated the efficacy of MPSS compared with no pharmacologic treatment.26 Based on the randomized controlled trials, there was no effect of MPSS on motor function at 6 weeks, 6 months, or 12 months (Figure 2). Likewise, in the observational study, there was no difference between those who did and did not receive MPSS in terms of total motor recovery (13.7 vs 14.1, respectively; P = .43), upper extremity motor recovery (7.3 vs 6.4; P = .38), or lower extremity motor recovery (6.5 vs 7.7; P = .40). Pinprick sensation was significantly improved at 6 months in one randomized controlled trial (mean difference = 3.37; 95% CI = 0.75 to 5.99)23 but not in 2 other trials at 12 months (Figure 3).23,25 Similar results were seen for light touch (Figure 4).
Figure 2.
Motor scores, all patients. *Prospective cohort study with median follow-up of 127 and 117 days in the MPSS and control groups, respectively.
Figure 3.
Pinprick, all patients.
Figure 4.
Light touch, all patients.
What Is the Safety Profile of MPSS Compared With No Pharmacologic Treatment?
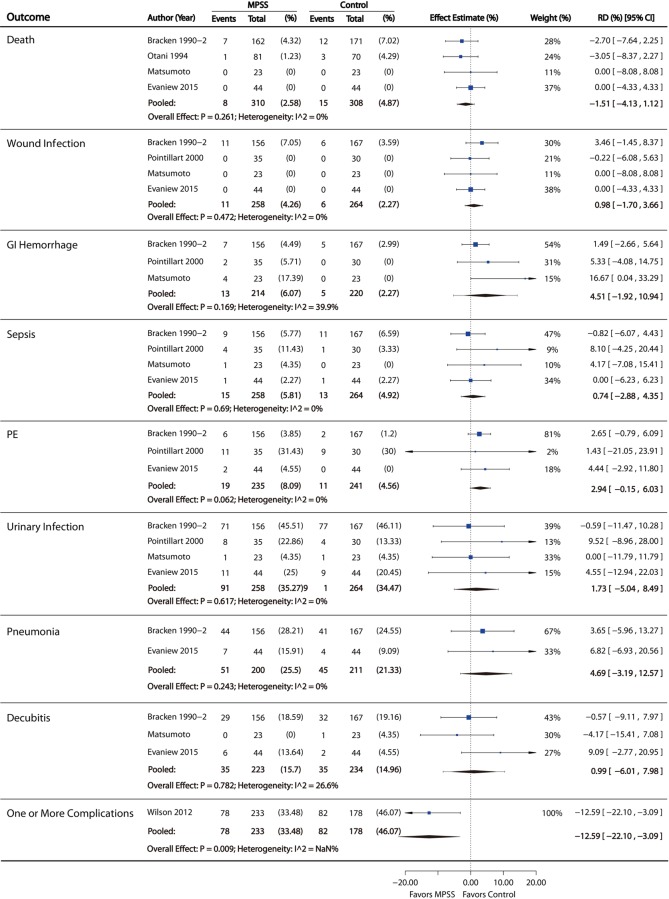
There was no statistical difference between groups in the pooled risk of death, wound infection, gastrointestinal hemorrhage, sepsis, pulmonary embolism, urinary tract infection, pneumonia, or decubiti. One prospective nonrandomized study evaluated the risk of one or more complications and found a lower risk in those receiving MPSS, after controlling for severity of injury and other baseline differences (risk difference = 12.6%, 95% CI = 3.1% to 22.1%; Figure 5). In one randomized controlled trial comparing 24-hour versus 48-hour infusion of MPSS, there was a significantly higher incidence of severe pneumonia (P = .02) in the 48-hour group. Additionally, there was an increased incidence of severe sepsis in the 48-hour group, though the difference between the 24-hour and 48-hour groups for this outcome was within the limits of chance (P = .07).29
Figure 5.
Complications.
What Is the Evidence That MPSS Has Differential Efficacy or Safety in Subpopulations?
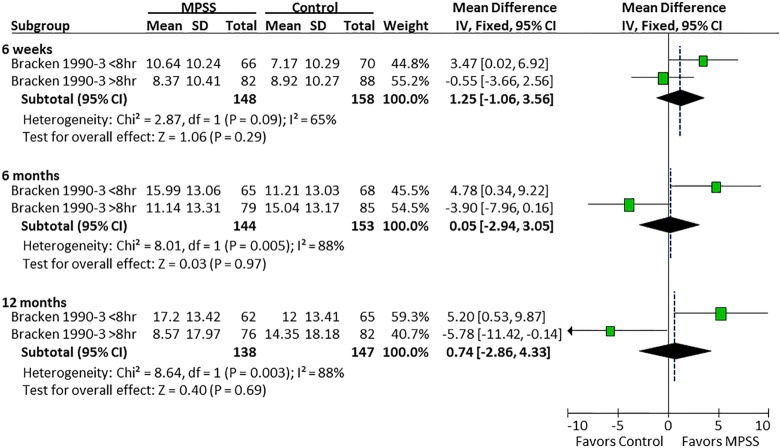
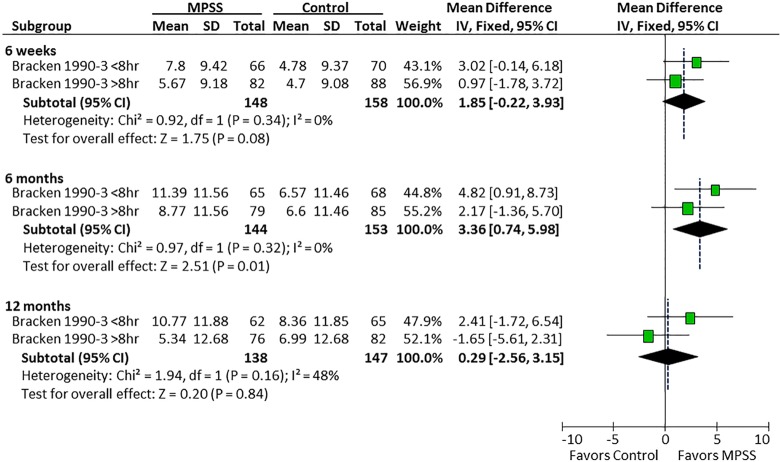
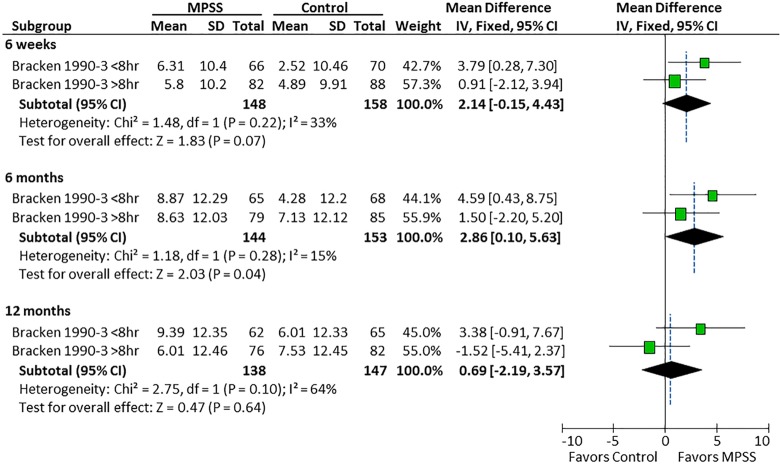
In the study by Bracken et al, there was a differential effect of MPSS on motor recovery compared with controls depending on the timing of MPSS administration. Patients receiving MPSS within 8 hours had a mean 4.8- and 5.2-point improvement in motor scores at 6 and 12 months follow-up compared with a mean 3.9- and 5.8-point deterioration when administered after 8 hours (Figure 6). There was no evidence of a differential effect of the timing of MPSS administration on pinprick or light touch (Figures 7 and 8).
Figure 6.
Timing of MPSS administration and motor scores.
Figure 7.
Timing of MPSS administration and pinprick.
Figure 8.
Timing of MPSS administration and light touch.
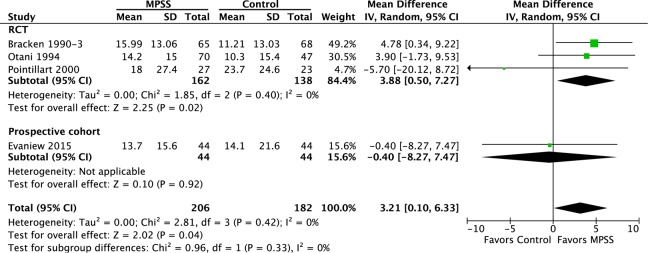
Two additional randomized controlled trials and one prospective observational study compared MPSS to a control in patients receiving treatment within 8 hours. Based on the randomized controlled trials, pooled results at final follow-up (6 or 12 months) demonstrated a modest improvement of 3.88 (95% CI = 0.50 to 7.27; P = .02) in mean motor scores in the MPSS group compared with the control group. When adding the results of the prospective cohort study (median follow-up of 127 and 117 days in the MPSS and control groups, respectively), this mean difference decreased to 3.21 (95% CI = 0.10 to 6.33; P = .04; Figure 9).
Figure 9.
Motor score in patients treated within 8 hours at final follow-up of 6-12 months. *Evaniew had a median follow-up of 122 days
Evidence Summary (Table 5)
Table 5.
Complications From the NASCIS III Trial Comparing 24-Hour Versus 48-Hour Infusion.
| 6-Week Complications | Combined 6- and 12-Month Complications | ||||
|---|---|---|---|---|---|
| 24-Hour Infusion | 48-Hour Infusion | 24-Hour Infusion | 48-Hour Infusion | ||
| Urinary tract infection | Mild-moderate | 34.4 | 38.3 | 53.1 | 49 |
| Severe | 0 | 0 | 0.8 | 3.3 | |
| Decubiti | Mild-moderate | 12.3 | 13.6 | 13.8 | 13.4 |
| Severe | 0.6 | 0.6 | 3.4 | 6 | |
| Other infection | Mild-moderate | 3.9 | 7.8 | 4.1 | 4 |
| Severe | 0 | 0 | 0.7 | 0.7 | |
| Phlebitis | Mild-moderate | 2.6 | 1.3 | 0.7 | 0 |
| Severe | 0 | 0 | 0 | 0 | |
| Incision, pin, halo infection | Mild-moderate | 1.9 | 2.6 | 1.4 | 0.7 |
| Severe | 0.6 | 1.9 | 0 | 0 | |
| Sepsis | Mild-moderate | 3.9 | 4.5 | 0 | 1.3 |
| Severe | 0.6 | 2.6 | 0 | 1.3 | |
| Adult RDS | Mild-moderate | 1.9 | 1.9 | 0 | 0 |
| Severe | 1.3 | 1.9 | 0.7 | 0 | |
| Atelectasis | Mild-moderate | 5.2 | 7.1 | 1.4 | 0.7 |
| Severe | 0 | 0 | 0.7 | 0 | |
| Other respiratory failure | Mild-moderate | 7.8 | 9.1 | 1.4 | 0 |
| Severe | 1.9 | 3.2 | 0 | 0.7 | |
| Pneumonia | Mild-moderate | 12.3 | 11 | 2.8 | 3.4 |
| Severe | 2.6 | 5.8 | 1.4 | 1.4 | |
| GI hemorrhage | Mild-moderate | 0 | 1.3 | 0 | 0 |
| Severe | 0 | 0.6 | 0 | 0 | |
| Thrombophlebitis | Mild-moderate | 2.6 | 4.5 | 2.1 | 2.7 |
| Severe | 0.6 | 0 | 1.4 | 0.7 | |
| Pulmonary embolus | Mild-moderate | 0 | 0.6 | ||
| Severe | 1.3 | 0.6 | |||
| Bradycardia | Mild-moderate | 2.6 | 0.6 | ||
| Severe | 1.3 | 0 | |||
| Tachycardia | Mild-moderate | 0.6 | 2.6 | ||
| Severe | 0 | 0 | |||
| Other arrhythmia | Mild-moderate | 0.6 | 1.9 | ||
| Severe | 0 | 0 | |||
| Paralytic ileus | Mild-moderate | 1.3 | 3.2 | ||
| Severe | 0 | 0.6 | |||
| Other complications | Mild-moderate | 11.7 | 18.2 | ||
| Severe | 4.5 | 5.8 | |||
Abbreviations: GI, gastrointestinal; RDS, respiratory distress syndrome.
There is moderate evidence that MPSS (compared with no treatment or placebo) administered according to the dose and duration of the NASCIS II protocol confers no benefit in motor recovery, pinprick, or light touch when initiated at indiscriminate time periods following acute SCI. However, there is moderate evidence of a small benefit in motor recovery when MPSS is administered within 8 hours of injury compared with no treatment. There is no difference between groups in the pooled risk of death, wound infection, gastrointestinal hemorrhage, sepsis, pulmonary embolism, urinary tract infection, pneumonia, or decubiti. The evidence for safety is moderate. There may be a higher incidence of severe pneumonia and increased incidence of severe sepsis when the duration of infusion increases from 24 hours to 48 hours.
Discussion
The primary goal of this systematic review was to determine the efficacy and safety of MPSS compared with no treatment or placebo. While the majority of primary research articles emanated from prospective randomized controlled trials, 2 observational studies also met eligibility criteria and were included in this review. With respect to the overall impact of MPSS, there were no differences in motor and sensory neurological recovery between patients treated with MPSS and those receiving placebo or no treatment. The overall strength of this conclusion was “Moderate,” meaning that, based on assessment of risk of bias and the strength of the overall body of evidence, we are moderately confident that the calculated effect estimate reflects the true estimate.
From the perspective of safety, when considering the NASCIS II 24-hour MPSS regimen, there was a trend toward reduced mortality in patients receiving MPSS as compared to no treatment. Similarly, with respect to complications, there were no significant differences between patients who received the 24-hour NASCIS II regimen of MPSS and those who did not. There were, however, trends toward an increased incidence of pulmonary embolism and gastrointestinal hemorrhage in patients treated with MPSS; the relative severity of these events and their impact on recovery and mortality is unknown. In general, when considering the 24-hour NASCIS II MPSS regimen, we can conclude with a moderate degree of confidence that there are no significant differences in rates of mortality or other complication between treated and untreated patients. Although not the primary focus of this review, compared to patients receiving the 24-hour NASCIS II regimen, patients treated with the 48-hour NASCIS III regimen experienced higher rates of severe sepsis (P = .02) and pneumonia (P = .07); this may represent a valid argument against the administration of the 48-hour regimen to SCI patients (Table 5).
We also evaluated outcomes based on time to drug administration and specifically examined the impact of MPSS within the first 8 hours of injury. The importance of this time window was established based on a subgroup analysis from the NASCIS II study: the authors reported a significantly larger improvement in long-term motor score recovery (an additional 4 points of improvement) in patients receiving the 24-hour MPSS compared to those receiving placebo. Critics of the NASCIS II study have commented on the potential bias associated with this “post hoc” retrospective subanalysis. In reality, however, the authors of the NASCIS II study indicate that an a priori hypothesis was made that the effects of MPSS are influenced by how quickly treatment is administered postinjury; that said, the specific method for selecting the 8-hour cutoff was not fully described in the primary manuscript text. However, in subsequent articles, rationale for selecting the 8-hour cutoff is further explained. In a commentary piece published in 2000 by Bracken, he indicates,
It is important that major analytic stratifications be part of the original proposal and such was the case in NASCIS 2, which proposed to the National Institutes of Health to analyze “time to loading dose.” With respect to how data should be categorized, it is perfectly acceptable to use statistical criteria (mean, median, mode) to operationalize a hypothesis about early versus late initiation of treatment because the variable distribution is almost always unknown before the trial is completed. This is particularly so when, as in the NASCIS trials, there was no a priori biologic rationale for defining a dichotomy. The whole hour close to the median (8.5 hours) was used because a more precise representation of time was unwarranted as we only wished to classify “early” versus “late” treatment. It is certainly an unacceptable analytic practice to analyze multiple cut-off points while presenting only the most favorable one; this was not done in the NASCIS.
These points are further supported by commentary in the original NASCIS II NIH grant proposal and in more recent Cochrane meta-analyses.30,31 While it remains unfortunate that the rationale provided above was not included in the primary NASCIS II manuscript, this justification should serve to establish the a priori nature of the “time to effect hypothesis,” and disabuse individuals of the notion that the 8-hour cutoff was chosen as a matter of convenience given the presence of a positive effect.
In summary, when considering all studies included in our meta-analysis, patients receiving MPSS within 8 hours of injury had significantly larger motor gains at a minimum of 6 months follow-up, with treated patients experiencing an additional 3.2 points of motor recovery (95% CI = 0.10 to 6.3). When considering only randomized data, this margin of benefit increased to an additional 3.8 points of motor recovery (95% CI = 0.50 to 7.27), with a “Moderate” degree of confidence in this estimate. Although the magnitude of the effect observed is ostensibly small, the clinical significance of such an improvement is unknown and likely varies from patient to patient depending on the specifics of the injury, including its severity and neurological level. Of relevance, in a recent survey study, SCI patients were presented with an objective summary of the potential risks and benefits of MPSS based on the trials discussed above.32 Overall, 41 of 69 respondents (59.4%) felt very strongly that the motor and sensory benefits observed with the NASCIS II MPSS dose administered within 8 hours of injury would be clinically important. Future work is needed to improve the interpretability of neurological measures in the context of SCI and to define what constitutes a clinically important change. It is, nonetheless, important to acknowledge that the statistically significant improvement in motor function demonstrated by the most rigorously performed clinical studies represents fewer than 5 motor points. Appreciating the small magnitude of this improvement is important for establishing realistic expectations about the neuroprotective efficacy of MPSS in acute SCI.
In addition to including individual studies, we also considered previous systematic reviews in order to gauge how other groups assessed the evidence on this topic. Across the 7 systematic reviews that met our inclusion criteria, conclusions and methodology varied substantially. With respect to methodological quality, as evaluated by the AMSTAR score, 3 reviews were rated as low quality, 3 were rated as medium quality, and 1 was rated as high quality. Of note, the single review deemed to be high quality (Cochrane meta-analysis authored by Bracken et al) supported a positive impact of the 24-hour NASCIS II MPSS regimen started within 8 hours of injury on long-term motor recovery, with overall results very similar to those observed in this review.
Recently a systematic review and meta-analysis by Evaniew et al, published after our inclusion dates, sought to determine the impact of MPSS on motor recovery and risk of adverse events.33 This systematic review concluded, in part, that the pooled evidence does not demonstrate a significant long-term benefit for MPSS in patients with acute SCI. Furthermore, the authors conclude that their findings support current guidelines against routine use; however, they also note that strong recommendations are not warranted due to limited confidence in the effect estimate. Our systematic review differs from theirs in 2 important areas. First, we pooled studies into a final follow-up group with 6- or 12-month follow-up, which included 3 studies: Otani et al (6-month follow-up), Pointillart et al (12-month follow-up), and Bracken et al (12-month follow-up). This seems reasonable given the profile of recovery for SCI patients, with the bulk of improvement occurring in the first 6 to 9 months postinjury.34 On the other hand, Evaniew et al defined 6-month follow-up as short-term and therefore did not include Otani et al in the long-term meta-analysis. As a result, they report a nonsignificant result in long-term motor improvement. Second, given that severity of injury has the largest prognostic effect on SCI recovery and may influence the decision on whether to administer MPSS, we only included observational studies that controlled for this potential confounder. Evaniew et al included observational studies that did not control for severity. Given that patients with a more severe injury may be more likely to receive MPSS, the effect of MPSS on prognosis would be underestimated in these studies.
Study Limitations
Several limitations of this review must be recognized. First, many of the included studies had methodological shortcomings and risk of bias that resulted in the downgrading of the overall strength of evidence. As a result, the overall strength of evidence was found to be moderate for all 3 key questions. Second, although other studies have evaluated other types of corticosteroids apart from MPSS, as well as other dosing regimens of MPSS, these were not included in this review; this decision was based primarily on a desire to focus on studies comparing MPSS to no treatment or placebo in order to obtain a better estimate of the independent effects of this medication. Last, when evaluating the impact of MPSS on certain subpopulations of patients, we chose to examine those receiving treatment within 8 hours of injury. Although this may be somewhat arbitrary from a biological perspective, it was chosen given the widespread adoption of this time window by clinicians throughout the world following the findings of the NASCIS II subanalysis. As a result, we felt it necessary to critically evaluate the existing evidence with respect to this time threshold.
Conclusions
When considering all time points of drug administration, there is moderate evidence that the 24-hour NASCIS II MPSS regimen has no impact on indices of long-term neurological recovery. However, there is moderate evidence of a small improvement in motor recovery when the same regimen is administered within 8 hours of injury. Although there is moderate evidence confirming the safety of the 24-hour regimen, there may be a higher incidence of infectious complications when the duration of infusion increases to 48 hours.
Acknowledgments
We would like to acknowledge Dr Jim Kutsogiannis and Dr Donald Griesdale for their review of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by AOSpine and the AANS/CNS Section on Neurotrauma and Critical Care. Dr Fehlings wishes to acknowledge support from the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration and the DeZwirek Family Foundation. Dr Tetreault acknowledges support from a Krembil Postdoctoral Fellowship Award.
References
- 1. Braughler J, Hall E. Effects of multi-dose methylprednisolone sodium succinate administration on injured cat spinal cord neurofilament degradation and energy metabolism. J Neurosurg. 1984;61:290–295. [DOI] [PubMed] [Google Scholar]
- 2. Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol. 1982;18:320–327. [DOI] [PubMed] [Google Scholar]
- 3. Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na++K+)-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg. 1982;57:247–253. [DOI] [PubMed] [Google Scholar]
- 4. Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: a systematic review of methylprednisolone. Altern Lab Anim. 2009;37:43–62. [DOI] [PubMed] [Google Scholar]
- 5. Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine (Phila Pa 1976). 2006;31:E250–E253. [DOI] [PubMed] [Google Scholar]
- 6. Hurlbert RJ. Methylprednisolone for the treatment of acute spinal cord injury: point. Neurosurgery. 2014;61(suppl 1):32–35. [DOI] [PubMed] [Google Scholar]
- 7. Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(suppl 1):36–42. [DOI] [PubMed] [Google Scholar]
- 8. Hurlbert R. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Nerosurg Spine. 2000;93:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(suppl 2):93–105. [DOI] [PubMed] [Google Scholar]
- 10. Hadley M, Walters B, Grabb P, et al. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50:563–572. [DOI] [PubMed] [Google Scholar]
- 11. Coleman WP, Geisler FH. Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. 2004;4:373–378. [DOI] [PubMed] [Google Scholar]
- 12. Kay ED, Deutsch A, Wuermser LA. Predicting walking at discharge from inpatient rehabilitation after a traumatic spinal cord injury. Arch Phys Med Rehabil. 2007;88:745–750. [DOI] [PubMed] [Google Scholar]
- 13. Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1999;80:1391–1396. [DOI] [PubMed] [Google Scholar]
- 14. Tee JW, Chan CH, Gruen RL, et al. Early predictors of health-related quality of life outcomes in polytrauma patients with spine injuries: a level 1 trauma center study. Global Spine J. 2014;4:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A (1):1–3. [PubMed] [Google Scholar]
- 16. West S, King V, Carey TS, et al. Systems to Rate the Strength of Scientific Evidence (Evidence Report/Technology Assessment No. 47) Rockville, MD: Agency for Healthcare Research and Quality; 2002. [PMC free article] [PubMed] [Google Scholar]
- 17. Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews (AHRQ Publication No. 10(14)-EHC063-EF) Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 18. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 20. Review Manager (RevMan) (Version 5.3) [Computer program]. Copenhagen, Denmark: The Nordic Cochrane Centre: The Cochrane Collaboration; 2014. [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 22. Bracken MB, Shepard MJ, Collins WF, Jr, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the Second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23–31. [DOI] [PubMed] [Google Scholar]
- 23. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. [DOI] [PubMed] [Google Scholar]
- 24. Otani K, Abe H, Kadoya S, Nakagawa H, Ikata T, Tominaga S. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui J. 1994;7:633–647. [Google Scholar]
- 25. Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71–76. [DOI] [PubMed] [Google Scholar]
- 26. Evaniew N, Noonan V, Fallah N, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multicenter spinal cord injury registry. J Neurotrauma. 2015;32:1674–1683. doi:10.1089/neu.2015.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976). 2001;26:426–430. [DOI] [PubMed] [Google Scholar]
- 28. Wilson JR, Arnold PM, Singh A, Kalsi-Ryan S, Fehlings MG. Clinical prediction model for acute inpatient complications after traumatic cervical spinal cord injury: a subanalysis from the Surgical Timing in Acute Spinal Cord Injury Study. J Neurosurg Spine. 2012;17(1 suppl):S46–S51. [DOI] [PubMed] [Google Scholar]
- 29. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 30. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;(1):CD001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bracken MB. National Acute Spinal Cord Injury Study 2. Washington, DC: National Institutes of Health; 1984. [Google Scholar]
- 32. Bowers CA, Kundu B, Rosenbluth J, Hawryluk GW. Patients with spinal cord injuries favor administration of methylprednisolone. PLoS One. 2016;11:e0145991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evaniew N, Belley-Cote EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma. 2016;33:468–481. doi:10.1089/neu.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fawcett J, Curt A, Steeves J, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic trials. Spinal Cord. 2007;45:190–205. [DOI] [PubMed] [Google Scholar]
- 35. Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury: a systematic review from a clinical perspective. Spinal Cord. 2000;38:273–286. [DOI] [PubMed] [Google Scholar]
- 36. Hurlbert RJ. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26(24 suppl):S39–S46. [DOI] [PubMed] [Google Scholar]
- 37. Hugenholtz H, Cass DE, Dvorak MF, et al. High-dose methylprednisolone for acute closed spinal cord injury: only a treatment option. Can J Neurol Sci. 2002;29:227–235. [DOI] [PubMed] [Google Scholar]
- 38. Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335–343. [DOI] [PubMed] [Google Scholar]
- 39. Botelho RV, Daniel JW, Boulosa JL, et al. Effectiveness of methylprednisolone in the acute phase of spinal cord injuries: a systematic review of randomized controlled trials [in Portuguese]. Rev Assoc Med Bras. 2009;55:729–737. [DOI] [PubMed] [Google Scholar]
- 40. Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the Third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699–706. [DOI] [PubMed] [Google Scholar]