Abstract
Study Design:
Systematic review.
Objectives:
The primary objective of this systematic review was to define the change in impairment, disability, and pain following surgical intervention in patients with degenerative cervical myelopathy (DCM). Secondary objectives included to assess the impact of preoperative disease severity and duration of symptoms on outcomes and to summarize complications associated with surgery.
Methods:
A systematic literature search was conducted to identify prospective studies evaluating the effectiveness and safety of operative treatment in patients with DCM. Outcomes of interest were functional status, disability, pain, and complications. The quality of each study was evaluated using the Newcastle-Ottawa Scale, and the strength of the overall body of evidence was rated using guidelines outlined by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) Working Group.
Results:
Of the 385 retrieved citations, 32 met inclusion criteria and are summarized in this review. Based on our results, pooled standard mean differences showed a large effect for improvement in Japanese Orthopaedic Association or modified Japanese Orthopaedic Association score from baseline at short-, medium-, and long-term follow-up: 6 to 12 months (1.92; 95% confidence interval [CI] = 1.41 to 2.43), 13 to 36 months (1.40; 95% CI = 1.12 to 1.67), and ≥36 months (1.92; 95% CI = 1.14 to 2.69) (moderate evidence). Surgery also resulted in significant improvements in Nurick, Neck Disability Index, and Visual Analogue Scale scores (low to very low evidence). The cumulative incidence of complications was low (14.1%; 95% CI = 10.1% to 18.2%).
Conclusion:
Surgical intervention for DCM results in significant improvements in functional impairment, disability, and pain and is associated with an acceptably low rate of complications.
Keywords: degenerative cervical myelopathy, surgery, outcomes, complications, severity
Introduction
Degenerative disease of the cervical spine is the most common indication for cervical spine surgery.1,2 Neurological consequences of such disease include radiculopathy and myelopathy, which result in progressive pain, disability, and diminished quality of life. The utility of nonoperative therapy for patients with degenerative cervical myelopathy (DCM) was previously investigated by Rhee et al3; this systematic review revealed a paucity of evidence supporting such management and recommended against nonoperative treatment in patients with moderate to severe disease.
When indicated, the goals of surgical intervention include decompressing the spinal cord, stabilizing the spinal column, and, if necessary, realigning the sagittal plane. The techniques required to accomplish these goals remain controversial and are often selected based on the nature of the underlying pathology, clinical presentation, the presence of sagittal plane deformity, and other patient and surgeon factors.
Despite the frequent application of surgery for this disease, there is a paucity of high-quality data supporting operative management in patients with DCM.4 An underpowered randomized controlled trial provided evidence that outcomes in patients with mild to moderate myelopathy do not differ between conservative and surgical treatment arms.5,6 Conversely, other observational cohort studies with large sample sizes reveal superior neurological recovery following surgical intervention compared to conservative management.7,8
The primary objective of this study was to define the change in impairment, disability, and pain following surgical intervention in patients with DCM. Secondary objectives included to evaluate the impact of preoperative disease severity and duration of symptoms on outcomes and to summarize treatment complications associated with surgery. Specifically, key questions for this systematic review were the following:
Key Question 1: What are the expected functional, disability, and pain outcomes following surgical intervention for DCM?
Key Question 2: Do these expected outcomes depend on preoperative disease severity or duration of symptoms?
Key Question 3: What are the complications associated with surgical intervention?
Materials and Methods
Electronic Literature Search
A systematic review of the literature was conducted to identify relevant articles published up to May 18, 2015. Electronic databases (PubMed, EMBASE, Cochrane Collaboration library) and reference lists of key articles were searched to identify prospective studies on adult patients with DCM (cervical spondylotic myelopathy or ossification of the posterior longitudinal ligament [OPLL]) that evaluated outcomes or safety of operative treatment. For Key Question 1, we included studies that assessed functional, disability, and quality of life outcomes following surgical intervention. For Key Question 2, we identified studies that evaluated the impact of preoperative disease severity and duration of symptoms on postoperative outcomes. For Key Question 3, we searched for studies that summarized complications associated with surgery. Table 1 displays the PICO table and highlights the inclusion and exclusion criteria for this review. Studies were excluded if fewer than 50 patients were included, the follow-up was less than 1 year, arthroplasty procedures were performed, and the outcome metrics did not include modified Japanese Orthopaedic Association (mJOA), Neck Disability Index (NDI), or Nurick scores. For studies comparing operative and nonoperative outcomes, we only extracted data from the surgical arm. For studies that compared different types of surgery (eg, anterior vs posterior), we combined the results from both approaches. Studies that did not report primary outcomes but did analyze complications were included for Key Question 3. Two investigators (EDB, JRD) reviewed the full texts of potential articles to obtain a final collection of studies.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion | Exclusions | |
|---|---|---|
| Patient |
|
|
| Intervention |
|
|
| Comparison |
|
|
| Prognostic factors |
|
|
| Outcomes |
|
|
| Study design |
|
|
Abbreviations: ACDF, anterior cervical discectomy and fusion; CSM, cervical spondylotic myelopathy; JOA, Japanese Orthopaedic Association score; mJOA, modified Japanese Orthopaedic Association score; NDI, Neck Disability Index; OPLL, ossification of the posterior longitudinal ligament.
Data Extraction
We extracted the following data from the included articles: study design, patient demographics, diagnosis, surgical approach, preoperative and postoperative neurological status (mJOA, NDI, Nurick, Visual Analog Scale [VAS] scores), and complications. Preoperative disease severity and duration of symptoms were recorded when available. Each citation was independently reviewed by 2 of the authors.
Study Quality and Overall Strength of Body of Literature
The risk of bias was appraised by 2 of the study authors using the modified Newcastle-Ottawa Scale.9 The evaluation for risk of bias is included in the supplemental material (available in the online version of the article).
Next, the overall quality of evidence with respect to each outcome was determined based on methodology outlined by the Grades of Recommendation Assessment, Development and Evaluation (GRADE) Working Group10,11 and recommendations from the Agency for Healthcare Research and Quality.12,13
The overall body of evidence is considered “Low” if all studies are observational. The quality of the body of evidence may be upgraded or downgraded depending on a number of factors. Criteria for downgrading 1 or 2 levels include (1) inconsistency of results, (2) indirectness of evidence, or (3) imprecision of the effect estimates (eg, wide variance). Alternately, the body of evidence may be upgraded 1 or 2 levels based on (1) large magnitude of effect or (2) dose-response gradient.
A quality level of “High” indicates high confidence that the true effect lies close to the estimate of effect. A “Moderate” quality level reflects moderate confidence in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. A “Low” quality level represents limited confidence in the effect estimate; the true effect may be substantially different from the estimate of the effect.11 “Very Low” ratings indicate very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. This rating may be used if there is no evidence or if it is not possible to estimate an effect.
Data Analysis
Outcomes were stratified by follow-up timing as short term (less than 12 months), medium term (13-36 months), and long term (>36 months). Effect measures were first defined as mean differences (MD) with missing standard deviations imputed using the highest value from included studies. Standardized mean differences (SMDs) were computed to reflect the heterogeneity of outcome measures for JOA and mJOA scores that are correlated but not identical.14 Using Cohen’s criteria,15 an SMD of 0.2 to 0.49 represents a “small” effect, 0.5 to 0.79 a “moderate” effect, and 0.8 or greater a “large” effect. When it was necessary to merge multiple surgical cohorts from a single article, the weighted average scores were calculated and Cohen’s approximation16 for combining standard deviations was used. Results were further stratified by study quality based on risk of bias (high or low) to determine if there were qualitative and quantitative differences.
Results
Study Selection
The search strategy yielded 385 relevant citations, of which 312 were excluded based on their title and/or abstract. Seventy-seven studies were selected for full-text review. Forty-five of these were excluded for the following reasons: retrospective study design, sample included both radiculopathy and myelopathy, desired outcomes or preoperative and postoperative comparisons were not available, fewer than 50 patients, duplicated results from a separate study, or follow-up period less than 1 year. An overview of our selection process is summarized in Figure 1. Thirty-two studies ultimately met the inclusion criteria and are summarized in this review.17–48 Study characteristics are provided in Table 2; sample sizes ranged from 52 to 479 patients, mean ages ranged from 46 to 65 years and males comprised between 34% and 94% of the study populations. Disease pathoanatomy was also variable, with 9 studies including patients with OPLL (range = 6.6% to 33.6% of patients); of the remaining studies, 12 specifically excluded OPLL patients and 6 did not specify whether or not OPLL patients were included. Surgical approaches expectedly varied across studies: 7 included anterior decompression and fusion techniques only18,20,30,35,37,42,43,45; 12 included posterior decompression techniques that were either motion-preserving (laminectomy or laminoplasty) or used fusion24,25,27,28,31,33,34,36,39,40,44,46,47; and 8 studies incorporated both techniques.17,19,21–23,26,29,32,38,41,48
Figure 1.

Overview of search strategy.
Table 2.
Characteristic of Included Studies Evaluating Operative Treatment for Degenerative Cervical Myelopathy.
| Author (Year); Study Design | Operative Treatment | Patient Characteristicsa | Baseline Severity (Mean) | Symptom Duration (Mean) | Follow-up, % Followed | Outcome Measures |
|---|---|---|---|---|---|---|
| Bapat (2008); Prospective case series | Anterior or posterior decompression | N = 129 Mean age: 49.3 ± 11.4 years Male: 84.5% Diagnosis of OPLL: 0% | mJOA: 10.4 ± 3.33 | 5.1 ± 5.7 months | Mean 33.6 months (range 24-60) 77.7% (129/166) | • mJOA • Nurick • Safety |
| Chen (2013); RCT | ACDF with titanium (n = 29) or PEEK cages (n = 31) | N = 60 Mean age: 46.5 yearsb Male: 55% Diagnosis of OPLL: 0% | NR | NR | Mean 99.7 months (range 86-116) 75% (60/80) | • JOA score • NDI score |
| Cheung (2008); Prospective case series | ACDF using autologous ICBG (n = 27); laminoplasty (n = 28) | N = 55 Mean age: 64 ± 11 years Male: 63.6% Diagnosis of OPLL: unknown | JOA: 10.0 ± 3.5 | 17 ± 30 months | Mean 54 ± 26 months %NR | • JOA |
| Chibbaro (2009); Prospective case series | Multilevel oblique corpectomy without fusion | N = 268 Mean age: 58 years (range 29-83) Male: 60% Diagnosis of OPLL: unknown | mJOA: 8.1 NDI: 55.2 | 9.6 months (range 4-33) | 96 months %NR | • mJOA • NDI • Safety |
| Fehlings (2012)c; Prospective case series | ACDF or ACCF with instrumentation (n = 176); laminectomy and fusion or laminoplasty (n = 107); circumferential (n = 19) | N = 302 Mean age: 57 years (range 29-86) Male: 58.9% Diagnosis of OPLL: unknown | mJOA: 12.8b | NR | 24 months 91.1% (275/302) | • Safety (perioperative and delayed complications) |
| Fehlings (2013)c; Prospective case series | ACDF or ACCF with instrumentation (n = 169); laminectomy and fusion or laminoplasty (n = 95); circumferential (n = 14) | N = 278 Mean age: 56.3 ± 11.7 years Male: 59.4% Diagnosis of OPLL: unknown | mJOA: 12.9 (95% CI 12.5-13.2) Nurick: 3.1 ± 0.97 NDI: 41.8 ± 20.8 | 25.8 ± 45.5 months | 12 months 93.5% (260/278) | • mJOA • Nurick • NDI |
| Fehlings (2015); Prospective case series | Anterior (ACDF, corpectomy) (n = 276); posterior (laminectomy with or without instrumented fusion, laminoplasty) (n = 191); circumferential (n = 11) | N = 479 Mean age: 56.4 ± 11.9 years Male: 64.7% Diagnosis of OPLL: 28.2% | mJOA: 12.5 (95% CI 12.2-12.8) Nurick: 3.3 (95% CI 3.2-3.4) NDI: 36.4 (95% CI 34.3-38.4) | 27.0 ± 34.7 months | 12 months 84.3% (404/479) 24 months 81.6% (391/479) | • mJOA • Nurick • NDI • 30-meter walk test |
| Hirai (2011); Prospective comparative | ACCF (n = 39); laminoplasty using Miyazaki and Kirita method (n = 47) | N = 86 Mean age: 60.3 yearsb Male: 73.3% Diagnosis of OPLL: 0% | JOA: 9.8b | 10.8 monthsb | 60 months 91.5% (86/95) | • JOA • Safety |
| Hu (2014); Prospective comparative | Open-door laminoplasty with suture anchor (n = 30) or titanium miniplate (n = 25) fixation | N = 55 Mean age: 55.6 ± 4.1 years Male: 54.5% Diagnosis of OPLL: 0% | JOA: 8.3b | 17.6 ± 3.6 months | Mean 20.9 monthsb 82.1% (55/67) | • JOA • Safety |
| Kaneyama (2010); Prospective comparative | Open-door laminoplasty, (n = 73) or double-door laminoplasty (n = 73) | N = 146 Mean age: 64.1 yearsb Male: 73.9% Diagnosis of OPLL: 21.2% | JOA: 9.95b | NR | 2 weeks 100% (146/146) | • C5 palsy |
| Karpova (2013)c; Prospective case series | ACDF or ACCF (n = 42); laminectomy and fusion or laminoplasty (n = 17); and circumferential (n = 2) | N = 61 Mean age: 56.2 ± 11.9 years Male: 69% Diagnosis of OPLL: 6.6% | mJOA: 12.8 ± 2.7 | 21.1 ± 18.2 months | 12 months 94% (61/85) | • mJOA • Safety |
| Kimura (2014); Prospective case series | Double-door laminoplasty | N = 116 Mean age: 63.3 ±12.7 years Male: 67.2% Diagnosis of OPLL: 33.6% | JOA: 11 | 34.3 ± 47.2 months | Mean 35 ± 13 months 94.3% (116/123) | • JOA • Safety |
| Kommu (2014); Prospective case series | ACCF with plating (n = 14); laminectomy with (n = 5) and without (n = 40) instrumented fusion; laminoplasty (n = 3); circumferential (n = 1) | N = 63 Mean age: 51.1 years (range 30-80) Male: 77.8% Diagnosis of OPLL: 0% | Nurick: 2.82 | Motor symptoms: 7.1 months Sensory symptoms: 9.6 months | 24 months %NR | • Nurick • Safety |
| Lian (2010); RCT | Noncontiguous ACDF plus discectomy using PEEK cages or titanium mesh (n = 55); continuous ACDF using titanium mesh (n = 50) | N = 105 Mean age: 60.2 years (range 38-78) Male: 60% Diagnosis of OPLL: 0% | JOA: 8.9b | NR | Mean 31.5 months (range 24-48) 95.5% (105/110) | • JOA • VAS (pain) • Safety |
| Machino (2013); Prospective case series | Double-door laminoplasty | N = 520 Mean age: 62.2 years Male: 63.7% Diagnosis of OPLL: 0% | JOA: 10.4 ± 2.8 | 20.1 ± 32.0 months | Mean 33.3 months (range 12-90) 91.1% (520/571) | • JOA • Safety |
| Moussellard (2014); Prospective case series | ACCF (n = 7); ACDF (n = 4); laminectomy and fusion (n = 27); laminoplasty and fusion (n = 28); circumferential (n = 1) | N = 67 Mean age: 61.6 ± 12.1 years Male: 67% Diagnosis of OPLL: 0% | JOA: 11.5 ± 2.6 | 23.5 ± 25.3 months | 24 months %NR | • JOA • 30-meter walk test |
| Nakashima (2014); RCT | Open-door (n = 44) or French-door (n = 46) laminoplasty | N = 90 Mean age: 63.0 yearsb Male: 63.3% Diagnosis of OPLL: 23.3% | JOA: 9.0b | NR | Mean 28.9 monthsb 95.7% (90/92) | • JOA • Infection • C5 palsy |
| Ohashi (2014)/Katsumi (2012)d; Prospective comparative | En bloc laminoplasty with prophylactic bilateral C4-C5 foraminotomy | N = 121 Mean age: 64 ± 10.2 years Male: 71.9% Diagnosis of OPLL: 17.4% | JOA: 11.6 ± 2.7 | NR | 24 months 85.8% (121/141) | • JOA • VAS (pain) • Safety |
| Riew (2008); Prospective case series | ACDF (Prestige ST trial) | N = 52 Mean age: 46.0 ± 8.5 years Male: 40.4% Diagnosis of OPLL: 0% | NDI: 53.5 ± 16.9 | NR | 24 months 71.2% (37/52) | • NDI • VAS (pain) • Safety |
| Sasai (2003); Prospective comparative | Midsagittal laminoplasty with (n = 11) and without (n = 63) microcervical foraminotomy; en bloc laminoplasty with microcervical foraminotomy (n = 37) | N = 111 Mean age: 63 years Male: 61.3% Diagnosis of OPLL: 25.3% | JOA: 10.7b | NR | Postoperative (overall mean 39 months) 100% (111/111) | • C5 palsy |
| Setzer (2009); Prospective case series | ACDF using titanium cages | N = 60 Mean age: 61.5 ± 14.6 years Male: 66.7% Diagnosis of OPLL: unknown | mJOA: 10.32 ± 3.6 | 22.0 ± 30.5 months | Mean 18.8 ± 4.6 months %NR | • mJOA |
| Suri (2003); Prospective case series | ACDF/ACCF or laminectomy/laminoplasty | N = 146 Mean age: 47 years (range 17-76) Male: 79.5% Diagnosis of OPLL: unknown | Nurick: 3.19b | 11.7 months (range 1.5-120) | 24 months %NR | • Nurick |
| Suzuki (2009); Prospective comparative | Double-door laminoplasty | N = 98 Mean age: 59.6 years Male: 70.4% Diagnosis of OPLL: 20.4% | JOA: 11.1 ± 0.4 | 17.1 monthsb | Mean 81.6 months (range 60-144) 84.5% (98/116) | • JOA |
| Tanaka (2006); Prospective case series | Open-door laminoplasty; with additional foraminotomy (n = 18) | N = 62 Mean age: 64 years (range 32-89) Male: 75.8% Diagnosis of OPLL: 17.7% | JOA: 9.9 (range 5-15.5) | NR | 14 months (range 12-28) %NR | • JOA • Safety |
| Tetreault (2013)c; Prospective case series | Anterior (ACDF or ACCF with instrumentation) (n = 169); posterior (laminectomy and fusion or laminoplasty) (n = 95); circumferential (n = 14) | N = 272 Mean age: 56.5 ± 11.5 years Male: 58.8% Diagnosis of OPLL: 8.8% | mJOA: 12.7 ± 2.6 | 23.8 ± 34.6 months | 12 months 79.8% (217/272) | • mJOA |
| Thakar (2009, 2012); Prospective case series | Uninstrumented ACCF using iliac or fibular bone graft | N = 70 Mean age: 51.9 ± 9.9 years Male: 94.3% Diagnosis of OPLL: unknown | Nurick: 3.13 ± 0.91 | 15.2 ± 4.5 months | 18 months (range 12-22) %NR | • Nurick • VAS (pain) • Safety |
| Wang (2015); Prospective comparative | Double-door laminoplasty using Kurokawa method (n = 64) or modified Kurokawa method (n = 88) | N = 152 Mean age: 57.2 ± 19 years Male: 74.3% Diagnosis of OPLL: 0% | JOA: 8.91 ± 2.79 NDI: 25.52 ± 4.13 | NR | Mean 52 ± 33 months 100% (152/152) 36-60 months 53.3% (81/152) ≥60 months 46.7% (71/152) | • JOA • NDI • VAS (pain) |
| Ying (2007); RCT | ACCF with preserved posterior vertebral wall with ICBG or cages (n = 89); conventional ACCF with ICBG or cages (n = 89) | N = 178 Mean age: 49.0 yearsb Male: 65.7% Diagnosis of OPLL: 0% | JOA: 12.92b | NR | 12 months 78.7% (140/178) | • JOA • Safety |
| Yoshida (2013); Prospective case series | Expansive laminoplasty | N = 369 Mean age: 65 years (range 27-87) Male: 61.2% Diagnosis of OPLL: 0% | JOA: 9.2b | NR | Mean 54.1 months (range 24-97) 81.8% (369/451) | • JOA |
| Zong (2014); Prospective comparative | Posterior decompression (not specified) | N = 396 Mean age: 60.2 yearsb Male: 33.6% Diagnosis of OPLL: unknown | mJOA: 7.44b NDI: 42.97b | 12.8 monthsb | 24 months 64.9% (396/610) | • mJOA • NDI • VAS (pain) |
Abbreviations: ACCF, anterior cervical corpectomy and fusion; ACDF, anterior cervical discectomy and fusion; ICBG, iliac crest bone graft; JOA, Japanese Orthopaedic Association score; mJOA, modified Japanese Orthopaedic Association score; NDI, Neck Disability Index; NR, not reported; OPLL, ossification of the posterior longitudinal ligament; RCT, randomized controlled trial; VAS, visual analog scale for pain.
aWith the exception of Fehlings (2012, 2013, 2015), Riew (2008), Tetreault (2013), Wang (2015), and Ying (2007), demographics are reported only for patients that were analyzed (ie, those after loss-to-follow-up).
bFor studies that only provided demographic data stratified by treatment or other comparison groups, weighted means were calculated to provide an estimate for the entire study population.
cSame study population (multicenter AOSpine North American Study): Fehlings (2012) was included for perioperative and delayed complications; Fehlings (2013) included primary outcome results and provided data regarding the association of preoperative disease severity on change in impairment; Karpova (2013) analyzed data from a single center and provided data regarding the impact of preoperative symptom duration on change in impairment; and Tetreault (2013) provided supplemental modeling data using preoperative disease severity and symptom duration as predictors of achieving a mJOA ≥ 16.
dThis was a comparative study using a historical control. Only the treatment group enrolled prospectively is included in our analysis.
Effect of Surgery on Functional Impairment, Disability, and Pain
Japanese Orthopaedic Association Score
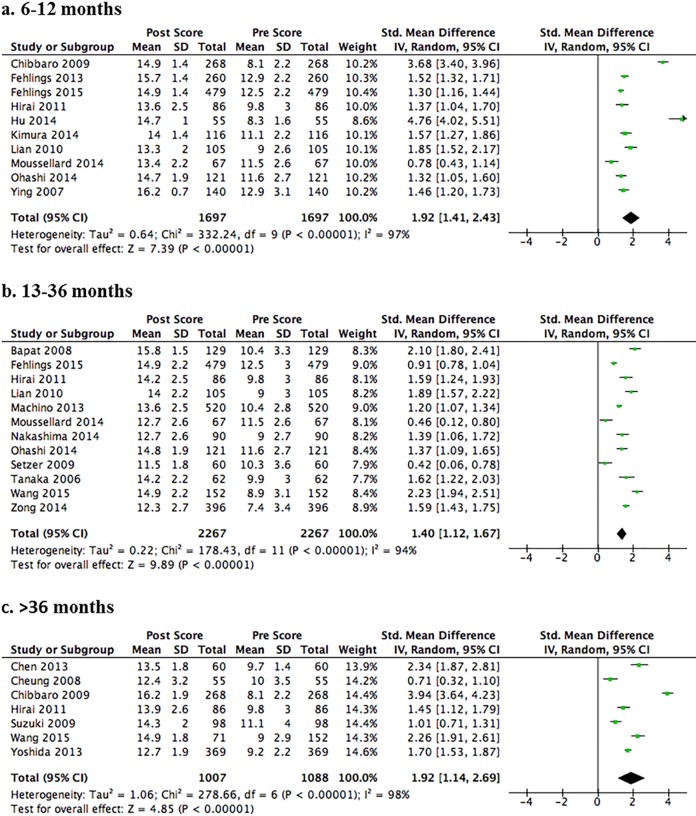
Twenty-one studies reported change in JOA or mJOA scores in patients with moderate to severe DCM (mean baseline JOA = 7.4 to 12.9); follow-up duration ranged from 12 to 98 months.17–20,22–24,28,30–34,37,39,40,44–48 The risk of bias was low or moderately low in 14 studies17,18,22,23,28,30,31,33,34,39,44–46,48 and moderately high in 7 because of undefined or low follow-up rates.19,20,24,32,37,40,47 A strong effect for improvement in function was observed at each time point (P < .001): the pooled SMD across 10 studies was 1.92 (95% confidence interval [CI] = 1.41 to 2.43) at 12-months follow-up (Figure 2a)20,22–24,28,30,32,34,45,48; 1.40 (95% CI = 1.12 to 1.67) across 12 studies at 13- to 36-months follow-up (Figure 2b)17,23,30–34,37,40,44,47,48; and 1.92 (95% CI = 1.41 to 2.69) across 7 studies at 36-months follow-up (Figure 2c).18–20,23,39,44,46
Figure 2.
Forest plots and meta-analysis of postoperative Japanese Orthopaedic Association (JOA) scores among patients with degenerative cervical myelopathy. Significant improvements in JOA scores are observed following surgery at short (Panel A, 6-12 months), medium (Panel B, 13-36 months), and long (Panel C, >36 months) term follow-up.
Neck Disability Index and Nurick Grade
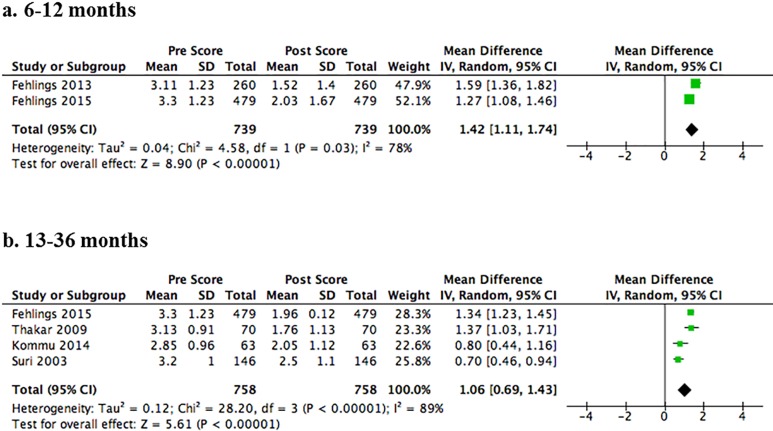
Seven studies assessed change in NDI scores (follow-up duration ranged from 12 to 99.7 months)18,20,22,35,44,47,48 and 5 studies evaluated change in Nurick grade (follow-up duration ranged from 12 to 24 months).22,29,38,42,48 The risk of bias was low or moderately low in 4 studies reporting NDI18,22,44,48 and in 2 studies reporting Nurick grade.22,48 The remaining studies were classified as high or moderately high risk of bias because of undefined or low follow-up rates.20,29,35,38,42,47 A strong effect for improvement was observed at each time point (P < .001) for both NDI and Nurick scores. For up to 12-months follow-up, the pooled MD for NDI was 18.02 (95% CI = 11.02 to 25.02) across 5 studies (Figure 3a)20,22,35,44,48 and 1.42 (95% CI = 1.11 to 1.74) for Nurick across 2 studies (Figure 4a).22,48 In the medium term (13-36 months), the pooled MD for NDI was 19.71 (14.01 to 25.42) across 5 studies (Figure 3b)20,35,44,47,48 and 1.06 (95% CI = 0.69 to 1.43) for Nurick across 4 studies (Figure 4b).29,38,42,48 In the long term (over 36 months), the pooled MD for NDI was 23.21 (95% CI = 11.84 to 34.58) across 2 studies (Figure 3c).18,20
Figure 3.
Forest plots and meta-analysis of postoperative Neck Disability Index (NDI) scores among patients with degenerative cervical myelopathy. Significant improvements in NDI scores are observed following surgery at short (Panel A, 6-12 months), medium (Panel B, 13-36 months), and long (Panel C, >36 months) term follow-up.
Figure 4.
Forest plots and meta-analysis of postoperative Nurick scores among patients with degenerative cervical myelopathy. Significant improvements in Nurick scores are observed following surgery at short (Panel A, 6-12 months) and medium (Panel B, 13-36 months) term follow-up.
Visual Analog Scale for Pain
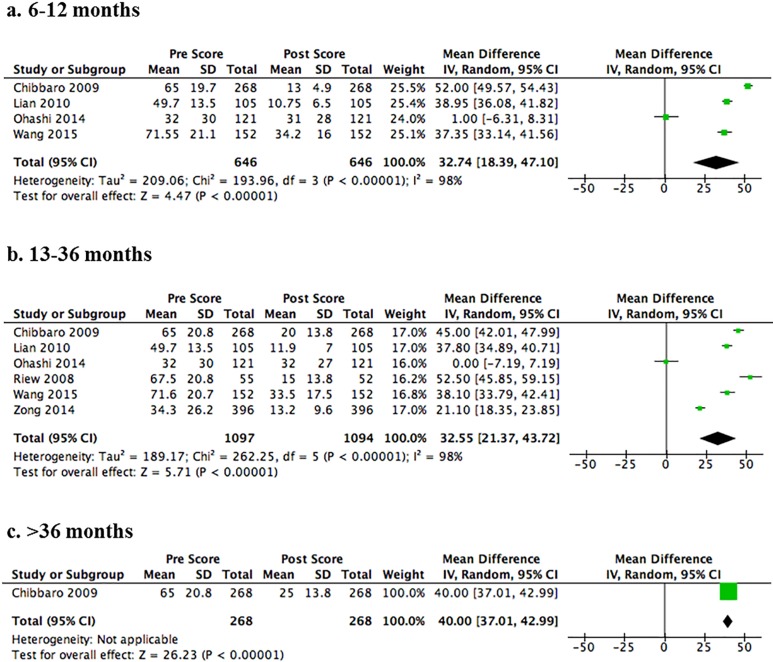
Five studies reported change in VAS scores with follow-up duration ranging from 6 to 72 months.20,30,34,44,47 The risk of bias was low or moderately low in 3 studies,30,34,44 and moderately high in 2 studies because of undefined or low follow-up rates20,47 and/or concerns regarding selection bias.47 A strong effect was seen for improvement in pain at each time point (P < .001): the pooled MD was 32.74 (95% CI = 18.39 to 47.10) across 4 studies at 12-months follow-up (Figure 5a)20,30,34,44 and 32.55 (95% CI = 21.37 to 43.72) across 6 studies at 13- to 36-months follow-up (Figure 5b).20,30,34,35,44,47 The MD from one study was 40.00 (95% CI = 37.01 to 42.99) at greater than 36-months follow-up (Figure 5c).20
Figure 5.
Forest plots and meta-analysis of postoperative Visual Analog Scale (VAS) scores among patients with degenerative cervical myelopathy. Significant improvements in VAS scores are observed following surgery at short (Panel A, 6-12 months), medium (Panel B, 13-36 months), and long (Panel C, >36 months) term follow-up.
Effect of Preoperative Duration of Symptoms and Myelopathy Severity on Surgical Outcomes
Four studies stratified their sample based on preoperative duration of symptoms (<6, 6-12, >12 months19; <12 or >12 months26,39; or <12, 12-24, and >24 months38) (Table 3). Patients in these subgroups exhibited similar functional improvements as assessed by JOA or mJOA scores.19,26,39 A fifth study by Tetreault et al evaluated whether duration of symptoms is a significant predictor of a postoperative mJOA score ≥16.41 Based on their results, the odds of achieving a mJOA ≥ 16 decreased by 22% when a patient moved from a shorter to a longer duration of symptoms group (≤3; >3, ≤6; >6, ≤12; >12, ≤24; >24 months) (adjusted odds ratio: 0.78; 95% CI = 0.61 to 0.997; P = .048).
Table 3.
The Impact of Preoperative Duration of Symptoms on Japanese Orthopaedic Association (JOA) and Nurick Outcomes at 3 Different Follow-up Periods.
| Author and Symptom Duration | n | Preoperative JOA Score (95% CI or ±SD) | JOA Change Score (95% CI) | Nurick Improved (%)a |
|---|---|---|---|---|
| Short-term F/U (12 months) | ||||
| Karpova (2013) | ||||
| <12 months | NR | 13.5b (12.6, 14.1) | 3.1b (2.5, 3.7) | |
| >12 months | NR | 11.9b | 2.6b (2.0, 3.2) | |
| Suzuki (2009)c | ||||
| <12 months | 65 | 11.2 ± 3.0 | 3.4 (2.1, 4.7) | |
| >12 months | 33 | 11.1 ± 3.0 | 2.8 (1.4, 4.2) | |
| Medium-term F/U (24-36 months) | ||||
| Suzuki (2009) | ||||
| <12 months | 65 | 11.2 ± 3.0 | 3.4 (2.4, 4.6) | |
| >12 months | 33 | 11.3 ± 3.0 | 2.7 (1.2, 3.8) | |
| Suri (2003) | ||||
| <12 months | 86 | 58.1% | ||
| 12-24 months | 31 | 71.0% | ||
| >24 months | 29 | 51.7% | ||
| Long-term F/U (54-60 months) | ||||
| Suzuki (2009) | ||||
| <12 months | 65 | 11.2 ± 3.0 | 3.4 (2.4, 4.6) | |
| >12 months | 33 | 11.2 ± 3.0 | 2.4 (1.2, 3.8) | |
| Cheung (2008) | ||||
| <6 months | NR | 8.6 ± 3.4 | 2.9 | |
| 6-12 months | NR | 10.3 ± 3.9 | 3.1 | |
| >12 months | NR | 10.6 ± 3.6 | 1.0 |
Abbreviations: CI, confidence interval; F/U, follow-up; JOA, Japanese Orthopaedic Association Score; NR, not reported; SD, standard deviation.
aProportion of patients that improved by 1 or more Nurick grades at follow-up.
bmJOA scores.
cValues estimated from Figure 4 in the Suzuki 2009 article; 95% confidence intervals calculated from estimate.
Two studies compared postoperative outcome in cohorts of varying preoperative myelopathy severities (Table 4).20,22 Two other studies examined the association between baseline severity scores and surgical outcomes.37,41 With respect to change in mJOA, Fehlings et al22 reported improvements across all levels of impairment; patients with severe myelopathy (mJOA < 12), however, demonstrated the greatest functional change (4.91, 95% CI = 4.34 to 5.49), whereas those with mild disease (mJOA ≥ 15) achieved the least amount of improvement (1.29, 95% CI = 0.70 to 1.87; Table 4). Conversely, Chibbaro et al20 reported that a similar percentage of patients with either moderate (mJOA = 10-13) or severe (mJOA = 5-9) myelopathy exhibited improvement on the mJOA scale at long-term follow-up (96 months). In a study by Tetreault et al,41 the odds of achieving a mJOA ≥ 16 at 1-year following surgery was 1.22 times greater for every 1-point increase in preoperative mJOA score. A complementary result was observed in a study by Setzer et al,37 who reported that patients with more severe preoperative impairment were less likely to exhibit a postoperative improvement of 2 or more points on the mJOA scale at 18-months follow-up (odds ratio = 0.72; 95% CI = 0.66 to 0.92).37
Table 4.
Modified Japanese Orthopedic Association, Neck Disability Index, and Nurick Outcomes Based on Preoperative Myelopathy Severity.
| Author | n | mJOA Change Score (95% CI) | Improved mJOA (%) | NDI Change Score (95% CI) | Nurick Change Score (95% CI) |
|---|---|---|---|---|---|
| Short-term F/U (12 months) | |||||
| Fehlings (2013) | |||||
| Mild (mJOA ≥15) | 78 | 1.29 (0.70, 1.87) | 12.05 (7.76, 16.34) | 1.54 (1.22, 1.86) | |
| Moderate (mJOA 12-14) | 105 | 2.58 (2.07, 3.09) | 9.79 (5.90, 13.68) | 1.51 (1.22, 1.81) | |
| Severe (mJOA <12) | 77 | 4.91 (4.34, 5.49) | 12.53 (8.05, 17.02) | 1.74 (1.41, 2.08) | |
| Long-term F/U (96 months) | |||||
| Chibbaro (2009) | |||||
| Moderate (mJOA 10-13) | 90 | 86.7 | |||
| Severe (mJOA 5-9) | 178 | 86.5 |
Abbreviations: CI, confidence interval; F/U, follow-up; mJOA, modified Japanese Orthopaedic Association Score; NDI, Neck Disability Index; NR, not reported; SD, standard deviation.
Complications
Pooled estimates of complications across included studies are presented in Table 5 in order of decreasing frequency. The cumulative incidence of complications is low (14.1%; 95% CI = 10.1% to 18.2%). Mortality was reported in 0.3% of patients (95% CI = 0.0% to 0.5%). Neurological complications included worsening of myelopathy (1.3%; 95% CI = 0.5% to 2.1%), laryngeal nerve injury/dysphagia (2.2%; 95% CI = 1.4% to 3.0%), and C5 radiculopathy or palsy (1.9%; 95% CI = 1.4% to 2.4%). Infection was infrequent and reported in 1.5% of patients (95% CI = 1.0% to 2.1%). Instrumentation or graft complication occurred in 2.0% of cases (95% CI = 1.3% to 2.7%).
Table 5.
The Cumulative Incidence of Complications.
| Complications | Number of Studies and References | n/N | Cumulative Incidence % | 95% CI |
|---|---|---|---|---|
| Axial pain | 3 [21, 30, 45] | 33/585 | 5.6 | 3.8, 7.5 |
| Laryngeal nerve injury/dysphagia | 8 [17, 20, 21, 23, 29, 30, 43, 45] | 26/1182 | 2.2 | 1.4, 3.0 |
| Instrumentation/graft complication | 9 [17, 18, 21, 24, 30, 35, 43, 45, 48] | 28/1411 | 2.0 | 1.3, 2.7 |
| C-5 radiculopathy/palsy | 15 [17, 20, 21, 23-25, 27, 28, 30, 31, 33, 36, 40, 43, 48] | 50/2661 | 1.9 | 1.4, 2.4 |
| Pseudarthrosis | 7 [17, 18, 21, 23, 30, 34, 45] | 17/954 | 1.8 | 0.9, 2.6 |
| Infection (deep and superficial) | 10 [20, 21, 24, 27, 29-31, 33, 43] | 32/2074 | 1.5 | 1.0, 2.1 |
| Adjacent segment disease (symptomatic) | 2 [17, 21] | 6/404 | 1.5 | 0.3, 2.7 |
| Reoperation/revision | 7 [20, 21, 23, 29, 30, 32, 35] | 13/943 | 1.4 | 0.6, 2.1 |
| Dural tear/CSF leak | 11 [20, 21, 24, 27, 28, 30-33, 43, 45] | 26/1893 | 1.4 | 0.8, 1.9 |
| Worsening of myelopathy | 2 [21, 48] | 10/781 | 1.3 | 0.5, 2.1 |
| Hematoma | 7 [21, 24, 30, 32, 43, 45, 48] | 11/1237 | 0.9 | 0.4, 1.4 |
| Radiculopathy/palsy (not C5) | 3 [21, 36, 43] | 4/464 | 0.9 | 0.0, 1.7 |
| Neurologic deterioration/new deficit | 3 [17, 21, 31] | 9/969 | 0.9 | 0.3, 1.5 |
| Delayed wound healing/dehiscence | 2 [21, 32] | 3/369 | 0.8 | 0.0, 1.7 |
| Dysphonia | 3 [21, 23, 48] | 6/867 | 0.7 | 0.1, 1.2 |
| Postoperative deformity | 2 [17, 21] | 2/404 | 0.5 | 0.0, 1.2 |
| Death | 6 [17, 18, 21, 30, 32, 48] | 3/1162 | 0.3 | 0.0, 0.5 |
| Stroke/transient ischemic attack | 3 [21, 31, 43] | 3/873 | 0.3 | 0.0, 0.7 |
| Esophageal injury | 2 [23, 30] | 0/191 | 0.0 | 0.0, 2.9 |
| Other | 7 [17, 20, 21, 23, 32, 43, 48] | 51/1382 | 3.7 | 2.7, 4.7 |
| Cardiopulmonary | 1 [21] | 10/302 | 3.3 | 1.3, 5.3 |
| Fracture | 1 [27] | 3/141 | 2.1 | 0.0, 4.5 |
| Bed sore | 1 [17] | 1/129 | 0.8 | 0.0, 2.3 |
| Spinal cord injury | 1 [24] | 0/55 | 0.0 | 0.0, 0.0 |
| Any complicationa | 1 [21] | 40/283 | 14.1 | 10.1, 18.2 |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; n, number of complications; N, total number of patients.
aOnly reported for anterior and posterior surgery; 19 patients who had circumferential decompression were not included.
Evidence Summary
The evidence summary is presented in Table 6.
Table 6.
Evidence Summary.
| Studies; N [References] | Strength of Evidence | Conclusions/Comments | |
|---|---|---|---|
| Key Question 1: What are the expected functional, disability and pain outcomes following surgical intervention for DCM? | |||
| JOA/mJOA | 6-12 months 10 studies [20, 22-24, 28, 30, 32, 34, 45, 48] (N = 1697) 13–36 months 12 studies [17, 23, 30-34, 37, 40, 44, 47, 48] (N = 2267) ≥36 months 7 studies [18-20, 23, 39, 44, 46] (N = 1088) | Moderatea | Surgical intervention resulted in improved JOA/mJOA scores at all time points assessed. Pooled standardized mean differences showed a large effect for improvement in function from baseline at short term (10 studies, SMD 1.92), medium term (12 studies, SMD 1.40), and long term (7 studies, SMD 1.92) follow-up. |
| NDI | 6-12 months 5 studies [20, 22, 35, 44, 48] (N = 1211) 13-36 months 5 studies [20, 35, 44, 47, 48] (N = 1347) ≥36 months 2 studies [18, 20] (N = 328) | Very Lowb | Surgical intervention resulted in improved NDI scores at all time points assessed. Pooled mean differences showed clinically meaningful improvement in disability from baseline at short term (5 studies, MD 18.02), medium term (5 studies, MD 19.71), and long term (2 studies, MD 23.21) follow-up. |
| Nurick | 6-12 months 2 studies [22, 48] (N = 739) 13-36 months 4 studies [29, 38, 43, 48] (N = 758) | Low | Surgical intervention resulted in improved Nurick scores at short-term and medium-term assessments. Pooled mean differences showed clinically meaningful improvement in disability from baseline at short term (2 studies, MD 1.42) and medium term (4 studies, MD 1.06) follow-up. |
| Pain (VAS 100-point scale) | 6-12 months 4 studies [20, 30, 34, 44] (N = 646) 13-36 months 6 studies [20, 30, 34, 35, 44, 47] (N = 1097) ≥36 months 1 study [20] (N = 268) | Moderatea 6-12 months Very Lowb 13-36 and ≥36 months | Surgical intervention resulted in improved pain scores at all time points assessed. Pooled mean differences showed clinically meaningful improvement in pain from baseline at short term (4 studies, MD 32.7), medium term (6 studies, MD 32.5), and long term (1 study, MD 40.0) follow-up. |
| Key Question 2: Do these expected outcomes depend on preoperative disease severity or duration of symptoms? | |||
| Preoperative Duration of Symptoms | |||
| JOA improvement | 12 months 2 studies [26, 39] (N = NC) 24-36 months 1 study [39] (N = 98) 54-60 months 2 studies [19, 39] (N = NC) | Very Lowc | Change in neurological status following surgical intervention does not depend on preoperative duration of symptoms. Patients with a longer duration of symptoms (≥12 months) had similar neurological improvement as those with a shorter duration of symptoms (<12 months) at all follow-up periods. |
| Success (mJOA ≥16) | 1 study [41] (N = 272) F/U 12 months | Low | The odds of a “successful” outcome following surgical intervention depend on preoperative duration of symptoms. The odds of achieving a mJOA ≥16 decreased by 22% when a patient moves from a shorter to a longer duration of symptoms group (≤3; >3, ≤6; >6, ≤12; >12, ≤24; >24 months). |
| Preoperative Myelopathy Severity | |||
| JOA improvement | 1 study [22] (N = 260) F/U 12 months | Moderated | Change in neurological status following surgical intervention depends on preoperative disease severity. Less improvement on the mJOA was observed among patients with milder symptoms at presentation (preoperative mJOA ≥15: 1.29 [95% CI 0.70, 1.87]; preoperative mJOA 12-14: 2.58 [2.07, 3.09]; preoperative mJOA <12: 4.91 [4.34, 5.49]). |
| Success mJOA ≥16 at F/U; 2-point improvement in mJOA | 2 studies [37, 41] (N = 332) F/U 12 months | Low | The odds of a “successful” outcome following surgical intervention depends on preoperative disease severity. The odds of achieving a mJOA ≥16 were 1.22 times greater for every 1-point increase in preoperative mJOA score. |
| % improved mJOA | 1 study [20] (N = 268) F/U 96 months | Very lowb | Likelihood of neurological improvement following surgical intervention does not depend on preoperative disease severity. Similar fractions of patients improved by 1 mJOA point when comparing patients with moderate (mJOA 10-13, 86.7%) and severe (mJOA 5-9, 86.5%) disease. |
| NDI improvement | 1 study [22] (N = 260) F/U 12 months | Very lowc | Similar improvements in NDI across preoperative disease severities following surgical intervention. At 12-months follow-up, NDI changes were 12.1 (mild mJOA ≥15), 9.8 (moderate mJOA 12-14), and 12.5 (severe mJOA <12). |
| Nurick improvement | 1 study [22] (N = 260) F/U 12 months | Very lowc | Similar improvements in Nurick scores across preoperative disease severities following surgical intervention. At 12-months follow-up, the Nurick score changes were 1.6 (mild mJOA ≥15), 1.5 (moderate mJOA 12-14), and 1.7 (severe mJOA <12). |
| Key Question 3: What are the complications associated with surgical intervention? | |||
| C5 radiculopathy or palsy | 15 studies [17, 20, 21, 23-25, 27, 28, 30-32, 36, 40, 42, 48] (N = 2661) | Low | Pooled cumulative incidence of C5 radiculopathy or palsy is 1.9% (95% CI 1.4, 2.4). |
| Infection | 10 studies [20, 21, 24, 27, 29-31, 33, 42, 48] (N = 2074) | Low | Pooled cumulative incidence of infection is 1.5% (95% CI 1.0, 2.1) |
| Reoperation | 7 studies [20, 21, 23, 29, 30, 32, 35] (N = 943) | Very lowe | Pooled cumulative incidence of reoperation 1.4% (95% CI 0.6, 2.1) |
| Dural tear/CSF leak | 11 studies [20, 21, 24, 27, 28, 30-34, 43, 45] (N = 1893) | Very lowc | Pooled cumulative incidence of dural tear is 1.4% (95% CI 0.8, 1.9) |
| Worsening of myelopathy | 2 studies [21, 48] (N = 781) | Very lowc | Pooled cumulative incidence of worsening of myelopathy is 1.3% (95% CI 0.5, 2.1) |
| Death | 6 studies [17, 18, 21, 30, 31, 48] (N = 1162) | Very lowc | Pooled cumulative incidence of mortality is 0.3% (95% CI 0.0, 0.5) |
| Fracture | 1 study [27] (N = 141) | Very lowc | Pooled cumulative incidence of fracture is 2.1% (95% CI 0.0, 4.5) |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; F/U, follow-up; JOA, Japanese Orthopaedic Association score; mJOA, modified Japanese Orthopaedic Association score; NDI, Neck Disability Index.
aUpgraded 1 for magnitude of effect.
bDowngraded 1 for serious risk of bias.
cDowngraded 1 for serious risk of imprecision.
dUpgraded 1 for dose response.
eDowngraded 1 for serious risk of bias and imprecision.
Effect of Surgery on Functional Impairment, Disability and Pain
There is moderate strength of evidence that surgical intervention for DCM results in significant improvements in function at short-, medium-, and long-term follow-up as assessed by JOA or mJOA scores. Clinically meaningful improvements in both the NDI and Nurick scores were also observed following surgery. However, the strength of evidence for these findings was rated as very low for NDI and low for Nurick score. Finally, based on moderate evidence, surgical intervention results in significant improvements in pain scores at 6- to 12-months follow-up.
Effect of Preoperative Duration of Symptoms and Myelopathy Severity on Surgical Outcomes
There is very low strength of evidence that preoperative duration of symptoms is not associated with surgical outcomes, evaluated by change in JOA or mJOA scores between patients with a duration of symptoms greater than or less than 12 months. There is low strength of evidence that the odds of achieving a postoperative mJOA ≥ 16 decrease as duration of symptoms increases.
There is moderate strength of evidence that improvements on the mJOA are less for patients with mild myelopathy than those with moderate or severe disease. There is low strength of evidence that the odds of achieving a postoperative mJOA ≥ 16 decrease as preoperative myelopathy severity increases. Based on very low strength of evidence, improvement in NDI and Nurick scores do not depend on preoperative disease severity.
Complications
Surgical complications occur infrequently in patients treated surgically for DCM. C5 radiculopathy or palsy and surgical site infection were reported in fewer than 2% of patients. The strength of evidence for these estimates is low. The cumulative incidence of reoperation, dural tears, worsening of myelopathy, and death was less than 1.5% and was 2% for instrumentation failure. The strength of evidence to support these estimates is very low.
Discussion
The management of DCM remains controversial with limited evidence available to the clinician and patient to make informed decisions. The only randomized controlled trial5,6 concluded that there is no difference in mJOA scores between patients with “milder” DCM (mJOA ≥ 12) that receive operative versus nonoperative care. This is in contrast to results reported in other observational studies,7,8 which suggest that surgery leads to superior functional recovery. These conflicting results highlight the importance of patient selection and identifying ideal surgical candidates. Further controversy arises because there are a variety of surgical techniques available to the surgeon to accomplish the objectives of spinal cord decompression, spinal column stabilization, and, if necessary, spinal realignment. Based on a study by Fehlings et al, patients treated anteriorly or posteriorly exhibit similar improvements22; however, selection criteria between approaches remain controversial and is the topic of a current randomized controlled trial of ventral versus dorsal surgery.49
There is a lack of evidence on the comparative effectiveness of surgery and nonoperative treatment in patients with DCM. As a result, this systematic review aims to summarize data from prospective studies that report changes in outcomes and rates of complications associated with surgical intervention.
The first objective of this review was to determine the change in functional impairment, disability, and pain following surgical management. Based on our conclusions, there is moderate evidence to suggest that surgery results in significant gains in mJOA or JOA scores at short-, medium-, and long-term follow-up. These reported improvements exceed the minimum clinically important difference for this metric,50,51 suggesting that patients undergoing surgery are expected to exhibit functional gains in addition to disease stabilization. Furthermore, based on low evidence, surgery also results in significant improvements in Nurick scores at short- and medium-term follow-up. The mJOA has been adopted as the standard for evaluating functional impairment in patients with DCM; this scale is responsive to change, especially in patients with moderate and severe myelopathy.52 Reported improvements on the Nurick Grade were less than those observed on the mJOA; however, this is likely because this scale only has 6 categories and because it is inherently less sensitive.
The second objective of this review was to evaluate whether duration of symptoms or preoperative myelopathy severity were associated with surgical outcomes. Based on our findings, operative management results in significant functional improvements in patients with both a “short” and “long” duration of symptoms and in patients with mild, moderate, and severe disease. The clinical prediction rule proposed by Tetreault et al,41 however, suggested that the likelihood of achieving a postoperative mJOA ≥ 16 decreases with a longer duration of symptoms and a lower baseline severity score. The authors of this study acknowledged that patients with severe myelopathy are, in part, less likely to attain a postoperative mJOA ≥ 16 as this would require a ≥5-point improvement; to address this limitation, a second model was developed to predict a postoperative mJOA ≥ 12 and also included baseline severity score as a relevant factor.48 Future studies are required to validate these associations and should use outcome measures that are better suited to assess patients with mild myelopathy. In a recent review, Kalsi-Ryan et al53 suggested that ancillary measures be used in combination with the mJOA or JOA scale to assess impairment in patients with DCM; these include tools that focus on upper extremity function (Graded Redefined Assessment of Strength Sensibility and Prehension and grip dynamometer), gait (GAITRite analysis and 30-meter walk test), and balance (Berg Balance Score).
The third objective of this review was to evaluate the incidence of surgical complications. The quality of evidence for our conclusions was low to very low, due to risk of bias and imprecision. The imprecision of our estimates likely results from the variability of definitions across studies; this highlights a major knowledge gap and a need to develop a classification system for surgical complications. Nevertheless, reported cumulative incidences were low for perioperative mortality (0.3%), recurrent laryngeal nerve injury or dysphagia (2.2%), and C5 radiculopathy or palsy (1.9%). Nationwide estimates of perioperative morbidity have previously been presented by Shamji et al54,55; their results indicated that, among 8548 patients with diffuse cervical spondylosis, rates of mortality were 0.33% and 0.69% in patients approached anteriorly and posteriorly, respectively. Dysphagia was reported in 3.63% of patients approached anteriorly and 1.87% of patients approached posteriorly. Posterior surgery was associated with a more frequent need for transfusion (1.38% anteriorly, 7.20% posteriorly) and a higher rate of clinically significant hematoma (0.80% anteriorly, 2.12% posteriorly).
The cost-effectiveness of surgery must also be considered when developing treatment protocols. Two studies were identified that evaluated the cost-utility of surgery in Canadian patients enrolled in the AOSpine North America and/or International studies. The first study (based on the North America study) estimated the cost of surgery to be $21 066.44, with an incremental cost-utility ratio (ICUR) of $32 916 per quality adjusted life year (QALY).56 A second study by Witiw et al (based on the North America and International studies) conducted a more rigorous cost-utility analysis using a 2-arm, Markov State Transition model where values for subjects undergoing surgery were compared with estimated counterfactual outcomes of initial conservative management.57 In a primary model, the lifetime ICUR was determined to be $11 496/QALY gained for surgical intervention, an estimate considered very cost-effective according to criteria defined by the World Health Organization. Further testing using a Monte Carlo probabilistic sensitivity analysis revealed that 97.9% of estimates fell within the WHO threshold, suggesting robustness to variability in the parameter estimates. To supplement this testing, a highly conservative assumption that individuals undergoing initial nonoperative management would not experience any neurologic decline over their lifetime was added to the model. In this scenario, the ICUR was calculated as $20 548/QALY gained with 94.7% of estimates falling within the WHO threshold; this finding further supports the cost-effectiveness of surgical intervention. Unfortunately, these analyses only explored the cost-effectiveness of surgery in Canada and did not stratify their sample based on preoperative myelopathy severity.
Limitations
A substantial limitation of this systematic review is that the impact of surgical approach on recovery was not studied. Surgical interventions vary in both approach (anterior or posterior) and objectives (decompression, stabilization, realignment); as a result, data may be too heterogeneous to conduct a meta-analysis. Among anterior surgical approaches, a previous systematic review58 demonstrated heterogeneous neurological, pain, and alignment outcomes among multiple discectomy, corpectomy, and hybrid procedures. Among posterior surgical approaches, a previous systematic review by Yoon et al59 reported that both laminoplasty and laminectomy and fusion procedures result in functional improvement. Unfortunately, the comparative effectiveness and safety of these and other approaches have not been rigorously addressed.
Conclusions
Based on our conclusions, in patients with DCM, surgery prevents further disease progression and also results in significant gains in functional impairment, disability, and pain. A shorter duration of symptoms and less severe myelopathy preoperatively are both important predictors of achieving a postoperative mJOA ≥ 16. Finally, surgery for DCM is a relatively safe treatment option, with a cumulative incidence of complications estimated at 14.1%.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by AOSpine and also received funding from the Cervical Spine Research Society (CSRS). Dr Fehlings wishes to acknowledge support from the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration and the DeZwirek Family Foundation. Dr Tetreault acknowledges support from a Krembil Postdoctoral Fellowship Award.
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Cowan JA, Jr, Dimick JB, Wainess R, et al. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59:15–20. doi:10.1227/01.NEU.0000219836.54861.CD. [DOI] [PubMed] [Google Scholar]
- 2. Patil PG, Turner DA, Pietrobon R. National trends in surgical procedures for degenerative cervical spine disease: 1990-2000. Neurosurgery. 2005;57:753–758. [PubMed] [Google Scholar]
- 3. Rhee JM, Shamji MF, Erwin WM, et al. Nonoperative management of cervical myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38:S55–S67. doi:10.1097/BRS.0b013e3182a7f41d. [DOI] [PubMed] [Google Scholar]
- 4. Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40:E675–E693. doi:10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 5. Kadanka Z, Bednarik J, Novotny O, Urbánek I, Dušek L. Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur Spine J. 2011;20:1533–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadanka Z, Bednarik J, Vohanka S, et al. Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur Spine J. 2000;9:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine (Phila Pa 1976). 2000;25:670–676. [DOI] [PubMed] [Google Scholar]
- 8. Yoshimatsu H, Nagata K, Goto H, et al. Conservative treatment for cervical spondylotic myelopathy. Prediction of treatment effects by multivariate analysis. Spine J. 2001;1:269–273. [DOI] [PubMed] [Google Scholar]
- 9. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 2, 2017.
- 10. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 12. West S, King V, Carey TS, et al. Systems to Rate the Strength of Scientific Evidence (Evidence Report/Technology Assessment No. 47). Rockville, MD: Agency for Healthcare Research and Quality; 2002. [PMC free article] [PubMed] [Google Scholar]
- 13. Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews (AHRQ Publication No. 10(14)-EHC063-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. http://www.effectivehealthcare.ahrq.gov/. Accessed May 2, 2017. [PubMed] [Google Scholar]
- 14. Kato S, Oshima Y, Oka H, et al. Comparison of the Japanese Orthopaedic Association (JOA) score and Modified JOA (mJOA) score for the assessment of cervical myelopathy: a multicenter observational study. PLoS One. 2015;10:e0123022 doi:10.1371/journal.pone.0123022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang MH, Fossel AH, Larson MG. Comparisons of five health status instruments for orthopedic evaluation. Med Care. 1990;28:632–642. [DOI] [PubMed] [Google Scholar]
- 16. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 17. Bapat MR, Chaudhary K, Sharma A, Laheri V. Surgical approach to cervical spondylotic myelopathy on the basis of radiological patterns of compression: prospective analysis of 129 cases. Eur Spine J. 2008;17:1651–1663. doi:10.1007/s00586-008-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J. 2013;22:1539–1546. doi:10.1007/s00586-013-2772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheung WY, Arvinte D, Wong YW, et al. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy: a prospective study. Int Orthop. 2008;32:273–278. doi:10.1007/s00264-006-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chibbaro S, Mirone G, Makiese O, George B. Multilevel oblique corpectomy without fusion in managing cervical myelopathy: long-term outcome and stability evaluation in 268 patients. J Neurosurg Spine. 2009;10:458–465. doi:10.3171/2009.1.spine08186. [DOI] [PubMed] [Google Scholar]
- 21. Fehlings MG, Smith JS, Kopjar B, et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. J Neurosurg Spine. 2012;16:425–432. doi:10.3171/2012.1.spine11467. [DOI] [PubMed] [Google Scholar]
- 22. Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651–1658. doi:10.2106/jbjs.l.00589. [DOI] [PubMed] [Google Scholar]
- 23. Hirai T, Okawa A, Arai Y, et al. Middle-term results of a prospective comparative study of anterior decompression with fusion and posterior decompression with laminoplasty for the treatment of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2011;36:1940–1947. doi:10.1097/BRS.0b013e3181feeeb2. [DOI] [PubMed] [Google Scholar]
- 24. Hu W, Shen X, Sun T, Zhang X, Cui Z, Wan J. Laminar reclosure after single open-door laminoplasty using titanium miniplates versus suture anchors. Orthopedics. 2014;37:e71–e78. [DOI] [PubMed] [Google Scholar]
- 25. Kaneyama S, Sumi M, Kanatani T, et al. Prospective study and multivariate analysis of the incidence of C5 palsy after cervical laminoplasty. Spine (Phila Pa 1976). 2010;35:E1553–E1558. doi:10.1097/BRS.1550b1013e3181ce1873d. [DOI] [PubMed] [Google Scholar]
- 26. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38:392–400. doi:10.1097/BRS.0b013e3182715bc3. [DOI] [PubMed] [Google Scholar]
- 27. Katsumi K, Yamazaki A, Watanabe K, Ohashi M, Shoji H. Can prophylactic bilateral C4/C5 foraminotomy prevent postoperative C5 palsy after open-door laminoplasty? A prospective study. Spine (Phila Pa 1976). 2012;37:748–754. doi:10.1097/BRS.1090b1013e3182326957. [DOI] [PubMed] [Google Scholar]
- 28. Kimura A, Endo T, Inoue H, Seichi A. Preoperative predictors of patient satisfaction with outcome after cervical laminoplasty. Global Spine J. 2014;4:77–82. doi:10.1055/s-0034-1366973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kommu R, Sahu BP, Purohit AK. Surgical outcome in patients with cervical ossified posterior longitudinal ligament: a single institutional experience. Asian J Neurosurg. 2014;9:196–202. doi:10.4103/1793-5482.146602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lian XF, Xu JG, Zeng BF, Zhou W, Kong WQ, Hou TS. Noncontiguous anterior decompression and fusion for multilevel cervical spondylotic myelopathy: a prospective randomized control clinical study. Eur Spine J. 2010;19:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Machino M, Yukawa Y, Hida T, et al. Modified double-door laminoplasty in managing multilevel cervical spondylotic myelopathy: surgical outcome in 520 patients and technique description. J Spinal Disord Tech. 2013;26:135–140. doi:10.1097/BSD.0b013e31823d848b. [DOI] [PubMed] [Google Scholar]
- 32. Moussellard HP, Meyer A, Biot D, Khiami F, Sariali E. Early neurological recovery course after surgical treatment of cervical spondylotic myelopathy: a prospective study with 2-year follow-up using three different functional assessment tests. Eur Spine J. 2014;23:1508–1514. doi:10.1007/s00586-014-3315-x. [DOI] [PubMed] [Google Scholar]
- 33. Nakashima H, Kato F, Yukawa Y, et al. Comparative effectiveness of open-door laminoplasty versus French-door laminoplasty in cervical compressive myelopathy. Spine (Phila Pa 1976). 2014;39:642–647. doi:10.1097/brs.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 34. Ohashi M, Yamazaki A, Watanabe K, Katsumi K, Shoji H. Two-year clinical and radiological outcomes of open-door cervical laminoplasty with prophylactic bilateral C4-C5 foraminotomy in a prospective study. Spine (Phila Pa 1976). 2014;39:721–727. doi:10.1097/brs.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 35. Riew KD, Buchowski JM, Sasso R, Zdeblick T, Metcalf NH, Anderson PA. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am. 2008;90:2354–2364. doi:10.2106/jbjs.g.01608. [DOI] [PubMed] [Google Scholar]
- 36. Sasai K, Saito T, Akagi S, Kato I, Ohnari H, Iida H. Preventing C5 palsy after laminoplasty. Spine (Phila Pa 1976). 2003;28:1972–1977. doi:10.1097/01.brs.0000083237.94535.46. [DOI] [PubMed] [Google Scholar]
- 37. Setzer M, Vrionis FD, Hermann EJ, Seifert V, Marquardt G. Effect of apolipoprotein E genotype on the outcome after anterior cervical decompression and fusion in patients with cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:659–666. doi:10.3171/2009.7.spine08667. [DOI] [PubMed] [Google Scholar]
- 38. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33–45. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34:2874–2879. doi:10.1097/BRS.0b013e3181bb0e33. [DOI] [PubMed] [Google Scholar]
- 40. Tanaka N, Nakanishi K, Fujiwara Y, Kamei N, Ochi M. Postoperative segmental C5 palsy after cervical laminoplasty may occur without intraoperative nerve injury: a prospective study with transcranial electric motor-evoked potentials. Spine (Phila Pa 1976). 2006;31:3013–3017. doi:10.1097/01.brs.0000250303.17840.96. [DOI] [PubMed] [Google Scholar]
- 41. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95:1659–1666. doi:10.2106/JBJS.L.01323. [DOI] [PubMed] [Google Scholar]
- 42. Thakar S, Christopher S, Rajshekhar V. Quality of life assessment after central corpectomy for cervical spondylotic myelopathy: comparative evaluation of the 36-Item Short Form Health Survey and the World Health Organization Quality of Life–Brief. J Neurosurg Spine. 2009;11:402–412. doi:10.3171/2009.4.spine08749. [DOI] [PubMed] [Google Scholar]
- 43. Thakar S, Rajshekhar V. Evaluation of pain as a preference-based health status measure in patients with cervical spondylotic myelopathy undergoing central corpectomy. Acta Neurochir. 2012;154:335–340. doi:10.1007/s00701-011-1229-5. [DOI] [PubMed] [Google Scholar]
- 44. Wang L, Wei FX, Liu S, et al. Can modified Kurokawa’s double-door laminoplasty reduce the incidence of axial symptoms at long-term follow-up? A prospective study of 152 patients with cervical spondylotic myelopathy. J Spinal Disord Tech. 2015;28:E186–E193. [DOI] [PubMed] [Google Scholar]
- 45. Ying Z, Xinwei W, Jing Z, et al. Cervical corpectomy with preserved posterior vertebral wall for cervical spondylotic myelopathy: a randomized control clinical study. Spine (Phila Pa 1976). 2007;32:1482–1487. doi:10.1097/BRS.0b013e318068b30a. [DOI] [PubMed] [Google Scholar]
- 46. Yoshida G, Kanemura T, Ishikawa Y, et al. The effects of surgery on locomotion in elderly patients with cervical spondylotic myelopathy. Eur Spine J. 2013;22:2545–2551. doi:10.1007/s00586-013-2961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zong Y, Xue Y, Zhao Y, et al. Depression contributed an unsatisfactory surgery outcome among the posterior decompression of the cervical spondylotic myelopathy patients: a prospective clinical study. Neurol Sci. 2014;35:1373–1379. doi:10.1007/s10072-014-1714-8. [DOI] [PubMed] [Google Scholar]
- 48. Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976). 2015;40:1322–1328. doi:10.1097/BRS.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 49. Ghogawala Z, Benzel EC, Heary RF, et al. Cervical spondylotic myelopathy surgical trial: randomized, controlled trial design and rationale. Neurosurgery. 2014;75:334–346. doi:10.1227/NEU.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Auffinger BM, Lall RR, Dahdaleh NS, et al. Measuring surgical outcomes in cervical spondylotic myelopathy patients undergoing anterior cervical discectomy and fusion: assessment of minimum clinically important difference. PLoS One. 2013;8:e67408 doi:10.1371/journal.pone.0067408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tetreault L, Nouri A, Kopjar B, Côté P, Fehlings MG. The minimum clinically important difference of the modified Japanese Orthopaedic Association Scale in patients with degenerative cervical myelopathy. Spine (Phila Pa 1976). 2015;40:1653–1659. doi:10.1097/BRS.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 52. Kopjar B, Tetreault L, Kalsi-Ryan S, Fehlings M. Psychometric properties of the modified Japanese Orthopaedic Association Scale in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2015;40:E23–E28. doi:10.1097/BRS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 53. Kalsi-Ryan S, Singh A, Massicotte EM, et al. Ancillary outcome measures for assessment of individuals with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S111–S122. doi:10.1097/BRS.0b013e3182a7f499. [DOI] [PubMed] [Google Scholar]
- 54. Shamji MF, Cook C, Tackett S, Brown C, Isaacs RE. Impact of preoperative neurological status on perioperative morbidity associated with anterior and posterior cervical fusion. J Neurosurg Spine. 2008;9:10–16. doi:10.3171/SPI/2008/9/7/010. [DOI] [PubMed] [Google Scholar]
- 55. Shamji MF, Cook C, Pietrobon R, Tackett S, Brown C, Isaacs RE. Impact of surgical approach on complications and resource utilization of cervical spine fusion: a nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J. 2009;9:31–38. doi:10.1016/j.spinee.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 56. Fehlings MG, Jha NK, Hewson SM, Massicotte EM, Kopjar B, Kalsi-Ryan S. Is surgery for cervical spondylotic myelopathy cost-effective? A cost-utility analysis based on data from the AOSpine North America prospective CSM study. J Neurosurg Spine. 2012;17:89–93. doi:10.3171/2012.6.aospine111069. [DOI] [PubMed] [Google Scholar]
- 57. Witiw CD, Tetreault LA, Smieliauskas F, Kopjar B, Massicotte EM, Fehlings MG. Surgery for degenerative cervical myelopathy: a patient centered quality of life and health economic evaluation. Spine J. 2017;17:15–25. doi:10.1016/j.spinee.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 58. Shamji MF, Massicotte EM, Traynelis VC, Norvell DC, Hermsmeyer JT, Fehlings MG. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl 1): S195–S209. doi:10.1097/BRS.0b013e3182a7eb27. [DOI] [PubMed] [Google Scholar]
- 59. Yoon ST, Hashimoto RE, Raich A, Shaffrey CI, Rhee JM, Riew KD. Outcomes after laminoplasty compared with laminectomy and fusion in patients with cervical myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S183–S194. doi:10.1097/BRS.0b013e3182a7eb7c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.